Abstract
The avian basilar papilla (BP) is a likely homolog of the auditory sensory epithelium of the mammalian cochlea, the organ of Corti. During mammalian development Fibroblast growth factor receptor-3 (Fgfr3) is known to regulate the differentiation of auditory mechanosensory hair cells (HCs) and supporting cells (SCs), both of which are required for sound detection. Fgfr3 is expressed in developing progenitor cells (PCs) and SCs of both the BP and the organ of Corti; however its role in BP development is unknown. Here we utilized an in vitro whole organ embryonic culture system to examine the role of Fgf signaling in the developing avian cochlea. SU5402 (an antagonist of Fgf signaling) was applied to developing BP cultures at different stages to assay the role of Fgf signaling during HC formation. Similar to the observed effects of inhibition of Fgfr3 in the mammalian cochlea, Fgfr inhibition in the developing BP increased the number of HCs that formed. This increase was not associated with increased proliferation, suggesting that inhibition of the Fgf pathway leads to the direct conversion of PCs or supporting cells into HCs, a process known as transdifferentiation. This also implies that Fgf signaling is required to prevent the conversion of PCs and SCs into HCs. The ability of Fgf signaling to inhibit transdifferentiation suggests that its down-regulation may be essential for the initial steps of HC formation, as well as for the maintenance of SC phenotypes.
Keywords: Fgf, transdifferentiation, hair cell, basilar papilla, supporting cell, SU5402
1. Introduction
Similar to the mammalian organ of Corti, the avian basilar papilla (BP) contains mechanosensory hair cells (HCs) which act as the primary transducers of auditory stimuli. While birds are able to replace damaged HCs through a regenerative process (Corwin et al., 1988; Cotanche, 1987; Ryals et al., 1988), HC loss in the mammalian auditory system is permanent. In both sensory epithelia, HCs are surrounded by supporting cells (SCs) to form a mosaic that spans the proximal-to-distal and neural-to-abneural axes, and previous lineage tracing experiments have demonstrated that HCs and SCs are derived from a common progenitor pool (Fekete et al., 1998). It has been suggested that HC formation during regeneration may share similarities with HC formation during development, and studies have identified many aspects of HC development that are recapitulated during regeneration (Cotanche et al., 2010; Levic et al., 2007; Stone et al., 2007). Thus, a full characterization of the process of HC and SC development and the genes that are responsible for their differentiation from a common precursor pool should provide insights into the process of regeneration.
In the chick, outgrowth of the BP from the otic vesicle is evident by embryonic day 5 (E5), and HCs begin to differentiate around E6 (Bartolami et al., 1991; Cotanche et al., 1983) even though terminal mitosis will not be complete until E8.5 (Katayama et al., 1989). The initial progenitor cell (PC) population forms a pseudostratified epithelium with multiple layers of disorganized nuclei (Fig. 1A). As the distal-to-proximal gradient of HC differentiation begins, the epithelium reorganizes (Fig. 1B) to form a single lumenal layer of HCs with several layers of SCs more basally located (Cotanche et al., 1984).
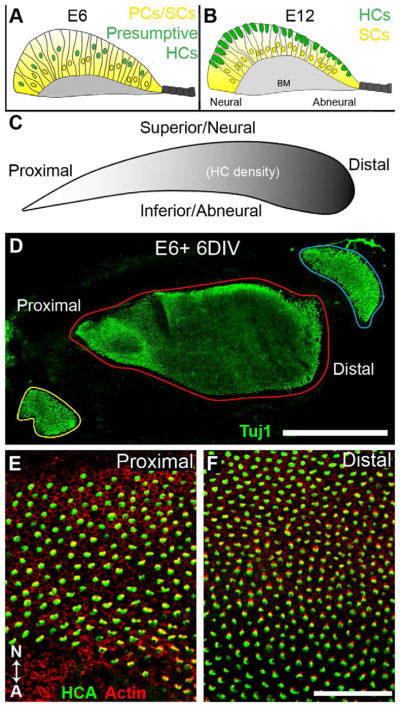
Figure 1. Development of the basilar papilla in vivo and in vitro.
(A,B) Schematic cross-sections through the developing basilar papilla (BP) at the indicated time points. At E6, the BP is comprised of a population of progenitor cells (PCs) that have yet to differentiate. Presumptive hair cell nuclei (HCs, green) are located at more lumenal positions within the epithelium. By E12, HCs (green) are located at the lumenal surface while supporting cell nuclei (SCs, yellow) are located closer to the basement membrane (BM) although their cell bodies extend processes forming contacts with both surfaces. Neural and abneural axes are indicated for orientation. (C) Schematic of the BP at approximately E12. Two axes of the BP, proximal-distal and neural-abneural are indicated. Shading represents the gradient of HC density (darker regions are higher density). (D) An E6 BP culture after 6 days in vitro (DIV) on a Millipore-tissue-tek membrane. HCs have been labeled with the Tuj1 antibody. The three sensory organs within the original explant are outlined in different colors. The vestibular sacculus is near the proximal end of the BP (yellow), the auditory BP is in red and the vestibular lagena is in blue. While the overall growth of the BP is somewhat restricted, the general shape is consistent with the shape of a normal BP. (E,F) High magnification views of the proximal (E) and distal (F) regions of an E6 explant maintained for 4 DIV showing the normal change in HC density between the proximal and distal regions as well as the different HC morphologies across the neural-abneural axis (indicated by N-A). Scale bars: D, 500 μm; E and F, 50 μm.
Recent work has demonstrated that formation of the PC population, often referred to as prosensory cells in mammals, is dependent on expression of the transcription factor Sox2 and activation of the Notch signaling pathway (Basch et al., 2011; Daudet et al., 2005; Daudet et al., 2007; Kiernan et al., 2005; Yamamoto et al., 2011), while subsequent development of some PCs into HCs requires expression of another transcription factor, Atoh1 (Bermingham et al., 1999; Woods et al., 2004). In both mammals and birds, expression of Sox2 is maintained in the SC population into adulthood (Dabdoub et al., 2008; Neves et al., 2007). Sorting of PCs into hair cells and supporting cells is mediated through a lateral inhibitory pathway in which differentiating HCs inhibit neighboring PCs from developing into HCs through activation of the Notch-Delta signaling pathway (Adam et al., 1998; Daudet et al., 2005; Daudet et al., 2007). A similar mode of regulation has been demonstrated in the mouse cochlea (Lanford et al., 1999; Lewis et al., 1998). However, data from both species suggest that Notch-Delta signaling alone is not sufficient to fully explain the generation of the HC-SC mosaic, and published reports suggest that the Fibroblast growth factor (Fgf) signaling pathway may also contribute to this patterning (Chen et al., 1999; Hayashi et al., 2007; Jacques et al., 2007; Mueller et al., 2002; Puligilla et al., 2007; Shim et al., 2005).
The Fgf family includes more than 20 secreted ligands and 4 membrane-bound receptors (each with multiple splice variants) that play important roles in cell survival, proliferation, migration, and differentiation (Hebert, 2011). During inner ear formation Fgf signaling has been shown to regulate otic placode induction as well as early events in otocyst formation (Schimmang, 2007). Later in development, Fgf ligands and their receptors have also been shown to regulate the formation of auditory HCs. In particular, in the developing murine cochlea Fgf20 has been suggested to signal through Fgfr1 to promote early HC specification (Hayashi et al., 2008; Pirvola et al., 2002). Fgfr3 is also expressed in the mouse cochlear sensory epithelium (Peters et al., 1993) and inhibition either in vivo or in vitro results in the loss of SCs and the formation of ectopic HCs (Colvin et al., 1996; Hayashi et al., 2007; Jacques et al., 2007; Mueller et al., 2002; Puligilla et al., 2007) suggesting that one effect of Fgfr3 activation is to prevent HC differentiation. In both the developing organ of Corti and the BP, undifferentiated progenitor cells express fgfr3 (Bermingham-McDonogh et al., 2001); but as HC differentiation begins (at approximately E6 in the BP) the expression of fgfr3 becomes restricted to the SC layer. In chick, fgfr3 expression persists in SCs and is maintained into adulthood. However, if a regenerative response is induced in the BP as a result of HC loss, fgfr3 becomes down-regulated in SCs. This down-regulation is transient, and once HC regeneration is complete fgfr3 expression in the SCs returns to normal levels (Bermingham-McDonogh et al., 2001). These results suggest that Fgfr3 activation within SCs of the BP may act to block the differentiation of cochlear progenitors and/or SCs into HCs. The aim of the present study was to test this hypothesis using an in vitro preparation of the developing BP coupled with pharmacological inhibition of Fgfr activity.
2. Materials and methods
2.1 Chick embryos
Fertilized eggs from White Leghorn chickens (Gallus gallus domesticus) were obtained from CBT Farms (Chestertown, Maryland) or McIntyre Poultry and Fertile Eggs (Lakeside, California) at zero, three or six days post-fertilization and incubated until specific stages between E5 and E12. Embryos were removed from their eggs, developmentally staged (Hamburger et al., 1951) and then decapitated. All embryos were terminated prior to hatching. All protocols for embryo use were approved by the Animal Care and Use Committees at the NIH, the University of Maryland and the University of California San Diego.
2.2 Basilar papilla cultures
Developing basilar papillae (BPs) were dissected from embryonic chicks at specific gestational ages between E5 and E8 as follows: the bony labyrinth was visualized through the ventral aspect of the skull and the cartilaginous capsule surrounding the duct of the basilar papilla was removed. Next, the overlying membrane was peeled away to expose the sensory epithelium; in E5–E6 dissections this included tissue that would form the sacculus, the BP, and the macula lagena, as these structures are in close approximation at these stages. The underlying mesenchyme and developing auditory-vestibular ganglion were removed to free the epithelium. The dissected BPs and associated tissue were placed onto Matrigel-coated (diluted to 5% to prevent activity of endogenous aminoglycoside antibiotics, which are ototoxic; BD Biosciences, San Jose, CA) Millipore tissue-tek permeable membrane inserts that were then placed into 35 mm culture dishes containing 0.8 mL of culture media (Medium-199, Sigma-Aldrich) with 10% FBS (Sigma), 1% N2 supplement (Gibco) and ciprofloxacin (10 μg/mL). Membrane inserts were placed in contact with the surface of the culture media. This technique allowed BP explants to be maintained at an air-media interface with the resulting surface tension acting to hold the tissue in a flat orientation (Stone et al., 1991). Cultures were maintained for 3–10 days in vitro (DIV). Fgf signaling was antagonized by adding 10 μM SU5402 (Santa Cruz Biotech, Santa Cruz, California) to the culture media. Control media contained an equivalent amount of the carrier, DMSO. At the conclusion of each experiment, explants were fixed in 4% PFA for 30 minutes then rinsed in PBS. For all experiments, a minimum of 3 independent culture sets with at least 6 explants per treatment condition were analyzed.
2.3 Immunohistochemistry
The same labeling protocol was used for all BP tissue. Samples were permeabilized in 0.5% PBS-triton (PBST) for one hour followed by one hour of blocking in 10% goat or horse serum in PBST. All cultures were incubated in primary antibodies for 3 hours at room temperature or over night at 4°C. Antibody labeling was visualized using secondary antibodies directed against the primary host species that were conjugated to Alexa 488, 546 or 633 (1:1000; Invitrogen). The following primary antibodies were used at the indicated dilutions: Myosin7a, 1:1000, Proteus Biosciences; HCA, 1:10 (a gift from Guy Richardson, University of Sussex, UK); Tuj1 (anti-β-tubulin III), 1:500, Sigma; Sox2, 1:200, Santa Cruz Biotechnology; activated caspase 9, 1:100, Cell Signaling Technology. Alexa-conjugated (488, 546 or 633) Phalloidin (Invitrogen) was used at 1:200. DAPI (1:3000; Roche) was used to stain cell nuclei. For identification of BrdU-positive cells, tissue was placed into 1N HCl for 30 minutes following fixation, tissue was then incubated in anti-BrdU antibody (1:50 in PBST, BD Biosciences) over night, and secondary antibody detection was performed as described above.
2.4 Quantification of cell density and epithelial thickness
Embryonic BP cultures were established such that one ear from each embryo was maintained under control conditions while the contralateral ear was treated with SU5402. Once fixed, explants were immuno-labeled to detect class III β tubulin (the Tuj1 antigen) in HCs and to detect Sox2 in SCs. Z-stack confocal images were obtained from the proximal, middle and distal regions of each sample using a Zeiss LSM510 laser scanning microscope with the Z-plane beginning at the tips of the stereocilia and ending below the level of supporting cell nuclei. HC and SC densities were determined by placing a scale box measuring 50×100 μm (approximately 10% of the total surface area of each region) over the image and counting the number of cells of each cell type inside the box. Three non-overlapping boxes were counted for each region. Three or more independent culture sets were quantified for each experiment. HC and SC numbers were then averaged for each condition. Nuclear density was analyzed in these explants by labeling nuclei with DAPI then comparing similar regions from control and SU5402-treated explants as described above. Zeiss Image Browser software was used to quantify epithelial thickness; measurements were obtained by analyzing confocal images in the z-plane, spanning from the lumenal surface of the HCs through the last Sox2-positive SC nuclei. The Student’s t-test was used for all statistical analysis.
2.5 BrdU analysis
To identify cells undergoing mitosis, BrdU (3 μg/mL; BD Biosciences) was added to the culture media of some control and SU5402-treated explants. For the quantification of BrdU-incorporation by hair cells, samples were double-labeled with antibodies to BrdU and either HCA (a HC-specific antigen) or Sox2. The same imaging process was used as described in section 2.4. The total number of BrdU-positive cells per explant that were also positive for either HCA or Tuj1 was determined (n=6 explants analyzed per condition).
2.6 Measurement of hair cell diameters and surface areas
To determine HC diameter in control and SU5402-treated cultures, BP cultures were labeled with Phalloidin to mark the cell boundaries and Tuj1 or HCA to identify HCs. Z-stack confocal images were generated beginning at the lumenal surface and extending down to the level of the supporting cell nuclei, at 1 μm intervals. For each sample, the composed Z-stack was analyzed to qualitatively assess the depth in the epithelium at which HC diameters were greatest. Measurements of the cross-sectional area of each HC were then obtained from the depth at which HC diameter was largest using Zeiss LSM Image Analysis software. Measurements were obtained from the proximal and distal regions of the BP in control and SU5402-treated samples. To determine the total surface area of cultured BPs, low magnification images were taken of whole explants that had been immuno-reacted for Tuj1 or HCA to identify the HC domain. Zeiss LSM Image Browser software was then used to measure the total surface area of the BP.
3. Results
3.1 Inhibition of Fgf signaling in the developing basilar papilla results in supernumerary hair cells
As discussed, the pattern of fgfr3 expression in the developing BP, along with its known role in the mammalian cochlea, suggests that it may play a role in inhibiting progenitor cells from developing as HCs. To test this hypothesis, a modification of the in vitro culture system for the chicken BP developed by Stone and Cotanche (1991) was used; with this method, the time course and pattern of HC differentiation proceeds as in vivo. Briefly, the ventral out-pocketing of the otocyst was dissected from E6-E7 chick embryos and placed in a Millipore filter cup with the lumenal surface oriented up (see section 2.2 in Materials and Methods for details). After 5 or 6 days in culture, explants were fixed and HCs were visualized with one of the following HC markers: anti-β-tubulin III (Tuj1), Myosin7a or the hair cell antigen (HCA). Tuj1 labeling indicated relatively normal HC pattern formation in control explants (Fig. 1C, D). At this stage of development, the sacculus (located at the proximal end of the cochlear duct), the BP, and macula lagena (located at the distal end of the cochlear duct) were easily distinguished from one another (Fig. 1D). In these explants, HCs were clearly present along the entire length of the BP and the normal proximal-to-distal gradients of HC morphology and HC density were evident (Fig. 1C, E, F).
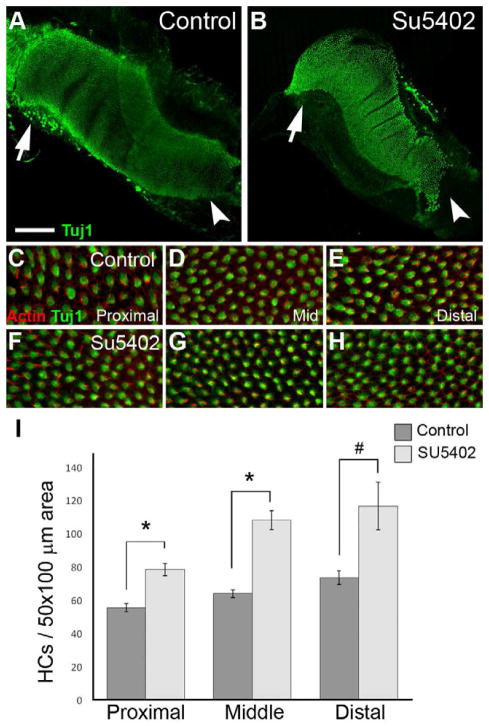
At E6, HC and SC differentiation from the pool of sensory progenitor cells is just beginning (Cohen et al., 1978; Cotanche et al., 1983). To determine if Fgf signaling regulates the number of cells that develop as HCs, explants were maintained in media containing 10 μM SU5402, a potent and specific pharmacological inhibitor of Fgfr signaling (Mohammadi et al., 1997; Mueller et al., 2002). Treatment with SU5402 for 5 DIV beginning on E5 or E6 resulted in a significant increase in HC density along the length of the BP compared to control explants (Fig. 2). In SU5402 treated cultures, HCs were clearly smaller and appeared to be present at a higher density than in controls in all three regions of the BP (Fig. 2C-H). Quantification of HC density indicated a significant increase in the number of HCs (by approximately 30%) at each position along the BP (Fig. 2I). While the density of HCs was significantly greater in SU5402-treated samples, there was no significant difference in the overall surface area of the sensory epithelium between control and SU5402-treated explants (n=8 control and n=9 SU5402-treated explants measured), further indicating an overall increase in the number of HCs by Fgfr inhibition. In addition to HC quantification, we also analyzed nuclear density in control and SU5402-treated BP cultures. While a clear increase in DAPI-positive nuclei was evident in the upper HC layer of the epithelium, no difference was observed in the deeper layers of control compared to SU5402 samples (See Supplementary Figure). During normal development most HC differentiation occurs between E6 and E8, and by E9 most HCs have differentiated. When treatment with SU5402 was initiated at E9, a similar increase in HC density was observed (data not shown) suggesting that the temporal window of sensitivity to Fgf signaling extends to later periods of BP development.
Figure 2. SU5402 treatment beginning at E6 results in a significant increase in hair cell density.
(A,B) Low-magnification images of control (A) and SU5402-treated (B) BP explants, established on E6 and maintained for 5 DIV. HCs are labeled with Tuj1 (green). Arrow heads indicate the proximal regions and arrows indicate the distal regions. (C–H) High-magnification images from proximal, middle and distal regions of control (C–E) and SU5402-treated (F–H) BPs. Note the increased HC density across all three regions of the BP in the SU5402 treated explants. (I) HC density was determined as the number of HCs within a 50×100 μm box placed at different positions (proximal, middle, distal) along the BP. Exposure to SU5402 leads to a 30% or greater increase in the number of HCs per unit area in each region of the BP. (*) p<0.0005; (#) p<0.02. Scale bar in A and B: 200 μm.
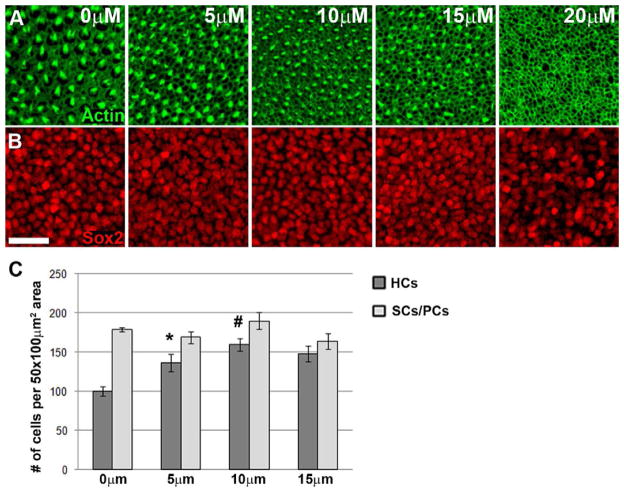
Moreover, the increase in HC density following treatment with SU5402 was dose dependent (Fig. 3A). At 5 μM, HC density increased by approximately 37%, a significant increase compared to controls (p=0.02); at 10 μM, density increased by almost 60% compared to controls (Fig. 3C). At 15 μM, HC density was slightly less than at 10 μM (although this difference was not significant) and at 20 μM few HCs could be detected and cell death was observed, suggesting significant toxicity at this dose.
Figure 3. SU5402-induced increase in hair cell density is dose dependent.
(A) High-magnification surface views of BP explants maintained in various concentrations of SU5402; images were taken at a position located 1/3 of the total length of the BP from the proximal end, at the midpoint between the neural-abneural axis. Actin in the HC membranes and stereocilia is labeled with phalloidin (green). Control (0 μM) is shown at left and increasing doses (5, 10, and 15 μM) are shown adjacent. HC density increases up to 10 μM; at 15 μM density is greater than at 5 μM yet less than at 10 μM, and HC death occurs at 20 μM. (B) Supporting cell/progenitor cell (SC/PC) nuclei from the same tissue samples as in A are shown at the level below the HC nuclei; no change in the number of Sox2-positive cells (red) is apparent. Scale bar is the same for all: 20 μm. (C) Graph illustrating the changes in HC and SC/PC density at different concentrations of SU5402; (*) P=0.02 for 0 vs. 5 μM; (#) P<0.0003 for 0 vs. 10 μM.
To determine whether the increase in HC density could be accounted for by a corresponding change in the number of SCs or progenitor cells, the number of Sox2-positive cells was determined following SU5402-treatment. During early development Sox2 is known to be expressed in all progenitor cells of the BP; it eventually is down-regulated in differentiating HCs but is maintained by SCs (Neves et al., 2007). Tuj1 expression is one of the earliest markers of a differentiating HC (Molea et al., 1999), therefore cells that are Tuj1-negative and Sox2-positive can be considered to be SCs or progenitor cells. Unfortunately there is no marker available to clearly distinguish between differentiated SCs and undifferentiated progenitor cells (PCs), thus we will refer to these cells as SCs/PCs. To determine if the increase in HC density is coupled to changes in the number of SCs/PCs, Sox2 was used to label and count the number of SCs/PCs. (Fig. 3B). Quantification could identify no significant change in the number of Sox2-positive cells at doses between 0 and 15 μM (Fig. 3C).
3.2 SU5402-induced hair cells are not the direct result of increased mitosis
To identify if the increase in HC density was the result of enhanced proliferation induced by SU5402, the mitotic tracer BrdU was added to SU5402 or control media for the entire culture period starting at E6. Tissue was fixed after 5 DIV and the explants were labeled for nuclear incorporation of BrdU (Fig. 4A). Results indicated no difference in the number of BrdU-positive HCs between control (Fig. 4B; with an average of 2.4 BrdU+ HCs per culture) and SU5402-treated (Fig. 4C, 2.2 HCs per culture) explants (n=6 explants counted per condition). However, a slight increase in BrdU-positive cells was observed in the SC/PC layer (data not shown).
Figure 4. SU5402 treatment does not result in BrdU incorporation in developing HCs.
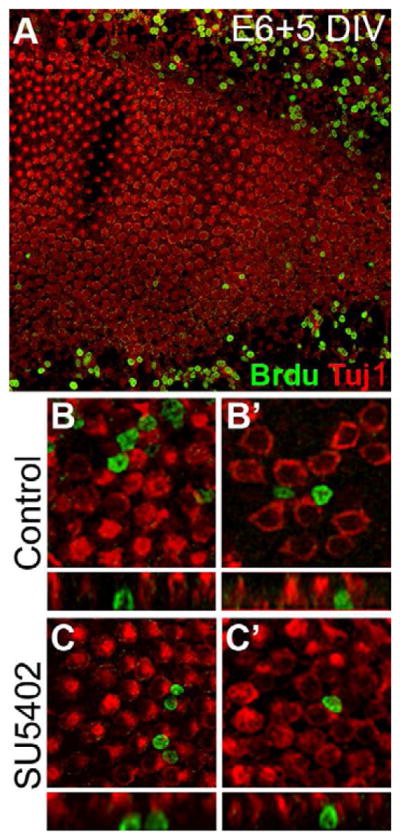
(A) Low-magnification view of an embryonic BP culture established at E6 and maintained for 5 DIV with SU5402 shows BrdU incorporation (green) in the nuclei of mesenchymal cells outside the epithelium but few BrdU-positive cells within the sensory epithelium; HCs are identified by Tuj1 expression (red). High agnification images of two control (B, B′) and two SU5402-treated (C, C′) BP cultures shows that HC nuclei are not positive for BrdU incorporation. Lumenal surfaces are shown above, and confocal-generated Z-stack cross sections of the same BrdU-positive cells are shown below.
3.3 Inhibition of Fgfr activity produces new hair cells in the developing BP
The lack of mitotically generated HCs indicated by the results of the BrdU experiment described in section 3.2 suggested that the source of additional HCs in SU5402-treated cultures was likely due to an increase in the number of post-mitotic SCs/PCs that adopted a HC fate. During normal development of the BP, PCs have processes that span the entire depth of the epithelium forming contacts with both the lumenal and basal surfaces; these cells are also characterized by having centrally placed nuclei (Whitehead et al., 1985). As HC differentiation begins, nuclei migrate toward the lumenal surface and retract their basally extended processes. In contrast, differentiating SCs maintain contact with both surfaces (Stone et al., 2000). Thus newly differentiating HCs should have a spindle-shaped morphology, intermediate between SCs and HCs (Umemoto et al., 1995), with nuclei located below the level of the normal HC layer. To determine if treatment with SU5402 results in the ectopic differentiation of SCs/PCs into HCs, we examined the cellular mosaic along the basal-to-lumenal axis using confocal Z-stack projections in E6 explants that had been maintained for 5 DIV. Following SU5402-treatment, a reduction in the surface area of SCs was apparent at the lumenal surface of the BP (Fig. 5A and B). Moreover, similar images captured at the level of the HC nuclei indicated the presence of immature, potentially newly differentiated HCs. In controls, the HC nuclear layer was comprised of uniformly sized, Tuj1-positive HC nuclei surrounded by projections from SCs (Fig. 5C). In contrast, within the HC nuclear layer of SU5402-treated BPs, many narrow Tuj1-positive profiles could be observed interspersed between other HC nuclei (Fig. 5D). Focusing downward into the epithelium revealed that these profiles were actually processes from HCs with more deeply situated nuclei (schematics in Fig. 5G and H show the approximate position of the images in Fig. 5C and D within the z-plane of the epithelium and generalized drawings of the basic types of HCs within these explants). These results suggest that these cells represent differentiating HCs that are still relatively immature.
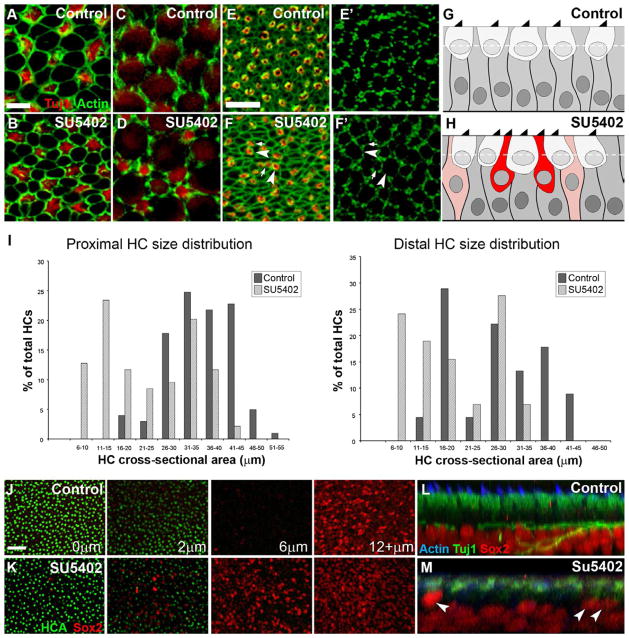
Figure 5. Inhibition of Fgf signaling induces developing progenitor cells/supporting cells to differentiate into hair cells.
(A,B) High magnification lumenal surface views of control E6 +5 DIV (A) and SU5402-treated (B) explants demonstrating higher HC (Tuj1, red) density and a reduction in the size of lumenal SC/PC projections (actin shown in green) following Fgfr inhibition (B) compared to control (A). (C,D) High magnification z-slices at the level of the HC nuclei of control (C) and SU5402-treated (D) explants indicating the presence of many small HCs deep within the epithelium of SU5402-treated explants and not in control. At this level, large HC nuclei are visible and only thin projections of the new HCs are visible as bright red circles (Tuj1), as their nuclei are situated below those of the larger HCs. (E-F′) Surface (E,F) and nuclear-level (E′,F′) views of control (E,E′) and SU5402-treated (F,F′) BP explants established at E6 and maintained for 5 DIV demonstrating the increased number of HC-HC contacts between small (arrows) and large (arrowheads) HCs (HCA, red; actin, green) following Fgfr inhibition. (G,H) Generalized cartoon renderings of control (G) and SU5402-treated (H) BP explants; the dashed white lines approximate the level in the epithelium depicted in C and D. Red cells represent the newly formed HCs with deeply situated nuclei; pink cells represent those undergoing differentiation into HCs. Schematics do not reflect actual nuclear density. (I) HC size distribution in the embryonic BP following SU5402 treatment. HC sizes were measured as the cross-sectional area through the widest point of the HC in the z-plane in control and SU5402 treated samples that were established at E9 and maintained for 5 DIV. HCs were binned in groups based on the size of their cross sectional area, each bin represents a range of 5 μm2; the number of HCs was then plotted as a percentage of the total number of HCs measured (n= 90 HCs per region of the cochlea). Distribution of HC sizes from the proximal 2/3 of the cochlea is shown at left and HCs in the distal 1/3 are shown at right. (J,K) Sox2 expression (red) in the lumenal HC (HCA, green) region of E6 explants maintained for 48 hours in vitro in either control (J) or SU5402-treated (K) media. Far left panels are lumenal surface views (at 0 μm depth in the epithelium) and the right panels are sections at the indicated depths from the lumenal surface. Far right panels are bright point projections starting at a depth of 12 μm and extending down through the region of Sox2 expression (12+ μm). (L,M) Optical z-cross sections of control (L) and SU5402-treated (M) E9 BP explants maintained for 6 DIV. HCs are labeled with Tuj1 (green) and SC/PC nuclei with anti-Sox2 (red). Stereociliary bundles are marked with phalloidin (actin, blue); arrow heads point to Sox2-positive nuclei in the HC region. Scale bars: A–D, 5 μm; E–F′, 10 μm; J and K, 20 μm.
During early stages of HC development in the BP (prior to E9), HCs are often observed to be in direct contact with one another, i.e. forming HC-HC contacts, however at later stages (E12) these contacts are rarely observed (Goodyear et al., 1997). This suggests that it likely takes a few days for Notch signaling and lateral inhibition to become fully established and uniformly separate HCs. Fgfr inhibition in embryonic BPs established at E6 and maintained for 5 DIV resulted in disorganized HC patterning and an increase in apparent HC-HC contacts compared to controls (Fig. 5E compared to F). In controls, very few of these “HC-HC” contacts are present (Fig. 5E), and most HCs appear to be of uniform size when viewed below the surface of the epithelium at the level of the HC nuclei (Fig. 5E′). In the many cases of HC-HC contacts in SU5402-treated cultures (Fig. 5F), however, it appears that one large HC may be directly adjacent to a smaller HC when viewed at the nuclear level (Fig. 5F′), further suggesting that the two HCs are not of comparable ages. Finally, measurements of epithelial thickness in the lumenal-basal z-plane identified no significant change between control and SU5402-treated samples (control: mean thickness=18.9±2.9, n=15; SU5402: mean thickness=20.5±4.3, n=18; standard deviation is shown), suggesting the presence of smaller cells.
To further examine if treatment with SU5402 leads to an increase in immature-appearing, small HCs, HC diameters were measured at their widest point (a few micrometers below the lumenal surface) and were quantified at different positions along the proximal-to-distal axis of the BP in E9 cultures maintained for 5 DIV. Because HCs located in the distal third of the BP are significantly smaller than HCs located in the middle and proximal thirds at this stage, data from distal regions was grouped separately (Fig. 5I). In controls, HCs located in the middle and proximal thirds of the BP showed a roughly normal distribution around an average diameter of 35.49 μm. In contrast, in SU5402-treated explants the average HC diameter in the proximal and middle thirds was shifted to 23.11 μm, representing a significant decrease (p<0.005). Moreover, HC diameters showed a bimodal distribution with approximately 50% of the cells normally distributed around the same mean as in control, but with the remaining 50% of cells normally distributed around a lower mean centered between 10 and 15 μm (Fig. 5I). Since these explants were established at E9, and most HC formation in the proximal and middle thirds of the BP is complete by this stage, these results suggest that the population of smaller cells may represent younger, newly formed HCs that have begun to differentiate over the course of the experiment. Similarly, the average diameter of HCs in the distal region of control explants was 27.80 μm while in SU5402-treated explants the diameter was 18.79 μm, also a significant decrease (p<0.005). In this region of the BP, a normal distribution of HC shapes was not observed in either the control or SU5402-treated explants, which may reflect the relative level of immaturity in this region of the BP at the time of these experiments.
3.4 SU5402 induces transdifferentiation of Sox2-positive cells into HCs
The transcription factor Sox2 is initially expressed by all PCs in the developing BP but as development proceeds expression is down-regulated in HCs while it is maintained in SCs (Neves et al., 2007). Thus the presence of Sox2-positive nuclei within the HC layer might indicate newly differentiated HCs. While control explants established at E6 and maintained for 48 hours in vitro showed a clear separation of HCs and Sox2-positive cells into two distinct layers (Fig. 5J), treatment with SU5402 during the same time period resulted in a substantial increase in the number of Sox2-positive nuclei within the upper HC layer (Fig. 5K). During later stages of development, if treatment with SU5402 induces the transdifferentiation of SCs into HCs, then some HCs should show residual expression of Sox2. To confirm this, Sox2 expression was examined at the mid-point between the proximal-distal and neural-abneural axes in E9 control and SU5402-treated explants fixed at a stage equivalent to E15, a time point and region in which most normal HC differentiation has been completed. In controls, Sox2 expression was strictly limited to the SC layer (Fig. 5L), whereas in SU5402-treated samples, cells positive for Sox2 were observed in both the SC and the HC layer (Fig. 5M) suggesting that SCs may be undergoing transdifferentiation into HCs.
3.5 Fgfr inhibition leads to the formation of immature hair cells during advanced stages of BP development
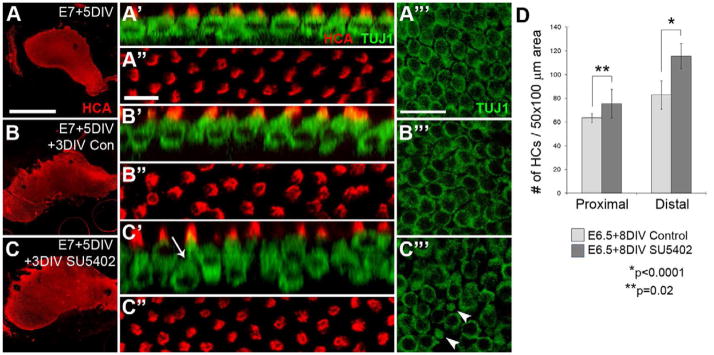
To determine if Fgf signaling may play a role in preventing the spontaneous transition of SCs into HCs, Fgfr activity was inhibited in later-stage embryonic sensory epithelia. By E12, the nearly full complement of HCs is present along the length of the BP (Tilney et al., 1986) and HCs begin to show signs of maturity such as polarization of stereocilia bundles (Cohen et al., 1978) and activation of mechanotransduction channels (Si et al., 2003). While our attempts to establish explants at this late stage resulted in significant HC death, an alternative approach in which explants were established at a young age (E6–E8) then maintained in vitro until the equivalent of E12 proved highly successful. Explants established between E6-E8 and fixed after 5 DIV (at an age equivalent to E12 or greater) looked healthy and viable, indicated by strong labeling for HC markers and a normal distribution and patterning of HCs in both the proximal and distal regions (Fig. 6A). If these explants were maintained for an additional 3 days (for a total of 8 DIV, to an age equivalent to E15+), the epithelia appeared very similar to explants fixed after only 5 DIV (compare Fig. 6A to B), although they were larger and appeared to have a slightly reduced HC density. The basis for the change in density is unclear, but given that there was no observed increase in cell death (data not shown) the change is most likely the result of ongoing growth of the HCs and expansion of the epithelium (Tilney et al., 1986) during the additional 3 DIV.
Figure 6. Inhibition of Fgfr activity promotes HC formation in more mature basilar papillae.
(A) Whole mount image of an E7 BP maintained for 5 DIV; HC stereociliary bundles are immuno-labeled with HCA (red) and HC bodies labeled with Tuj1 (green). At this time point HCs appear healthy and relatively mature in confocal z-stack cross sections (A′) as well as in surface views of the HC bundles (A″). At this stage, all HC nuclei are within the same z plane (A‴). (B) Whole mount image of an E7 BP from the same culture set as the sample shown in A, “pre-incubated” for 5 DIV then maintained in control media for an additional 3 days prior to fixation. HCs appear healthy and mature in z-stack cross sections and in surface preparations (B′ and B″, respectively) with evenly distributed, although slightly larger, nuclei (B‴) by comparison with samples fixed 3 days earlier (A‴). (C) In an explant from the same culture set that had been “pre-incubated” for 5 DIV followed by 3 DIV in SU5402, HC nuclei are located at different levels within the lumenal-to-basal axis (C′, arrow points to a deeply situated long-necked HC), and HC density is increased (C″) compared to controls (B″). A single confocal section through the HC nuclear layer confirms the presence of HCs with nuclei situated deeper in the epithelium (arrows in C‴). All high-magnification images were taken at the midpoint between the neural-abneural and proximal-distal axes. (D) A significant increase in HC density was present in the distal (*p<0.0001) and proximal (**p=0.02), region of the explants in the presence of SU5402. Scale bars: A, same for column, 500 μm; A″, same for column, 10 μm; A‴, same for column, 20 μm.
Next, the effects of inhibiting Fgfr signaling were examined by treating E7+5DIV explants with SU5402 for 3 DIV. Consistent with experiments at earlier time points, a significant increase in HC density was observed (Fig. 6C,D). Rather than being organized into a single layer as in controls (Fig. 6B′), HCs were observed to be stacked upon one another (Fig. 6C′) and those in the lower layer extended long, thin processes up to the lumenal surface (see arrow in Fig. 6C′, and arrowheads in Fig. 6C‴). The average number of HCs per 50×100 μm area in explants treated with SU5402 for 3 days beginning at E12-equivalent showed a significant increase in HC density in both the distal (39.6% increase; n=6 explants counted, *p<0.001) and proximal (18.7% increase; n=6 explants counted, **p=0.02) regions compared to controls (Fig. 6D). To confirm that the increased HC density was not the result of a change in the overall size of the explants, the total surface area of the HC region of the BP was measured in both control (n= 5) and SU5402-treated (n=3) explants. No significant change was detected suggesting that the increase in HC density was not the result of reduced epithelial size. Moreover, the approximate 30% increase observed in the proximal region in these late-stage cultures is similar to the increase observed in young BP explants. Similar to treatment with SU5402 at early stages, BrdU incorporation was not observed in HCs of these late-stage cultures (data not shown), suggesting that new HCs are the product of transdifferentiation.
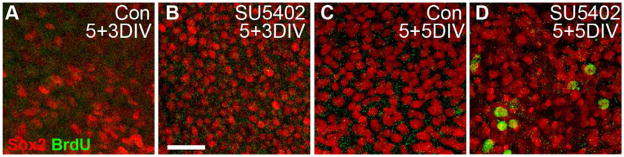
Analysis of BrdU incorporation within the SCs of these late-stage explants revealed no apparent difference in the number of mitotic cells between control and SU5402-treated epithelia after a 3 day exposure to SU5402 (Fig. 7A and B). After an additional 5 DIV, however, an increase in Sox2-postive/BrdU-positive cells was observed in SU5402-treated explants compared to controls (Fig. 7C and D).
Figure 7. Sox2-positive cells re-enter the cell cycle following SU5402-induced transdifferentiation.
(A–D) High magnification bright-point projections of the Sox2-positive (red) nuclear supporting cell layer of BP explants that were established at E6.5, maintained for 5 DIV, then treated with BrdU and either control media (A and C) or SU5402 (B and D) for an additional 3 or 5 DIV (A and B or C and D, respectively). No BrdU incorporation (green) was observed in the Sox2-positive SCs (red) after an additional 3 DIV in either treatment condition (A,B). Similarly, after an additional 5 DIV, no Sox2/BrdU-positive cells were apparent in control explants (C), however many Sox2/BrdU-positive cells were observed in SU5402-treated explants (D). Scale bar: 20 μm.
4. Discussion
The chicken basilar papilla, and all other vertebrate sensory HC-containing epithelia, is comprised of a mosaic of HCs and SCs. The basic process in which this mosaic develops is thought to be largely conserved in all vertebrates. Signaling through Sox2 and the Notch pathway leads to the formation of a prosensory domain with subsequent up-regulation of the bHLH transcription factor Atoh1 in cells that will develop as HCs although there is still some debate regarding the extent of Atoh1 signaling (Bermingham et al., 1999; Chen et al., 2002; Woods et al., 2004). A second round of Notch signaling then mediates the formation of a mosaic of HCs and SCs (Brooker et al., 2006; Daudet et al., 2005; Kiernan et al., 2005; Lanford et al., 1999). However, studies in both the developing mammalian cochlea and the regenerating adult BP have suggested that Notch signaling alone is not sufficient to account for all HC/SC patterning decisions (Daudet et al., 2009; Doetzlhofer et al., 2009; Hayashi et al., 2007; Puligilla et al., 2007). In particular, Notch activity does not maintain SC quiescence in the post-embryonic BP (Daudet et al., 2009). Similarly, during regeneration of zebrafish lateral line HCs, Notch1 has been shown to regulate proliferation and the number of new HCs that form (Ma et al., 2008), however this study also suggested that a complementary, as yet unidentified pathway, likely acts upstream of Notch signaling to maintain SCs in a quiescent state in which they retain the potential to become HCs.
The results presented here are consistent with a role for Fgf signaling in regulating HC fate in the chick BP. While the specific Fgf ligand and receptor pairs that may mediate this process cannot be determined from the results presented here, data from the literature suggests fgfr3 as a good candidate receptor. In mammals, Fgfr3 is broadly expressed in prosensory cells but becomes down-regulated in cells that will differentiate into HCs. Moreover, Fgfr3 expression is only maintained in a subset of SCs unique to the mammalian cochlea, the pillar cells (Mueller et al., 2002; Pirvola et al., 2002), which have been shown to retain the ability transdifferentiate into HCs, at least through the early post-natal period (Kiernan et al., 2005; White et al., 2006). Similarly, in the BP all SCs maintain fgfr3 expression throughout adulthood, unless a regenerative response is induced, in which case fgfr3 is transiently down-regulated in SCs as well (Bermingham-McDonogh et al., 2001). This expression pattern suggested that fgfr3 may play an in inhibitory role in HC formation during both development and repair. Consistent with this hypothesis, in the present study in vitro treatment of developing BPs with the Fgfr antagonist SU5402 resulted in a significant increase in HC numbers. This increase, measured as a function of HC density, was observed in all regions of the BP and could be induced during early or mid-developmental stages (E5–E14). Moreover, we observed supernumerary HCs of various sizes and different developmental stages, indicated by their positioning within the lumenal-to-basal plane of the epithelium, regardless of whether treatment was started during the early or late stages of BP development. The presence of seemingly immature HCs interspersed among more mature HCs suggests that inhibition of Fgf signaling leads to an increase in the number of SCs/PCs that differentiate into HCs and that blocking Fgf signaling may remove an inhibitory signal which is necessary to prevent excess HC formation. Furthermore, the presence of Sox2 positive HCs in some SU5402-treated cultures that were fixed at an in vitro age equivalent to E15, a time point at which initial HC formation is complete, demonstrates that SCs may be undergoing direct transdifferentiation into HCs as a result of Fgfr inhibition. It is possible however, that some cells appear smaller due to crowding within the epithelium, and their nuclei may fail to complete their lumenal migration due to this crowding, however their overall morphology and positioning, along with the expression of Sox2 in some of these cells, suggests that they differentiated more recently than the original population of HCs.
The lack of BrdU incorporation within SU5402-induced HCs, or other cells within the sensory epithelium of BP explants, suggests that cellular proliferation is not required for their formation, consistent with the hypothesis that these HCs are derived from SCs/PCs within the epithelium. However, it is important to consider that at the earliest stages tested in these experiments (E6), PCs within the developing BP are still actively proliferating in vivo and therefore some might be expected to incorporate BrdU. Therefore, the lack of BrdU-positive HCs suggests that overall levels of mitosis may be reduced in these cultures. It has been reported that non-mitotic HC formation is favored in an in vitro model of regeneration in the post–hatch BP (Shang et al., 2010), and it is possible that our in vitro model may inherently favor non-mitotic development as well. However, we did observe a slight increase in proliferation within the SC/PC population in SU5402-treated explants. This is likely a secondary response to SU5402 treatment and/or the differentiation of progenitors which leads to prolonged or increased proliferation within the progenitor population.
In older epithelia (E12+), when most normal HC production is complete (Tilney et al., 1986), the increase in HC density resulting from the inhibition of Fgf signaling is likely attributable to the direct transdifferentiation of SCs into HCs. Alternatively, it is possible that a subpopulation of undifferentiated progenitor cells is still present within the epithelium at E12 and some of these cells, in addition to SCs, may contribute to new HCs at later stages. Unfortunately, there are currently no specific markers which can be used to distinguish differentiated SCs from PCs. Within these older SU5402-treated explants, the lack of BrdU incorporation by Sox2-positive cells after 3 DIV, and the subsequent increase in BrdU after 5 DIV is similar to that observed during HC regeneration in which SC proliferation does not occur until a few days after the initial round of transdifferentiation (Roberson et al., 2004). This suggests that Fgfr inhibition may induce the transdifferentiation of some SCs into HCs, which subsequently induces some of the remaining SCs to re-enter the cell cycle to restore the number of SCs. It is likely that these mitotic SCs do not subsequently differentiate into HCs as there is an over-abundance of HCs in these SU5402-treated explants, unlike in regenerating BPs where transdifferentiation of these proliferating SCs is necessary to restore the normal complement of HCs.
The expression of fgfr3 in embryonic and mature SCs of the BP and its transient reduction during HC regeneration (Bermingham-McDonogh et al., 2001) is consistent with our hypothesis that blocking Fgf signaling at any stage can push SCs or PCs out of Fgf-induced stasis enabling them to undergo transdifferentiation into HCs. The persistent expression of fgfr3 in adult tissue suggests that fgfr3, or at least Fgf signaling, may continue to prevent transdifferentiation even in the mature BP. This conclusion is consistent with data from the mammalian auditory epithelium where Fgfr3 is initially expressed in a broad pool of progenitors that will develop as both outer HCs and SCs, but later becomes restricted to the pillar cells (Jacques et al., 2007; Mueller et al., 2002). Inhibition of Fgfr3 signaling (Colvin et al., 1996; Hayashi et al., 2007; Mueller et al., 2002; Puligilla et al., 2007) or its ligand Fgf8 (Jacques et al., 2007) results in a decrease in the number of pillar cells, and in Fgfr3 mutant mice, results in an increase in HCs (Hayashi et al., 2007; Puligilla et al., 2007). Conversely, in vitro activation of Fgfr3 in the organ of Corti increases the number of SCs and/or undifferentiated progenitor cells at the expense of HCs, while transient activation likely holds progenitors temporarily in an undifferentiated state (Jacques et al., 2007). Similarly, ectopic SCs (specifically pillar cells) have been reported in both a mouse model expressing a ligand-independent, constitutively active form of Fgfr3(P244R), as well as in a null mutant of the Fgf signaling antagonist Sprouty2 (Mansour et al., 2009; Shim et al., 2005). These data are consistent with the hypothesis that Fgfr3 activation acts to prevent auditory PCs from differentiating into HCs, and therefore regulates the number of each cell type that forms.
Of all the SC types in the organ of Corti, only pillar cells maintain Fgfr3 expression through adulthood, suggesting that they may retain some similarities with SCs of the BP. In addition to maintaining Fgfr3 expression, pillar cells have also been shown to maintain the most developmental plasticity compared to other SCs of the mammalian cochlea (Jacques et al., 2007; White et al., 2006), suggesting that the presence of Fgfr3 may act as a competency factor to prevent terminal differentiation thus allowing cells to switch fate. Interestingly, a study in the mammalian cochlea has demonstrated that the HC inhibitory effect of Fgfr3 in pillar cells is mediated through Notch-independent induction of Hey2 expression, a known Notch target, which acts by repressing Hes5, an inhibitor of Atoh1 (Doetzlhofer et al., 2009). Furthermore, it has been suggested that pillar cells may express low-levels of Atoh1 (Matei et al., 2005; Yang et al., 2010), although current studies have not identified atoh1 in SCs of the BP except during regenerative transdifferentiation (Cotanche et al., 2010). While the expression of c-hey2 has yet to be studied in the BP, future studies aimed at examining its expression and function might yield important insights into potential differences between avian SCs and the extent to which they may differentially respond to Notch signaling and/or a Hey2-Fgf mode of regulation.
Despite the discussion above, the specific Fgf receptors and ligands that regulate SC and HC formation in the BP, have yet to be conclusively determined. While the pattern of fgfr3 expression is consistent with the responses that were observed in vitro, SU5402 has equivalent inhibitory effects on all four Fgfrs (Mohammadi et al., 1997). Both fgfr1 and fgfr2 have been shown to be transcribed within the adult chicken inner ear during regeneration (Pickles et al., 1997), although the developmental expression pattern and the specific cell types which express these receptors has not been characterized, and data is lacking for fgfr4. In mice, Fgfr2 is not expressed within the cochlear prosensory domain, and while Fgfr2-IIIb null mutants fail to develop a cochlear sensory epithelium, the overall gross morphological defects in the inner ears of these embryos (Pirvola et al., 2000) suggest that the lack of HCs and SCs is due to disturbances in otocyst formation and not direct effects on sensory cell development. Fgfr1, however, has been shown to be required for normal mammalian HC development (Pirvola et al., 2002), although activation of this receptor, likely by Fgf20 (Hayashi et al., 2008), positively promotes HC formation whereas Fgfr3 activation is inhibitory to HC formation (Jacques et al., 2007). In addition to its pattern of expression, Fgfr3 has been shown to regulate cellular differentiation in other systems such as in developing lens fibers (Govindarajan et al., 2001), further supporting a similar role in the inner ear.
The potential endogenous Fgf ligands that may activate Fgfrs within the BP, however, have been less studied. In the organ of Corti, Fgf8 is the reported ligand for Fgfr3 activation - its secretion by the inner hair cells regulates the timing and differentiation of pillar cells and outer hair cells (Jacques et al., 2007; Mueller et al., 2002; Pirvola et al., 2002). However, unlike the avian vestibular system in which HCs do express fgf8, auditory HCs do not express this ligand (Sanchez-Calderon et al., 2002; Sanchez-Calderon et al., 2004). Fgf9, which is also a potent activator of Fgfr3 (Ornitz et al., 1996) and has been reported to be expressed in the mammalian cochlea and utricle (Pirvola et al., 2004; Sajan et al., 2007), is expressed in the avian inner ear as well (Alvarado et al., 2011), although Fgf9 null mutants showed no defects in the organ of Corti (Pirvola et al., 2004). Thus this family of Fgf ligands, which also includes Fgf16 and Fgf20, is one possible candidate for Fgfr activation in the developing and mature BP and should be explored further. Additionally, in mammals, Fgf20 has been shown to signal through Fgfr1 to regulate initial HC formation (Hayashi et al., 2008; Pirvola et al., 2002) and it is possible that this pathway may also function within the BP, although, as described above, the apparent effects of Fgf20/Fgfr1 interactions as inducers of HC formation suggest that this ligand-receptor pair likely does not play an inhibitory role on HC formation in the chick BP. Fgf10 expression has also been reported in and surrounding the developing organ of Corti (Pirvola et al., 2000) and in the developing chick inner ear (Pujades et al., 2006), although cochlear HC patterning in an Fgf10 null mutant appeared relatively normal (Pauley et al., 2003). Fgf10, however, has been implicated in cross-regulation with Bmp signaling, and Bmp4 is known to influence organ of Corti formation (Ohyama et al., 2010; Puligilla et al., 2007) as well as HC formation in the chick (Li et al., 2005; Pujades et al., 2006; Wu et al., 1996) thus future studies should be aimed at identifying the expression of BMPs and their relationship with Fgf signaling in the developing and regenerating BP.
Overall, current research supports the hypothesis that HCs may secrete Fgf ligands that can bind to and activate Fgfr3 in neighboring SCs/PCs, thereby preventing those cells from differentiating into HCs. In mammals, this interaction may only be required during embryonic development, while in birds, loss of this interaction, as would occur following HC death, could act as a trigger to initiate a regenerative response. Future studies to characterize the developmental expression profiles for all of the Fgf receptors and ligands in the BP and organ of Corti would help clarify our understanding of how this complex pathway regulates the generation of HCs and SCs. In addition, the identification and functional characterization of the factors that regulate Fgfr3 expression within the BP is needed. In the developing lymphatic system, the homeodomain transcription factor Prox1 has been shown to regulate the expression of Fgfr3 (Shin et al., 2006), and in the developing BP, the onset of c-prox1 expression corresponds with that of fgfr3 around E5 (Stone et al., 2003). Expression of c-prox1 is eventually restricted to SCs (Stone et al., 2003) suggesting that it may act to regulate expression of fgfr3within the BP as well. Similar to Fgfr3, it has been suggested that Prox1 may serve to antagonize the HC phenotype in the mammalian cochlea (Kirjavainen et al., 2008); however, the Fgfr3 knockout and the Prox1 null mutant phenotypes were not identical although both resulted in the formation of some additional outer hair cells. Finally, the antagonistic Sprouty family of genes (Mason et al., 2006; Shim et al., 2005), as well as other negative regulators of Fgf signaling, should also be investigated to provide an overall understanding of the role of Fgf signaling during avian HC development and regeneration.
Supplementary Material
High-magnification lumenal and sub-lumenal views of E6.5 BP explants maintained for 5 DIV and immunostained with Tuj1to label HCs (green) and DAPI to label cell nuclei (blue). Compared to control explants (A′), SU5402-treated samples (B′) show an increase in overlapping nuclei in the upper HC layer (identified by large round nuclei). In the deeper SC layer (indicated by smaller, ovoid nuclei), no obvious difference was observed (compare A″ to B″). Scale bar: 20 μm.
Highlights.
We investigated the function of Fgf signaling in the developing basilar papilla (BP).
Fgf receptor activity was inhibited in cultured E5–E12 developing BPs with SU5402.
Early Fgfr inhibition resulted in the conversion of progenitor cells into hair cells.
Late-stage Fgfr inhibition induced supporting cell transdifferentiation.
Fgf signaling prevents conversion of supporting/progenitor cells into hair cells.
Acknowledgments
We would like to thank Douglas Cotanche and Jennifer Stone for reading an earlier version of the manuscript and providing very helpful comments. We would like to thank Dr. Guy Richardson at the University of Sussex for kindly providing the HCA antibody. Some of the images were generated at the UCSD Shared Microscopy Facility, Cancer Center specialized support grant P30 CA23100. This research was supported by funds from the intramural program at the National Institute on Deafness and other Communication Disorders (M.W.K.).
Abbreviations
- BP
Basilar papilla
- HC
Hair cell
- SC
Supporting cell
- PC
Progenitor cell
- DIV
Days in vitro
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bonnie E. Jacques, Email: bjacques@ucsd.edu.
Alain Dabdoub, Email: adabdoub@ucsd.edu.
Matthew W. Kelley, Email: kelleymt@nidcd.nih.gov.
References
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–54. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Alvarado DM, Hawkins RD, Bashiardes S, Veile RA, Ku YC, Powder KE, Spriggs MK, Speck JD, Warchol ME, Lovett M. An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci. 2011;31:4535–43. doi: 10.1523/JNEUROSCI.5456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolami S, Goodyear R, Richardson G. Appearance and distribution of the 275 kD hair-cell antigen during development of the avian inner ear. J Comp Neurol. 1991;314:777–88. doi: 10.1002/cne.903140410. [DOI] [PubMed] [Google Scholar]
- Basch ML, Ohyama T, Segil N, Groves AK. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci. 2011;31:8046–58. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001;238:247–59. doi: 10.1006/dbio.2001.0412. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–86. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chen L, Adar R, Yang X, Monsonego EO, Li C, Hauschka PV, Yayon A, Deng CX. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Invest. 1999;104:1517–25. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cohen GM, Fermin CD. The development of hair cells in the embryonic chick’s basilar papilla. Acta Otolaryngol. 1978;86:342–58. doi: 10.3109/00016487809107513. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–95. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Sulik KK. Early differentiation of hair cells in the embryonic chick basilar papilla. A preliminary report. Arch Otorhinolaryngol. 1983;237:191–5. doi: 10.1007/BF00453723. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Sulik KK. The development of stereociliary bundles in the cochlear duct of chick embryos. Brain Res. 1984;318:181–93. doi: 10.1016/0165-3806(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hear Res. 2010;266:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–78. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–21. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. Pattern formation in the basilar papilla: evidence for cell rearrangement. J Neurosci. 1997;17:6289–301. doi: 10.1523/JNEUROSCI.17-16-06289.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–27. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–33. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–9. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM. FGFs: Neurodevelopment’s Jack-of-all-Trades - How Do They Do it? Front Neurosci. 2011;5:133. doi: 10.3389/fnins.2011.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–9. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Katayama A, Corwin JT. Cell production in the chicken cochlea. J Comp Neurol. 1989;281:129–35. doi: 10.1002/cne.902810110. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kirjavainen A, Sulg M, Heyd F, Alitalo K, Yla-Herttuala S, Moroy T, Petrova TV, Pirvola U. Prox1 interacts with Atoh1 and Gfi1, and regulates cellular differentiation in the inner ear sensory epithelia. Dev Biol. 2008;322:33–45. doi: 10.1016/j.ydbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–92. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci U S A. 2007;104:19108–13. doi: 10.1073/pnas.0705927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–63. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SL, Twigg SR, Freeland RM, Wall SA, Li C, Wilkie AO. Hearing loss in a mouse model of Muenke syndrome. Hum Mol Genet. 2009;18:43–50. doi: 10.1093/hmg/ddn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–60. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Molea D, Stone JS, Rubel EW. Class III beta-tubulin expression in sensory and nonsensory regions of the developing avian inner ear. J Comp Neurol. 1999;406:183–98. [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–77. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–51. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–7. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–15. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–30. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Pickles JO, van Heumen WR. The expression of messenger RNAs coding for growth factors, their receptors, and eph-class receptor tyrosine kinases in normal and ototoxically damaged chick cochleae. Dev Neurosci. 1997;19:476–87. doi: 10.1159/000111245. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol. 2004;273:350–60. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–80. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–34. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair-cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–17. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–71. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sajan SA, Warchol ME, Lovett M. Toward a systems biology of mouse inner ear organogenesis: gene expression pathways, patterns and network analysis. Genetics. 2007;177:631–53. doi: 10.1534/genetics.107.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martin-Partido G, Hidalgo-Sanchez M. Differential expression of Otx2, Gbx2, Pax2, and Fgf8 in the developing vestibular and auditory sensory organs. Brain Res Bull. 2002;57:321–3. doi: 10.1016/s0361-9230(01)00725-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martin-Partido G, Hidalgo-Sanchez M. Otx2, Gbx2, and Fgf8 expression patterns in the chick developing inner ear and their possible roles in otic specification and early innervation. Gene Expr Patterns. 2004;4:659–69. doi: 10.1016/j.modgep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51:473–81. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J. Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J Assoc Res Otolaryngol. 2010;11:203–22. doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–64. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–84. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci. 2003;23:10815–26. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell differentiation in the developing chick cochlea and in embryonic cochlear organ culture. J Comp Neurol. 1991;314:614–25. doi: 10.1002/cne.903140315. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Temporal, spatial, and morphologic features of hair cell regeneration in the avian basilar papilla. J Comp Neurol. 2000;417:1–16. doi: 10.1002/(sici)1096-9861(20000131)417:1<1::aid-cne1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–47. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. Expression of Prox1 defines regions of the avian otocyst that give rise to sensory or neural cells. J Comp Neurol. 2003;460:487–502. doi: 10.1002/cne.10662. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Saunders JS, DeRosier DJ. Actin filaments, stereocilia, and hair cells of the bird cochlea. III. The development and differentiation of hair cells and stereocilia. Dev Biol. 1986;116:100–18. doi: 10.1016/0012-1606(86)90047-3. [DOI] [PubMed] [Google Scholar]
- Umemoto M, Sakagami M, Fukazawa K, Ashida K, Kubo T, Senda T, Yoneda Y. Hair cell regeneration in the chick inner ear following acoustic trauma: ultrastructural and immunohistochemical studies. Cell Tissue Res. 1995;281:435–43. doi: 10.1007/BF00417861. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ear: a study with the electron microscope. Neuroscience. 1985;14:277–300. doi: 10.1016/0306-4522(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–8. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–62. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353:367–79. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010;48:407–13. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-magnification lumenal and sub-lumenal views of E6.5 BP explants maintained for 5 DIV and immunostained with Tuj1to label HCs (green) and DAPI to label cell nuclei (blue). Compared to control explants (A′), SU5402-treated samples (B′) show an increase in overlapping nuclei in the upper HC layer (identified by large round nuclei). In the deeper SC layer (indicated by smaller, ovoid nuclei), no obvious difference was observed (compare A″ to B″). Scale bar: 20 μm.