Abstract
Since several different pathways are involved in cerebral ischemia/reperfusion injury, combination therapy rather than monotherapy may be required for efficient neuroprotection. In this study, we examined the protective effects of an apoptosis inhibitor Gly14-humanin (HNG) and a necroptosis inhibitor necrostatin-1 (Nec-1) on hypoxia/ischemia/reperfusion injury. Cultured mouse primary cortical neurons were incubated with Nec-1, HNG or both in a hypoxia chamber for 60 min. Cell viability was determined by MTS assay at 24 h after oxygen-glucose deprivation (OGD) treatment. Mice underwent middle cerebral artery occlusion for 75 min followed by 24 h reperfusion. Mice were administered HNG and/or Nec-1 (i.c.v.) at 4 h after reperfusion. Neurological deficits were evaluated and the cerebral infarct volume was determined by TTC staining. Nec-1 or HNG alone had protective effects on OGD-induced cell death. Combined treatment with Nec-1 and HNG resulted in more neuroprotection than Nec-1 or HNG alone. Treatment with HNG or Nec-1 reduced cerebral infarct volume from 59.3 ± 2.6% to 47.0 ± 2.3% and 47.1 ± 1.5%, respectively. Combined treatment with HNG and Nec-1 improved neurological scores and decreased infarct volume to 38.6 ± 1.5%. In summary, we demonstrated that the combination treatment of HNG and Nec-1 conferred synergistic neuroprotection on hypoxia/ischemia/reperfusion injury in vitro and in vivo. These findings provide a novel therapeutic strategy for the treatment of stroke by combining anti-apoptosis and anti-necroptosis therapy.
Keywords: Necrostatin-1, humanin, necroptosis, apoptosis, neuroprotection
1. Introduction
Stroke is the third leading cause of death, behind heart diseases and cancer, in the United States (Rosamond et al., 2007). Intravenous administration of the tissue plasminogen activator (t-PA) is the only therapy approved by the Food and Drug Administration (FDA) for the treatment of acute ischemic stroke within 3 h of symptom onset (1995). However, only a small percentage of patients with ischemic stroke are treated with t-PA for its narrow therapeutic window and contraindications of thrombolytic therapy (Wardlaw et al., 1997). Therefore, there is an urgent need for additional safe and effective treatments for ischemic stroke.
Humanin (HN) is a newly identified 24-amino acid anti-apoptotic peptide well known for its activity to suppress neuronal cell death induced by AD-related insults such as amyloid beta (Aβ) toxicity (Tajima et al., 2002). In our previous study, a highly potent HN variant Gly14-HN (replacement of the 14th amino acid serine with glycine, HNG) protected against cerebral ischemia/reperfusion injury in a mouse model (Xu et al., 2006a). Specifically, HNG reduced infarct volume, improved neurological deficits, and decreased the number of apoptotic neurons. The mechanism by which HNG achieves these effects involves decreasing cleaved poly(ADP-ribose)-polymerase (PARP, a marker of caspase activity), inhibiting extracellular signal regulated kinase (ERK) activation (Xu et al., 2006a), and activating PI3K/Akt signal pathways (Xu et al., 2008).
Recently, Degretev et al reported a novel type of cell death called necroptosis (Degterev et al., 2005). Importantly, they identified a specific necroptosis inhibitor necrostatin-1 (Nec-1) that reduced the infarct volume in a cerebral ischemia/reperfusion mouse model even when it was administered 6 h after reperfusion (Degterev et al., 2005). In a previous study, we showed that Nec-1 protects against glutamate-induced necroptosis in hippocampal HT-22 cells (Xu et al., 2007). These findings suggest that necroptosis exists in cerebral ischemia/reperfusion injury and that Nec-1 could represent a potential therapeutic intervention against this type of injury. Degterev et al. further indicated that RIP1 kinase is the cellular target for the anti-necroptosis activity of Nec-1 (Degterev et al., 2008). Our previous data also showed that Nec-1 inhibits BNIP3 translocation to inner membrane of mitochondria and indirectly blocked PARP/AIF-mediated cell death (Xu et al., 2007; Xu et al., 2010).
Since several different pathways are involved in cerebral ischemia/reperfusion injury, combination therapy rather than monotherapy may be required for efficient neuroprotection. (Gladstone et al., 2002; Grotta, 2002; Lo et al., 2003). Previous studies in animal models of stroke revealed pharmacological synergy by using two neuroprotective agents (Ma et al., 1998; Onal et al., 1997; Xu et al., 2006b). In this study, we designed a cocktail of an apoptosis inhibitor HNG and a necroptosis inhibitor Nec-1 that simultaneously acts on distinct cell death pathways in vitro and in vivo.
2. Results
HNG and Nec-1 have synergistic protective effect on OGD-induced neuronal death in vitro
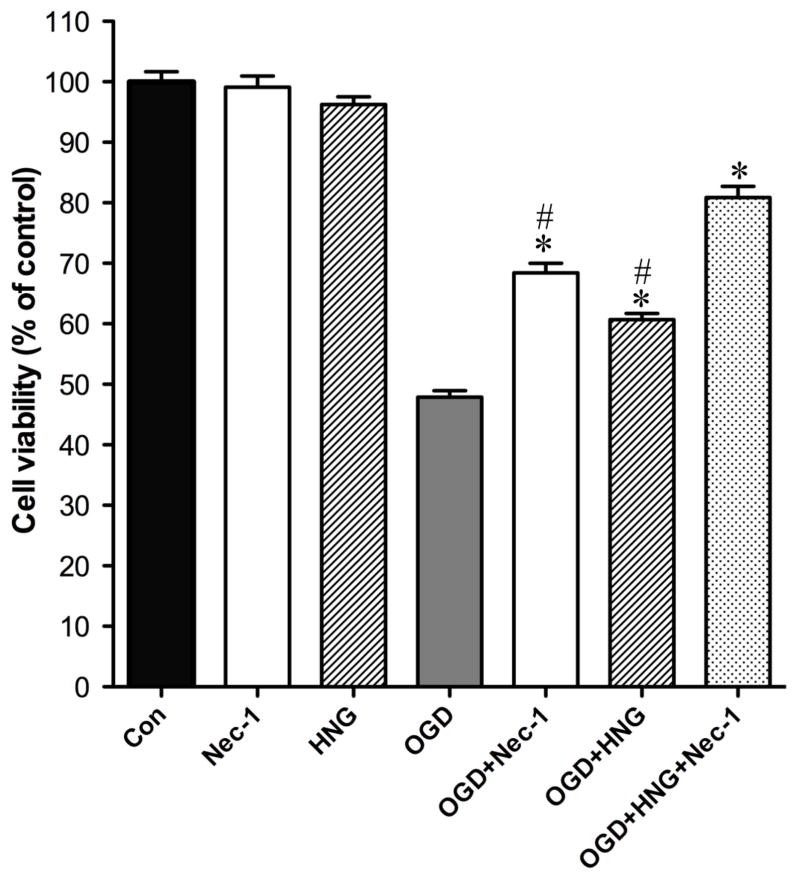
Cultured cortical neurons were subjected to oxygen-glucose deprivation (OGD) treatment at day 10 after culture. HNG alone (0.2 μM) or Nec-1 alone (25 μM) treatment had no effect on cell viability of normal primary cortical neurons (Figure 1). OGD-induced damage decreased cell viability to 47.9 ± 1.1% of the control. Nec-1 or HNG treatment significantly increased cell viability to 68.4 ± 1.6 % and 60.7 ± 1.0 % of the control, respectively (P<0.05). The combination of Nec-1 and HN increased cell viability to 80.8 ± 1.9 % of the control (P<0.05, versus other groups). These data indicated that Nec-1 and HNG have synergistic protective effect on OGD-induced cell death.
Figure 1.
Protective effect of Nec-1 and HNG on oxygen-glucose deprivation (OGD)-induced cell death in primary cortical neurons. Primary cortical neurons were used to perform OGD at DIV 10. Neurons were washed with HBSS and incubated with vehicle, Nec-1 (25 μM), HNG (0.2 μM), or both in a hypoxia chamber for 60 min. Cell viability was determined by MTS assay 24 h after reperfusion. Control neurons were exposed to oxygenated HBSS containing 5.5 mM glucose in normoxic conditions during the same time as the OGD culture. Bars represent mean ± SEM of 8 samples. *P<0.01, versus OGD group; #, P<0.01, versus OGD+HNG+Nec-1 group.
HNG and Nec-1 have additive protection on cerebral ischemia/reperfusion injury in vivo
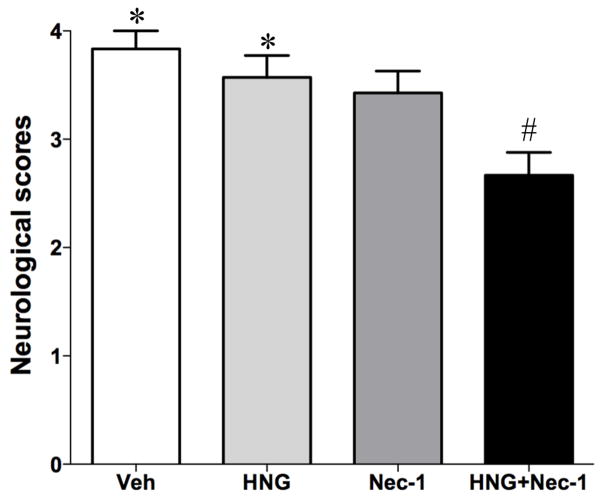
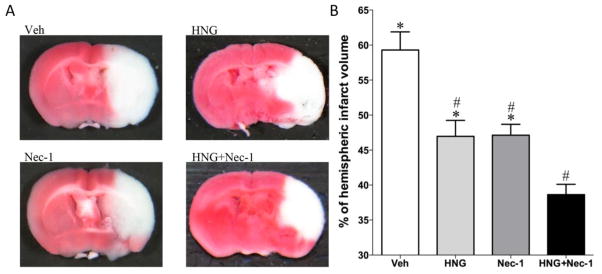
In this study, we examined the protective effects of HNG and Nec-1 on cerebral ischemia/reperfusion injury. Mice were treated with HNG and/or Nec-1 at 4 h after reperfusion. This time point was chosen because our previous study showed that HNG is effective at 4 h but not 6 h after ischemia/reperfusion (Xu et al., 2006a). Neurological deficits were evaluated by a scoring system. There was no significant difference between the HNG alone or Nec-1 alone group compared with the saline-treated group (P>0.05 in neurological scores). However, the combination of HNG and Nec-1 treatment significantly decreased neurological deficits when compared with saline-treated vehicle group (P<0.05, Figure 2). TTC staining demonstrated that HNG or Nec-1 alone reduced cerebral infarct volume from 59.3 ± 2.6% to 47.0 ± 2.3% and 47.1 ± 1.5%, respectively (P<0.05, Figure 3). HNG and Nec-1 treatment decreased the infarct volume to 38.6 ± 1.5%, which was significantly different from the HNG alone group or Nec-1 alone group (P<0.05, Figure 3).
Figure 2.
Protective effects of HNG and Nec-1 on neurological scores after cerebral ischemia/reperfusion injury. Mice were treated with HNG (0.1 μg in 5 μl saline and 1 μl DMSO) and/or Nec-1 (2.6 μg in 1 μl DMSO and 5 μl saline) after 75 min of ischemia and 4 h of reperfusion. Neurological deficits of the mice in four groups were evaluated. Bars represent mean ± SEM of 6–7 samples. *P<0.05, versus HNG+Nec-1 group; #, P<0.05, versus vehicle (Veh) group.
Figure 3.
Protective effects of HNG and Nec-1 on infarct volume. Mice were treated with HNG (0.1 μg in 5 μl saline and 1 μl DMSO) and/or Nec-1 (2.6 μg in 1 μl DMSO and 5 μl saline) after 75 min of ischemia and 4 h of reperfusion. Cerebral infarct volume was determined by TTC staining 24 h after reperfusion. (A) Representative pictures of different treatments. (B) Quantitative analysis of cerebral infarct volume in four groups was performed. Bars represent mean ± SEM of 6–7 samples. *P<0.05, versus HNG+Nec-1 group; #, P<0.05, versus vehicle (Veh) group.
3. Discussion
In this study, we investigated the efficacy of a drug cocktail treatment of two protective agents, HNG and Nec-1. These agents target different forms of cell death (apoptosis and necroptosis). In cultured primary cortical neurons, HNG and Nec-1 protected against OGD-induced cell death (Figure 1). When the drugs were administered 4 h after transient cerebral ischemia/reperfusion, the cocktail significantly decreased infarct size and neurological deficits in mice (Figures 2 and 3).
Since multiple pathways lead to neuronal cell death in stroke, it may be optimal to design therapies that target multiple pathways. HNG is a potent anti-apoptosis agent that inhibits caspase activity and decreases cleaved PARP level after ischemia injury (Xu et al., 2006a). Nec-1 is a necroptosis inhibitor that acts on a caspase-independent pathway and leads to the inhibition of BNIP3 and PARP-1/AIF-mediated cell death (Xu et al., 2007). Moreover, Nec-1 increases cellular glutathione in physiological and pathological conditions to prevent free radical formation. In this study, combined HNG/Nec-1 therapy conferred synergistic neuroprotection, validating the idea that inhibiting multiple pathways of neuronal cell death is a valuable strategy. HNG and Nec-1 have no toxic effects on normal cells in vitro as well as no side effects in animals (Degterev et al., 2005; Xu et al., 2006a), suggesting that this combined HNG/Nec-1 treatment could be a clinically useful option. These findings suggest a promising new therapeutic strategy for stroke by using a combination of anti-apoptosis and anti-necroptosis therapy. Further studies will need to be done to explore the therapeutic potential of this cocktail.
4. Methods and Materials
Materials and Animals
Humanin (HNG) was a product from Peptide International, Inc. (Lexington, KY). Nec-1 was obtained from Chembridge Corporation (San Diego, CA). CellTiter 96* nonradioactive cell proliferation assay (MTS assay) kit was purchased from Promega Corporation (Madison, WI).
Male CD-1 mice, 25–30g, were purchased from Harlan (Indianapolis, IN). All animal procedures were approved by the University Committee on Animal Care and Use of East Tennessee State University.
Middle cerebral artery occlusion model
We used an intraluminal occlusion method with subsequent reperfusion as described previously (Xu et al., 2006a). Briefly, the right common carotid artery, the right external carotid artery, and the internal carotid artery were exposed through a ventral midline neck incision. A 6-0 nylon monofilament (Ethicon, Ethicon Inc., Somerville, NJ) coated with silicon resin (Heraeus, Kulzer, Germany) was introduced into the right external carotid artery and advanced until a faint resistance was felt. Reperfusion was achieved by withdrawing the suture after 75 min of occlusion. Body temperature was maintained at 36.5–37.5°C by using a heating pad and a lamp throughout the procedure from the start of the surgery until the animals recovered from anesthesia. Occlusion and reperfusion of the middle cerebral artery was monitored by a laser Doppler blood flowmeter (Periflux 5010, PERIMED, Sweden) positioned 1 mm posterior and 3 mm lateral to the bregma bilaterally. In our study, any mouse with incomplete reperfusion or SAH examined before TCC was excluded from this study.
Animal experimental groups and agent administration
In each experiment, animals were randomly divided into four groups (n=6–7): (1) vehicle-treated I/R group, the mice were administered intraventricularly 5 μl saline and 1 μl dimethyl sulfoxide (DMSO); (2) HNG-treated I/R group, the mice were treated with 0.1 μg HNG in 5 μl saline and 1 μl DMSO; (3) Nec-1-treated I/R group, the mice were treated with 2.6 μg Nec-1 in 1 μl DMSO and 5 μl saline ; (4) HNG and Nec-1-treated I/R group, the mice were treated with 0.1 μg HNG in 5 μl saline and 2.6 μg Nec-1 in 1 μl DMSO. HNG, Nec-1, or vehicle was administered at 4 h after reperfusion. For the injection into the lateral ventricle, a small burr hole was made in the parietal region (0.5 mm posterior and 1.0 mm lateral to the bregma on the left side). A 28G needle on a Hamilton syringe was inserted into the left lateral ventricle 2.5 mm in depth. We verified the correct injection site by using Evans’ blue. No mice died of intraventricular injection or a drug side effect. We measured regional CBF immediately before injection surgery and before MCAO surgery and found that the injection did not cause regional CBF change.
Neurological deficit scoring evaluation
Neurological deficits were measured according to the following graded scoring system 24 h after the MCAO. The modified scoring system was based on a 5-point scale system described (Yang and Betz, 1994): 0, no deficit; 1, flexion of the contralateral torso and forelimb; 2, turning to the ipsilateral side when held by tail; 3, leaning to affected side; 4, no spontaneous locomotor activity. If no deficit was observed after MCAO, the animal was removed from further study.
Evaluation of infarct volume
After neurological evaluation, all animals were anesthetized, decapitated and the brains were removed. The brains with subarachnoid hemorrhage and/or clot formation in the MCA were eliminated from the analysis in this study. All brains were sliced into 1 mm sections. Slices were incubated for 30 min in a 0.1% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO) at 37°C and then fixed in 10% buffered formaldehyde solution. For analysis, the sections were photographed by a high-resolution digital camera (Nikon Coolpix 5700). The cross-sectional area of infarction in the right MCA territory of each brain slice was determined with a computerized image analysis system (AlphaEase Image Analysis Software V 3.1.2). The total mean infarct area of each section was calculated as the average of the area on its rostral and the caudal surface. The hemispheric lesion volume was calculated by multiplying the area by the thickness of slices. The areas of the infarcted tissue and the areas of both hemispheres were calculated for each brain slice. The percentage of hemispheric infarction volume was calculated as described (Giffard and Swanson, 2005).
Primary mouse cortical neuron culture and the induction of oxygen-glucose deprivation (OGD)
Embryonic day 16-18 mice were obtained from anesthetized pregnant CD-1 mice. Skin, skull, and meninges were carefully removed and cerebral cortices were isolated from the mouse brains. Cerebral cortices were dissociated with papain (8.2 U/ml, Worthington Biochemical, Lakewood, NJ) for 30 min at 37°C. Fetal bovine serum and trypsin inhibitor were used to stop the digestion. Tissues were then triturated with a Pasteur pipette. Freshly dissociated cells were seeded at 2×105 cells/cm2 into 96-well plastic plates coated with L-polyornithine (10 μg/ml) and incubated in Neurobasal medium (Invitrogen, Carlsbad, CA) containing 2% B-27 supplement, Glutamax (1:100) (Invitrogen), penicillin, and streptomycin at 37°C. The medium was changed 24 h after plating and half of the medium was changed every 3 d. OGD experiments were conducted on the DIV 10. Immunohistochemical analysis of neuronal marker protein gene product 9.5 (Chemicon International, Inc., Temecula, CA) revealed that the neurons were 90% pure. Neurons in 96-well plate were rinsed with 1x Hank’s balanced salt solution (HBSS, Invitrogen) containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM HEPES, 30 μM glycine, pH 7.4. Cultured cortical neurons were incubated with HNG (0.2 μM) and/or Nec-1 (25 μM) in pre-gassed HBSS buffer and then the plate was placed in a Billups-Rothenberg modular incubator chamber (Del Mar, CA), flushed with a mixed gas of 5% CO2 and 95% N2 for 10 min. The chamber was then sealed and placed into a humidified incubator at 37°C. After 60 min in the hypoxic chamber, the OGD treatment was stopped by replacing HBSS with Neurobasal medium supplemented with B27 containing HNG and/or Nec-1. The plate was placed back to normoxic conditions and incubated for 24 h for the cell viability determination. Control culture plate was exposed to oxygenated HBSS containing 5.5 mM glucose in normoxic conditions during the same time as the OGD culture.
Cell viability assay
Cell viability was assessed by the ability of the viable cells to metabolize 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)2-H-tetrazolium, inner salt (MTS), as described previously (Cory et al., 1991). At 24 h after OGD treatment, 10 μl of MTS solution (5 mg/ml) was added to each well, and the cells were maintained in the growth medium for 3 h at 37°C. Absorbance was subsequently measured at 490 nm. Untreated cells were considered as the control, and the growth medium without cells in the presence of MTS solution was used as a solution background. Cell viability was calculated as the percentage of the untreated controls. For the MTS assay, there were eight samples in each group, and the experiment was repeated at least 3 times.
Statistical analysis
All data are expressed as mean ± SEM. Differences among groups were compared by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test if there was a significant difference between groups. Differences were deemed statistically significant if P <0.05.
Acknowledgments
This study was supported by grants from HL087271 and HL096099, American Heart Association-Southeast Affiliate, and VA Merit Review (to BHLC). This study was also supported by the grant 30700245 from the National Natural Science Foundation of China (to XX).
Abbreviations
- Nec-1
necrostatin-1
- HNG
Gly14-humanin
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)2-H-tetrazolium, inner salt
- OGD
oxygen-glucose deprivation
- TTC
2,3,5-triphenyltetrazolium chloride
- ERK
extracellular signal regulated kinase,
- TNFα
tumor necrosis factor-α
- MCAO
middle cerebral artery occlusion,
- DMSO
dimethyl sulfoxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 3.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 4.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giffard RG, Swanson RA. Ischemia-induced programmed cell death in astrocytes. Glia. 2005;50:299–306. doi: 10.1002/glia.20167. [DOI] [PubMed] [Google Scholar]
- 6.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 7.Grotta J. Neuroprotection is unlikely to be effective in humans using current trial designs. Stroke. 2002;33:306–7. [PubMed] [Google Scholar]
- 8.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Endres M, Moskowitz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol. 1998;124:756–62. doi: 10.1038/sj.bjp.0701871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onal MZ, Li F, Tatlisumak T, Locke KW, Sandage BW, Jr, Fisher M. Synergistic effects of citicoline and MK-801 in temporary experimental focal ischemia in rats. Stroke. 1997;28:1060–5. doi: 10.1161/01.str.28.5.1060. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 12.Tajima H, Niikura T, Hashimoto Y, Ito Y, Kita Y, Terashita K, Yamazaki K, Koto A, Aiso S, Nishimoto I. Evidence for in vivo production of Humanin peptide, a neuroprotective factor against Alzheimer's disease-related insults. Neurosci Lett. 2002;324:227–31. doi: 10.1016/s0304-3940(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Warlow CP, Counsell C. Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet. 1997;350:607–14. doi: 10.1016/s0140-6736(97)03022-5. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006a;37:2613–9. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–14. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BH. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. 2008;1227:12–8. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Chua CC, Zhang M, Geng D, Liu CF, Hamdy RC, Chua BH. The role of PARP activation in glutamate-induced necroptosis in HT-22 cells. Brain Res. 2010;1343:206–12. doi: 10.1016/j.brainres.2010.04.080. [DOI] [PubMed] [Google Scholar]
- 18.Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res. 2006b;1102:154–62. doi: 10.1016/j.brainres.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 19.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–64. doi: 10.1161/01.str.25.8.1658. discussion 1664–5. [DOI] [PubMed] [Google Scholar]