Abstract
Objective
It is known that hyperplastic polyps are more difficult to detect than adenomatous polyps at CT colonography (CTC) and it has been theorized that this is because hyperplastic polyps are flatter. Using automated software that computes polyp height, we determined whether hyperplastic colonic polyps on CTC are indeed flatter than adenomatous polyps of comparable width.
Materials and Methods
1186 screening patients at 3 medical centers underwent oral contrast-enhanced CTC and same-day optical colonoscopy (OC) with segmental unblinding. 185 of the patients had at least one hyperplastic or adenomatous polyp 6–10 mm in size visible at CTC, where size was determined by a calibrated guidewire at OC. To assess flatness, the heights of the polyps at CTC were measured using a validated automated software program. Heights and height-to-width ratios of the hyperplastic polyps were compared to those of the adenomatous polyps using a t-test (two-tailed, unpaired, unequal variance).
Results
There were 176 adenomatous and 83 hyperplastic polyps visible at segmentally unblinded OC. The fraction of these polyps that were measurable at CTC using the automated software was not significantly different for adenomatous versus hyperplastic polyps (158/176, 89.8% versus 73/83, 83.9%, p=0.2). The average height-to-width ratios using automated width measurements were 15% less for hyperplastic polyps: 0.39±0.20 (n=158) and 0.33±0.19 (n=73) for adenomatous and hyperplastic polyps, respectively (p=0.03). When polyps of comparable OC size or CTC width were considered, the heights of hyperplastic polyps were up to 27% less than those of adenomatous polyps.
Conclusions
For 6–10 mm polyps of a given size as determined by optical colonoscopy or a given width at CT colonography, hyperplastic polyps tend to be flatter (i.e., have lower height) compared to adenomatous polyps.
Keywords: CT, colon, Colon cancer, Automated size measurement
INTRODUCTION
Most colorectal cancers are thought to arise from malignant transformation of adenomatous polyps [1]. Consequently, the adenomatous polyp is the primary target for colorectal cancer screening with CT colonography [2–4]. However, non-adenomatous polyps, the most common of which are hyperplastic polyps, may be incidentally detected at CT colonography and lead to unnecessary polypectomy. Fortunately, non-adenomatous polyps are more difficult to detect at CT colonography [5]. The reasons for this lower sensitivity are not fully understood. Subjective visual inspection of non-adenomatous polyps at colonography and colonoscopy suggests that such polyps are more frequently flat and more easily compressed during gaseous distention of the colon [5, 6].
Using a validated automated quantitative assessment of polyp height and width, we determined whether hyperplastic colonic polyps on CTC are objectively flatter than adenomatous polyps of comparable width [7, 8].
MATERIALS AND METHODS
Written informed consent for retrospective research was obtained from all patients during the initial study [9]. The initial study was approved by the institutional review board and the retrospective study used data that was exempt. Both studies complied with the Health Insurance Portability and Accountability Act. The research was performed in part using software donated by Viatronix (Stony Brook, NY). Researchers who were not Viatronix board members had full control over the data.
Patient population (Figure 1)
Figure 1.
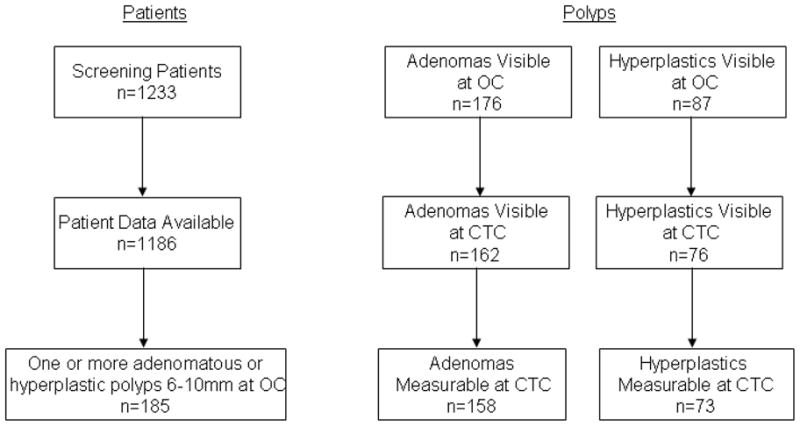
Patient and Polyp Flowchart
The patient population consisted of 1233 screening patients who underwent same day CT colonography and segmentally unblinded optical colonoscopy. Full CT colonography and optical colonoscopy data were available for 1186 of these patients. 185 of these patients had one or more adenomatous or hyperplastic polyps measuring from 6 to 10 mm at optical colonoscopy and visible at CTC and formed the study cohort. The average patient age was 59.4±7.2 years (range 46 to 79). There were 124 men and 61 women.
Patient preparation
Patients underwent a 24-hour clear-liquid diet colonic preparation that included orally administered laxatives [90 mL of sodium phosphate (Fleet 1 preparation; Fleet Pharmaceuticals, Lynchburg, VA) and 10 mg of bisacodyl [10]] and oral contrast agents in divided doses [500 mL of barium sulfate (Scan C, Lafayette Pharmaceuticals, Lafayette, Ind; 2.1% by weight) and 120-mL diatrizoate meglumine and diatrizoate sodium (Gastrografin; Bracco Diagnostics, Princeton, NJ)] [9].
CT Scanning
CT scanning was performed in both the supine and prone positions during a single breathhold following patient-controlled insufflation of the colon with room air via a smallflexible rectal catheter. The CT scanning parameters were 1.25-to-2.5-mm collimation, a table speed of 15 mmper second, a reconstruction interval of 1 mm, tube current and voltageof 100 mAs and 120 kVp on a four or eight-channel CT scanner (Light Speed or LightSpeedUltra, General Electric Medical Systems). The CTC scans were prospectively interpreted by board certified radiologists prior to optical colonoscopy.
Optical Colonoscopy
OC was performed by one of 17 experienced colonoscopists. The colonoscopists measured polyp size using a calibrated guidewire. OC was performed using segmental unblinding, in which CTC findings were revealed during OC to create an enhanced reference standard. Polyp type was determined by histopathology.
Assessment of Polyp Shape
Both the radiologists and colonoscopists prospectively provided a subjective assessment of polyp shape (sessile, pedunculated or flat) using generally accepted criteria. Sessile polyps were those having a broad base of attachment and a height greater than or equal to ½ the width of the base. Pedunculated polyps were those having a visible stalk. Flat polyps were those having a height less than ½ the width of the base. For pedunculated polyps, the polyp size was that of the head of the polyp excluding the stalk. Polyps described by the radiologists as “oval”, “ovoid” or “round” were included in the sessile category.
Polyp Identification
Using the optical colonoscopy findings, polyps located at optical colonoscopy were matched to polyps identified at CT colonography. Polyps were matched if they were within one colonic segment and 50% of the size of the polyp found at optical colonoscopy. The matching was done by research trainees under the supervision of a board certified radiologist. For each matched polyp, the location of a voxel within the polyp was recorded in a computer file. The matching accuracy for the more difficult to match 6 – 9 mm polyps was subsequently reviewed by a second board certified radiologist. If the second radiologist disagreed with a match, the disagreement was resolved upon further review of the polyp by the first radiologist.
Automated Assessment of Polyp Height and Width
To assess flatness, the heights of the polyps at CTC were measured using a validated automated software program [7, 8]. The program uses a topographical height map to locate the base of the polyp and then computes the height as the perpendicular distance from the base to the tip of the polyp. For polyps measurable on both supine and prone scans, one of the two measurements was chosen randomly per polyp.
Because OC size measurements could incorporate height as well as width, the analysis was repeated using a validated automated measurement of polyp width that computes the width as the average circumferential span of the polyp base [8]. Automated width measurements could fall outside the 6 – 10 mm range depending upon polyp shape and measurement uncertainties at CTC and OC. The height-to-width ratio (height/width) was also calculated.
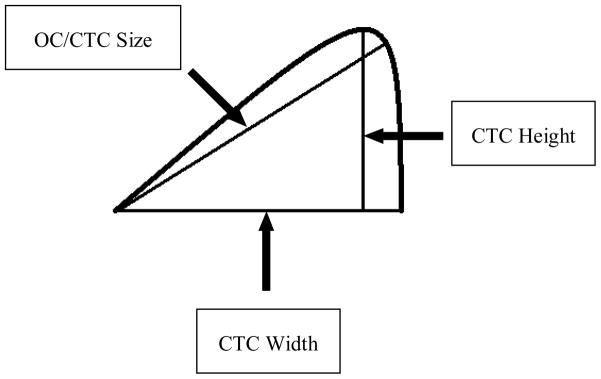
The previously reported 95% Bland-Altman limits of agreement (mm) comparing manual and automated polyp measurements were (−1.5 mm, 1.8 mm) for height and (−4.3 mm, 1.7 mm) for width [8, 11]. The OC and CTC size measurements are shown schematically in Figure 2.
Figure 2.
Schematic of polyp size measurements. The polyp shape (curved line) is intentionally skewed to exaggerate differences in the measurements, but polyps can have shapes similar to that shown. “OC Size” is the measurement of polyp size at optical colonoscopy that best approximates the maximum polyp size. “CTC Width” is the maximum width of the base of the polyp. “CTC Height” is the maximum height measured perpendicular to the base.
Relationship of Polyp Height to Radiologists’ and Colonoscopists’ Assessments of Polyp Shape
To ascertain the relationship between physicians’ assessments of polyp shape and automated measurement of polyp height, the average polyp heights were calculated for each grouping by polyp shape (flat, sessile, pedunculated). False negative polyps at prospective radiologist interpretation were excluded.
Statistical analysis
Mean values were compared using a t-test (two-tailed, unpaired, unequal variance) or ANOVA (Microsoft Excel). Fractions were compared using the Fisher exact test using an online calculator [12].
RESULTS
The size distribution of the polyps seen at OC and CTC are shown in Table 1. The proportion of polyps that were adenomas at OC and CTC were similar (176/263, 66.9 % vs. 162/238, 68.1%). Automated height and width measurements could be obtained for 231 (97.1%) of the 238 polyps visible at CTC (all but 4 adenomatous (three 6 mm and one 7mm in size at OC) and 3 hyperplastic polyps (one 6 mm and two 8 mm in size at OC)). For the 231 polyps, 199 (137 adenomas, 62 hyperplastics) supine and 197 (133 adenomas, 64 hyperplastics) prone measurements could be obtained (112 adenomas, 53 hyperplastics), and of these one random measurement per polyp was used of which 116 (81 adenomas, 35 hyperplastics) were on the supine and 115 (77 adenomas, 38 hyperplastics) were on the prone CTC (p=NS for comparing ratios of adenomas to hyperplastics on supine versus prone). The fraction of measurable polyps that were measurable on only one scan (supine or prone) was not significantly different for adenomatous compared to hyperplastic polyps (46/158, 29.1% versus 20/73, 27.4%, p=0.9). The distribution of adenomatous and hyperplastic polyps was not significantly different for patients scanned at 2.5 or 1.25 mm collimations (fraction at 2.5 mm collimation: 100/158 for adenomatous and 53/73 for hyperplastic polyps, p=0.2). The fraction of polyps visible at segmentally unblinded OC that were measured was not significantly different for adenomatous versus hyperplastic polyps, either for comparisons by size (0.1 ≤p ≤0.8) or for all sizes (158/176, 89.8% versus 73/87, 83.9%, p=0.2; Figure 1).
Table 1.
Size Distribution of Polyps
| Visible at OC (n) | Visible at CTC (n) | |||
|---|---|---|---|---|
| OC Size (mm) | Adenoma | Hyperplastic | Adenoma | Hyperplastic |
| 6 | 75 69.4% |
33 30.6% |
67 70.5% |
28 29.5% |
| 7 | 38 63.3% |
22 36.7% |
35 64.8% |
19 35.2% |
| 8 | 34 63. 0% |
20 37.0% |
33 63.5% |
19 36.5% |
| 9 | 10 71.4% |
4 28.6% |
9 75% |
3 25% |
| 10 | 19 70.4% |
8 29.6% |
18 72% |
7 28% |
| Total | 176 66.9% |
87 33.1% |
162 68.1% |
76 31.9% |
Data are numbers (%) of polyps visible at segmentally-unblinded optical colonoscopy (OC) and upon retrospective review of CT colonography (CTC). Polyp histologies other than adenomatous or hyperplastic (including one cancer) were excluded.
The average height of the adenomatous polyps was 0.3 mm greater than that of the hyperplastic polyps (2.9±1.2 mm [n=158] vs. 2.6±1.1 mm [n=73], p = .03). The 0.4 mm decreased width for adenomatous polyps was not statistically significant (8.0±2.4 mm [n=158] vs. 8.4±2.3 mm [n=73], p=0.26). The average height-to-width ratios using automated width measurements were 15% less for hyperplastic polyps: 0.39±0.20 (n=158) and 0.33±0.19 (n=73) for adenomatous and hyperplastic polyps, respectively (p=0.03). For polyps of a given size as determined by optical colonoscopy or a given width at CT colonography, hyperplastic polyps tended to be flatter (i.e., had lower height) by 2 – 27% on average compared to adenomatous polyps (p=NS for all but one size group).
Comparison of the polyp heights grouped by polyp shape showed that pedunculated polyps tended to have the greatest average heights, followed by sessile and then flat polyps, both at the radiologists’ and colonoscopists’ assessments (Table 2). The height-to-width ratios followed the same pattern. Of note, the radiologists called nearly twice as many polyps flat as did the colonoscopists. 78/158 (49%) of the adenomatous and 46/73 (63%) of the hyperplastic polyps had a height of 2 mm or less and/or a height-to-width ratio of less than 1/3 (p=.07).
Table 2.
Comparison of Automated Polyp Height at CTC and Radiologists’ and Colonoscopists’ Prospective Assessments of Polyp Shape at CTC and OC
| Radiologist | Colonoscopist | |||||
|---|---|---|---|---|---|---|
| Flat | Sessile | Pedunculated | Flat | Sessile | Pedunculated | |
| Height | 2.1±0.9 [35] |
2.8±1.2 [141] |
4.0±0.9 [28] |
2.1±1.0 [16] |
2.8±1.2 [156] |
3.4±1.2 [32] |
| Height-to-Width Ratio | 0.28±0.21 [35] |
0.37±0.18 [141] |
0.54±0.21 [28] |
0.29±0.29 [16] |
0.37±0.18 [156] |
0.46±0.21 [32] |
Heights are in millimeters (mean±st. dev.). Height-to-width ratios are dimensionless. Data include both adenomatous and hyperplastic polyps. Numbers of polyps are in brackets.
N=204 for both the radiologists and the colonoscopists. 25 false negative polyps at prospective radiologist interpretation are not included in this table. For radiologists and colonoscopists, there were statistically significant differences in the heights and height-to-width ratios for the different polyp shapes (ANOVA, p < 0.001 for radiologists for both measures; p = 0.002 and 0.01, respectively, for colonoscopists).
Abbreviations: CTC = CT colonography; OC = optical colonoscopy
The heights and height-to-width ratios of adenomatous polyps found (true positives) or missed (false negatives) at the initial prospective radiologist’s interpretation of the CTC images were similar, but those of hyperplastic polyps trended lower for missed polyps (Table 3).
Table 3.
Polyp Height and Detectability at Radiologists’ Prospective Interpretation of CTC Images
| Polyps Found at Prospective Interpretation | Polyps Not Found at Prospective Interpretation | |||
|---|---|---|---|---|
| Adenoma | Hyperplastic | Adenoma | Hyperplastic | |
| Height | 2.9±1.2 [146] |
2.7±1.1 [64] |
3.0±1.3 [12] |
1.9±1.0 [9] |
| Height-to-Width Ratio | 0.39±0.20 [146] |
0.34±0.20 [64] |
0.35±0.20 [12] |
0.25±0.15 [9] |
Heights are in millimeters (mean±st. dev.). Numbers of polyps are in brackets. For adenomas in different groups, p=0.87. For hyperplastics, p=0.07.
Height-to-width ratios are dimensionless. For adenomas in different groups, p=0.46. For hyperplastic polyps, p=0.14).
Representative polyps and their automated height and width measurements are shown in Figure 3 and Figure 4.
Figure 3.
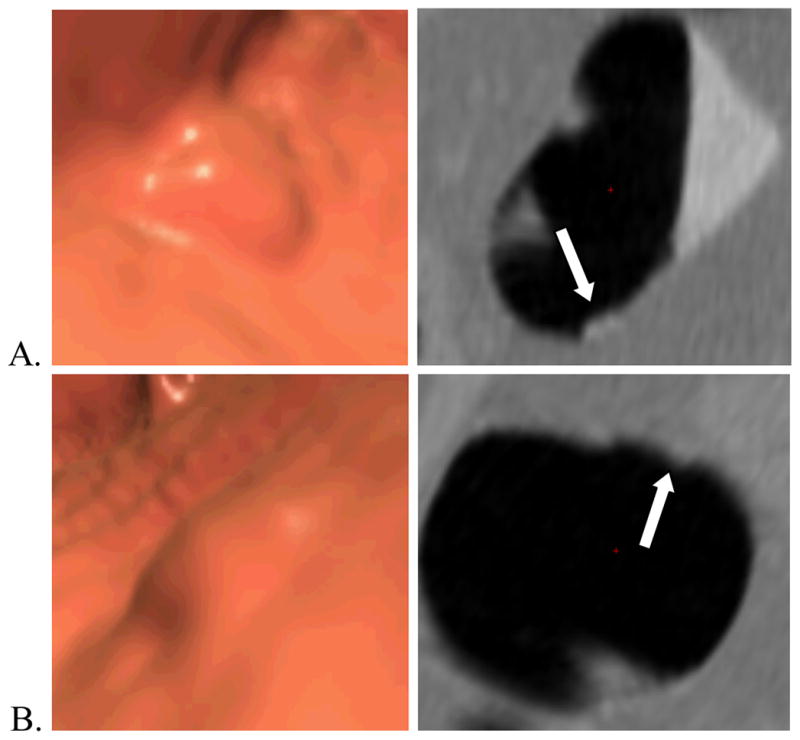
Example of a hyperplastic polyp (arrows). Three-dimensional endoluminal (left) and two-dimensional cross-sectional (perpendicular to centerline) (right) (A) prone and (B) supine CT colonography images of a 8 mm flat hyperplastic polyp (white arrows) in the transverse colon of a 58-year-old man. The polyp’s widths and heights computed by automated software are (A) 6.4 and 3.5 mm and (B) 8.3 and 1.9 mm. For this polyp, there was a substantial change in both width and height between the supine and prone scans suggesting pliability.
Figure 4.
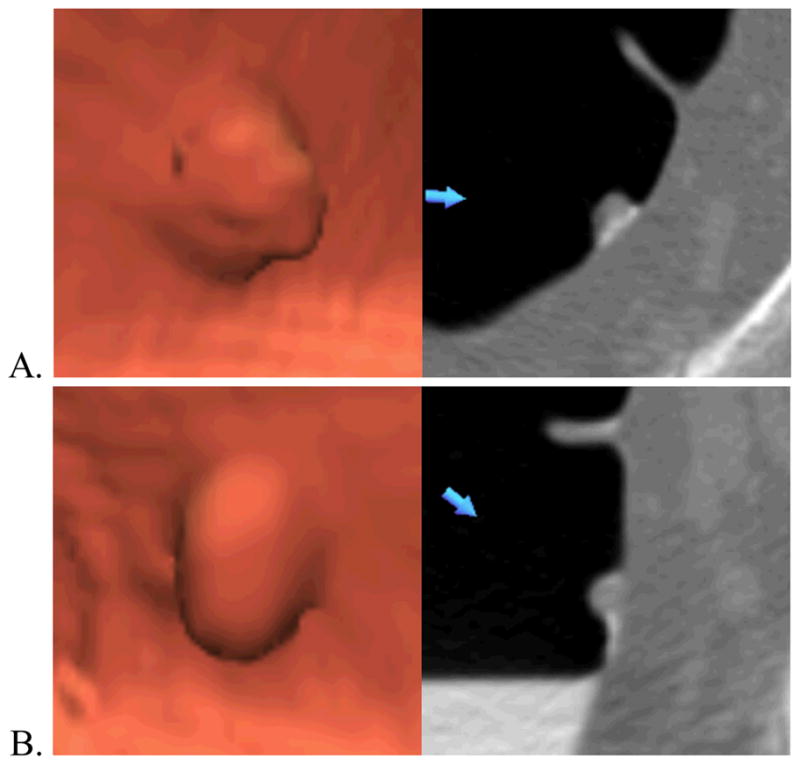
Example of an adenomatous polyp. Three-dimensional endoluminal (left) and two-dimensional transverse (right) (A) prone and (B) supine CT colonography images of a 8 mm sessile adenomatous polyp (blue arrows) in the rectum of a 66-year-old man. The polyp’s widths and heights computed by automated software are (A) 10.0 and 5.0 mm and (B) 10.1 and 5.0 mm. Unlike the hyperplastic polyp in Figure 3, for this polyp there was no change in either width or height between the supine and prone scans suggesting lack of pliability.
DISCUSSION
We found that hyperplastic polyps were up to 27% flatter than adenomatous polyps of comparable size. On average, the height-to-width ratio was 15% less for hyperplastic polyps. The nominal height differences were small but substantial when considered as a fraction of average polyp height. The height differential between adenomatous and hyperplastic polyps was statistically significant for all polyps. Hyperplastic polyps missed at prospective radiologist interpretation tended to be flatter than hyperplastic polyps that were found. Since it is known that flatter polyps are more difficult to detect at CT colonography, these findings may explain why hyperplastic polyps are detected with lower sensitivity than adenomatous polyps.
Due to substantial overlap in the height distributions, height was not diagnostic for individual polyps. This result was expected since adenomatous and hyperplastic polyps are known to be indistinguishable at CTC.
Pedunculated polyps had the greatest height, followed by sessile and flat polyps. These results were consistent between radiologists’ and colonoscopists’ assessments of polyp shape. Of note, the mean CTC polyp heights were less than half the optical colonoscopy polyp size for all shape categories. This finding calls into question the definition of polyp flatness (height less than half width) used by many authors since in our study more than half of polyps fit that definition. Based on our findings, a more appropriate definition of a 6–10 mm flat polyp would be a polyp having a height of 2 mm or less or a height-to-width ratio of less than 1/3. However, 49% of medium-sized adenomatous and 63% of medium-sized hyperplastic polyps would fit either or both of these two definitions. The C-RADS uses a similar definition of a flat lesion (“<3 mm of vertical elevation above the colonic mucosa”) [13].
We confirmed that the proportions of measurable (at CTC) to total (at OC) adenomatous and hyperplastic polyps were not significantly different. This finding reduces the possibility of selection bias for non-flat polyps. We also confirmed that there were similar proportions of adenomatous and hyperplastic polyps measureable on only one view and that the proportions of adenomas to hyperplastics were similar on the supine versus prone views, reducing the possibility of bias for polyps visible on only one view. There were also similar proportions of adenomatous and hyperplastic polyps on the scans with 2.5 mm and 1.25 mm collimations, reducing the possibility of a bias due to slice collimation.
A number of investigators have developed methods of automated polyp size measurement [14–18]. These methods assessed polyp width or a combination of width and height, but not height alone, since the focus was to obtain a measurement comparable to the size measurements determined at optical colonoscopy. The height map algorithm used in this paper was tested on 226 polyps and found to agree well with manual assessment of polyp height [7, 8].
The detection of flat and/or hyperplastic colorectal lesions at CTC has been the subject of several investigations. In the recently reported ACRIN CTC trial, the sensitivity for detecting patients with large adenomas was 90% and with all histologic types of polyps was 87% [19]. Since large hyperplastic polyps were 1/6th as frequent as adenomas in that study, it may be inferred that the sensitivity for detecting them was approximately 70–75% (an estimate, since per polyp sensitivity was not provided for hyperplastic or other nonadenomatous polyps in that study). In one study, flat colorectal lesions were more difficult to detect at CT colonography, and those less than 2 mm in height were not detectable at all [20]. In another study, the sensitivities for detecting flat lesions (a combination of adenomatous and hyperplastic lesions) ranged from 15 to 65% for three radiologists [21]. The authors used 5-mm collimation and either single- or four-detector row CT scans. Five-mm collimation and single-detector scanning likely explain the low sensitivities. In another study, the sensitivity for detecting hyperplastic flat lesions 6 mm or greater was 76.7% compared to 82.8% for flat adenomas and 86.2% for non-flat adenomas [22]. The potential underreporting of flat polyps at colonoscopy has been the subject of recent interest and has raised the concern that the true sensitivity for detecting such polyps at CTC may be even lower than that reported since optical colonoscopy is the gold standard for CTC clinical trials [23]. Flat polyps were defined to be those with height less than one half the width in [20–23].
Non-adenomatous polyps are also more difficult to detect than adenomatous polyps at optical colonoscopy. In one study, using back-to-back colonoscopies, 28% of non-adenomatous polyps, including hyperplastic polyps, were missed at the first colonoscopic examination [24]. In a meta-analysis of tandem colonoscopy, 22% of adenomas and 27% of non-adenomas were missed but this difference was not statistically significant [25]. In a recently reported study, 31% of all hyperplastic polyps were missed at optical colonoscopy, compared to a miss rate of 20% for all adenomas [26].
The relative difficulty of detecting hyperplastic polyps is potentially beneficial to patients because hyperplastic polyps have little or no malignant potential and detecting such polyps could lead to unnecessary polypectomy. There is evidence that some hyperplastic polyps may become cancerous through the serrated polyp pathway [27, 28]. In our experience, however, serrated polyps tend to be large and distinct from the subcentimeter hyperplastic polyps in our study.
While it is not known why hyperplastic polyps would be flatter than adenomatous polyps, a theory relating to their internal structure has been proposed. In this theory, hyperplastic polyps may be softer than adenomatous polyps and when the colon is insufflated, they may flatten out [29, 30]. This “disappearing phenomenon” has been used to predict the histologic type of diminutive polyps in the rectum and rectosigmoid colon at optical colonoscopy, with a sensitivity of 100% and specificity of 46.7% for polyps that completely disappeared [6]. The author of that study hypothesized that the disappearing phenomenon related to the structure of hyperplastic polyps that is based on a delay in shedding of surface epithelial cells, as opposed to excess cellular proliferation that is found in adenomas [6, 31]. We attempted to ascertain whether polyp height was dependent upon degree of colonic distention at CTC, but because colonic distention was generally good, there were an insufficient number of polyps in less well-distended colon segments to serve as an informative comparison group (data not presented).
The difficulty of detecting flat polyps on CTC likely relates to their poor visual conspicuity. In a study of four radiologist observers who reviewed 29 proven polyps at CTC, visual conspicuity was found to be correlated with polyp height but not polyp width [32]. This finding was consistent with the observation that the greater the polyp height, the greater the shadows and highlights in 3D endoluminal renderings where the light source is directed close to the plane of the colonic surface [32]. Less conspicuous, flatter, polyps were in general more difficult to detect by computer-aided detection software [32].
The current study has several limitations. First, the automated algorithm does not differentiate polyp head and stalk for pedunculated polyps, potentially increasing the assessment of automated width. We focused on 6–10 mm polyps rather than larger polyps and masses because larger polyps and masses tend to form more complex shapes for which height is more difficult to determine. Also, hyperplastic polyps larger than 10 mm are relatively uncommon.
In conclusion, hyperplastic polyps are quantitatively flatter then adenomatous polyps; this finding may relate to their internal structures and may explain why hyperplastic polyps are more difficult to detect than adenomatous polyps at CTC.
Acknowledgments
We thank Dr. William Schindler for supplying CT colonography data and Dr. Andrew Dwyer for critical review of the manuscript. This research was supported by the Intramural Research Programs of the NIH Clinical Center.
Footnotes
Presented at the 2008 RSNA Scientific Meeting
References
- 1.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ. Initial management and follow-up surveillance of patients with colorectal adenomas. Gastroenterol Clin North Am. 1997;26:85–101. doi: 10.1016/s0889-8553(05)70285-5. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz AJ, Winawer SJ. Management of colorectal polyps. CA Cancer J Clin. 1997;47:93–112. doi: 10.3322/canjclin.47.2.93. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien M, Winawer SJ, Waye JD. Colorectal polyps. In: Winawer SJ, Kurtz RC, editors. Gastrointestinal cancer. Chapter 3. New York: Gower Medical Pub; 1992. [Google Scholar]
- 5.Pickhardt PJ, Choi JR, Hwang I, Schindler WR. Nonadenomatous polyps at CT colonography: prevalence, size distribution, and detection rates. Radiology. 2004;232:784–790. doi: 10.1148/radiol.2323031614. [DOI] [PubMed] [Google Scholar]
- 6.Bertoni G, Sassatelli R, Conigliaro R, et al. Visual “disappearing phenomenon” can reliably predict the nonadenomatous nature of rectal and rectosigmoid diminutive polyps at endoscopy. Gastrointest Endosc. 1994;40:588–591. doi: 10.1016/s0016-5107(94)70259-4. [DOI] [PubMed] [Google Scholar]
- 7.Yao J, Frentz S, Li J, Summers R. Polyp height and width measurement using topographic height map. Progress in Biomedical Optics and Imaging -Proceedings of SPIE. 2008 [Google Scholar]
- 8.Yao J, Li J, Summers RM. Employing topographical height map in colonic polyp measurement and false positive reduction. Pattern Recognition. 2009;42:1029–1040. doi: 10.1016/j.patcog.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 10.Pickhardt PJ, Choi JH. Electronic cleansing and stool tagging in CT colonography: advantages and pitfalls with primary three-dimensional evaluation. AJR Am J Roentgenol. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 12.Langsrud O. [Date Accessed: March 31, 2009];Fisher’s Exact Test. http://www.langsrud.com/fisher.htm.
- 13.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JG, Booya F, Melton Z, et al. Automated polyp measurement with CT colonography: preliminary observations in a phantom colon model. AJR Am J Roentgenol. 2007;188:945–952. doi: 10.2214/AJR.06.1169. [DOI] [PubMed] [Google Scholar]
- 15.van Wijk C, Florie J, Nio CY, et al. Protrusion method for automated estimation of polyp size on CT Colonography. Am J Roentgenol. 2008;190:1279–1285. doi: 10.2214/AJR.07.2865. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SA, Slater A, Halligan S, et al. CT colonography: automated measurement of colonic polyps compared with manual techniques--human in vitro study. Radiology. 2007;242:120–128. doi: 10.1148/radiol.2421052068. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JY, Kim MJ, Kim SS. Manual and automated polyp measurement comparison of CT colonography with optical colonoscopy. Acad Radiol. 2008;15:231–239. doi: 10.1016/j.acra.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Yao J, Summers RM. Adaptive deformable model for colonic polyp segmentation and measurement on CT colonography. Med Phys. 2007;34:1655–1664. doi: 10.1118/1.2717411. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SH, Ha HK, Kim AY, et al. Flat polyps of the colon: detection with 16-MDCT colonography--preliminary results. AJR Am J Roentgenol. 2006;186:1611–1617. doi: 10.2214/AJR.04.1889. [DOI] [PubMed] [Google Scholar]
- 21.Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdom Imaging. 2002;27:292–300. doi: 10.1007/s00261-001-0171-z. [DOI] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol. 2004;183:1343–1347. doi: 10.2214/ajr.183.5.1831343. [DOI] [PubMed] [Google Scholar]
- 23.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies [see comments] Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 25.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 26.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–290. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien MJ. Hyperplastic and serrated polyps of the colorectum. Gastroenterology Clinics of North America. 2007;36:947–968. doi: 10.1016/j.gtc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3–47. doi: 10.1016/j.gie.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 29.Cravens E, Lehman GA. Disappearing rectal polyps. Gastrointest Endosc. 1991;37:88–91. doi: 10.1016/s0016-5107(91)70635-4. [DOI] [PubMed] [Google Scholar]
- 30.Waye JD, Bilotta JJ. Rectal hyperplastic polyps: now you see them, now you don’t--a differential point. Am J Gastroenterol. 1990;85:1557–1559. [PubMed] [Google Scholar]
- 31.Hayashi T, Yatani R, Apostol J, Stemmermann GN. Pathogenesis of hyperplastic polyps of the colon: a hypothesis based on ultrastructure and in vitro cell kinetics. Gastroenterology. 1974;66:347–356. [PubMed] [Google Scholar]
- 32.Summers RM, Frentz SM, Liu J, et al. Conspicuity of colorectal polyps at CT colonography: visual assessment, CAD performance, and the important role of polyp height. Acad Radiol. 2009;16:4–14. doi: 10.1016/j.acra.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]