Abstract
Background
Human metapneumovirus (HMPV) is a paramyxovirus with multiple genetic lineages that is a leading cause of acute respiratory disease. Several RT-PCR assays have been described based on limited available sequence data.
Objectives
To develop a broadly reactive real-time RT-PCR assay for HMPV that allows for a rapid, sensitive, and specific detection in a clinical or research setting.
Study Design
Three published assays for HMPV were modified based on analysis of multiple HMPV sequences obtained from GenBank. Original and modified assays were tested against prototype HMPV strains from each genetic sublineage, multiple isolates of HMPV from different years, a collection of clinical specimens, and commercial validation panels.
Results
A number of potential sequence mismatches with diverse HMPV strains were identified. Modifications were made to oligonucleotides to improve annealing efficiency. Primers and probes based on newer sequence data offered enhanced detection of all subgroups, especially for low titer specimens. The new primers and probe detected multiple clinical isolates of HMPV collected over a twenty-year period. The modified assay improved detection of HMPV in a panel of clinical specimens, and correctly identified HMPV samples in two commercial validation sets.
Conclusions
We report a modified real-time RT-PCR assay for HMPV that detects all genetic lineages with high sensitivity.
Keywords: Human metapneumovirus, diagnostics, real-time RT-PCR
BACKGROUND
Human metapneumovirus (HMPV) is a paramyxovirus isolated from children with lower respiratory infection in the Netherlands in 20011. HMPV is a leading cause of acute respiratory infection (ARI) in children and adults, with symptoms similar to those caused by other respiratory viruses2-14. Several RT-PCR assays to detect HMPV have been published14-21. However, there are two major genetic lineages of HMPV, each with minor sublineages that exhibit substantial diversity22-28. Published assays have been based on limited sequence data and some vary in sensitivity between viral lineages16,21. Due to the differential transcription of paramyxoviruses, N is the most highly transcribed gene30 and both HMPV F and N genes are relatively conserved23,24,27,31. Thus, the fusion (F) and nucleocapsid (N) genes have been widely used as RT-PCR targets14-21,29.
OBJECTIVES
We sought to develop an improved HMPV real-time RT-PCR assay based on the most recent available sequence data to enhance detection of diverse strains of HMPV from all four lineages.
STUDY DESIGN
Sequence analysis
Published full-length and partial N and full-length F gene sequences were obtained from GenBank or strains sequenced in our laboratory8,12,27,31,32. Viral sequences and published primer and probe sequences were aligned with MacVector 11 (MacVector). GenBank sequences used are listed in Supplemental Table 1. Primers and probe were designed using Primer Express 2.0 (Applied Biosystems).
Viruses, clinical specimens, and reference panels
Plaque-purified prototype HMPV strains were cultured and titered on LLC-MK2 cells33,34. Viruses isolated in the Vanderbilt Vaccine Clinic8,12 were passaged until cytopathic effects were visible and confirmed by immunofluorescence using anti-HMPV antisera33. Clinical specimens were obtained from adult and pediatric patients with ARI. Nasal and throat swabs were collected and combined in transport medium (Beckton Dickinson) and aliquoted into MagMAX Lysis/Binding Solution Concentrate (Applied Biosystems) or MagNAPure LC Total Nucleic Acid Isolation Kit lysis/binding buffer (Roche), snap frozen, and stored at −80°C. Reference proficiency panels were obtained from the Quality Control For Molecular Diagnostics (QCMD)35,36 and extracted by the same methods.
RNA extraction
RNA was extracted from thawed specimens according to the manufacturer’s protocols for the MagMAX-96 Viral RNA Isolation Kit (Applied Biosystems) or MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche).
Real-time RT-PCR assay
One-step reactions were prepared using AgPath-ID One-Step RT-PCR kit (Ambion) according to the manufacturer’s instructions with 1 μM forward and reverse primers and 0.25 μM probe. Cycling parameters were 50°C × 30 min, 95°C × 10 min and 45 cycles of 95°C × 15 sec and 60°C × 30 sec, with fluorescence data collected during the 60°C annealing/extension step. Primer/probe sets were also tested using SuperScript III Platinum One-Step qRT-PCR kit (Invitrogen) according to the manufacturer’s instructions with the same primer/probe concentrations; results were similar to the AgPath-ID kit (not shown). Amplifications were performed using the StepOne Plus (Applied Biosystems). All clinical specimens were tested in a separate real-time RT-PCR assay for RNAse P to ensure RNA integrity and exclude PCR inhibition19. To generate RNA runoff transcripts, target genes were cloned into pGEM (Promega) under a T7 promoter, transcribed in vitro from HincII-digested plasmids using T7 RNApol (NEB), purified, and quantified by spectrophotometry. RNA transcripts were used as positive controls and nuclease-free water as negative control.
RESULTS
Sequence analysis
One hundred twenty full-length F sequences and a published F-targeted assay were aligned (Supplemental Figure 1)19. While the target sequences were generally conserved, there were several polymorphisms detected in numerous strains of HMPV. Thus, nucleotide changes were introduced into the forward and reverse primers as shown in Table 1 to increase degeneracy. The probe sequence was reversed and complemented to increase the number of cytosine residues, and extended slightly to increase the Tm.
Table 1.
Primer and probe sequences tested.
| Assay | Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Probe (5′ to 3′) | Ref. |
|---|---|---|---|---|---|
| NL-N | N | CATATAAGCATGCTATATTAAAAGAGTCTC | CCTATTTCTGCAGCATATTTGTAATCAG | TGYAATGATGAGGGTGTCACTGCGGTTG | 21 |
| NL-N 2 | N | CATAYAARCATGCTATATTAAAAGAGTCTC | CCTATYTCWGCAGCATATTTGTAATCAG | CAACHGCAGTRACACCYTCATCATTRCA | * |
| CDC | F | CAAGTGTGACATTGCTGAYCTRAA | ACTGCCGCACAACATTTAGRAA | TGGCYGTYAGCTTCAGTCAATTCAACAGA | 19 |
| CDC 2 | F | CAARTGYGACATTGMTGAYCTRAA | AYTGCCGCACAACATTTAGRAA | CTTCTGTTGAATTGACTGAAGCTRACRGCCA | * |
| UR 2 | N | CATGCTATATTAAAAGAGTCTCA | TCWGCAGCATATTTGTAATCAG | CAACHGCAGTRACACCYTCATCAATGCA | 14 * |
| VU | N | ATGTCTCTTCAAGGGATTCACC | ATYTCTTGYTGCAATGATGARG | TCATAYAARCATGCTATATTAAAAGAGTCTCARTACACA | § |
Primers were purchased from Invitrogen and were normal phase chromatography desalted. Probes were purchased from Operon and were reverse phase high performance liquid chromatography (HPLC) purified. Probes were 5′-labeled with 6-FAM and 3′-labeled with Black Hole Quencher-1 (BHQ-1).
= modified from published sequences as described in text; UR = University of Rochester; NL = Netherlands; VU = Vanderbilt University
= this study
H = A, C, or T; R = A or G; W = A or T; Y = C or T
Three hundred fifty-two partial or full N sequences were aligned with published real-time RT-PCR assays targeting the N gene14-18,20,21. Many of these were partial and non-overlapping, so that the number of viral sequences compared against any single primer/probe ranged from 45 to 85 (not shown). Some published assays had several mismatches with diverse viruses and were not pursued further. However, two published assays were generally well matched to N sequences (Supplemental Figures 2 and 3)14,21. One of these (UR for) contained a “CG” that was likely a typographic error in the published manuscript14, since all N sequences encoded “GC” at this position. This and other nucleotide modifications were made to increase degeneracy or adjust Tm. Both the NL-N and UR probes were reversed and complemented to increase the number of cytosine residues (Table 1).
Assay performance against 4 HMPV prototypes
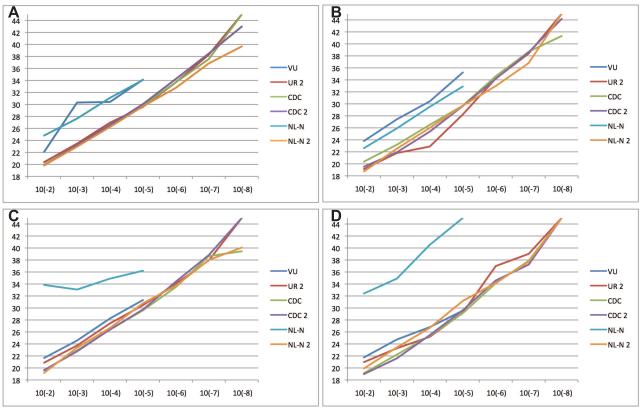
We prepared serial ten-fold dilutions of RNA extracted from each of four fully sequenced HMPV prototypes representing each of the four subgroups: TN96-12 (A1), TN94-49 (A2), TN98-242 (B1), and TN99-419 (B2)27,28. The infectious titer of the stocks ranged from 1.4 × 106 to 3.7 × 106 pfu/ml. We tested each of the primer/probe sets initially against dilutions of viral RNA from 10−2 to 10−5 (Figure 1A-D). All six assays reacted against all four subgroups, though the original NL-N assay performed poorly against subgroup B viruses and the VU assay was less sensitive for subgroup A viruses. The other assays tested exhibited linear performance and were tested at dilutions from 10−2 to 10−8 (Figure 1A-D). In this series of experiments, all four assays performed well. The NL-N 2 assay exhibited detection at slightly lower Ct for A1, A2, and B1 viruses, with the original CDC assay also performing well against B1 virus.
Figure 1. Performance of assays tested against prototype HMPV strains.
Plots of cycle threshold (CT) versus RNA serial 10-fold dilutions. A) TN96-12 A1. B) TN94-49 A2. C) TN98-242 B1. D) TN99-419 B2. Y axis = CT, x-axis = dilution.
Performance against clinical specimens and clinical isolates
We tested the NL-N 2 assay against a panel of 222 clinical specimens collected from adults hospitalized with ARI37-39 that had been tested previously with the original NL-N assay. The original assay detected three (1.4%) HMPV-positive specimens; the NL-N 2 assay detected 11 (5%) HMPV-positive specimens. We also tested the NL-N and NL-N 2 assay against a collection of 222 specimens obtained from infants with ARI40,41. The original NL-N assay detected two (1%) HMPV-positive specimens, while the NL-N 2 assay detected seven (3.2%) HMPV-positive specimens. All clinical specimens tested positive for RNAse P. Finally, we tested the NL-N 2 assay against a panel of 42 cultured HMPV isolates collected between 1982 to 2003, comprising all four subgroups, and the assay detected all isolates. The NL-N 2 assay did not react with RNA extracted from cultured isolates or clinical specimens positive for influenza viruses A/B, human rhinovirus, respiratory syncytial virus, and parainfluenza viruses 1-3 (not shown). The NL-N 2 assay was tested against serial 10-fold dilutions of RNA runoff transcripts and the limit of detection was <50 copies.
Performance against External Quality Assessment (EQA) panels
Two previous QCMD panels were available for HMPV testing, the 2006 MPV.RSV06 and 2009 MPV.RSVRNA09 (www.qcmd.org)40, 42. The 2006 EQA panel comprised cultured A1 and B1 viruses at dilutions from 10−3 to 10−7; all were detected by the NL-N 2 assay except one sample containing A1 at 2×10−6 dilution. This sample tested negative in all three QCMD in-house reference laboratories and was only detected by 27% of QCMD participating laboratories. The 2009 EQA panel comprised A1 and B2 viruses at dilutions from 10−4 to 10−6; all were detected by the NL-N 2 assay, including low titer specimens that were infrequently detected by other QCMD participating laboratories. RSV-positive specimens and negative controls in both EQA panels tested negative for HMPV with the NL-N 2 assay.
DISCUSSION
Human metapneumovirus is a leading cause of ARI in children and adults worldwide. Reliable diagnosis for clinical or research use is dependent on genome detection methods, since the virus is difficult to culture and there are no commercially available rapid antigen tests. Here we report a modified HMPV real-time RT-PCR assay based on more recently available sequence data. HMPV, like other RNA viruses, exhibits a substantial rate of mutation due to the error-prone RNA polymerase44 and thus significant genetic diversity22, 23, 27, 28, 31. The increase in available HMPV sequences facilitates the analysis of multiple strains to optimize oligonucleotide design.
A strength of this study is the use of sequences from disparate geographic sites and times, dating back to 1982. While HMPV, like other paramyxoviruses, undergoes mutations and genetic variation, these nucleotide changes do not result in progressive antigenic “drift” over time24,31,45,46. Thus, sequence-based assays for HMPV targeting conserved regions are likely to remain capable of detecting future circulating strains. Further, N is the most highly conserved HMPV gene27.
There are limitations to our study. We did not test all published primer/probe sets, and some might have performed well despite nucleotide mismatches; in silico analysis does not always predict in vitro performance. However, we tested assays that have been used in multiple studies of HMPV epidemiology14,19,21. All assays performed well, though with sequence-based modifications the performance was enhanced. We tested these primer/probe sets with two chemistries with similar results, though some viral assays exhibit extreme variability in performance with different chemistries47. Validation with specific instruments and kits is necessary for all molecular assays.
In summary, we developed an improved HMPV real-time RT-PCR assay based on comparison of hundreds of HMPV gene sequences. The assay exhibited linear performance against all four viral subgroups, detected viruses from diverse lineages over time, enhanced detection of HMPV in clinical specimens, and performed well in EQA proficiency panels. This assay should be useful for ongoing epidemiology studies.
Supplementary Material
Supplemental Figure 1. Aligned partial HMPV F sequences with original and modified primers and probes. All oligonucleotide sequences are shown in coding sense for ease of alignment; actual primer and probe sequences are listed in Table 1. Published assay = CDC; modified = CDC 2. Only the portion of the F sequence alignment around the assay target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.
Supplemental Figure 2. Aligned partial HMPV N sequences with original and modified forward primers. All oligonucleotide sequences are shown in coding sense for ease of alignment; primer sequences are listed in Table 1. Published assays = NL-N, UR; modified = NL-N 2, UR 2. Only the portion of the N sequence alignment around the forward primer target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.
Supplemental Figure 3. Aligned partial HMPV N sequences with original and modified probes and reverse primers. All oligonucleotide sequences are shown in coding sense for ease of alignment; 5′ to 3′ primer and probe sequences are listed in Table 1. Published assays = NL-N, UR; modified = NL-N 2, UR 2. Only the portion of the N sequence alignment around the probe and reverse primer target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.
ACKNOWLEDGMENTS AND COI
The authors thank Marie R. Griffin for providing specimens from clinical research studies.
Financial support: Supported by NIH AI-82417 and AI-085062 to JVW; Vanderbilt CTSA grant NIH/NCRR UL1-RR024975, Thrasher Research Fund Clinical Research Grant (TVH), NIH AI-077930 (TVH), NIH U01 HL-072471 (TVH); AI074863 (HKT); AI-25462 (KME).
Footnotes
Conflict of interest information: KME has received funding from the NIH and the CDC to evaluate the impact of influenza vaccines and study new influenza vaccines. HKT has received research funds from sanofi pasteur. JVW serves on the Scientific Advisory Board of Quidel.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 3.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Hara M, et al. Human metapneumovirus infection in Japanese children. J Clin Microbiol. 2004;42:126–32. doi: 10.1128/JCM.42.1.126-132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdam AJ, Hasenbein ME, Feldman HA, Cole SE, Offermann JT, Riley AM, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–6. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 6.Mullins JA, Erdman DD, Weinberg GA, Edwards K, Hall CB, Walker FJ, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerging infectious diseases. 2004;10:700–5. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 8.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englund JA, Boeckh M, Kuypers J, Nichols WG, Hackman RC, Morrow RA, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–9. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–57. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloots TP, Mackay IM, Bialasiewicz S, Jacob KC, McQueen E, Harnett GB, et al. Human metapneumovirus, Australia, 2001-2004. Emerg Infect Dis. 2006;12:1263–6. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JV, Wang CK, Yang CF, Tollefson SJ, House FS, Heck JM, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–95. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G, De Serres G, Hamelin ME, Cote S, Argouin M, Tremblay G, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44:1152–8. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 14.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouscambert-Duchamp M, Lina B, Trompette A, Moret H, Motte J, Andreoletti L. Detection of human metapneumovirus RNA sequences in nasopharyngeal aspirates of young French children with acute bronchiolitis by real-time reverse transcriptase PCR and phylogenetic analysis. J Clin Microbiol. 2005;43:1411–4. doi: 10.1128/JCM.43.3.1411-1414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cote S, Abed Y, Boivin G. Comparative evaluation of real-time PCR assays for detection of the human metapneumovirus. J Clin Microbiol. 2003;41:3631–5. doi: 10.1128/JCM.41.8.3631-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dare R, Sanghavi S, Bullotta A, Keightley MC, George KS, Wadowsky RM, et al. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol. 2007;45:548–52. doi: 10.1128/JCM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins MJ, Redmond C, Shaw JM, Hart IJ, Hart CA, Smyth RL, et al. Detection and characterisation of human metapneumovirus from children with acute respiratory symptoms in north-west England, UK. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;42:273–9. doi: 10.1016/j.jcv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, Anderson LJ, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–82. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay IM, Jacob KC, Woolhouse D, Waller K, Syrmis MW, Whiley DM, et al. Molecular assays for detection of human metapneumovirus. J Clin Microbiol. 2003;41:100–5. doi: 10.1128/JCM.41.1.100-105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maertzdorf J, Wang CK, Brown JB, Quinto JD, Chu M, de Graaf M, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–6. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastien N, Liu L, Ward D, Taylor T, Li Y. Genetic variability of the G glycoprotein gene of human metapneumovirus. J Clin Microbiol. 2004;42:3532–7. doi: 10.1128/JCM.42.8.3532-3537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boivin G, Mackay I, Sloots TP, Madhi S, Freymuth F, Wolf D, et al. Global genetic diversity of human metapneumovirus fusion gene. Emerging infectious diseases. 2004;10:1154–7. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Graaf M, Osterhaus AD, Fouchier RA, Holmes EC. Evolutionary dynamics of human and avian metapneumoviruses. J Gen Virol. 2008;89:2933–42. doi: 10.1099/vir.0.2008/006957-0. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro N, Ebihara T, Endo R, Ma X, Kikuta H, Ishiko H, et al. High genetic diversity of the attachment (G) protein of human metapneumovirus. J Clin Microbiol. 2004;42:3406–14. doi: 10.1128/JCM.42.8.3406-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay IM, Bialasiewicz S, Jacob KC, McQueen E, Arden KE, Nissen MD, et al. Genetic diversity of human metapneumovirus over 4 consecutive years in Australia. The Journal of infectious diseases. 2006;193:1630–3. doi: 10.1086/504260. [DOI] [PubMed] [Google Scholar]
- 27.Piyaratna R, Tollefson SJ, Williams JV. Genomic analysis of four human metapneumovirus prototypes. Virus Res. 2011 doi: 10.1016/j.virusres.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Hoogen BG, Herfst S, Sprong L, Cane PA, Forleo-Neto E, de Swart RL, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–66. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuypers J, Wright N, Corey L, Morrow R. Detection and quantification of human metapneumovirus in pediatric specimens by real-time RT-PCR. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005;33:299–305. doi: 10.1016/j.jcv.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins PL, Wertz GW. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:3208–12. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CF, Wang CK, Tollefson SJ, Piyaratna R, Lintao LD, Chu M, et al. Genetic diversity and evolution of human metapneumovirus fusion protein over twenty years. Virol J. 2009;6:138. doi: 10.1186/1743-422X-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JV, Edwards KM, Weinberg GA, Griffin MR, Hall CB, Zhu Y, et al. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–8. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tollefson SJ, Cox RG, Williams JV. Studies of culture conditions and environmental stability of human metapneumovirus. Virus Res. 2010;151:54–9. doi: 10.1016/j.virusres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. Journal of virology. 2005;79:10944–51. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace PS. Linkage between the journal and Quality Control Molecular Diagnostics (QCMD) J Clin Virol. 2003;27:211–2. doi: 10.1016/s1386-6532(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 36.Loens K, van Loon AM, Coenjaerts F, van Aarle Y, Goossens H, Wallace P, et al. Performance on a multipathogen External Quality Assessment (EQA) panel by different mono- and multiplex nucleic acid amplification tests. J Clin Microbiol. 2011 doi: 10.1128/JCM.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poehling KA, Talbot HK, Williams JV, Zhu Y, Lott J, Patterson L, et al. Impact of a school-based influenza immunization program on disease burden: comparison of two Tennessee counties. Vaccine. 2009;27:2695–700. doi: 10.1016/j.vaccine.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talbot HK, Poehling KA, Williams JV, Zhu Y, Chen Q, McNabb P, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27:1923–7. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talbot HK, Williams JV, Zhu Y, Poehling KA, Griffin MR, Edwards KM. Failure of routine diagnostic methods to detect influenza in hospitalized older adults. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010;31:683–8. doi: 10.1086/653202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali SA, Gern JE, Hartert TV, Edwards KM, Griffin MR, Miller EK, et al. Real-World Comparison of Two Molecular Methods for Detection of Respiratory Viruses. Virol J. 2011;8:332. doi: 10.1186/1743-422X-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartert TV, Carroll K, Gebretsadik T, Woodward K, Minton P. The Tennessee Children’s Respiratory Initiative: Objectives, design and recruitment results of a prospective cohort study investigating infant viral respiratory illness and the development of asthma and allergic diseases. Respirology. 2010;15:691–9. doi: 10.1111/j.1440-1843.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khuri-Bulos N, Williams JV, Shehabi AA, Faouri S, Al Jundi E, Abushariah O, et al. Burden of respiratory syncytial virus in hospitalized infants and young children in Amman, Jordan. Scand J Infect Dis. 2010;42:368–74. doi: 10.3109/00365540903496544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–32. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 44.Drake JW. Rates of spontaneous mutation among RNA viruses. Proc Natl Acad Sci U S A. 1993;90:4171–5. doi: 10.1073/pnas.90.9.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rima BK, Earle JA, Baczko K, ter Meulen V, Liebert UG, Carstens C, et al. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78(Pt 1):97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- 46.Tamin A, Rota PA, Wang ZD, Heath JL, Anderson LJ, Bellini WJ. Antigenic analysis of current wild type and vaccine strains of measles virus. J Infect Dis. 1994;170:795–801. doi: 10.1093/infdis/170.4.795. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–9. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Aligned partial HMPV F sequences with original and modified primers and probes. All oligonucleotide sequences are shown in coding sense for ease of alignment; actual primer and probe sequences are listed in Table 1. Published assay = CDC; modified = CDC 2. Only the portion of the F sequence alignment around the assay target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.
Supplemental Figure 2. Aligned partial HMPV N sequences with original and modified forward primers. All oligonucleotide sequences are shown in coding sense for ease of alignment; primer sequences are listed in Table 1. Published assays = NL-N, UR; modified = NL-N 2, UR 2. Only the portion of the N sequence alignment around the forward primer target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.
Supplemental Figure 3. Aligned partial HMPV N sequences with original and modified probes and reverse primers. All oligonucleotide sequences are shown in coding sense for ease of alignment; 5′ to 3′ primer and probe sequences are listed in Table 1. Published assays = NL-N, UR; modified = NL-N 2, UR 2. Only the portion of the N sequence alignment around the probe and reverse primer target region is shown. Sequences are arranged from top to bottom grouped as A1, A2, B1 and B2 sublineages.