Abstract
Several lines of evidence have implicated a direct reciprocal interaction between serotonin and nitric oxide. The goal of this investigation was, therefore, to examine the co-expression of tryptophan hydroxylase (TPH; the rate limiting enzyme for the synthesis of serotonin) and neuronal nitric oxide synthase (nNOS) in the ascending cortical projecting raphe nuclei (B6–B9 subgroups), as compared to the descending spinal cord projecting raphe nuclei (B1–B3 subgroups). Our data demonstrated that: 1) a significant number of raphe-cortical projecting neurons was identified not only in the midline subgroup of dorsal raphe (DR; B6,7) but also in the median raphe (MR; B8), as well as in the supralemniscal nucleus (SLN; B9); 2) serotonergic cortical projecting neurons from these three raphe nuclei exhibited a high (>80%) percentage of co-expression with nNOS immunoreactivity; 3) similarly, serotonin transporter (SERT) immunoreactive fibers in the medial prefrontal cortex (mPFC) were also double-labeled with nNOS immunoreactivity; 4) in contrast, the descending spinal cord projecting raphe nuclei revealed only TPH but not nNOS immunoreactivity. Our present findings suggest the existence of a direct interaction between serotonin and nitric oxide (NO) in the ascending cortical projecting raphe system. In contrast, a different strategy appears to operate the descending spinal cord projecting raphe system.
Keywords: Medial prefrontal cortex, dorsal raphe, median raphe, supralemniscal nucleus
Introduction
Traditionally, nine brainstem serotonin-containing cell groups, which were termed B1–B9, contain most of serotonin positive neurons in the central nervous system (Dahlstrom and Fuxe, 1964, 1965). Although rostral raphe nuclei, i.e., dorsal raphe (DR; B6, 7), median raphe (MR; B8) and supralemniscal nucleus (SLN; B9), have been reported projecting to various cortical areas (Moore et a., 1978; O’Hearn and Molliver, 1984; Johnson and Ma, 1993), DR and MR are considered as major serotonergic sources to the cortex (Azmitia and Segal, 1978; Steinbusch et al., 1980; Vertes and Martin, 1988; Tork 1990). In contrast, SLN has received much less attention and has been regarded earlier as a minor serotonergic input to the cortex (Takeuchi et al., 1982; O’Hearn and Molliver, 1984). However, recent evidences have shown a large number of serotonergic cells within SLN in different species (Hornung and Fritschy, 1988; Baker et al., 1991; Vertes and Crane, 1997), and the SLN in rat, for example, has been shown containing as many serotonergic neurons as the MR (~4,000 neurons), and it constitutes about 1/3 of the number of serotonin positive neurons in the DR (~15,000 neurons) (Vertes and Crane, 1997). Unfortunately, the precise functional role of SLN has yet to be addressed.
Neurochemically, nitric oxide (NO) is another neurotransmitter that has been noticed in raphe nuclei, especially in the rostral raphe groups. More specifically, several studies have suggested that a rather high percentage (60–90%) of the midline serotonergic DR neurons co-expresses NO immunoreactivity (Johnson and Ma, 1993; Wang et al., 1995; Xu and Hokfelt, 1997). In contrast, DR neurons in the lateral wing subregion express either serotonin or NO immunoreactivity, but not both in the same cell (Dun et al., 1994; Wotherspoon et al., 1994). Similarly, our laboratory has reported that DR provides not only serotonergic but also nitrergic inputs to the rodent trigeminal somatosensory system (Simpson et al., 2003). In particular, the majority of cortical projecting neurons in the midline DR contain both serotonin and neuronal nitric oxide synthase (nNOS), while sub-cortical projecting DR neurons in the lateral wing subregion express either serotonin or nNOS, but not both immunoreactivities. These findings provide the first clue regarding the divergence of serotonin and NO impacting upon cortical verse sub-cortical targets. In addition to DR, other raphe nuclei such as MR and SLN have also been shown containing nitrergic cells (Johnson and Ma, 1993). At present, whether MR or SLN also provides such dual sources of chemical markers to cortex remains to be elucidated.
Functionally, NO has been implicated in a variety of functions including vasodilation, synaptic plasticity, and neuroprotection/degeneration (see review: Dawson and Snyder, 1994; King, 2003). However, several studies have also suggested that NO may have an anti-depressant-like activity (Jeffery and Funder, 1996; Harkin et al., 1999). Recently, a direct interaction between serotonin transporter and nNOS in the same cell has been reported (Chanrion et al., 2007; Garthwaite, 2007). This new line of evidence suggests that the anti-depressant-like action of NO may derive from the serotonergic raphe neurons, which in term affect the limbic cortical structures.
Medial prefrontal cortex (mPFC) is one of the well known cortical regions involved in stress related activities (Larsen and Divac, 1978; Brito et al., 1982), and has also been recognized as the cognitive center of the cortex regulating individual’s responses to emotional stress (Amodio and Frith, 2006; Radley et al., 2006). In addition, in spite of the global projection from DR to the cortex, mPFC is the only cortical structure projecting back to the DR (Peyron et al., 1998; Vertes 2004). Such cortical input has been reported to modulate the DR neuronal responsiveness to different stressors, i. e., controllable vs uncontrollable (Amat et al., 2005). So far, DR and MR cortical projecting fibers have been demonstrated in the mPFC (Vertes and Martin 1988). However, the projection from SLN to mPFC is currently not known.
In order to better understand the unique role of serotonin and nNOS co-expression within cortical projecting raphe nuclei, we were also interested in determining the neurochemical identities of descending spinal cord projecting raphe neurons in the nuclei of raphe pallidus (B1), raphe obscurus (B2), raphe magnus (B3) (for review: Jacobs and Azmitia, 1992). A small population of NO positive cells has been identified in these three caudal raphe nuclei, whereas very few, if any, serotonin positive neurons co-express nNOS immunoreactivity (Johnson and Ma, 1993; Dun and Snyder, 1994; Leger et al., 1998).
The goal of the present study was, therefore, to determine: 1) the co-expression of serotonin transporter (SERT) and/or nNOS immunoreactive fibers in the mPFC; 2) the prevalence of raphe-mPFC projecting neurons expressing TPH and/or nNOS immunoreactivities; 3) the presence and/or absence of descending spinal cord projecting raphe neurons containing TPH and/or nNOS immunoreactivities. To address these issues, retrograde tracer Fluoro-Gold (FG) was injected into the mPFC or spinal cord, and tissues were processed for tryptophan hydroxylase (TPH; Ota et al., 1992) and nNOS (Moncada et al., 1991) immunoreactivities. Double immunostainings for both SERT and nNOS antibodies were also conducted in the mPFC in order to examine the co-labeling of these two markers within the same axon.
Materials and Methods
Subjects
A total of 8 female Long-Evans hooded rats weighting 250–300 g were used for the present study. All animals were treated according to guidelines approved by the Institutional Animal Care and Use Committee and conformed to NIH Standards for the Care and Use of Animals in Research.
Surgery and Tracer Injection
Animals were anesthetized by intraperitoneal administration of Nembutal (50mg/kg). Subjects were then positioned in a stereotaxic apparatus so that the bregma and lambda suture marks were oriented in the same horizontal plane. For mPFC injections (n=6), the skull above the mPFC was removed and retrograde tracer FG (10% in saline, Fluorochrome Inc.) was deposited. A 10 μl Hamilton syringe was employed and 0.2–0.4 μl of tracer were injected for a period of 15–30 minutes. Coordinates for the placement of tracer agent were derived from the atlas of Paxinos and Watson (1986) and were as follows: mPFC [AP: +2.0 to +3.5 mm; ML: 0.8 mm; VD: 3.0–4.5 mm (from the pia surface)]. For spinal cord injections (n=2), a small amount (0.2 ul) of FG was injected by exposing the spinal cord at the upper lumbar levels. After a small amount (0.2 ul) of FG was injected into the upper lumbar levels, rats were sacrificed 10 days later instead of 7 days in order to allow FG longer time to transport and accumulate in target cells.
After a survival period of 7–10 days, animals were again deeply sedated with Nembutal (100 mg/kg) and perfused through the ascending aorta with saline followed by 3.5% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Brains were removed immediately and stored at 4 °C overnight in the same fixative with 25% sucrose. The next day tissue was sectioned in the coronal plane on a freezing AO microtome to a thickness of 40 μm.
Immunofluorescent Staining
In order to visualize TPH or nNOS immunoreactive neurons in the raphe system, every third section through the raphe nuclear complex were incubated in either the monoclonal TPH antiserum (1: 1-2000; Mouse; MAB5278; Millipore), polyclonal nNOS antiserum (1:1000; Rabbit; 24287; Immunostar) or both antisera at 4 °C in PBS with 0.3% Triton and 1% bovine serum albumin (BSA) overnight. The next day, sections were exposed to anti-mouse and/or anti-rabbit conjugated Cy-2 or Cy-3 (1:200; Jackson Immunoresearch) for one hour at the room temperature in the dark. Colors were coded in a complementary fashion, so that a red fluorophore (Cy-3) represented TPH containing neurons and a green fluorophore (Cy-2) elucidated nNOS profiles, or vice versa. Similar procedures were also conducted for monoclonal SERT (1:1000; Mouse; MAB1564; Millipore) and/or polyclonal nNOS co-labeling. In order to reveal SERT or nNOS positive fibers, sections were incubated in the primary antibodies for three days at 4 °C in PBS with 0.3% Triton and 1% BSA. Tissues were rinsed in PBS three times (10 minutes for each) between every step and then linked with appropriate fluorophores as described previously. Finally, tissues were mounted on gelatin-coated slides and air dried. The slides were then cover-slipped with DPX and examined using epifluorescent microscope. Additional experiments were also conducted for the control of cross-reactivity or labeling biases. In brief, sections were processed according to the previous protocol with the omission of selective primary antiserum, and the labeling was found to be neurochemically specific.
Data Analysis
A Nikon epifluorescent microscope (E800) that was equipped with appropriate filters was employed to survey neuronal labeling and fiber staining. Every third section through the raphe complex from B1 to B9 (total 180 sections) or mPFC (total 30 sections) was placed in serial order and then examined for the number of retrogradely and immunohistochemically labeled raphe cells in the raphe nuclear complex or fibers in the mPFC. Photomicrographs were digitally acquired by using a Photometrics Coolsnap ES CCD camera attachment (Roper Scientific) and MetaMorph imaging software (Universal Imaging Systems). Pictures were aligned to illustrate the overlap of fluorescent markers. Individual tissue sections were photographed under different wavelengths to record the distribution of TPH and/or nNOS containing raphe cells or SERT and/or nNOS containing fibers in the mPFC. Typically, 20 sections of DR (total ~60), 16 sections of MR (total ~48) and 12 sections of SLN (total ~36) from each case were counted for the neuronal double-staining analysis, and 10 sections in the mPFC for the fiber double-staining analysis. The other two thirds of sections were processed for either single immunofluorescent staining or control immunofluorescent staining.
Results
Injection sites restricted to the mPFC
mPFC is a cortical structure located medially, and a small volume of FG (0.2–0.4 ul) was injected into the mPFC for each case by using a 10 μl Hamilton syringe. With this approach, the spread of tracer to other hemisphere could be mainly avoided. A representative example of such injection site and schematic drawings of injection sites were shown in Figure 1. To further rule out extensive tracer spread, we also examined the relative proportion of FG retrogradely labeled cells in ipsilateral vs contralateral locus coeruleus (LC), since previous study has suggested a predominant ipsilateral labeling pattern following retrograde tracer injections into the cortex (Simpson et al., 1997). From four out of six cases, FG retrograde labeled cells were found mainly in the ipsilateral LC. Once we could assure that FG tracer was properly placed into one side of the mPFC and cases were then further semi-quantitatively analyzed.
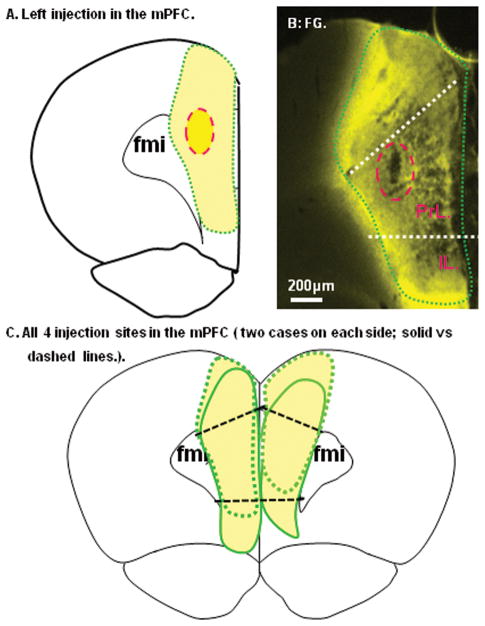
Figure 1.
Reconstruction of the Fluoro-Gold (FG) injection site in the medial prefrontal cortex (mPFC) from a represent case was demonstrated in A. Low power photomicrograph of same case was shown in B. Note that the injection site (red circle) and the tracer diffusion zone (Green circle) were restricted in the mPFC area. All four FG injection sites were further demonstrated in C. fmi: forceps minor of corpus callosum; IL: Infralimbic cortex; PrL: Prelimbic cortex.
Neurochemical identity of DR-mPFC projecting neurons
In order to demonstrate the topographic distribution pattern of serotonergic raphe groups in the rat brainstem, the combination of a sagittal view and corresponding coronal sections of TPH immunoreactive raphe nuclei from B1 to B9 were used in Figure 2. For example, DR (B6, 7) contained the most densely concentrated serotonin cells, particularly at the middle region and extended laterally into the lateral wing subgroup (Fig. 2E). DR can be further divided into three subsectors as defined by previous study, i.e., rostral and middle (B7), caudal (B6) (Abrams et al., 2004). In the present study, FG retrogradely labeled neurons were noted primarily in the midline subgroups of DR and exhibited a strong ipsilateral preference, especially in the middle and caudal levels but it was not so obvious in the rostral level of DR (Fig. 3).
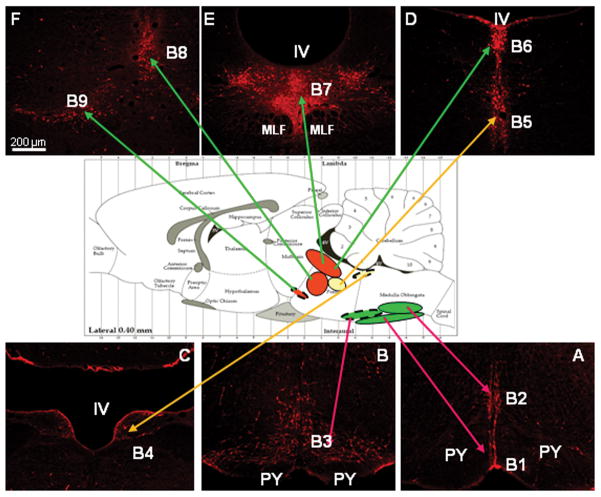
Figure 2.
Color photographs and schematic sagittal drawing demonstrating serotonergic nuclei [revealed with tryptophan hydroxylase (TPH) immunofluorescent staining linked to Cy3-red] in the rat brain. Caudally, the raphe nuclei (shown in green; A and B): B1 (raphe pallidus), B2 (raphe obscurus) and B3 (raphe magnus) projecting to the spinal cord. In the mid level, the target of two raphe nuclei (shown in yellow; C and D): B4 and B5 (pontine raphe nucleus), has yet to be determined. Rostrally, raphe nuclei (shown in red; D, E and F): B6 and B7 (dorsal raphe-DR), B8 (median raphe-MR) and B9 (supralemniscal nucleus-SLN) sending serotonergic afferents to the cortex. IV: IVth ventricle aperture; MLF: medial longitudinal fasciculus; PY: pyramidal tract.
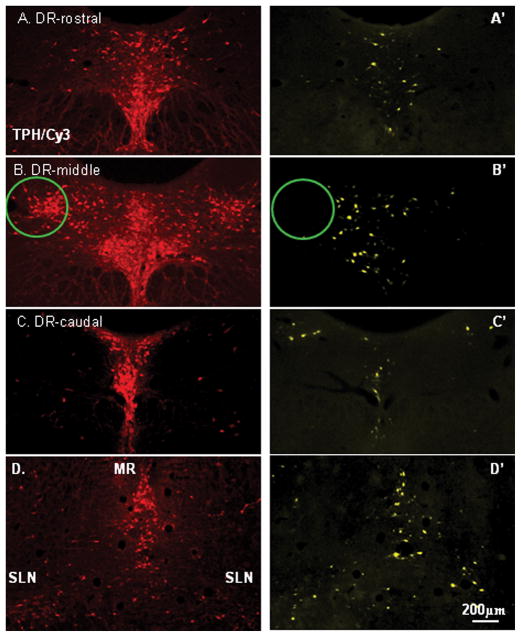
Figure 3.
Color photographs demonstrating rostral raphe complex [A–D; tryptophan hydroxylase (TPH); Cy3-red] and corresponding Fluoro-Gold (FG) retrogradely labeled raphe-mPFC projecting neurons (yellow) in dorsal raphe (DR; A′–C′), median raphe (MR; D′) and supralemniscal (SLN; D′). Note that there was no FG labeled neurons in the lateral wing of the DR (circles). Scale bar: 200 μm.
To further determine the incidence of FG retrogradely labeled DR neurons containing serotonin and/or NO, TPH and nNOS immunofluorescent stainings were conducted. Typically, a total of 60 sections from the DR region were collected and every third of them were processed and quantified for double-immunofluorescent stainings. An average of ~ 400 DR-mPFC projecting neurons per case was counted. The majority of DR-mPFC projecting neurons were ipsilateral to the tracer injection side, especially in the middle level. To further illustrate dual chemical expressions of DR-mPFC projecting neurons, an example in the mid level of DR was shown in Figure 4 (left column) and many FG retrogradely labeled neurons expressed both TPH and nNOS immunoreactivities (marked by arrows). Based on our semi-quantitative analysis (n=4), our data (see Table 1) revealed that an average of ~ 74% and 66% of FG retrogradely labeled neurons expressed either TPH or nNOS immunoreactivity. This was calculated by adding (“TPH only”) or (“nNOS only”) neurons to (“TPH and nNOS”), respectively. Furthermore, among all FG retrogradely labeled serotonergic neurons (“TPH and nNOS” plus “TPH only”), out data showed that ~ 84% of these serotonergic neurons were also nNOS positive. This was calculated by “TPH and nNOS” divided by “TPH and nNOS” plus “TPH only”.
Figure 4.
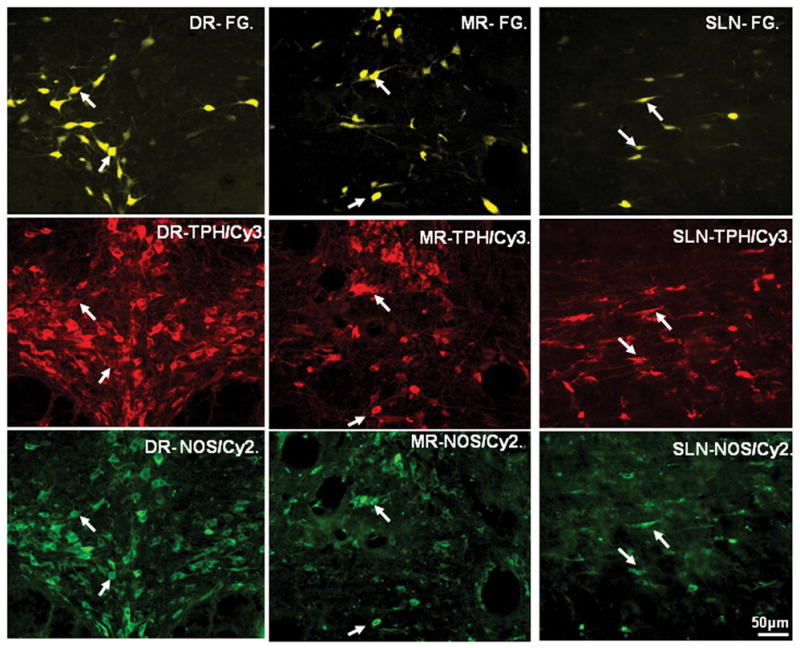
Color photographs demonstrating Fluoro-Gold (FG) retrogradely labeled dorsal raphe- (DR-), median raphe- (MR-) and supralemniscal- (SLN-) medial prefrontal cortex (mPFC) projecting neurons (upper row; FG-yellow) co-expressing both tryptophan hydroxylase (TPH; middle row; Cy3-red) and neuronal nitric oxide synthase (nNOS; bottom row; Cy2-green) immunoreactivities. Note that many retrogradely labeled neurons in these three raphe nuclei contained both TPH and nNOS (arrows) immunoreactivities. Scale bar: 50 μm.
Table 1.
DR-mPFC projecting neurons co-express both TPH and nNOS immunoreactivity.
| Case # | A: Total number FG retrogradely labeled neurons in DR | B: TPH only FG(+) TPH(+) nNOS(−) # and % |
C. TPH & nNOS FG(+) TPH(+) nNOS(+) # and % |
D. nNOS only FG(+) TPH(−) nNOS(+) # and % |
E. Neither FG(+) TPH(−) nNOS(−) # and % |
|---|---|---|---|---|---|
| 1 | 301 | 39; 13% | 192; 64% | 10; 3% | 60; 20% |
| 2 | 220 | 19; 9% | 146; 66% | 4; 2% | 51; 23% |
| 3 | 652 | 103; 20% | 365; 56% | 30; 5% | 154; 24% |
| 4 | 479 | 43; 10% | 313; 65% | 23; 5% | 100; 21% |
| Mean # | 413 | 51; 12% | 254; 62% | 17; 4% | 91; 22% |
[~74% of DR-mPFC projecting neurons are TPH positive (B+C); ~66% of DR-mPFC projecting neurons are nNOS postive (C+D); ~84% of FG retrogradely labeled serotonergic DR-mPFC projecting neurons also contain nNOS immunoreactiviy (C/B+C). DR: dorsal raphe; mPFC: medial prefrontal cortex; TPH: tryptophan hydroxylase; nNOS: neuronal nitric oxide synthase; FG: Fluoro-Gold.]
Neurochemical identity of MR-mPFC projecting neurons
MR (B8 subgroup) includes a dorsal division, which is more congregated along the midline, and a ventral division, which extends more laterally (Fig. 2F or 3D). Typically, FG retrogradely labeled MR-mPFC projecting neurons were located along the midline and did not show any regional specificity or laterality (Fig. 3D). On average, ~ 140 MR-mPFC projecting neurons per case were observed for TPH and nNOS double-immunofluorescent stainings.
With respect to neurochemical identities, our data showed that ~75% and 71% of FG retrogradely labeled MR-mPFC projecting neurons were TPH or nNOS positive, respectively (Table 2). The example illustrated in Figure 4 (middle column) showed that many FG retrogradely labeled neurons in MR contained both TPH and nNOS immunoreactivities (marked by arrows). Similarly, ~83% of FG retrogradely labeled serotonergic neurons also co-expressed nNOS immunoreactivity.
Table 2.
MR-mPFC projecting neurons co-express both TPH and nNOS immunoreactivity.
| Case # | A. Total number FG retrogradely labeled neurons in DR | B. TPH only FG(+) TPH(+) nNOS(−) # and % |
C. TPH & nNOS FG(+) TPH(+) nNOS(+) # and % |
D. nNOS only FG(+) TPH(−) nNOS(+) # and % |
E. Neither FG(+) TPH(−) nNOS(−) # and % |
|---|---|---|---|---|---|
| 1 | 106 | 14; 13% | 69; 65% | 8; 8% | 15; 14% |
| 2 | 83 | 9; 11% | 56; 67% | 4; 5% | 14; 17% |
| 3 | 229 | 29; 13% | 136; 59% | 20; 9% | 44; 19% |
| 4 | 139 | 19; 14% | 82; 59% | 16; 5% | 22; 16% |
| Mean # | 139 | 18; 13% | 86; 62% | 12; 9% | 24; 17% |
[~75% of MR-mPFC projecting neurons are TPH positive (B+C); ~71% of MR-mPFC projecting neurons are nNOS postive (C+D); ~83% of FG retrogradely labeled serotonergic MR-mPFC projecting neurons co-express nNOS immunoreactivity (C/B+C). MR: median raphe; mPFC: medial prefrontal cortex; TPH: tryptophan hydroxylase; nNOS: neuronal nitric oxide synthase; FG: Fluoro-Gold.]
Neurochemical identity of SLN-mPFC projecting neurons
SLN (B9 subgroup) has a crescent-shaped appearance and taperes laterally with pointed tail (Fig. 2F). These TPH positive neurons exhibits spindle morphology and oriented predominant mediolaterally. An average of ~140 FG retrogradely labeled SLN-mPFC projecting neurons per case was identified for double-immunofluorescent stainings.
Neurochemically, FG retrogradely labeled SLN-mPFC projecting neurons had the highest percentage of TPH and nNOS co-expression. As shown in Fig. 4 (right column), almost all FG retrogradely labeled neurons in the SLN demonstrated both TPH and nNOS immunoreactivity. On average, our data (Table 3) revealed that ~92% and ~86% of FG labeled SLN-mPFC projecting neurons were TPH or nNOS immunoreactive, respectively. Again, a high percentage (~91%) of FG retrogradely labeled serotonergic neurons co-expressed nNOS immunoreactivity.
Table 3.
SLN-mPFC projecting neurons co-express both TPH and nNOS immunoreactivity.
| Case # | A. Total number FG retrogradely labeled neurons in DR | B. TPH only FG(+) TPH(+) nNOS(−) # and % |
C. TPH & nNOS FG(+) TPH(+) nNOS(+) # and % |
D. nNOS only FG(+) TPH(−) nNOS(+) # and % |
E. Neither FG(+) TPH(−) nNOS(−) # and % |
|---|---|---|---|---|---|
| 1 | 80 | 3; 4% | 72; 90% | 2; 3% | 3; 4% |
| 2 | 63 | 4; 6% | 57; 90% | 1; 2% | 1; 2% |
| 3 | 244 | 28; 11% | 197; 81% | 6; 2% | 13; 5% |
| 4 | 154 | 8; 5% | 130; 84% | 2; 1% | 14; 9% |
| Mean # | 135 | 11; 8% | 114; 84% | 3; 2% | 8; 6% |
[~92% of SLN-mPFC projecting neurons are TPH positive (B+C); ~86% of SLN-mPFC projecting neurons are nNOS postive (C+D); ~91% of FG retrogradely labeled serotonergic SLN-mPFC projecting neurons also contain nNOS immunoreactivity (C/B+C). SLN: supralemniscal nucleus; mPFC: medial prefrontal cortex; TPH: tryptophan hydroxylase; nNOS: neuronal nitric oxide synthase; FG: Fluoro-Gold.]
Co-expression of SERT and nNOS immunoreactivities in the mPFC
Since our retrograde labeling combined with TPH and nNOS immunofluorescent stainings revealed a rather high percentage of co-expression, it was conceivably that most serotonergic fibers in the mPFC might also express nNOS immunoreactivity. As shown in figure 5, nearly every SERT positive fibers in mPFC contained nNOS immunoreactivity. Among these double-labeled fibers, many of them were concentrated in the layer I and VI, and they were running parallel with the pia surface or the white matter, respectively. In contrast, nNOS positive interneurons and their profiles in the mPFC were not overlapped with TPH immunoreactive labeling (marked by arrows).
Figure 5.
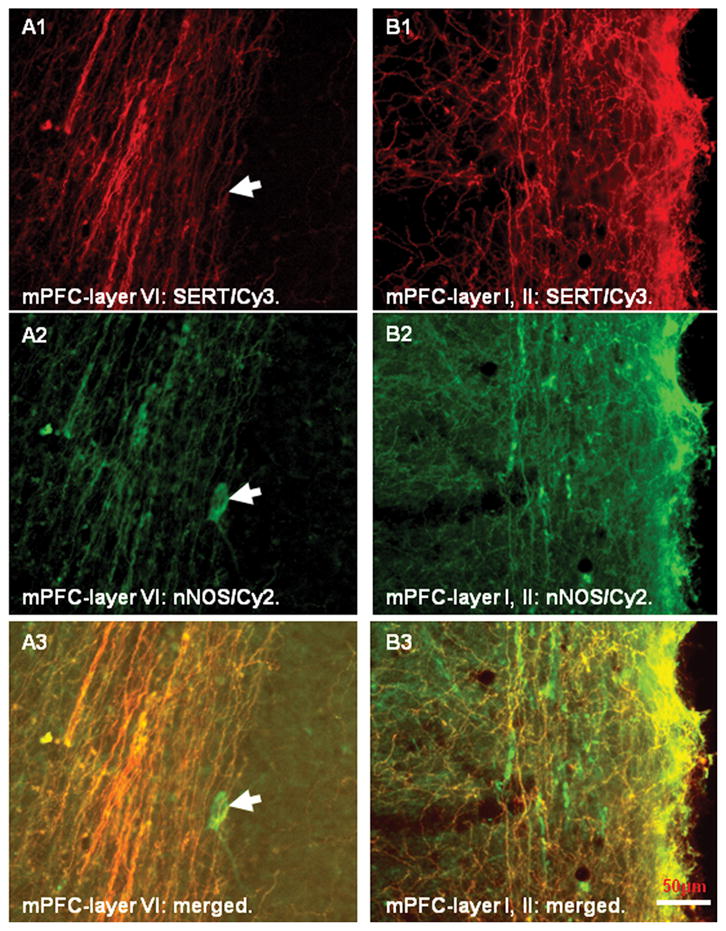
Color photomicrographs demonstrating serotonin transporter (SERT) positive fibers (A1–B1; Cy3-red) and neuronal nitric oxide synthase (nNOS) positive profiles (A2–B2; Cy2-green) in the medial prefrontal cortex (mPFC). Note that both immunostainings exhibited similar distribution patterns in the mPFC and most fibers were double-labeled with SERT and nNOS immunoreactivities (A3–B3; merged). In addition, nNOS positive neurons and their processes in the cortex were also identified but none of them contained SERT immunoreactivity (arrows in A1–A3). Scale bar: 50 μm.
Neurochemical identity of spinal cord raphe projecting neurons in B1, B2 and B3
Nucleus raphe pallidus (B1), raphe obscurus (B2) and raphe magnus (B3) are located in the most caudal part of the raphe complex (fig. 2A and 2B). In order to reveal the co-expression of TPH and nNOS immunoreactivities in these three caudal raphe nuclei, tissues were also processed for these two markers. Our data demonstrated that TPH positive neurons in all three caudal raphe nuclei tended to be more numerous and larger as compared to adjacent nNOS positive cells and none of them contained both markers (Figure 6). Following FG tracer injections into the spinal cord, we noted that the tracer spread throughout the entire cord and caused extensive necrosis. From these two cases, FG retrogradely labeled neurons were found in all three nuclei. Interestingly, numerous retrogradely labeled neurons were noted to contain TPH immunoreactivity, but none of them co-expressed nNOS immunoreactivity. An example of such labeling pattern in B3 subgroup was shown in Figure 7. Finally, it was important to note that FG retrogradely labeled neurons were not observed in caudal raphe nuclei following tracer injections into the mPFC, suggesting the dichotomy of the ascending vs the descending sources of raphe projecting systems.
Figure 6.
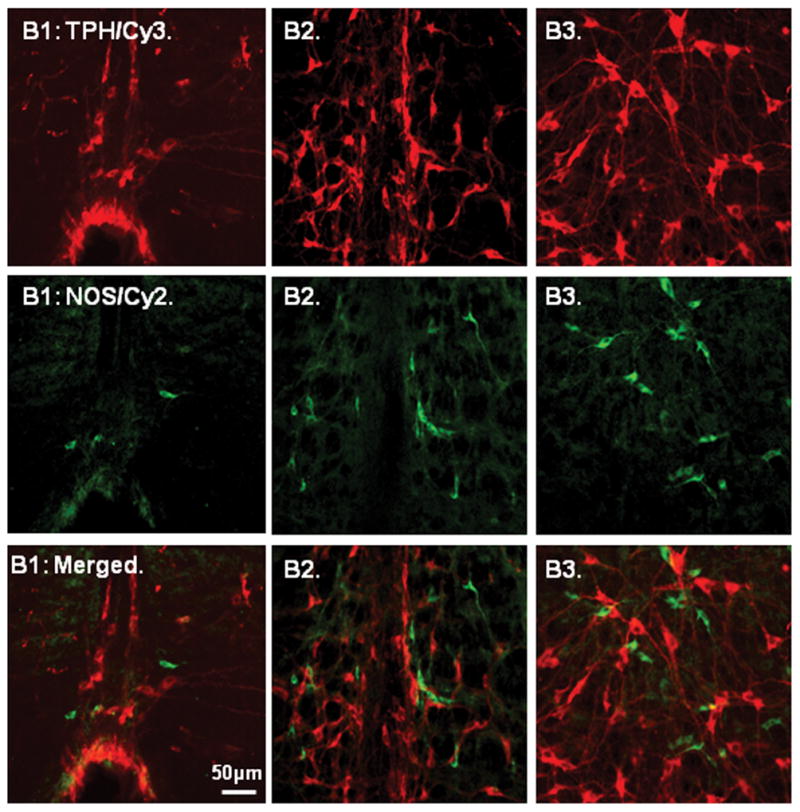
Color photographs demonstrating the relationship between tryptophan hydroxylase (TPH; upper row; Cy3-red) and neuronal nitric oxide synthase (nNOS; middle row; Cy2-green) positive neurons in the nucleus raphe pallidus (B1), raphe obscurus (B2) and raphe magnus (B3). Note that several small nNOS positive neurons were scattered and adjacent to the medium sized TPH positive neurons, but none contained TPH immunoreactivity (bottom row; merged). Scale: 50 μm.
Figure 7.
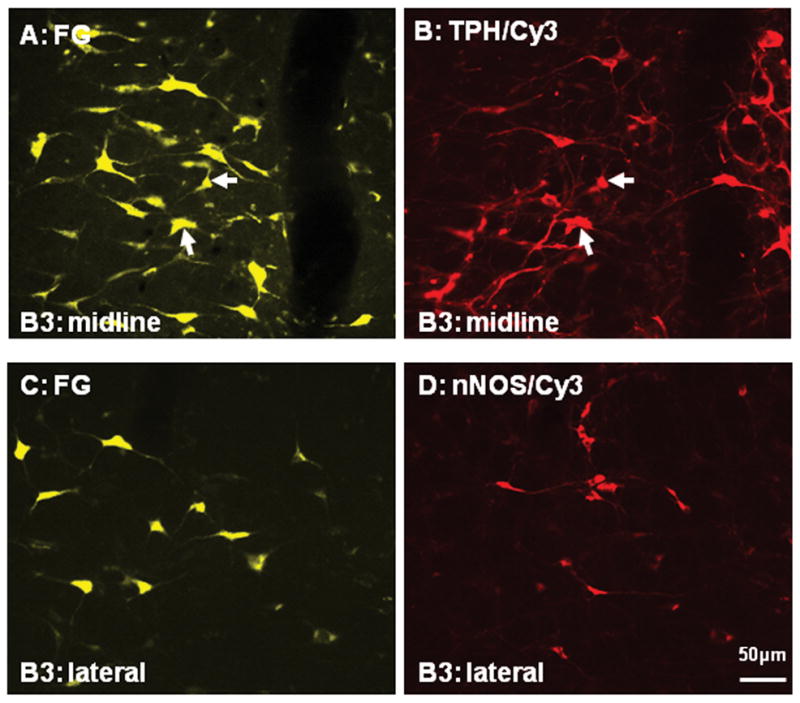
Color photographs demonstrating the relationship between Fluoro-Glod (FG; A and C) retrogradely labeled neurons in the nucleus raphe magnus (B3) and the corresponding tryptophan hydroxylase (TPH; B; Cy-3) or neuronal nitric oxide synthase (nNOS; D; Cy-3) immunostaining, respectively. Note that a few retrogradely labeled neurons in A contained TPH immunoreactivity (arrows in A and B), but none of retrogradely labeled neurons in C was double-labeled with nNOS immunoreactivity (C and D). Scale: 50 μm.
Discussion
The present study was undertaken to better our understanding about how raphe nuclear complex might selectively exert their effects upon cortical vs subcortical targets. More specifically, our data suggested that cortical projecting serotonergic neurons (B6–B9) exhibited a rather high percentage (>80%) of nNOS co-expression. In addition, most SERT positive fibers in the mPFC were also co-expressed with nNOS immunoreactivity, supporting the co-existence of nitric oxide and serotonin in the ascending raphe system. In contrast, all three descending raphe nuclei (B1, B2 and B3) expressed either TPH or nNOS immunoreactivity, but not both in the same cell. Furthermore, even though many FG retrogradely labeled neurons were TPH positive in these three caudal raphe nuclei, none of them expressed nNOS immunoreactivity, suggesting that these nNOS positive neurons were not spinal cord projecting cells. Based on available data, we concluded that raphe projections to the cortical vs the subcrotical targets were not only originated from different locations but also employed different neurotransmitter compositions. To our knowledge, the current investigation is the first to compare the differential neurochemical compositions of cortical ascending vs spinal cord descending raphe projecting systems.
Raphe projections to the mPFC
So far, several studies have suggested that DR is the major serotonergic nucleus projecting to the cortex while MR is considered as the minor source to the cortex (O’Hearn and Molliver, 1984; Waterhouse et al., 1986; Tork, 1990; Johnson and Ma, 1993). As for the rodent mPFC, previous studies have implicated a moderate density of MR projecting fibers, and a rather similar distribution pattern is also arising from DR (Vertes, 1991). Based on the relative numbers of retrogradely labeled neurons in the DR and MR following tracer injections in the mPFC, our present data revealed that about two to three folds of more DR neurons projecting to mPFC compared to MR. Since it is also known that DR contain about two to three times the number of neurons than MR (Vertes and Crane, 1997), therefore, it was tempting to suggest that both DR and MR exhibited relatively similar strength impacting upon the mPFC. Despite the fact that SLN contains similar number of serotonergic neurons compared to MR (Vertes and Crane, 1997), the role of SLN projection to the cortex is currently not known. Previous study reports a consistently low number of SLN projecting neurons (total of 420 cells in 15 animals) to various cortices, i.e., frontal, parietal and occipital cortex in comparison to the DR (total of 2639 cells) (O’Hearn and Molliver, 1984). Our present data indicated that the relative mean number of mPFC projecting neurons from DR (413), MR (139) and SLN (135) were almost parallel (i.e., ~3:1:1 ratio) with the relative number of serotonergic positive cells in these three raphe nuclei (DR-15,000; MR-4,100; SLN-4,600) (Vertes and Crane, 1997), further suggesting the possibility that SLN played an equally important role as MR and/or DR upon the mPFC.
An obvious and intriguing question is why mPFC receives afferent inputs from three separate raphe nuclei and each of them co-expresses similar percentage of TPH and nNOS immunoreactivities. One likely explanation is that these three raphe nuclei may exert different effects upon mPFC. So far, several lines of evidences have suggested such possibility. For example, previous studies have reported that DR and MR projecting fibers located in the same cortical area demonstrate different patterns (Kohler et al., 1980; Kosofsky and Molliver, 1987) and can be selectively destroyed by certain psychotropic amphetamine derivatives, suggesting different properties of these two raphe projecting fibers (Mamounas and Molliver, 1988). Under stress conditions, DR facilitates the function of the Hypothalamic-Pituitary-Adrenal (HPA) axis, while MR inhibits the function of the HPA axis via different pathways (Lowry et al., 2002). Furthermore, based on selective lesions made in DR and MR, Kusljic et al., 2003 have also reported that these two raphe nuclei play different roles in the pathphysiology of mental diseases such as Schizophrenia (Kusljic et al., 2003). At present, much less is known about the functional role of SLN. Previous retrograde studies have shown a pronounced serotonergic SLN projection to the pontine reticular formation (PRF) (Semba, 1993; Vertes and Crane, 1997), thus, SLN may play a modulatory role in PRF, a brainstem nucleus which is known to involve in sleep state control.
Raphe projections to the spinal cord
Classically, B1, B2 and B3 are identified as the major raphe-spinal projecting nuclei and have been regarded as the descending serotonergic systems with various emphases (Jacobs and Azmitia, 1992). Serotonin has also been considered as a vital neurotransmitter critically involved in spinal-mediated processes, including locomotion (Barbeau and Rossignol 1991; Schmidt and Jordan 2000) and nociception (Willis and Westlund 1997). In particular, Serotonin neurons in B1 and B2 target sympathetic preganglionic neurons in the intermediolateral cell column, contributing to autonomic regulation (Allen and Cechetto, 1994: Jacobs et al., 2002). On the other hand, serotonergic projections from B3 synapse in the dorsal horn, causing inhibition or facilitation of pain signaling depending on receptor types (Calejesan et al., 1998; Bardin et al., 2000). Furthermore, these serotonergic descending projections also provide excitatory input to ventral horn α-motoneurons to modulate motor function (Saruhashi et al., 1996).
Like rostral raphe nuclei, these caudally-located raphe nuclei are also not composed by homogeneous serotonergic cells and more than half of spinal cord projecting neurons are not serotonin positive (Jones and Light, 1992). Small populations of NO positive cells have been identified in all three nuclei, but very few, if any of them, contain serotonin immunoreactivity (Johnson and Ma, 1993; Dun et al., 1994; Leger et al., 1998). Our present data indicated the existence of nNOS positive neurons within caudal raphe nuclei, but none of them was double-labeled with retrograde tracer or co-expressed with TPH immunoreactivity. In addition, even though most nNOS positive neurons were adjacent to TPH positive neurons, most of them were smaller and exhibited different morphology from serotonergic neurons. Obviously, nNOS positive neurons in caudal raphe nuclei played different roles compared to nNOS positive neurons in rostral raphe nuclei, in which the majority of the raphe-cortical projecting neurons contained both nNOS and TPH immunoreactivities.
Serotonin and nitric oxide co-localization in rostral raphe nuclei
Previous studies have reported the co-expression of serotonin and NO in the rostral raphe nuclei, especially the DR midline subgroup which projects to cortical areas (Johnson and Ma, 1993; Dun et al., 1994; Leger et al., 1998; Simpson et al., 2003), whereas no such cells were detected in the subcortical projecting subgroups in the lateral wing subgroups (Simpson et al., 2003). Our current study confirmed that a high percentage (>80%) of DR-mPFC projecting serotonergic neurons also co-expressed nNOS immunoreactivity from midline DR subregion. Furthermore, our present data revealed that a majority of MR-mPFC and SLN-mPFC projecting neurons also contained these two neurotransmitters. Interestingly, a previous study reports that only ~10% of serotonergic neurons in the SLN histochemically stain for NADPHd (Johnson and Ma, 1993). Such discrepancy may be due to the differences in techniques being conducted. In particular, our current investigation was conducted with double immunofluorescent approaches, while the former study was conducted with the histochemical and immunocytochemical combined techniques. Even though NOS has been fully accounted for the NADPHd staining in the brain and peripheral tissues (Dawson et al., 1991; Hope et al., 1991), the current immunofluorescent approaches appeared to reveal a highly enhanced sensitivity.
Functional considerations
The presence of nNOS immunoreactivity within TPH positive neurons in raphe nuclei as well as co-expression of nNOS and SERT positive fibers in the cortex supported the hypothesis that NO co-localized with serotonin and could be co-released at the synaptic terminals. At present, several studies have, indeed, implicated such interactions. For instance, inhibition of serotonin synthesis has been shown to increase nNOS activity in DR, thus, producing more NO at their target sites (Tagliaferro et al., 2001, 2003). On the other hand, the use of NO donors to increase NO volume reduces serotonin effects by transforming serotonin into an inactive form or by inactivating TPH enzyme activity to produce less serotonin (Kuhn and Arthur, 1996; Fossier et al., 1999; Prast and Philippu, 2001). The nNOS inhibitors have also been demonstrated to be able to produce anti-depressant-like effects in the forced swim test in the rat, and this behavioral effect has been demonstrated previously following animals exposed to selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (Jefferys and Funder, 1996; Harkin et al., 1999). Recently, a reciprocal functional connection between serotonin and NO in the same cell has been implicated via SERT and nNOS activities (Chanrion et al., 2007; Garthwaite 2007). In particular, serotonin uptake induces NO production from nNOS which is physically linked to SERT, through a calmodulin-dependent mechanism. On the other hand, the binding of nNOS to the SERT carboxyl terminus decreases SERT trafficking to the plasma membrane and thereby reduces serotonin uptake. Furthermore, NO increases the SERT activity by phosphorylating SERT and increases serotonin uptake. Obviously, interactions between serotonin and NO are modulated by various mechanisms. Needless to say, DR, MR and SLN might affect the cortical structures such as mPFC by modulating a double-neurotransmitter system, i.e., both serotonin and nitric oxide within the same structural profile. In contrast, caudal serotonergic raphe nuclei, i.e., B1, B2 and B3, might modulate subcortical structures such as spinal cord through different mechanisms.
Acknowledgments
This research was supported by NIH grants RR017701 (KLS) and EUREKA MH084194 (RCSL); NSF grant RIMI P20MD002725(YLU).
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann NY Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Serotonergic and nonserotonergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8 (3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith C. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Azmitia E, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Baker KB, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RGH, Tork I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Bardin L, Schmidt J, Alloui A, Eschalier A. Effect of intrathecal administration of serotonin in chronic pain models in rats. Eur J Pharmacol. 2000;409:37–43. doi: 10.1016/s0014-2999(00)00796-2. [DOI] [PubMed] [Google Scholar]
- Brito GNO, Thomas GJ, Davis BJ, Gingold SI. Prelimbic cortex, mediodorsal thalamus, septum and delayed alternation in rats. Exp Brain Res. 1982;46:52–58. doi: 10.1007/BF00238097. [DOI] [PubMed] [Google Scholar]
- Calejesan AA, Chang MH, Zhou M. Spinal serotonergic receptors mediate facilitation of a nociceptive reflex by subcutaneous formalin injection into the hind paw in rats. Brain Res. 1998;798:46–54. doi: 10.1016/s0006-8993(98)00394-1. [DOI] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci. 2007;104 (19):8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62 (Suppl 232):1–55. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of vulvospinal neuron systems. Acta Physiol Scand. 1965;64 (Suppl 247):1–36. [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Forstermann U. Nitric oxide synthase immunoreactivity in rat pontine medullary neurons. Neurosci. 1994;59:429–445. doi: 10.1016/0306-4522(94)90607-6. [DOI] [PubMed] [Google Scholar]
- Fossier P, Blanchard B, Ducrocq C, Leprince C, Tauc L, Baux G. Nitric oxide transforms serotonin into an inactive form and this affects neuromodulation. Neurosci. 1999;93:597–603. doi: 10.1016/s0306-4522(99)00165-7. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Neuronal nitric oxide synthase and the serotonin transporter get harmonious. Proc Natl Acad Sci. 2007;104 (19):7739–7740. doi: 10.1073/pnas.0702508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin AJ, Bruce KH, Craft B, Paul IA. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur J Pharmacol. 1999;372:207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigger KM, Vincent SR. Neuronal NADPH-diaphorase is a nitric oxide synthase. Proc Natl Acad Sci. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM. Serotonergic system in the brainstem of the marmoset: A combined immunocytochemical and 3-dimensional reconstruction study. J Comp Neurol. 1988;270:471–487. doi: 10.1002/cne.902700402. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jefferys D, Funder J. Nitric oxide modulates retention of immobility in the forced swimming test in rats. Eur J Pharmacol. 1996;295:131–135. doi: 10.1016/0014-2999(95)00655-9. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ma PM. Localization of NADPH diaphorase activity in monoaminergic neurons of the rat brain. J Comp Neurol. 1993;332:391–406. doi: 10.1002/cne.903320402. [DOI] [PubMed] [Google Scholar]
- Jones SL, Light AR. Serotonergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. J Comp Neurol. 1992;322:599–610. doi: 10.1002/cne.903220413. [DOI] [PubMed] [Google Scholar]
- King SB. The nitric oxide producing reaction of hydroxyurea. Curr Med Chem. 2003;10:437–452. doi: 10.2174/0929867033368213. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Haglund L, Steinbusch HWM. Immunohistochemical localization of serotonin nerve terminals in the lateral entorhinal cortex of the rat: demonstration of two separate patterns of innervation from the midbrain raphe. Anat Embryol. 1980;160:1221–1299. doi: 10.1007/BF00301855. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotonergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal raphe and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr Inactivation of brain tryptophan hydroxylase by nitric oxide. J Neurochem. 1996;67:1072–1077. doi: 10.1046/j.1471-4159.1996.67031072.x. [DOI] [PubMed] [Google Scholar]
- Kusljic S, Copolov DL, Van den Buuse M. Differential role of serotonergic projections arising from the dorsal and median raphe nuclei in locomotor hyperactivity and prepulse inhibition. Neuropsychopharmacol. 2003;28:2138–2147. doi: 10.1038/sj.npp.1300277. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Divac I. Selective ablations within the prefrontal cortex of the rat and performance of delayed alternation. Physiol Psychol. 1978;6:15–17. [Google Scholar]
- Leger L, Charnay Y, Burlet S, Gay N, Schaad N, Bouras C, Cespuglio R. Comparative distribution of nitric oxide synthase and serotonin-containing neurons in the raphe nuclei of four mammalian species. Histochem Biol. 1998;110:517–525. doi: 10.1007/s004180050313. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurons: Implications for control of the Hypothalamic-Pituitary-Adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Molliver ME. Evidence for dual serotonergic projections to the neocortex: Axons form the dorsal and median raphe nuclei are differentially vulnerable to the neurotoxin P-chloroamphentamine (PCA) Exp Neurol. 1988;102:23–36. doi: 10.1016/0014-4886(88)90075-1. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Ota M, Dostert P, Hamanaka T, Nagatsu T, Naoi M. Inhibition of tryptophan hydroxylase by (R)- and (S)-1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (salsolinols) Neuropharmacol. 1992;31:337–341. doi: 10.1016/0028-3908(92)90065-w. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, second edition. Sydey: Academic press; 1986. [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neurosci. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;13:26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Semba K. Aminergic and cholinergic afferents to REM sleep induction regions of the pontine reticular formation in the rat. J Comp Neurol. 1993;330:543–556. doi: 10.1002/cne.903300410. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RCS, Waterhouse BD. Lateralization and functional organization of the locus Coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol. 1997;385:135–147. [PubMed] [Google Scholar]
- Simpson KL, Waterhouse BD, Lin RCS. Differential expression of nitric oxide in serotonergic projection neurons: neurochemical identification of dorsal raphe inputs to rodent trigeminal somatosensory target. J Comp Neurol. 2003;466:495–512. doi: 10.1002/cne.10912. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Vander Kooy D, Verhofstad AA, Pellegrino A. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci Lett. 1980;19:137–142. doi: 10.1016/0304-3940(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Ramos AJ, Lopez-Costa JJ, Lopez EM, Saavedra JP, Brusco A. Increased nitric oxide synthase activity in a model of serotonin depletion. Brain Res Bull. 2001;54:199–205. doi: 10.1016/s0361-9230(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Ramos AJ, Lopez-Costa JJ, Lopez EM, Bursco A. Changes in the postnatal development on nitric oxide system induced by serotonin depletion. Brain Res Dev Brain Res. 2003;146:39–49. doi: 10.1016/j.devbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kimura H, Sano Y. Immunocytochemical demonstration of the distribution of serotonin neurons in the brainstem of the rat and cat. Cell tissue Res. 1982;224:247–267. doi: 10.1007/BF00216872. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci. 1990;600:9–35. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Distribution, quantification and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997;378:411–424. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- Wang Q-P, Guan JL, Nakai Y. Distribution and synaptic relation of NOS neurons in the dorsal raphe nucleus: a comparison to 5-HT neurons. Brain Res Bull. 1995;37:177–187. doi: 10.1016/0361-9230(94)00277-8. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Mihailoff GS, Baack JC, Woooodward DJ. Topographical distribution of dorsal and median raphe neurons projecting to motor, sensorimotor and visual cortical areas in the rat. J Comp neurol. 1986;249:460–481. doi: 10.1002/cne.902490403. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon G, Albert M, Battray M, Priesthy JV. Serotonin and NADPH diaphoresis in the dorsal raphe nucleus of the adult rat. Neurosci Lett. 1994;173:31–36. doi: 10.1016/0304-3940(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Xu Z-Q, Hokfelt T. Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat. 1997;13:169–187. doi: 10.1016/s0891-0618(97)00043-4. [DOI] [PubMed] [Google Scholar]