Abstract
Rett Syndrome is a neurological disorder caused by mutations in the X-linked MECP2 gene. Mouse models where Mecp2 is inactivated or mutated recapitulate several features of the disorder and have demonstrated a requirement for the protein to ensure brain function in adult mice. We deleted the Mecp2 gene in ∼80% of brain cells at three postnatal ages to determine whether the need for MeCP2 varies with age. Inactivation at all three time points induced Rett-like phenotypes and caused premature death of the animals. We find two threshold ages beyond which the requirement for MeCP2 markedly increases in stringency. The earlier threshold (8–14 weeks), when inactivated mice develop symptoms, represents early adulthood in the mouse and coincides with the period when Mecp2-null mice exhibit terminal symptoms. Unexpectedly, we identified a later age threshold (30–45 weeks) beyond which an 80% reduction in MeCP2 is incompatible with life. This finding suggests an enhanced role for MeCP2 in the aging brain.
INTRODUCTION
MeCP2 is a nuclear protein able to bind preferentially to methylated DNA (1). It is expressed at varying levels in many different organs, but is particularly abundant in the brain, where it has been shown, in neurons, to almost coat the chromosomes (2). The MECP2 gene is located on the X chromosome and a spectrum of disease-causing mutations has been described (3). Male patients with a mutation in MECP2 develop neonatal encephalopathy and do not survive past 2 years old (4,5). Females carrying one mutated allele of the gene show mosaic expression of MECP2 due to random X-inactivation in somatic tissues and develop Rett Syndrome (RTT), an autistic spectrum disorder that affects 1 in 12 500 girls (6). After 6–18 months of apparently normal postnatal development, the first manifestation of the syndrome is a ‘crisis’, often associated with decelerated head growth and a loss of acquired skills, such as the ability to speak or to walk. This episode is followed by the occurrence of diverse symptoms, which include stereotypic hand movements, balance and coordination defects, breathing abnormalities, mental retardation, as well as susceptibility to seizures and scoliosis (7–10). These symptoms stabilize during the stationary phase, which may persist for the lifetime of the patient. In some cases, however, there is a late motor deterioration characterized by increasing stiffness and coordination problems (11). Girls with RTT can survive into middle age or older, but require intensive support (12,13).
Mice carrying Mecp2 mutations provide useful models to study RTT. Mecp2-null male mice acquire neurological phenotypes reminiscent of RTT at ∼6 weeks after birth, including hypoactivity, abnormal gait, cognitive defects and breathing problems, leading to death at ∼6–12 weeks of age (14,15). Heterozygous female mice also develop RTT-like phenotypes, but much later than the Mecp2-null male mice (4–12 months of age). The symptoms are milder than in males and, as in humans, they stabilize allowing survival well into adulthood (14).
Because the onset of symptoms in RTT patients occurs during postnatal development, coinciding with a period of intense synaptogenesis (16), RTT has long been considered a neurodevelopmental disorder. In mouse models, however, symptoms arise either during adulthood for heterozygous females or after weaning (3 weeks) for null male mice, suggesting that MeCP2 is not required at a specific developmental stage (14). This notion is strongly supported by a study demonstrating that reactivation of MeCP2 in symptomatic adult mice that had developed in its absence resulted in reversal of the phenotype and re-establishment of health (17). Defects in long-term potentiation in the hippocampus (17) and abnormal neuronal morpho-anatomical parameters (Robinson et al., in press) are also reversed by MeCP2 restoration in adult mice. Finally, inactivation of the Mecp2 gene in adult (8-week-old) mice triggers the appearance of RTT-like phenotypes and death (18). These findings demonstrate that MeCP2 is required throughout adult life to maintain brain function.
Levels of MeCP2 protein in the rodent brain increase dramatically after birth, reaching a plateau at 5–10 weeks of age (2,19,20). A significant increase in MeCP2 expression has also been observed in the cerebellum between 6 weeks and adulthood (21). The onset of overt neurological symptoms coincides with this period (4–8 weeks). Given that MeCP2 protein levels appear to be highly regulated during postnatal development and early adulthood, we wanted to assess whether Mecp2 is required equally throughout life or whether there are specific phases when the presence of the protein is particularly important. To test this, Mecp2 expression was inactivated in Mecp2lox/y,CreESRT (lox/y,Cre) mice at 3, 11 and 20 weeks after birth, using the inducible Cre-ESR/Lox system, and the resulting phenotypes were monitored. We found that Mecp2 inactivation caused the appearance of RTT-like phenotypes and premature death, independent of the age at inactivation. More importantly, the time between inactivation and onset of symptoms and death differed when Mecp2 was deleted during postnatal development or during adulthood, revealing the existence of two sensitive age periods centred around 11 weeks old and 39 weeks old. Beyond each of these ages, the requirement for normal levels of MeCP2 becomes significantly more stringent.
RESULTS
Tamoxifen-induced recombination at the Mecp2 locus results in a significant decrease in MeCP2 expression
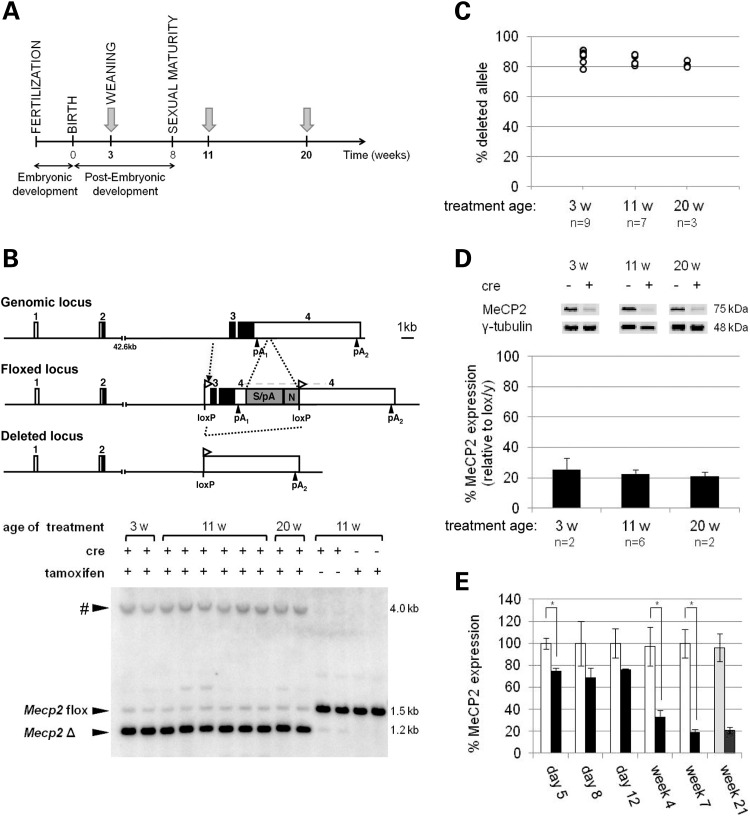
To assess the importance of Mecp2 at different ages during postnatal development and adulthood, lox/y,Cre mice and their control Mecp2lox/y (lox/y) littermates were treated with tamoxifen for 5 days at three postnatal ages: 3 weeks (weaning), 11 weeks and 20 weeks (Fig. 1A). Tamoxifen treatment enables the CreESRT protein to translocate into the nucleus where it causes recombination between loxP sites and consequent deletion of exons 3 and 4 of the floxed Mecp2 gene (Fig. 1B). Southern blots of genomic DNA isolated from brain tissue showed two fragments corresponding to floxed Mecp2 and the deleted alleles, indicating successful recombination in lox/y,Cre mice. Control littermates lacking the Cre transgene failed to delete the floxed allele, as did lox/y,Cre mice injected with corn oil alone (Fig. 1B). Blots showing Mecp2 deletion also displayed an unexpected 4 kb band, which was investigated further and found to be the result of recombination with a partial LoxP site at the 5′ end of the neo cassette (Supplementary Material, Fig. S1). The resulting Neo-deleted allele retains the human β-globin final intron and 3′UTR sequences introduced in the lox/y allele and is therefore also expected to express Mecp2 at the same level as the un-recombined allele. Quantification of recombination, taking the novel DNA fragment into account (see Materials and Methods), showed that 78–91% of lox/y,Cre brain cells contained the deleted allele after tamoxifen injection at all three time points. Average deletion frequencies were not significantly different between the three groups [Fig. 1C; 87% (3 weeks), 84% (11 weeks) and 82% (20 weeks); P> 0.05]. In approximate agreement with these values, tamoxifen-treated lox/y,Cre mice expressed MeCP2 protein at 21–25% of the level seen in lox/y controls (Fig. 1D). Immunofluorescence staining was compatible with the view that the frequency of MeCP2 loss in cortical neurons is similar to that measured in total brain DNA and protein (Supplementary material, Fig. S2). We infer that tamoxifen treatment had a similar effect on the MeCP2 protein levels in lox/y,Cre mice at all three time points (P> 0.05), as expected when using a ubiquitously expressed cre transgene (22), and therefore allows a valid comparison of phenotypic consequences between the three groups.
Figure 1.
Effect of tamoxifen treatment on MeCP2 expression. (A) Mice were injected with tamoxifen at 3 weeks old (lox/y n = 9; lox/y,Cre n = 12), at 11 weeks old (lox/y n = 6; lox/y,Cre n = 9) and at 20 weeks old (lox/y n = 11; lox/y,Cre n = 8). Each treatment week is shown as a grey arrow. (B) Maps of the wild-type genomic locus, the floxed locus and the deleted locus. pA, polyadenylation site; S/pA, splice/polyadenylation sequences from the human beta-globin gene; N, neomycin resistance gene. As shown on a representative Southern blot, independent of the age of treatment, lox/y,Cre mice injected with tamoxifen have deleted the floxed allele, in contrast with the lox/y mice or with lox/y,Cre mice injected with oil vehicle only. The symbol # represents a fragment of an unexpected size resulting from the existence of a residual loxP site (see Supplementary material, Fig. S1). (C) Tamoxifen treatment resulted in the deletion of the floxed allele in 78–91% of the brain cells of animals from each group of inactivation. Each circle represents a measurement from one animal. There was no significant difference between the average percentages of recombination measured from brains of lox/y,Cre mice injected with tamoxifen at the three different ages [ANOVA, F(2,16) = 3.125, P> 0.05]. (D) Deletion of the floxed allele triggered a significant decrease in MeCP2 expression giving ∼20% of control levels, independent of the age of inactivation. No statistical analysis is possible as n = 2. (E) Following the first day of tamoxifen injections, MeCP2 expression decreased and reached its final level after 4 weeks. Week 21 values represent mean % MeCP2 expression for the 20-week treatment group. Open bars: lox/y animals, filled bars: lox/y,Cre animals. Bars represent mean ± SEM. *P< 0.05.
Recombination in lox/y,Cre mice was completed by 8 days after the first tamoxifen injection (data not shown). At the protein level, however, the kinetics of loss were slower (Fig. 1E). When animals were treated at 20 weeks of age, MeCP2 was reduced by half between 2 and 4 weeks after the start of treatment, reaching its lowest level at 4–7 weeks. No further reduction was observed when brains from animals in the 20-week experimental cohort were analysed 21 weeks post-treatment. We conclude that MeCP2 protein persists after the loss of its gene, with an unexpectedly long half-life of ∼2 weeks.
Postnatal inactivation of Mecp2 induces the appearance of RTT-like phenotypes
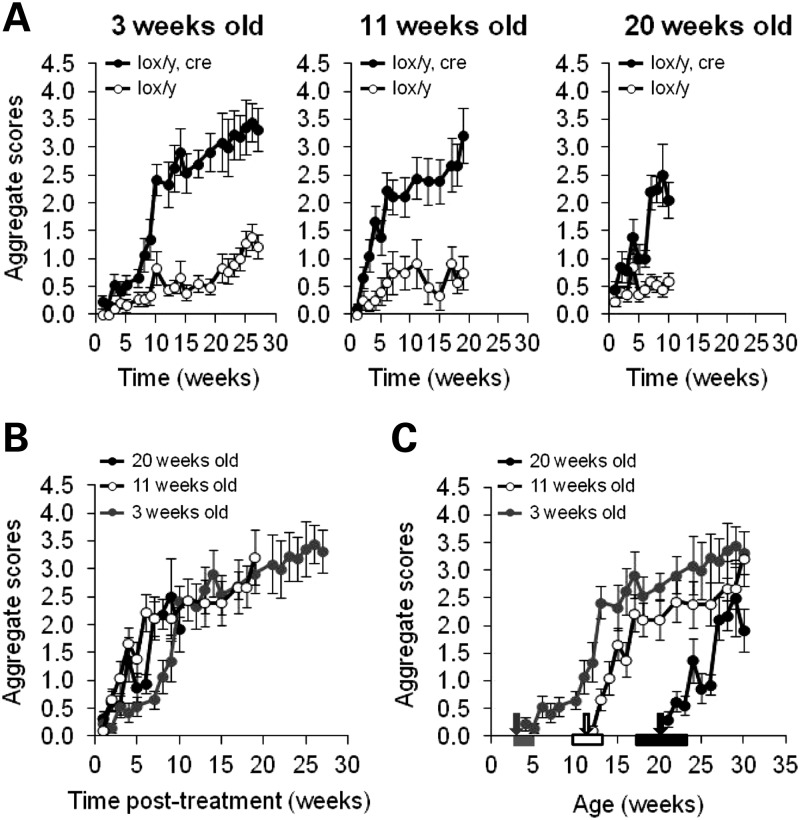
Following tamoxifen treatment, all mice were weighed and phenotypically scored once a week, as previously described (17). Lox/y,Cre animals in all three age groups developed RTT-like symptoms, including hypo-activity, altered gait, hind-limb clasping, tremors and, later on, breathing difficulties (Fig. 2A). The observed phenotypes closely resembled those seen in Mecp2−/y and Mecp2+/− animals (14). Control lox/y mice manifested mild RTT-like symptoms (open circles, Fig. 2A) due to reduced expression of the floxed Mecp2 allele, as previously described (23,24), and confirmed in this study (data not shown). Compared with their control littermates, lox/y,Cre mice acquired higher aggregate scores (Fig. 2A: 3 weeks, P < 0.0001; 11 weeks, P= 0.003; 20 weeks, P= 0.0081) and these also rose more rapidly than for lox/y animals (Fig. 2A: 3 weeks, P < 0.0001; 11 weeks, P < 0.0001; 20 weeks, P < 0.0001). Onset of symptoms could be attributed to inactivation of Mecp2, as lox/y,Cre and lox/y mice injected with oil vehicle at 11 weeks and lox/y mice injected with tamoxifen showed similar weak progression of phenotypic scores (Supplementary Material Fig. S3, P> 0.05). Thus, although the use of the hypomorphic Mecp2lox/y conditional allele in this and a previous study (18) was potentially problematic, scoring the animals blind to genotype revealed unambiguous increases in RTT-like symptoms in the inactivated animals compared with the background hypomorphic symptom level. This, together with the contrasting survival of lox/y and inactivated animals (see below), confirms the legitimacy of this model for the study of MeCP2 requirement. From the phenotypic data, we conclude that RTT-like symptoms of equivalent severity are triggered by reducing MeCP2 levels in mice aged 3, 11 or 20 weeks.
Figure 2.
Phenotypic analysis of Mecp2 inactivation. (A) Lox/y,Cre mice (filled circles) and lox/y mice (open circles) were weekly given a score reflecting their phenotype. Lox/y,Cre mice developed more severe RTT-like symptoms compared with their control littermates, independent of the age of Mecp2 inactivation [repeated-measures ANOVA, genotype effect; 3 weeks old, F(1,18) = 26.083, P < 0.0001; 11 weeks old, F(1,11) = 14.374, P = 0.003; 20 weeks old, F(1,15) = 9.316, P = 0.0081]. The severity of symptoms increased with time for both lox/y,Cre and hypomorphic lox/y mice [repeated-measures ANOVA, time effect; 3 weeks old, F(21,378) = 23.356, P< 0.0001; 11 weeks old, F(13,143) = 12.688, P< 0.0001; 20 weeks old, F(9,135) = 11.274, P< 0.0001], but the development of symptoms across time was faster and more pronounced for lox/y,Cre mice than for lox/y mice [repeated-measures ANOVA, interaction genotype-time post-treatment; 3 weeks old, F(21,378) = 6.945, P< 0.0001; 11 weeks old, F(13,143) = 4.721, P< 0.0001; 20 weeks old, F(9,135) = 6.872, P< 0.0001]. (B and C) Summary of data from tamoxifen-treated lox/y,Cre animals shown in (A), displayed with regard to time after treatment (B) or age of the animals (C). (B) By 9–10 weeks after tamoxifen injections, lox/y,Cre mice from the groups inactivated at 3 weeks old (grey circles, n = 12), at 11 weeks old (white circles, n = 9) and at 20 weeks old (black circles, n = 8) had developed symptoms of similar intensity. During the first 10 weeks, however, the severity and the onset of the symptoms were different in these three groups [repeated-measures ANOVA; group effect F(2,26) = 6.599, P = 0.0048; interaction age-time post-treatment F(12,156) = 2.218, P = 0.0133]. Precisely, the onsets of symptoms appearing in the groups inactivated at 11 and 20 weeks old were similar (Fisher's PLSD post hoc analysis, P = 0.5737), but both significantly differed from the onset of symptoms observed in the group inactivated at 3 weeks old (Fisher's PLSD post hoc analysis, 3 weeks old versus 11 weeks old P< 0.0001; 3 weeks old versus 20 weeks old P = 0.0002). (C) Evolution of the lox/y,Cre phenotype depending on age. Arrows indicate the average age of treatment (grey, 3 weeks old; white, 11 weeks old; black, 20 weeks old), and the rectangles of matching colours represent the ranges of ages of the mice when treated.
Although lox/y,Cre mice reached the same aggregate scores by 10 weeks after tamoxifen treatment, the timing of symptom onset was significantly different between the three inactivation groups (Fig. 2B; ANOVA age effect, P= 0.0048; interaction age-time post-treatment effect, P= 0.0133). Mice treated at 11 and 20 weeks old developed symptoms at the same time after injections (2–5 weeks from the start of injections, Fisher's PLSD post hoc analysis: P> 0.05), whereas mice treated at 3 weeks old displayed symptoms significantly later (8 weeks, Fisher's PLSD post hoc analysis: P < 0.01). Once overt neurological symptoms commenced, however, they developed at the same rate in all three groups (Fig. 2B). Given the ∼2week half-life of MeCP2, symptoms in 11- and 20-week groups approximately track the loss of the protein. Delayed symptom onset in the 3-week group was particularly evident when the scores were plotted as a function of group age rather than time post-tamoxifen treatment (Fig. 2C). It is possible that symptom onset in the 11- and 20-week treatment groups was more rapid because the animals were already symptomatic due to the hypomorphic lox/y allele. However, the 11-week animals from both genotype groups showed no phenotypic symptoms at the time of treatment, whereas both groups treated at 20 weeks of age were visibly symptomatic (Fig. 2A). Despite this difference in phenotypic status at the start of the experiment, 11- and 20-week groups nevertheless showed identical symptom progression. The implication of the results is that MeCP2 deficiency is relatively well tolerated in mice aged 11 weeks or less.
A prediction of this hypothesis is that inactivation earlier than 3 weeks would cause a correspondingly increased phenotypic delay from the time of drug treatment. To test this, we treated mice with tamoxifen neonatally (1 and 2 days post partum). Due to problems with treating such young animals, we administered the drug to the lactating dams, who then transferred it to their progeny via milk. Recombination achieved by this procedure (∼60%, data not shown) was less than that achieved by injection of older animals and, correspondingly, phenotypic scores plateaued at a lower level. The time of rapid symptom onset, however, was further delayed until the animals again reached ∼10 weeks of age (Supplementary Material Fig. S4), in support of the hypothesis that MeCP2 function becomes increasingly important at this developmental stage. An alternative hypothesis is that the delayed symptom onset during the post-natal period is due to the half-life of MeCP2 protein being longer at this stage than during adulthood. The finding that Mecp2 inactivation at 0 and 3 weeks both lead to a symptom transition at 11–14 weeks argues against this possibility, as levels of brain MeCP2 are very different at these two ages. Brain MeCP2 is very low at birth and rises rapidly during the first 3 weeks of life. It is unlikely that these distinct starting points could lead to the same 11–14 week symptom transition as a result of protein half-life alone.
Postnatal inactivation of Mecp2 causes a deficit in motor skills and learning
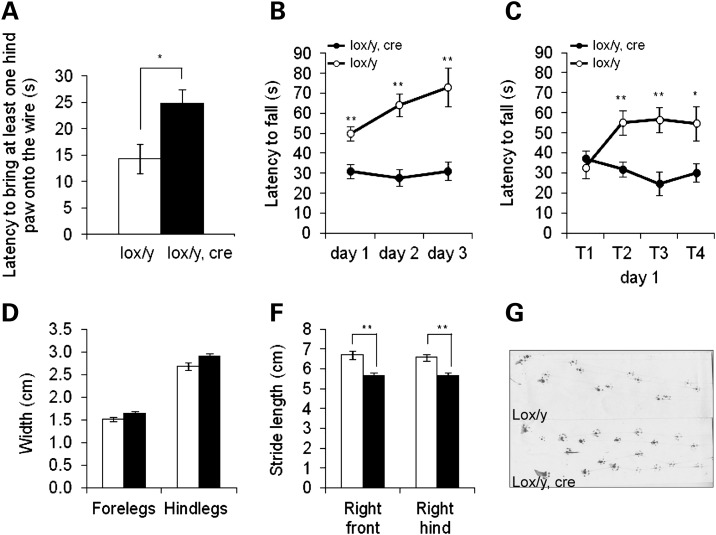
To analyse the phenotypic consequences of postnatal Mecp2 inactivation in more detail, lox/y,Cre and lox/y mice injected with tamoxifen at 3 weeks were evaluated in different motor coordination tasks 10 weeks after treatment. By this time, lox/y,Cre mice had developed significantly more severe symptoms than control littermates (Fig. 2A). In a wire suspension test, lox/y,Cre mice needed significantly more time to bring hind-paws up to the wire across three consecutive trials compared with lox/y mice (Fig. 3A; lox/y,Cre: 24.3 ± 2.9 s; lox/y: 14.3 ± 2.8 s; t-test: P= 0.0250), attesting to a motor coordination deficit. Motor coordination and learning were also assessed using the accelerating rotarod task. Latency to fall was significantly shorter for lox/y,Cre mice compared with controls (Fig. 3B, P= 0.0002). In addition, performances across the 3 days of trials differed between genotypes (P= 0.0024). During the first day of the task (Fig. 3C), all mice performed equally at the first trial, after which lox/y mice stayed significantly longer on the rod than the lox/y,Cre mice (P= 0.0026; Fisher's PLSD post hoc analysis, trial 1: P> 0.05; trial 2: P= 0.0038; trial 3: P= 0.002; trial 4: P= 0.0205). The results suggest that lox/y,Cre mice were unable to learn this motor task, but whether this was due to a learning deficit or to their altered motor coordination, as shown in the wire suspension test, remains unclear. Gait was analyzed using a footprint assay 12 weeks post-treatment. At this time, gait of lox/y,Cre mice was already altered as shown by the weekly scores (Supplementary Material, Fig. S5) and they exhibited a strong trend towards greater width between their fore- and hind-legs (P= 0.0534 and =0.0779, respectively) compared with the lox/y mice (Fig. 3D). Furthermore, the stride length of both the right fore- and right hind-legs was shorter in lox/y,Cre mice (Fig. 3E, P= 0.0004 and =0.0008, respectively). These differences cannot be attributed to a difference in size among the two genotypes, as the nose-to-base distance was similar between lox/y,Cre and lox/y mice (8.66 ± 0.15 and 8.74 ± 0.13 cm, respectively; t-test: P= 0.6903). Gait was therefore significantly altered after postnatal Mecp2 inactivation, with shorter steps and a wider distance between contra-lateral limbs (Fig. 3F), recapitulating the gait alterations observed in Mecp2-null male mice (15). Similar results were obtained with lox/y,Cre and lox/y mice treated with tamoxifen at 11 or 20 weeks, but altered motor function of the hypomorphic lox/y mice and obesity which developed in all experimental groups (Supplementary Material, Fig. S6) compromised interpretation of the data (data not shown). Nevertheless, our results strongly suggest that typical hallmarks of RTT, centred on deficient motor skills and learning, are recapitulated upon the postnatal inactivation of Mecp2.
Figure 3.
Assessment of motor abilities following Mecp2 inactivation. (A) Lox/y,Cre mice from the group inactivated at 3 weeks old (filled bar, n = 11) showed altered motor coordination compared with the lox/y mice (open bar, n = 9) with the wire suspension test (t-test P = 0.0250). Bars represent mean ± SEM. *P< 0.05. (B) Motor coordination and learning of lox/y,Cre mice (filled circles, n = 12) were also impaired in the accelerating rotarod task compared with their control littermates (open circles, n = 9), as shown by repeated-measures ANOVA [genotype effect, F(1,19) = 21.593, P = 0.0002]. Performances across the 3 days of trials improved for lox/y mice, but not for lox/y,Cre mice [repeated-measures ANOVA, interaction genotypes–trials, F(2,38) = 7.085, P = 0.0024]. Symbols represent mean ± SEM. **P < 0.01. (C) During the first day of the accelerating rotarod task, lox/y,Cre mice did not improve, in contrast with lox/y mice, even though all mice started with similar performances [repeated-measures ANOVA, genotype–trials interaction F(3,57) = 5.341, P = 0.0026; Fisher's PLSD post hoc analysis, trial 1: P = 0.4634; trial 2: P = 0.0038; trial 3: P = 0.002; trial 4: P = 0.0205]. Symbols represent mean ± SEM. *P< 0.05. **P< 0.01. (D–F) Lox/y,Cre mice (filled bars, n = 11) from the group inactivated at 3 weeks old exhibited an altered gait compared with lox/y controls (open bars, n = 9). (D) They displayed a tendency toward a wider distance between their fore-limbs (t-test, P = 0.0534) and their hind-limbs (t-test, P = 0.0779). (E) They had a shorter stride length (t-test, right fore-limbs P = 0004; right hind-limbs P = 0.0008). Bars represent mean ± SEM. **P< 0.01. (F) Examples of a footprint assay for both genotypes.
Postnatal inactivation of Mecp2 induces premature death
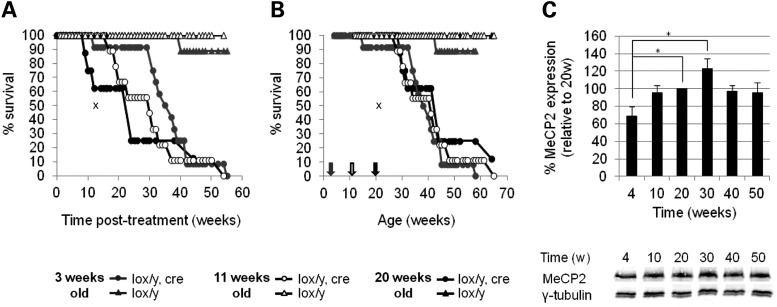
Lox/y,Cre mice exhibited a significantly shorter lifespan following tamoxifen treatment than their lox/y control littermates (Fig. 4A), but, unexpectedly, lox/y,Cre mice from the three inactivation groups had different median times between treatment and death. Mice in the group treated at 3 weeks survived for a median of 34.9 weeks, the 11-week group lived for 29.9 weeks and the 20-week group lived for 24.0 weeks. The significance of these differences was confirmed by a Kaplan–Meier survival analysis (Tarone–Ware, P= 0.041), confirming that the earlier Mecp2 was inactivated, the later after treatment animals died. Strikingly, plotting survival against the age of animals showed that lox/y,Cre mice died at approximately the same age, regardless of the age at which Mecp2 was inactivated (Fig. 4B). The median age at death for the 3-, 11- and 20-week groups was 38.5, 39.1 and 40.7 weeks, respectively, which is not significantly different by Kaplan–Meier survival analysis (Tarone–Ware, P= 0.9899). The results indicate that survival with this degree of MeCP2 deficiency becomes unsustainable at around 39 weeks of age.
Figure 4.
Survival analysis following Mecp2 inactivation. (A and B) Mecp2 inactivation (circles) triggers a significantly shortened survival compared with controls (triangles). (A) The time of death of lox/y,Cre mice after tamoxifen treatment is significantly different whether tamoxifen injections have been given at 3 (grey circles, n = 12), 11 (white circles, n = 9) or 20 (black circles, n = 8) weeks old, as shown by Kaplan–Meier survival analysis with the Tarone–Ware method (P = 0.041). (B) The age of death is, however, similar independently of the age of Mecp2 inactivation (Kaplan–Meier survival analysis with the Tarone–Ware method P = 0.9899). Arrows indicate the average age of treatment (grey, 3 weeks old; white, 11 weeks old; black, 20 weeks old). The cross symbol represents the median time post-treatment (A) or age (B) of death described in McGraw et al. (18). (C) MeCP2 expression in wild-type mice increases from 4 to 20 weeks old (t-test P= 0.0245), but is constant during adulthood (t-test, all P values > 0.05). Bars represent mean ± SEM. * P< 0.05.
The period at which MeCP2 function acquires functional importance in young adult mice (8–14 weeks of age) closely follows a large developmental increase in levels of brain MeCP2 during the first few weeks of life (2,19,20). To test whether the sensitivity to MeCP2 deficiency at ∼40 weeks is also associated with an increase in MeCP2 levels, we performed western blots on protein from brains of wild-type mice from age 4 weeks to 50 weeks (Fig. 4C). Following the dramatic increase in brain MeCP2 during the first few weeks of life, MeCP2 levels were relatively constant throughout adulthood (P> 0.05). Therefore, the period of hyper-sensitivity to MeCP2 levels at a post-natal age of around 39 weeks does not correlate with a change in levels of brain MeCP2.
DISCUSSION
RTT has traditionally been seen as a neurodevelopmental disorder, implying that MeCP2 is particularly important during development. In the past few years, however, increasing evidence has indicated that MeCP2 is required postnatally and during adulthood to maintain normal neuronal functions. First, the observation that female mice heterozygous for a Mecp2-null allele become symptomatic at 4–12 months of age, long after development is complete, argues against a purely developmental defect (14). This impression was reinforced by the finding that severe RTT-like symptoms could be reversed in adult male and female mice by restoration of MeCP2 (17). Conclusively, McGraw et al. (18) then found that numerous features observed in Mecp2-null male mice were recapitulated upon Mecp2 inactivation in 8–9-week-old mice, proving an on-going need for MeCP2 during adulthood. Our results confirm a lifelong need for the brain to express Mecp2.
McGraw et al. (18) found that mortality was equivalent between mice that inherited the Mecp2-null allele from the germ line and those where the gene was inactivated during adulthood, as in each case the median survival time after birth or after tamoxifen treatment was ∼13 weeks. A significant difference between that study and our results relates to survival. Not only did our tamoxifen-treated animals survive for well over 13 weeks (24–34 weeks), but also they lived for different periods of time depending on age at treatment. The reduced severity of phenotypes in our study is almost certainly due to a lower frequency of Mecp2 gene deletion. McGraw et al. (18) depleted brain MeCP2 levels to ∼5% of wild-type through 20 consecutive daily tamoxifen treatments, whereas our 5-day injection regimen achieved levels that were 20–25% of that in floxed Mecp2 brains. Since the floxed allele is hypomorphic, giving an average of 33% wild-type expression levels throughout the brain in our experiments (data not shown), we infer that MeCP2 levels in our animals were ∼8% of wild-type. That such a small difference in MeCP2 levels (5 versus 8%) has a significant impact on survival is unexpected and may be relevant to future therapeutic approaches to this condition. It is possible that a relatively incomplete restoration of the protein or its downstream functions may be of therapeutic benefit.
The milder phenotype and longer survival of the mice in the present study has allowed us to unmask two phenotypic thresholds during the mouse life cycle when the presence of MeCP2 assumes heightened importance (Fig. 5). The first transition centred at ∼11 weeks corresponds to the age of symptom onset in mice that suffer reduced Mecp2 either neonatally (0 week) or at weaning (3 weeks). Interestingly, this phase coincides with the period during which Mecp2-null male mice develop severe symptoms and die (14,15) (Fig. 5A). The data indicate that MeCP2 first becomes important several weeks after birth, at a time when major changes in the brain are occurring, including large-scale changes at synapses; notably refinement, reinforcement and maturation (16,19,20). Also at this time, MeCP2 protein reaches maximum levels in the brain and mice reach full sexual maturity.
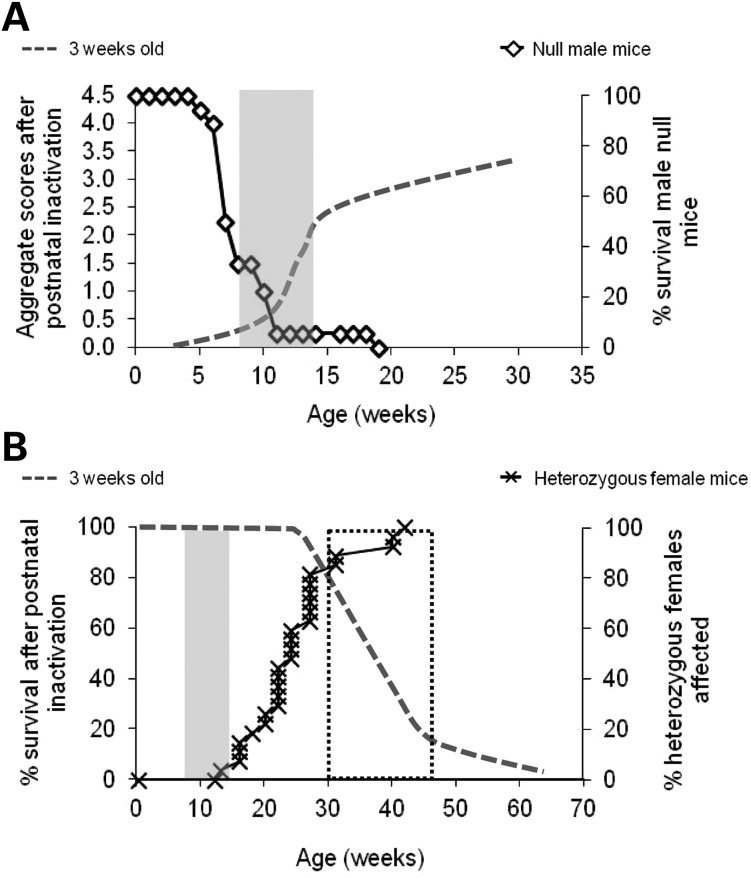
Figure 5.
Determination of two threshold periods important for MeCP2 functions. (A) The first time period (grey rectangle), between 8 and 14 weeks old, coincides with the period of rapid development of symptoms in mice that received tamoxifen injections at 3 weeks old (dotted line) and also with the period when Mecp2-null male mice die (open diamonds). (B) The second time period (dotted rectangle), between 30 and 45 weeks old, corresponds to the period of death for mice who underwent Mecp2 postnatal inactivation (dotted line) and to the period before which at least 80% of the heterozygous females (crosses) have already developed the first overt signs of the RTT-like phenotype and score ≥ 1 for any of the six categories of the phenotypic scoring system.
Mice with ∼8% of the wild-type level of expression survive the ∼11-week transition in MeCP2 sensitivity, but die at ∼39 weeks of age. Remarkably, all three inactivation groups showed similar survival, regardless of the time spent depleted of MeCP2. We conclude that a second phase of pronounced sensitivity to Mecp2 deficiency commences at this late age. There is independent evidence for this, as Guy et al. (14) observed that the fraction of Mecp2+/− females that were free of overt symptoms began to decline during this same period (30–45 weeks; Fig. 5B). In fact, the spectrum of phenotypes caused by various levels of MeCP2 deficiency can be explained if we assume that the threshold of MeCP2 expression required to allow proper functioning of neuronal networks increases stepwise at each transitional age. Absence of MeCP2 expression in null male mice means they do not survive the 11-week transition period. On the contrary, mice expressing 8% of wild-type MeCP2 protein develop neurological symptoms and survive the ∼11-week transition, but they are unable to survive beyond the second threshold at ∼39 weeks. Female mice heterozygous for a null mutation of Mecp2 also fit into this scenario, as their 50% level of MeCP2 expression (due to random X chromosome inactivation) allows them to traverse the ∼11-week time threshold without acquiring overt symptoms, but they develop RTT-like symptoms at 30–45 weeks. The appearance of more subtle phenotypes in younger heterozygous females (25,26) may be ascribed to the presence of null cells in the brain of these animals, in contrast to lox/y animals which express MeCP2 in all cells, albeit at a reduced level.
The 39-week threshold is not related to an obvious anatomical or physiological event, or to a change in MeCP2 expression, but may relate to the aging process. The reproductive efficiency of most mouse strains begins to drop after about 30 weeks of age (27), suggesting that reproductive senescence is underway by 39 weeks. It is possible that a decline in brain function also occurs near this time, although this has not been widely studied in the mouse. It may be relevant that RTT patients survive to a mean age of 40 years, which is significantly less than the average human lifespan (12,13). The physiological changes that accompany the aging process in humans and mice may place increased reliance on the presence of MeCP2 in the brain. Alternatively, deficiency of MeCP2 protein may accelerate neurological decline, resulting in a prematurely aged brain. Our findings set the scene for testing these and other hypotheses in order to gain insights into the molecular mechanisms underlying MeCP2 function and RTT syndrome.
MATERIALS AND METHODS
Animals
Male Mecp2lox/y,CreESRT and Mecp2lox/y littermates used in this study were obtained by crossing hemizygous CreESRT males (22) (Jackson Laboratory strain name:B6.Cg-Tg(cre/ESR1)5Amc/J, stock number 004682) with homozygous Mecp2lox/lox females (14) (Jackson Laboratory strain name: B6;129P2-Mecp2tm1Bird/J, stock number 006847).
Mice were maintained under standard conditions and in accordance with UK Home Office regulations and licences. Mice were carefully monitored for symptoms due to either the genetic mutation or the experimental treatment, and animals which exceeded the severity limit of the experiment were humanely culled.
Inactivation of the Mecp2 gene
Tamoxifen (Sigma) was used to induce CreESRT protein translocation into the nucleus, thereby triggering inactivation of Mecp2 in mice. Tamoxifen solution [20 mg/ml in corn oil (Sigma)] was prepared as previously described (17) and injected intraperitoneally at 100 mg/kg body weight/dose once a day for five consecutive days. Mecp2 was inactivated in three independent groups of mice, at three different ages: just after weaning at 3 weeks old (lox/y,Cre n = 12, lox/y n = 9), at 11 weeks old (lox/y,Cre n = 9, lox/y n = 6) and 20 weeks old (lox/y,Cre n = 8, lox/y n = 11). Control experiments were performed by injecting 11-week-old lox/y,Cre (n = 5) and lox/y (n = 8) mice with corn oil only, at the same volume/body weight used for the tamoxifen injections (5 μl/g body weight).
For neonatal inactivation, CreESRT males were mated with Mecp2lox/lox females. Females were allowed to give birth and then treated intraperitoneally with tamoxifen at 150 mg/kg body weight on days 1 and 2 post partum.
Southern blotting
To measure recombination of the Mecp2 floxed allele, high molecular weight genomic DNA was prepared from half mouse brains by standard procedures. DNA was digested with BamHI and NcoI and transferred to nylon membranes using a standard Southern blotting procedure. Blots were probed with a 1.1 kb NcoI–BamHI fragment (14). Recombination was quantified with a Storm PhosphorImager and ImageQuant software (Molecular Dynamics), and calculated as % recombination = (intensity of 1.2 kb deleted band ÷ total intensity of 1.2 kb + 1.5 kb + 4 kb bands) × 100. Animals showing a percentage of recombination inferior to 75% were excluded from the study.
Western blotting
Protein extracts for western blot analysis were prepared from half brains (∼60 × 106 cells). Whole cell lysates were prepared by homogenising brains on ice with 750 µl NE1 (20 mm HEPES, pH 7.9, 10 mm KCl, 1 mm MgCl2, 20% glycerol, 0.1% Triton X-100, 0.5 mm DTT and protease inhibitors). To release protein bound to DNA, samples were treated with 3 µl of Benzonase Nuclease (Sigma) for 15 min at room temperature. Seven hundred fifty micro litres of 2× sample buffer were added to each lysate. Samples were vortexed, boiled, aliquotted and stored at −20°C. Samples were run on SDS–PAGE gels and transferred to nitrocellulose membranes using standard procedures. Membranes were cut into two portions, blocked for 1 h in 5% milk in Tris-buffered saline and probed overnight at 4°C with an anti-MeCP2 antibody (Sigma, M6818, 1:1000) or an anti-gamma-tubulin antibody as a loading control (Sigma, T6557, 1:3000). Membranes were probed with anti-mouse secondary antibody (Licor Donkey anti-Mouse IRDye, 1:10 000) and analysed with a Licor scanner using Odyssey software (LI-COR Biosciences). MeCP2 levels were normalized to gamma-tubulin levels after background subtraction.
Phenotype assessment
Scoring of symptoms was performed blind to the genotype. Six parameters were examined at the same time each week (activity, gait, hind-limb clasping, tremor, breathing and general condition) and given a score between 0 and 2, as previously described (17). Given the slower and more subtle evolution of the phenotypes of inactivated lox/y,Cre mice compared with null male mice, this protocol was slightly modified by introducing two intermediate scores (0.5 and 1.5). Animals scoring 2 for breathing or general condition were automatically culled, and although for reasons of brevity animals are described in the text as having ‘died’, they were routinely culled at the point at which they were judged to have reached the severity limit of the experiment, according to the Home Office licence.
Behavioural characterization
Ten weeks after tamoxifen treatment, motor coordination and learning of lox/y,Cre and lox/y mice treated at 3 weeks were assessed using the wire suspension test and the accelerating rotarod test. The wire suspension test was conducted as previously described (28), using a thin horizontal wire (1.5 mm in diameter) 35 cm above the bench surface. Mice were allowed to grip the wire with their forepaws and the latency to bring at least one hind-paw up to the wire was recorded, for a maximum of 30 s. This experiment was repeated three times, with an inter-trial interval of 15 min. The mean of the three trials was calculated for each mouse. For the accelerating rotarod test, mice were submitted to a 4-day training protocol (29). They were first given a habituation pre-trial in which animals were placed on the rod rotating at a constant and low speed (4 rpm), for 30 s. If they fell, they were placed back on the rod in order to complete the 30 s pre-trial. Following habituation, mice were submitted to four training sessions per day, for three consecutive days. Each session consisted of placing the animal on the rod, which would accelerate from 4 to 40 rpm within 5 min, and was separated from the next session by an inter-trial interval of 1 h. Time spent on the rod was measured.
Twelve weeks post-treatment, the gait of the same mice was analysed using the footprint test (29). Briefly, mice were submitted to a training session in which they learnt how to walk along an illuminated runway in order to reach a dark, small box. Following this training session, paper was placed on the runway, and paws were painted in red (fore-paws) or green (hind-paws), using non-toxic paint (Snazaroo face paint), allowing a record to be kept of each trial. One set of footprints was collected for each mouse. From these footprints, the front- and hind-base width, as well as the stride length were measured, as previously described (29).
Statistical analysis
Data were analysed using Student's t-test or analysis of variance (ANOVA: one-way, two-way or repeated-measures analysis of variance as appropriate), with post hoc comparisons (Fisher's PLSD) when required (Statview 5.0, SAS Institute Inc.).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the Wellcome Trust (077224, 091580), the Medical Research Council, UK (G0800401), Action Medical Research in association with the Henry Smith Charity and the R S MacDonald Charitable Trust (SP4443), and the Rett Syndrome Research Trust (82561).
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Sabine Lagger and Robert Ekiert for critical reading of the manuscript and Alan McClure for assistance with animal husbandry.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lewis J.D., Meehan R.R., Henzel W.J., Maurer-Fogy I., Jeppesen P., Klein F., Bird A. Purification, sequence and cellular localisation of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 2.Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretti P., Zoghbi H.Y. MeCP2 dysfunction in Rett syndrome and related disorders. Cur. Opin. Genet. Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Schanen C., Houwink E.J., Dorrani N., Lane J., Everett R., Feng A., Cantor R.M., Percy A. Phenotypic manifestations of MECP2 mutations in classical and atypical Rett syndrome. Am. J. Med. Genet. A. 2004;126:129–140. doi: 10.1002/ajmg.a.20571. [DOI] [PubMed] [Google Scholar]
- 5.Villard L., Kpebe A., Cardoso C., Chelly P.J., Tardieu P.M., Fontes M. Two affected boys in a Rett syndrome family: clinical and molecular findings. Neurology. 2000;55:1188–1193. doi: 10.1212/wnl.55.8.1188. [DOI] [PubMed] [Google Scholar]
- 6.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong D.D. Neuropathology of Rett syndrome. Ment. Retard Dev. Disabil. Res. Rev. 2002;8:72–76. doi: 10.1002/mrdd.10027. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D.D. Neuropathology of Rett syndrome. J. Child. Neurol. 2005;20:747–753. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg B., Aicardi J., Dias K., Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 10.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien. Med. Wochenschr. 1966;116:723–726. [PubMed] [Google Scholar]
- 11.Chahrour M., Zoghbi H.Y. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Freilinger M., Bebbington A., Lanator I., De Klerk N., Dunkler D., Seidl R., Leonard H., Ronen G.M. Survival with Rett syndrome: comparing Rett's original sample with data from the Australian Rett Syndrome Database. Dev. Med. Child Neurol. 2010;52:962–965. doi: 10.1111/j.1469-8749.2010.03716.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirby R.S., Lane J.B., Childers J., Skinner S.A., Annese F., Barrish J.O., Glaze D.G., Macleod P., Percy A.K. Longevity in Rett syndrome: analysis of the North American Database. J. Pediatr. 2010;156:135–138 e1. doi: 10.1016/j.jpeds.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 15.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 16.Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGraw C.M., Samaco R.C., Zoghbi H.Y. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassel S., Revel M.O., Kelche C., Zwiller J. Expression of the methyl-CpG-binding protein MeCP2 in rat brain. An ontogenetic study. Neurobiol. Dis. 2004;15:206–211. doi: 10.1016/j.nbd.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Kishi N., Macklis J.D. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Mullaney B.C., Johnston M.V., Blue M.E. Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience. 2004;123:939–949. doi: 10.1016/j.neuroscience.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi S., McMahon A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 23.Kerr B., Alvarez-Saavedra M., Saez M.A., Saona A., Young J.I. Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Hum. Mol. Genet. 2008;17:1707–1717. doi: 10.1093/hmg/ddn061. [DOI] [PubMed] [Google Scholar]
- 24.Samaco R.C., Fryer J.D., Ren J., Fyffe S., Chao H.T., Sun Y., Greer J.J., Zoghbi H.Y., Neul J.L. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picker J.D., Yang R., Ricceri L., Berger-Sweeney J. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport. 2006;17:541–544. doi: 10.1097/01.wnr.0000208995.38695.2f. [DOI] [PubMed] [Google Scholar]
- 26.Stearns N.A., Schaevitz L.R., Bowling H., Nag N., Berger U.V., Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146:907–921. doi: 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Green E.L. Biology of the Laboratory Mouse. Dover Publications Inc; 1968. [Google Scholar]
- 28.Poirier R., Cheval H., Mailhes C., Charnay P., Davis S., Laroche S. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Front. Behav. Neurosci. 2007;1:6. doi: 10.3389/neuro.08.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galante M., Jani H., Vanes L., Daniel H., Fisher E.M., Tybulewicz V.L., Bliss T.V., Morice E. Impairments in motor coordination without major changes in cerebellar plasticity in the Tc1 mouse model of Down syndrome. Hum. Mol. Genet. 2009;18:1449–1463. doi: 10.1093/hmg/ddp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.