Abstract
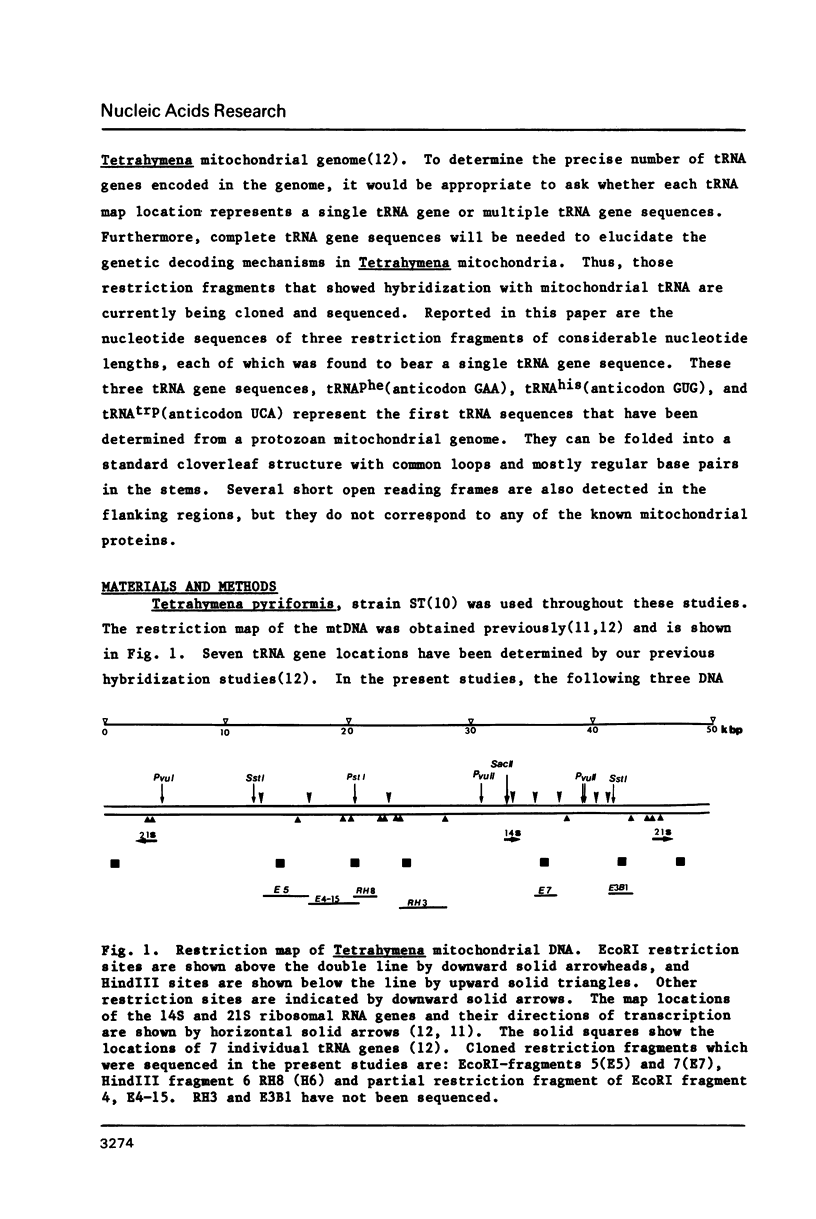
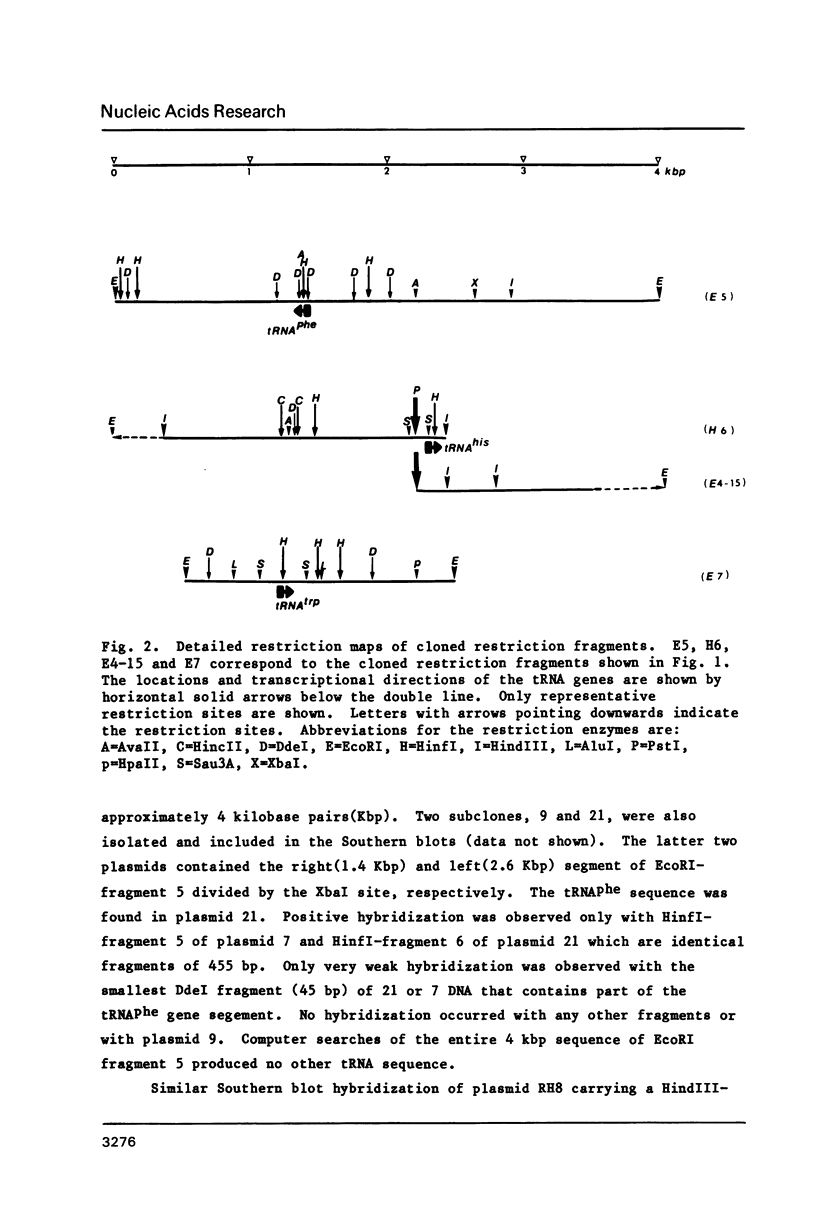
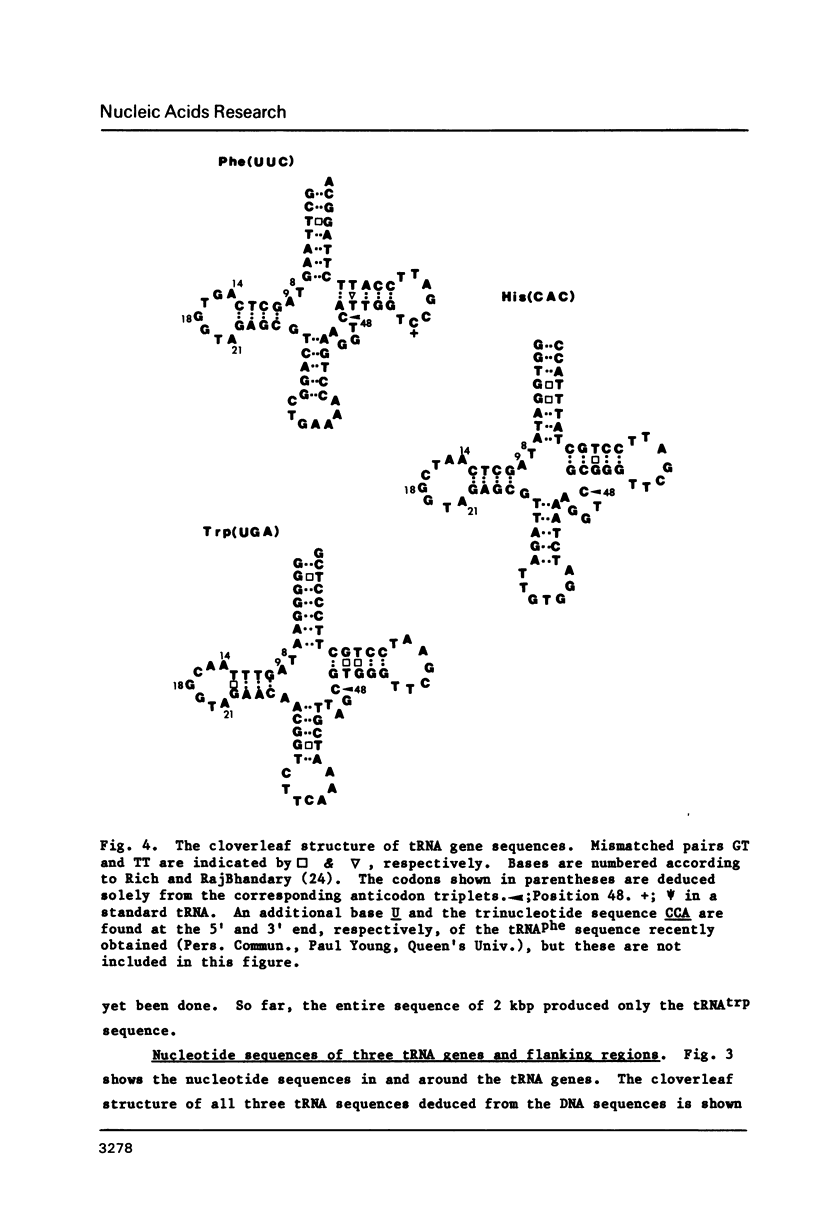
Nucleotide sequences of three cloned restriction fragments of Tetrahymena mtDNA which showed hybridization with mitochondrial tRNA have been determined. EcoRI fragment 5 (4.1 kbp) contains the tRNAphe gene sequence with anticodon GAA; Hind III fragment 6 (2.0 kbp) the tRNAhis with anticodon GTG; and EcoRI fragment 7 (1.9 kbp) the tRNAtrp with anticodon TCA. The CCA end is not encoded. All three tRNAs show usual features with common invariant and semi-invariant bases and can be folded into a cloverleaf structure with standard loops and regular base pairs in the stems. However, some minor irregular features are present including several GT pairs and an unmatched TT in the stems, and TCC instead of T psi C. All exhibit high G+C contents (about 50%); in contrast, the flanking regions are extremely A+T rich (about 80%). Several short coding frames can be deduced in these sequences, but their significance is not known.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Bonitz S. G., Coruzzi G., Nobrega M., Tzagoloff A. Transfer RNA genes in the cap-oxil region of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5017–5030. doi: 10.1093/nar/8.21.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlani R. E., Pentella C., Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: isolation of mitochondrial transfer ribonucleic acid mutants and characterization of transfer ribonucleic acid genes of Saccharomyces cerevisiae. J Bacteriol. 1980 Mar;141(3):1086–1097. doi: 10.1128/jb.141.3.1086-1097.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F., Meyer E. Does Paramecium primaurelia use a different genetic code in its macronucleus? Nature. 1985 Mar 14;314(6007):185–188. doi: 10.1038/314185a0. [DOI] [PubMed] [Google Scholar]
- Chi J. C., Suyama Y. Comparative studies on mitochondrial and cytoplasmic ribosomes of Tetrahymena pyriformis. J Mol Biol. 1970 Nov 14;53(3):531–556. doi: 10.1016/0022-2836(70)90082-3. [DOI] [PubMed] [Google Scholar]
- Chiu N., Chiu A., Suyama Y. Native and imported transfer RNA in mitochondria. J Mol Biol. 1975 Nov 25;99(1):37–50. doi: 10.1016/s0022-2836(75)80157-4. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wahleithner J. A., Wolstenholme D. R. Transfer RNA genes in Drosophila mitochondrial DNA: related 5' flanking sequences and comparisons to mammalian mitochondrial tRNA genes. Nucleic Acids Res. 1983 Apr 25;11(8):2411–2425. doi: 10.1093/nar/11.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. Genes for cytochrome c oxidase subunit I, URF2, and three tRNAs in Drosophila mitochondrial DNA. Nucleic Acids Res. 1983 Oct 11;11(19):6859–6872. doi: 10.1093/nar/11.19.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of sequences of tRNA genes. Nucleic Acids Res. 1983 Jan 11;11(1):r55–103. [PMC free article] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helftenbein E. Nucleotide sequence of a macronuclear DNA molecule coding for alpha-tubulin from the ciliate Stylonychia lemnae. Special codon usage: TAA is not a translation termination codon. Nucleic Acids Res. 1985 Jan 25;13(2):415–433. doi: 10.1093/nar/13.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köchel H. G., Lazarus C. M., Basak N., Küntzel H. Mitochondrial tRNA gene clusters in Aspergillus nidulans: organization and nucleotide sequence. Cell. 1981 Feb;23(2):625–633. doi: 10.1016/0092-8674(81)90158-6. [DOI] [PubMed] [Google Scholar]
- Larson R., Messing J. Apple II software for M13 shotgun DNA sequencing. Nucleic Acids Res. 1982 Jan 11;10(1):39–49. doi: 10.1093/nar/10.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C., Pham H. D., Donelson J. E. Sequence analysis of two yeast mitochondrial DNA fragments containing the genes for tRNA Ser UCR and tRNA Phe UUY. J Biol Chem. 1979 Nov 25;254(22):11735–11740. [PubMed] [Google Scholar]
- Newman D., Pham H. D., Underbrink-Lyon K., Martin N. C. Characterization of tRNA genes in tRNA region II of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5007–5016. doi: 10.1093/nar/8.21.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Rudman B. M., Barnett A. J. Deviation from the universal code shown by the gene for surface protein 51A in Paramecium. Nature. 1985 Mar 14;314(6007):188–190. doi: 10.1038/314188a0. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rüther U. pUR 250 allows rapid chemical sequencing of both DNA strands of its inserts. Nucleic Acids Res. 1982 Oct 11;10(19):5765–5772. doi: 10.1093/nar/10.19.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Preer J. R., Jr Mitochondiral DNA from protozoa. Genetics. 1965 Nov;52(5):1051–1058. doi: 10.1093/genetics/52.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. The genetic map of transfer RNA genes of yeast mitochondria: correction and extension. Mol Gen Genet. 1979 Mar 5;170(3):261–275. doi: 10.1007/BF00267059. [DOI] [PubMed] [Google Scholar]
- Wong J. F., Ma D. P., Wilson R. K., Roe B. A. DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res. 1983 Jul 25;11(14):4977–4995. doi: 10.1093/nar/11.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]


