Abstract
A short-term, localized outbreak of diatoms attached to live corals was observed along the coast of Sesoko Island, Okinawa, Japan in February, 2011. Diatoms are recognized as brown patches in the initial stage, becoming fluffy encrustations and resulting in complete or partial coral death. Attached diatoms, including Licmophora, Climacosphenia, Ardissonea and others, attached and overgrew exclusively Montipora corals, which are dominant corals in some parts of Sesoko reef. Heavily-covered colonies or branches died. The rate of affected corals reached 80% in the worst-affected area. Microscopic observation showed that most diatoms settled directly with polysaccharide stalks or pads onto the partly-bared skeleton of coral branches, although some settled on coral soft tissues. Although no similar phenomenon was reported from other areas of Japan, cold-water events might have important roles in coral weakening, as a consequence, enabling diatom attachment on corals, thus leading to coral death in this area.
Reef building corals are in crisis due to synergistic factors such as predation by crown-of-thorns starfish, coral bleaching driven by water temperature rises, a variety of increasing diseases, and ocean acidification1,2,3,4. In addition to predation by animals, overgrowth by animals and plants causes significant problems5,6. Algal (including cyanobacteria) blooms occur around coral reef areas, and are usually caused by excess terrigenous nutrient inputs into reefs7. Furthermore, it is predicted that the growth rates of corals will be decreased and that corals will be replaced with algae as a result of ocean acidification3.
Epizoic diatoms are distributed widely in both marine and freshwater environments, and some occur on substrata, including live hosts, plants and animals from porifera to vertebrata8. The present study describes a curious event in which coral colonies were covered by numerous attached diatoms on the reefs of Sesoko Island, Japan during the winter of 2011. This is the first report of a diatom outbreak on live coral surfaces resulting in coral death. Although this bloom was found in a restricted area, and the affected corals were exclusively Montipora spp., cold water stress could have been a causative agent in the outbreak. Our objective in this study is to describe the fundamental nature of the relationship between coral and attached diatoms, with some environmental measurements, to increase our understanding before the next outbreak occurrence. Attention must be focused on even such tiny algae (diatoms) which cause coral mortality.
Results
Coral colonies covered with sessile diatoms were noticed on 18th February, 2011 in a moat of Sesoko Island (Fig. 1). Individual diatoms were microscopic in size, but were obvious to the naked eye as brown patches when they created a dense cover. In advanced cases, coral colonies showing a fluffy appearance were found. We asked the members of the Japanese Coral Reef Society about this phenomenon, but similar observations were not provided from other areas in Japan, including Bise, Okinawa Island (adjacent to Sesoko Island), where huge numbers of branching Montipora colonies are distributed in the intertidal zone, as in Sesoko (pers. comm. from the staff of Okinawa Churaumi Aquarium). The corals affected at Sesoko Island were exclusively Montipora corals, especially branching M. digitata (Dana), M. samarensis Nemenzo and M. altasepta Nemenzo9,10. These congeners are distributed sympatrically, forming large assemblages in the shallow waters of the reef at depths of 1 to 2 m. Other montiporid corals, such as encrusting M. informis Bernard and foliose M. aequituberculata Bernard, were also observed to be affected by diatoms. However, the dominant acroporid or poritid corals and other corals were not affected, even when their colonies stood very close to infected Montipora colonies.
Figure 1. Map of the study site.
The spatial distribution of affected Montipora corals showed that this bloom was localized (Fig. 2). The area affected spread to about 1 km in length. In the area affected most heavily, the rate of affected corals attained was 81% for branching Montipora corals. Corals covered by diatom assemblages were observed from the moat to the reef front about 100 m from the shore. The rate of affected coral was higher for corals close to shore.
Figure 2. Sesoko Island.
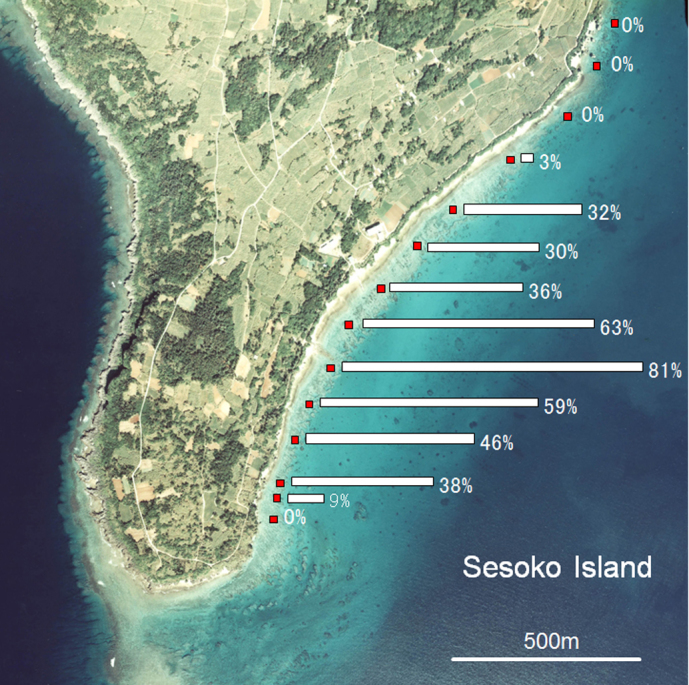
Horizontal bars indicate the rate of branching Montipora corals attached by sessile diatoms in April 2011.
Affected corals showed a variety of phases. In the initial stage, small aggregates of diatoms were visible on the colony surface as brown patches (Fig. 3A). Some corals showed mucus secretions, recognized as white strands (Fig. 3A), which included diatoms within them. It was difficult to judge the attachment of diatoms by the naked eye when diatoms were attached evenly on the coral surface (Fig. 3B). In the advanced stage, the coral surface showed a fluffy appearance, caused by numerous diatoms (Fig. 3C). Even in this stage, surviving coral polyps were observed. However, fully-covered parts of the colony eventually died. Although diatom detachment was observed when the corals were shaken in the initial stage, once the diatoms were securely attached to the coral skeleton, no detachment was observed, even after a heavy seas warning.
Figure 3. Diatoms covering Montipora coral.

A, Attached diatoms are visible as brown patches, and the coral secretes mucus strands against them. B, A coral branch covered by numerous diatoms (Licmophora). C, Advanced stage showing fluffy branches. D. Attached diatoms around the growing chip. E, Licmophora flabellata showing fan-like colony and branched mucilage stalks. F, Coral surface stained with Alcian blue, most diatoms are detached leaving pads on the protuberant bared skeleton.
On the basis of microscopic observation, the abundant diatoms on Montipora corals were identified as Licmophora flabellata (Carmichael ex Greville) C. Agardh (Fig. 3D-E), Climacosphenia moniligera Ehrenberg, Ardissonea fulgens (Greville) Grunow, Actinocyclus subtilis (Gregory) Ralfs and others. Of these diatoms, the most abundant species was L. flabellata, which formed dense mats, while other diatoms were also observed in the same space. The other diatoms observed are not described in the present study.
Alcian blue or toluidine blue staining were carried out to observe the extracellular polymeric substances (EPSs, composed of polysaccharides) that were secreted from attached diatoms, showing that the diatoms attached either onto the coral skeleton or to the soft tissues of the coral. EPSs, in the form of stalks or pads, were stained blue. For ethanol fixed/washed samples, some diatoms on soft tissues had become detached, leaving only EPSs on corals (Fig. 3F). It was difficult to remove tightly-attached diatoms using forceps, especially for tall stalks.
Changes in the rate of affected corals were surveyed weekly for randomly selected Montipora spp. colonies (84 to 98 colonies for each census) in the same area of Sesoko Island where the affected corals were first recognized, and also in the heavily covered site. The rate of affected coral colonies (divided into three categories as normal, covered by diatoms and dead) was almost constant during the observation period (Fig. 4). However, the rate of algal coverage within colonies increased with time, especially in the earlier observation period. Most affected branches/colonies were dead by 7th May (the final observation date before a strong typhoon on 28th May). Algal coverage on the coral colonies shifted from diatoms to filamentous green algae. Herbivorous fish such as surgeonfish were observed to graze on the tips of dead Montipora branches covered with filamentous green algae. Unfortunately, a strong typhoon hit Okinawa, including Sesoko Island, on 28th May, devastating fragile corals including branching Montipora spp. Thus, the periodical census was finished on 7th May. However, aggregations of attached diatoms (Licmophora and others) on branching Montipora colonies were found again in the same area on 27th February, 2012, although the number of affected colonies was small.
Figure 4. Changes in algal covers as a mean algal coverage (%) in whole colonies, and as three categories (% dead, % covered with diatoms, and % normal colonies).
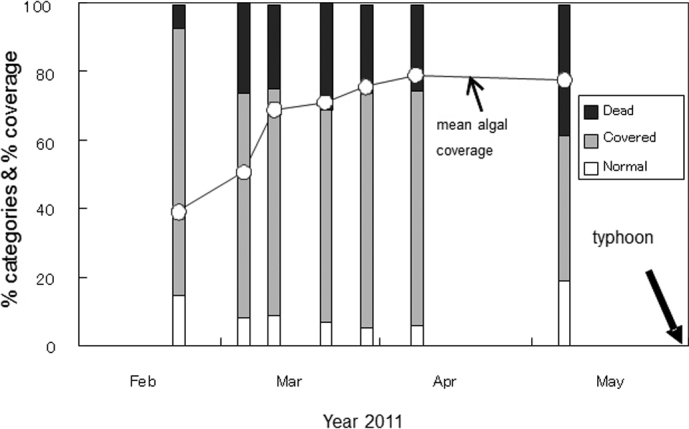
Periodical census started on Feb 20th and finished on 7th May before a strong typhoon on 28th May 8 in 2011.
The nutrient measurements (triplicate samples) from the reef front on 20th February, 2011 were 0.269 µmol for NH4, 0.323 µmol for NO3, 0.222 µmol for NO2 and <0.001 µmol for PO4. Those from the reef flat were 0.256 µmol for NH4, 0.575 µmol for NO3, 0.221 µmol for NO2 and <0.001 µmol for PO4, and those from the moat were 0.640 µmol for NH4, 0.850 µmol for NO3, 0.188 µmol for NO2 and <0.001 µmol for PO4.
Fig. 5 shows the change in water temperature in the moat at Sesoko throughout the year. In the winter of 2011, the water temperature was not only colder than that of the previous year, 2010, but was the coldest recorded for the past 20 years (unpublished data, Sesoko Station). In January and February of 2011, the water temperature dropped to less than 18°C several times.
Figure 5. Changes in water temperature in the moat of Sesoko Island from April, 2009 to May, 2010 (upper) and from July, 2010 to May, 2011 (lower).
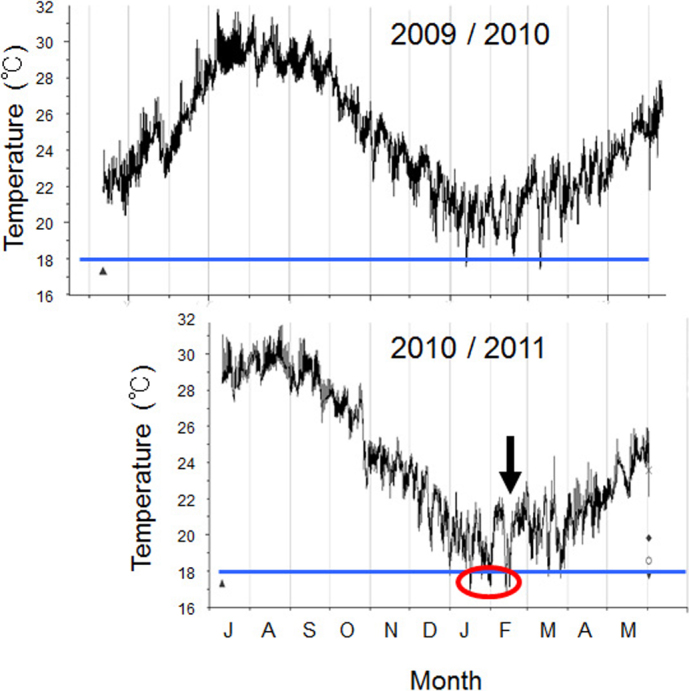
Data were retrieved from a logger every hour. Arrow indicates 18th February, 2011. Water temperature in the winter of 2011 was lower (encircled) than that in 2010.
Discussion
Diatoms play an important role in primary production in both marine and freshwater ecosystems. Although relationships between diatoms and associated hosts have been reported8, information about the detrimental effects of diatoms is scarce. In Okinawa, Japan, blue coral, Heliopora coerulea, covered by Licmophora sp. diatoms was reported in 2009 (Abe et al. in Japanese without English description) in the outer reef of Oura Bay (26°32′17′′ N, 128°04′47′′E) on the east coast of Okinawa Island. Partial bleaching of the blue coral caused by diatom attachment was found in September, and the coral recovered from bleaching in November as the diatoms disappeared. In the present study, diatoms attached tightly to montiporid corals in the winter and the overgrowth did not cause bleaching, but rather smothered and killed corals, suggesting that the mechanisms and fundamental features seem to differ between Heliopora and Montipora.
Coverage by diatoms would be problematic for corals, thus corals have to exclude diatoms by secreting mucus to entangle and remove them (Fig. 3A). It is probable that diatoms kill corals as a result of suffocation by forming dense aggregations rather than by generating hostile chemical substances. The aggregations of diatoms were not monospecific, with the relatively common species Licmophora flabellata, Climacosphenia moniligera, Ardissonea fulgens and Actinocyclus subtilis forming the algal assemblage. Periodical observations of algal coverage (Fig. 4) seemed to indicate that the initial attachment might have occurred in early February or January, or even much earlier, because entirely covered or dead colonies were found beginning on 18th February.
Even after strong wave action affected the Sesoko reefs, most diatoms did not detach from corals, indicating their high attachment strength. Marine fouling diatoms secrete extracellular polymeric substances (EPSs, polysaccharide) forming stalks (e.g. Licmophora), pads (e.g. Ardissonea), envelopes or tubes11,12,13, thus the diatoms on live Montipora corals could attach using EPSs and could withstand wave action when there were bare and rigid substrata.
Although Montipora corals attempted to exclude diatoms by secreting mucus on their surface, diatoms continued to grow and increased in number on live coral surfaces. Considering that (1) the bloom occurred over a narrow range, (2) diatoms did not necessarily spread over whole colonies with time, (3) diatoms appeared congener-specific, with not all coral species suffering this outbreak would have been established and initiated in a short period rather than being an infectious phenomenon among corals.
It is suggested that certain coincident events must have occurred between corals and diatoms to cause the algal bloom that resulted in coral mortality at Sesoko Island. Excessive nutrient input seems to be a possible cause; however, the nutrient levels measured on the Sesoko reef (reef front, reef flat and moat) were not elevated. Furthermore, the affected coral species were restricted to Montipora. It is likely that the sudden decrease in water temperature could be a causative environmental parameter. Severe cold-water events have been reported to cause bleaching or mortality of corals in Florida14,15 and on the Great Barrier Reef16,17,18. During cold-water events, Montipora corals would have been weakened first, and offered bare skeleton (especially for coenosteum papillae) as a substratum for diatoms in the narrow area of the Sesoko reef. Considering that both the coral species and prevailing time were restricted, similar events are not likely to happen every year. However, a small-scale aggregation of diatoms on Montipora colonies was observed also in the same area in the winter of 2012. Macroalgal-coral interactions have been considered in relation to algal phase shifts1 or the smothering of corals by algae19, unusual events observed on coral reefs should be recorded, even when such events are on a small-scale, found in a restricted area and caused by tiny diatoms.
Branching Montipora corals are common in some reefs in Okinawa, Japan. In addition to sexual reproduction20 they propagate clonally through fragmentation21, enabling them to form dense stands in reefs. M. digitata in Sesoko suffered severe bleaching in the summer of 199822 and declined significantly, but after a decade this species had recovered from a few remnant survivors, and was thus expressed as a short-term loser and long-term winner23. However, branching Montipora corals are also susceptible to bleaching in response to low water temperatures16. Mortality of corals caused by cold-water events has also been reported from Florida14,24,15. The present study showed a regional decrease in corals driven by both a sudden drop in water temperature and by diatom attachment to corals, which seemed to inhibit the recovery of corals. Our data suggest that even tiny unicellular diatom play a role in coral death. To evaluate the mechanisms of micro-algal blooms on specific live corals, continuous observations and experimental tests are required.
Methods
The study site, Sesoko Island (26°10′37″ N, 127°16′27″ E), located near Okinawa Island, Japan (Fig. 1), has a fringing reef. On the east coast of the island, corals are abundant, and some of them have been recovering from a severe bleaching event which occurred in 199823. A decade after the bleaching event, tabular Acropora coral had attained sizes of over 2 m in diameter. Furthermore, branching Montipora coral were forming dense populations in a moat, together with other dominant corals such as tabular acroporids (Family Acroporidae) and massive poritids (Family Poritidae).
Branching Montipora corals covered by attached diatoms were recognized for the first time on 18th February, 2011 in the moat of Sesoko Island, where the Sesoko Station of the University of the Ryukyus is located. Water temperatures have been monitored hourly both in the moat and on the reef slope with data loggers (HOBO® U-22, Onset Co.) since before the present outbreak. Nutrients (NH4, NO3, NO2, PO4) were measured using an NP autoanalyzer (SWAAT®, BL tech Co.) for seawater samples (three samples from each site) collected from the reef front, reef flat and moat on 20th February 2011, two days after the first notification.
Rates of diatom attachment were monitored weekly from February 20th to April 8th, and on May 7th for branching Montipora corals in the moat where this event was first recognized on 18th February, 2011. Attachment rate was judged by eye measurement for each colony and recorded as % cover of diatom. To elucidate the spatial distribution of affected corals, a survey along the east coast of the island was also conducted on 22nd April, 2011. Furthermore,monthly observations continued until March, 2012.
Microscopic observations using dissecting or digital microscopes (VHX-1000, Keyence Co.) were conducted on live or formalin-fixed coral samples. To observe how diatoms attached to corals with polysaccharide stalks or pads, Alcian blue or toluidine blue staining was conducted.
Author Contributions
HY found this phenomenon, conducted the fieldwork, analyzed the data, and wrote the manuscript. YM and HS observed and identified the diatoms. All authors reviewed and edited the manuscript.
Acknowledgments
We thank the staff of the Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus for the use of their facility. Coral sampling was conducted with the approval of Okinawa Prefecture, Japan. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas "Coral reef science for symbiosis and coexistence of human and ecosystem under combined stresses" (No. 20121002) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and "Development of technology for impacts mitigation and adaptation of climate change" program of the Ministry of Agriculture, Forestry and Fisheries, Japan.
References
- Hughes T. P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 (1994). [DOI] [PubMed] [Google Scholar]
- Harvell C. D., Mitchell C. E., Ward J. R., Altizer S., Dobson A. P., Ostfeld R. S. & Samuel M. D. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 (2002). [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O., Mumby P. J., Hooten A. J. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Koren O., Reshef L., Efrony R. & Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nature Rev. Micro. 5, 355–362 (2007). [DOI] [PubMed] [Google Scholar]
- Plucer-Rosario. The effect of substratum on the growth of Terpios, an encrusting sponge which kill corals. Coral Reefs 5, 197–200 (1987). [Google Scholar]
- Birrell C. L., McCook L. J., Willis B. L. & Diaz-Pulido G. A. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. An Ann. Review 46, 25–63 (2008). [Google Scholar]
- Paul V. J., Thacker R. W., Banks K. & Golubic S. Benthic cyanobacterial bloom impacts the reefs of South Florida (Broward Country, USA). Coral Reefs 24, 693–697 (2005). [Google Scholar]
- Totti C., Romagnoli T., De Stefano M., Di Camillo C. G. & Bavestrello G. The diversity of epizoic diatoms: relationships between diatoms and marine invertebrates. In J. Seckbach, Z Dubinsky (eds) Cellular origin, life in extreme habitats and astrobiology, Vol. 16, Part 6, 323–343 (2011).
- Veron J. E. N. Hermatypic corals of Japan. Aust. Inst. Mar. Sci. Monogr. Ser. 9, 234 pp. (1992). [Google Scholar]
- Veron J. E. N. Corals of the world. Vol. 1. Australian Institute of Marine Science, Townsville, 463 pp. (2000). [Google Scholar]
- Daniel G. F., Chamberlain A. H. L. & Jones E. B. G. Cytochemical and electron microscopical observations on the adhesive materials of marine fouling diatoms. Br. Phycol. J. 22, 101–118. (1987). [Google Scholar]
- Hoagland K. D., Rosowski J. R., Gretz M. R. & Roemer S. C. Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology. J. Phycol. 29, 537–566 (1993). [Google Scholar]
- Wustman B. A., Cretz M. R. & Hoagland K. D. Extracellular matrix assembly in diatoms (Bacillariophyceae) 1. A model of adhesives based on chemical characterization and localization of polysaccharides from the marine diatom Achnanthes longipes and other diatoms. Plant Physiol. 113, 1059–1069 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirman D., Schopmeyer S., Manzello D., Gramer L. J., Precht W. F. et al. Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PLoS one 6, e23047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella M. A., Ruzicka R. R., Kidney J. A., Morrison J. M. & Brinkhuis V. B. Cold-water event of January 2010 results in catastrophic benthic mortality on patch reefs in the Florida Keys. Coral Reefs 31, (2012)(in press). [Google Scholar]
- Saxby T., Dennison W. C. & Hoegh-Guldberg O. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 248, 85–97 (2003). [Google Scholar]
- Hoegh-Guldberg O., & Fine M. Low temperatures cause coral bleaching. Coral Reefs 23, 444 (2004). [Google Scholar]
- Hoegh-Guldberg O., Fine M., Skirving W., Johnstone R., Dove S. & Strong A. Coral bleaching following wintry weather. Limnol Oceanogr 50, 265–271 (2005). [Google Scholar]
- Tuya F., Haroun R. J., Boyra A. & Sanchez-Jerez P. Sea urchin Diadema antillarum: different functions in the structure and dynamics of reefs on both sides of the Atlantic. Mar. Ecol. Prog. Ser. 302, 307–310 (2005). [Google Scholar]
- Heyward A. J. & Babcock R. C. Self- and cross-fertilization in scleractinian corals. Marine Biology 90, 191–195 (1986). [Google Scholar]
- Highsmith R. C. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. 7, 207–226 (1982). [Google Scholar]
- Stimson J., Sakai K. & Sembali H. Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21, 409–421 (2002). [Google Scholar]
- van Woesik R., Sakai K., Ganase A. & Loya Y. Revisiting the winners and losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 434, 67–76 (2011). [Google Scholar]
- Kemp D. W., Oakley C. A., Thornhills D. J., Newcomb L. A., Schmidt G. W. & Fitt W. K. Catastrophic mortality on inshore coral reefs of the Florida Keys due to severe low-temperature stress. Global Change Biology 17, 3468–3477 (2011). [Google Scholar]


