Abstract
Aerobic metabolism is fundamental for almost all animal life. Cellular consumption of oxygen (O2) and production of carbon dioxide (CO2) signal metabolic states and physiological stresses. These respiratory gases are also detected as environmental cues that can signal external food quality and the presence of prey, predators and mates. In both contexts, animal nervous systems are endowed with mechanisms for sensing O2/CO2 to trigger appropriate behaviors and maintain homeostasis of internal O2/CO2. Although different animal species show different behavioral responses to O2/CO2, some underlying molecular mechanisms and pathways that function in the detection of respiratory gases are fundamentally similar and evolutionarily conserved. Studies of Caenorhabditis elegans and Drosophila melanogaster have identified roles for cyclic nucleotide signaling and the hypoxia inducible factor (HIF) transcriptional pathway in mediating behavioral responses to respiratory gases. Understanding how simple invertebrate nervous systems detect respiratory gases to control behavior might reveal general principles common to nematodes, insects and vertebrates that function in the molecular sensing of respiratory gases and the neural control of animal behaviors.
Keywords: oxygen, carbon dioxide, C. elegans, Drosophila, respiratory gases, animal behaviors
Introduction
The appearance of life on Earth caused dramatic changes in atmospheric O2 and CO2 concentrations. The atmosphere of pre-biotic Earth had little O2 and abundant CO2 (Lenton, 2003; Maina, 1998). The appearance of primitive single-celled organisms with a capacity for photosynthesis increased atmospheric O2, and in the presence of high O2 concentrations emerged more complex multicellular organisms that are capable of aerobic respiration. During aerobic respiration, glucose and O2 are metabolized to generate CO2, H2O and the universal currency of cellular energy, adenosine-5′-triphosphate (ATP). Over billions of years of evolution, O2 has become essential for most forms of animal life, and CO2 has become a near-ubiquitous metabolic by-product of cellular respiration (Lenton, 2003; Maina, 1998).
Changes either in internal or external concentrations of CO2 and O2 can carry crucial biological information. Because O2 and CO2 are fundamentally involved in metabolism, an organism experiences changes in internal concentrations of these two gases as cellular and organismal metabolism changes. Organisms that rely on aerobic respiration must avoid environments with low O2 and high CO2 concentrations. Also, changes in the environmental concentrations of respiratory gases can signal the presence of predators, mates and prey, as well as food quality (Guerenstein and Hildebrand, 2008; Luo et al., 2009; Scott, 2011). Accordingly, animals have evolved mechanisms for sensing both internal and external O2/CO2 concentrations to initiate and execute appropriate behavioral responses for optimal survival and reproduction. For example, large vertebrates have developed sophisticated neuronal circulatory and respiratory motor systems for controlling internal concentrations of O2/CO2. Animals small enough to exchange respiratory gases by passive diffusion do not require a respiratory motor organ. In its absence, however, the only way such animals can control internal concentrations of respiratory gases is to move to environments with desirable concentrations of respiratory gases.
Adaptive respiratory movements and foraging behaviors triggered by respiratory gases are examples of acute behavioral responses to O2 and CO2 that are mediated by gas-sensing neurons. Animal nervous systems also display homeostatic responses to long-term changes in environmental and internal O2 concentrations. For example, neurons in both vertebrates and invertebrates can respond to prolonged hypoxia by regulating the expression of genes that modulate neuronal survival and excitability (Bickler and Donohoe, 2002; Powell-Coffman, 2010). In general, acute and homeostatic responses to respiratory gas stimuli recruit distinct mechanisms. Acute responses can invoke a sensory cascade leading to activation of a specific motor program (Luo et al., 2009; Scott, 2011). By contrast, homeostatic responses can invoke changes in gene expression that leads to de novo protein synthesis and modification of cell metabolism, cell physiology, and neural synapses and circuits.
Studies of genetically tractable model organisms such as Drosophila melanogaster and Caenorhabditis elegans have discovered molecular mechanisms by which neurons sense acute and chronic changes in O2 and CO2 to control behavior. Because many of the molecular mechanisms uncovered by these studies are present in vertebrates, these studies might elucidate pathways that function in the neuronal control of the respiratory motor program and in homeostatic responses of the brain to hypoxia and hypercapnia in diverse organisms.
Mechanisms of CO2 sensing by invertebrate and vertebrate neurons
Although CO2 was long known to exert effects on neuronal physiology and activity, studies of invertebrate sensory neurons were the first to clearly identify receptor-type proteins that mediate the effects of CO2 on neurons. The olfactory system of D. melanogaster contains neurons that are highly specific to CO2 that drive an innate avoidance behavior (Suh et al., 2004). Subsequently, two receptor-like proteins, Gr21a and Gr63a, were shown to be necessary for activation of these olfactory neurons (Jones et al., 2007). Moreover, expression of Gr21a and Gr63a sufficed to convert olfactory sensory neurons into CO2-responders, suggesting that these two proteins might constitute a heteromeric receptor for CO2 or a CO2 metabolite. Subsequent to the discovery of CO2-responding neurons in the insect olfactory system, CO2-responding neurons were discovered in the insect gustatory system (Fischler et al., 2007). Interestingly, these neurons promote ingestive behaviors, indicating that while atmospheric CO2 is an aversive stimulus, aqueous CO2 (carbonation) is appetitive. The molecular receptors mediating activation of CO2-sensing gustatory neurons have not yet been identified.
D. melanogaster Gr21a and Gr63a proteins are part of an insect-specific family of putative odorant and gustatory receptor proteins that might constitute ligand-gated ion channels. Homologs are found in other insect species, such as the mosquito. In mosquitoes, Gr21a/Gr63a receptors are expressed in maxillary palps, not antennae, where they likely mediate attraction behavior to CO2 emitted by warm-blooded hosts (Jones et al., 2007). No homolog of the Gr21a/Gr63a receptor exists in mammals. CO2-sensing neurons of animals in non-insect phyla must, therefore, use different mechanisms to detect external and internal CO2. Studies of another invertebrate model organism, the nematode C. elegans, have revealed such a distinct mechanism.
C. elegans, like D. melanogaster, detect CO2 as an aversive environmental cue (Bretscher et al., 2008; Hallem and Sternberg, 2008). C. elegans have sensory neurons specialized for the detection of CO2, the BAG neurons (Hallem et al., 2011; Hallem and Sternberg, 2008), and these neurons are required for acute CO2 avoidance behavior. In addition to acute avoidance of CO2 stimuli, C. elegans can navigate to a preferred CO2 concentration in a CO2 gradient over longer periods of time. Navigation in a CO2 gradient does not strictly depend on the BAG neurons, and requires the function of multiple types of sensory neuron, which display distinct physiological responses to CO2 stimuli (Bretscher et al., 2008). Behavioral responses of C. elegans to CO2, therefore, are triggered either by specific activation of the BAG sensory neurons, or by activating a distributed neural circuit comprising multiple types of sensory neuron.
All C. elegans sensory neurons known to function in CO2-sensing use transduction pathways that generate cyclic nucleotides as second messengers to activate cyclic nucleotide-gated ion channels. In this regard, these C. elegans sensory neurons are similar to vertebrate olfactory sensory neurons and photoreceptors. The BAG neurons, which mediate acute CO2 avoidance, require a receptor-type guanylate cyclase, GCY-9, for CO2 detection (Hallem et al., 2011). GCY-9 is one of many integral membrane receptor-type proteins with cyclase homology domains encoded by the C. elegans genome (Yu et al., 1997). Recently, expression of GCY-9 was shown to be sufficient to confer CO2 sensitivity to other sensory neurons, suggesting that it acts as a receptor in BAG neurons for CO2 or a CO2 metabolite (Brandt et al., 2012). The molecular mechanism of CO2-sensing by C. elegans BAG neurons might be conserved between nematodes and mammals; the rodent olfactory system contains CO2-sensitive neurons that are marked by expression of a receptor-type cyclase, GC-D (Hu et al., 2007). On the basis of in vitro studies of its enzyme activity, GC-D has been proposed to act as a receptor for bicarbonate, a CO2 metabolite (Guo et al., 2009; Sun et al., 2009). If GC-D, as is GCY-9 in nematode sensory neurons, is necessary and sufficient for CO2 detection by olfactory neurons, there might be an evolutionarily ancient and conserved role for receptor-type cyclases in CO2 sensation.
C. elegans BAG neurons and vertebrate CO2-sensing neurons might also use related cell-fate specification pathways. BAG neurons require a conserved transcription factor, ETS-5, to promote expression of BAG-neuron-specific genes and to sense CO2 (Brandt et al., 2012; Guillermin et al., 2011). A likely mammalian homolog of ETS-5, Pet1, is likewise required for specification of serotonergic neurons in the vertebrate brainstem (Hendricks et al., 1999). In vitro some of these neurons are activated by CO2 (Richerson, 2004), and in vivo serotonergic neurons of the brainstem are required for the respiratory chemoreflex (Hodges et al., 2008; Ray et al., 2011). In C. elegans BAG neurons, ETS-5 directly regulates expression of the GCY-9 cyclase; it is possible that Pet1 likewise regulates the expression of a CO2 receptor in a subset of brainstem neurons (Brandt et al., 2012).
Hydration of CO2 generates carbonic acid and causes acidosis. In some instances, CO2 is detected via pH-sensing mechanisms. Studies of the rodent gustatory system revealed that, like insect gustatory neurons, some taste-receptor neurons of mammals are activated by aqueous CO2 i.e. carbonation (Chandrashekar et al., 2009). Carbonated solutions activate acid-sensitive sour-taste neurons, and this activation requires a cell surface-tethered isoform of carbonic anhydrase, an enzyme that catalyzes the reaction of CO2 with water to generate free protons (Chandrashekar et al., 2009).
Another example of CO2-sensing via acidosis is in the triggering by CO2 of an innate anxiety behavior of rodents: freezing. Rodent freezing behavior requires a central brain structure, the amygdala, which was recently shown to be acid-sensing (Ziemann et al., 2009). Exposure to a high-CO2 environment results in large changes in the pH of the amygdala in vivo and subsequent activation of amygdala neurons. The molecular basis of the acid-sensitivity of the amygdala has been identified: amygdala neurons express acid-sensitive ASIC channels that depolarize neurons upon activation. Although the ethological relevance of CO2-evoked freezing behavior is unclear, a relationship between internal CO2 levels and anxiety behaviors has been previously observed in the clinic: subjects diagnosed with anxiety disorders are prone to experiencing panic attacks in response to a respiratory CO2 challenge (Papp et al., 1993). The intrinsic sensitivity of the amygdala to acid stimuli might explain this connection between CO2 and panic attacks in human subjects, and might provide a mechanistic basis for a ‘suffocation-alarm’ theory of panic disorders (Klein, 1993; Ziemann et al., 2009).
Respiratory centers of the vertebrate brain are also activated by acidosis. Multiple types of brainstem neurons are activated by acid stimuli, including serotonergic neurons and neurons of the retrotrapezoid nucleus (Richerson, 2004; Spyer, 2009). In other chemosensitive brainstem areas, acidosis caused by increased CO2 levels activates pH-sensitive glia, which release ATP and trigger neuronal activity (Gourine et al., 2010; Gourine et al., 2005). How distinct brainstem cell-types detect acidosis, how the detection of CO2 by distinct brainstem circuits is integrated in in vivo, and whether CO2 regulates brainstem chemosensitive neurons in a pH-independent manner remain to be determined.
O2 sensing by invertebrate neurons
Behavioral studies of C. elegans have also led to the discovery of mechanisms by which neurons detect O2. C. elegans is a free-living nematode species that inhabits soils and microbe-rich environments in which O2 levels are usually far below the ambient level of 21% (Anderson and Ultsch, 1987; Felix and Braendle, 2010). Under laboratory conditions, C. elegans prefers O2 concentrations of 5% to 10%, and navigates in an O2 gradient to this preferred concentration range (Chang and Bargmann, 2008; Gray et al., 2004). Avoidance of high O2 concentrations by C. elegans requires four O2-sensing neurons: URXL/R, AQR and PQR. These neurons, like the CO2-sensing BAG neurons, use cyclic nucleotide signaling. O2-sensing neurons of C. elegans require both cyclic nucleotide-gated ion channels and a guanylate cyclase comprising subunits encoded by the genes gcy-35 and gcy-36. The GCY-35/GCY-36 cyclase is a cytoplasmic heme-containing enzyme that directly interacts with O2 (Gray et al., 2004). Expression of the GCY-35/GCY-36 cyclase in BAG neurons confers upon BAG neurons the ability to respond to hyperoxic stimuli (Zimmer et al., 2009), indicating that this enzyme is sufficient to mediate O2 detection when expressed in sensory neurons.
Genetic studies of hyperoxia avoidance by C. elegans have identified another gene that functions in O2 sensing. The neuronal globin GLB-5 functions in O2-sensing neurons and is required for behavioral discrimination between similar, high concentrations of O2 (Gray et al., 2004; McGrath et al., 2009; Persson et al., 2009). Animals carrying a polymorphism in the glb-5 locus cannot discriminate between 20% and 21% O2, likely as a consequence of loss of glb-5 function. GLB-5 functions in O2-sensing neurons, where it is required for their physiological activation by small increases in O2. Like the GCY-35/GCY-36 cyclase, GLB-5 contains a heme prosthetic group that directly interacts with O2. How GLB-5 changes the function of O2-sensing neurons to permit their activation by small changes in atmospheric O2 levels remains to be determined. The C. elegans genome encode more than 30 related globin genes, many of which are expressed by neurons, suggesting that many C. elegans circuits, even those not directly regulated by O2-sensing neurons, might be modulated by O2.
In addition to hyperoxia-avoidance behaviors, C. elegans displays an acute avoidance response to loss of O2 (Zimmer et al., 2009). This response requires the CO2-sensing BAG neurons and yet another guanylate cyclase, this one comprising GCY-31 and GCY-33 subunits. The GCY-31/GCY-33 cyclase is related to the GCY-35/GCY-36 cyclase and contains a heme group; unlike the GCY-35/GCY-36 cyclase, O2-binding is thought to inhibit the activity of the GCY-31/GCY-33 cyclase. Indeed, related invertebrate cyclases have been shown to be directly inhibited by O2 (see below).
Like studies of C. elegans, behavioral genetic studies of D. melanogaster have discovered roles for cytoplasmic guanylate cyclases with heme domains as molecular O2 sensors that control acute behavioral responses to changes in O2 levels (Morton, 2011). Three Drosophila GC subunits - Gcy89-Da, Gcy89-Db, and Gcy88E - can constitute a cyclase that directly binds O2 (Huang et al., 2007; Morton, 2004). These cyclase subunits are related to C. elegans GCY cyclases, and like their C. elegans counterparts, these D. melanogaster cyclases function in behavioral responses to changes in environmental O2 (Vermehren-Schmaedick et al., 2010). D. melanogaster O2-sensing neurons express different combinations of cyclase subunits and mediate responses to different O2 stimuli. Gcy89-Da-expressing neurons are required for responses to an O2 downshift from 16% to 11%; Gcy89-Db-expressing neurons mediate responses to an O2 upshift from 21% to 30% (Vermehren-Schmaedick et al., 2010). In both C. elegans and Drosophila, the O2-responding properties of neurons are largely determined by the expression of different cyclases. Other cell-intrinsic factors, which have not been defined, also contribute to the differential roles of O2-sensing neurons in O2-dependent behaviors (Vermehren-Schmaedick et al., 2010; Zimmer et al., 2009).
C. elegans show robust behavioral responses to O2 changes ranging from 5% to 21%. O2 levels below 5%, which are ethologically relevant to wild strains of C. elegans, can also have dramatic influences on animal behavior and physiology (Anderson and Ultsch, 1987; Powell-Coffman, 2010). Prolonged anoxia (0% O2) causes C. elegans to enter a behavioral state of “suspended animation” characterized by drastically reduced metabolic rates and locomotion speeds (Padilla et al., 2002). Brief exposure of C. elegans to anoxia and subsequent restoration of O2 levels (5%, 10%, or 20%) elicit robust locomotive behavioral responses that are independent of the known O2-sensing neurons (URXL/R, AQR, PQR) and the O2 sensors GCY-31/GCY-33 and GCY-35/GCY-36 (Ma et al., 2012). The molecular and cellular O2 sensors and the mechanisms for these anoxia/reoxygenation-induced behaviors are unknown.
Modulation of C. elegans behaviors by chronic hypoxia
The aerotaxis behavior of C. elegans can be modified by prior experience of hypoxia (Chang and Bargmann, 2008). Wild-type animals normally prefer O2 concentrations around 10%. By contrast, animals that have been cultivated in hypoxic conditions (48 hours at 0.5% O2) prefer lower O2 concentrations around 8%. This modification of C. elegans O2 preference by hypoxia experience requires the proline hydroxylase EGL-9 (Chang and Bargmann, 2008), which uses molecular O2 as a substrate for the hydroxylation of target proteins. The canonical target of EGL-9 in hypoxia pathways is the transcription factor HIF (Epstein et al., 2001), which is hydroxylated and degraded under normoxic conditions. Under hypoxic conditions, EGL-9 cannot efficiently hydroxylate HIF resulting in its accumulation and the activation of HIF target genes (Epstein et al., 2001). The C. elegans HIF homolog hif-1 is partly required for the hypoxia-induced change in aerotaxis behavior, and the hydroxylase EGL-9 is completely required indicating that HIF-1 is not the sole substrate of EGL-9 required for modification of aerotaxis behavior. Surprisingly, HIF-1 activation changes what neurons are required for hyperoxia avoidance, suggesting that the underlying aerotaxis neural circuit undergoes “reorganization” by hypoxia experience (Chang and Bargmann, 2008).
Chronic hypoxia and the HIF pathway also change a gustatory circuit in C. elegans. Under normoxic conditions, C. elegans is attracted to sodium chloride (NaCl), and this attraction requires the ASE chemosensory neurons (Bargmann et al., 1993). Prolonged hypoxia enhances NaCl chemotaxis through the HIF-1-dependent up-regulation of TPH-1, a biosynthetic enzyme for the neural modulator serotonin, in neurons that are not required for sensory processing under normoxic conditions (Pocock and Hobert, 2010). This remarkable finding demonstrates that hypoxia can regulate the neurotransmitter identity of neurons and demonstrates a specific mechanism by which hypoxia modifies neural circuits and behavior.
The C. elegans locomotory response to restoration of high O2 levels after brief exposure of anoxia (the so-called “O2-ON response”) is also modulated by prior exposure to hypoxia (Ma et al., 2012). Unlike naïve animals, which robustly accelerate when O2 is restored to normal levels, animals exposed to 0.5% O2 for 24 hours, followed by 2 hours of recovery at room air, show a suppressed O2-ON response. This hypoxia-induced suppression of the O2-ON response requires both HIF-1 and EGL-9. This suppression of behavior also requires a cysteine synthase or sulfhydrylase-like protein CYSL-1, which was identified from a screen for EGL-9 regulators. CYSL-1 functions by sequestering EGL-9 and thereby inhibiting EGL-9-mediated hydroxylation of HIF-1 during hypoxia. Interestingly, the interaction between EGL-9 and CYSL-1 is modulated by the gas hydrogen sulfide (H2S), which accumulates under hypoxic conditions because of reduced oxidation (Olson, 2011a, b). Prior experience of hypoxia might produce preconditioning effects that modulate the O2-ON response in response to anoxia/reoxygenation-induced cellular signals, analogous to alleviation of the reperfusion injury response by hypoxic preconditioning in mammals (Semenza, 2011a). In this context, EGL-9 acts as a homeostatic O2 sensor to control a transcriptional pathway to enable behavioral state changes in a hypoxia experience-dependent manner. The underlying mechanisms that trigger CYSL-1 regulation of the O2-sensing EGL-9 hydroxylase and how unidentified HIF-1 targets modify the acute locomotive O2-ON behavioral response await further investigation.
Mechanisms of O2 sensing by mammalian carotid bodies
Changes in environmental O2 can induce rapid behavioral responses in mammals, notably responses in the respiratory motor program. Hypoxia, for example, causes a rapid increase in the intensity and frequency of breaths, the hypoxic ventilatory response (HVR). In mammals, O2 levels in blood are sensed by chemoreceptors in the carotid body, a specialized tissue located near the bifurcation of the carotid artery that is innervated by fibers of the glossopharyngeal nerve (Prabhakar, 2006; Teppema and Dahan, 2010). Physiological responses of O2-sensitive cells of the carotid body have been extensively studied. Neuron-like cells of the carotid body release ATP in response to hypoxic stimuli, exciting neurons that express purinergic receptors and project to respiratory centers of the brainstem (Prabhakar, 2006; Teppema and Dahan, 2010). The molecular nature of the O2 sensor that functions in carotid body is not known. A list of candidates includes: O2-sensitive potassium channels, AMP-activated protein kinase (AMPK), plasma membrane bound NADPH oxidase, heme oxygenases and mitochondrial complex III (Olson, 2011a; Olson and Whitfield, 2010; Peng et al., 2010; Prabhakar, 2006).
Emerging lines of evidence support the hypothesis that O2-sensing by the carotid body recruits signaling by a gas messenger, hydrogen sulfide (H2S) (Li et al., 2010; Olson et al., 2006; Peng et al., 2010). Under normoxic conditions, endogenous H2S is produced by several thiol metabolic enzymes, including cystathionine γ-lyases (CSE) and cystathionine-beta-synthases (CBS), but is constantly oxidized, mainly in mitochondria, and remains at very low levels (Olson, 2011a; Singh et al., 2009). Under hypoxic conditions, H2S levels increase rapidly, and increases in H2S might depolarize O2-sensing cells of the carotid body either through inhibition of ATP-sensitive potassium channels or through activation of L-type Ca2+ channels (Li et al., 2010; Olson, 2011a; Peng et al., 2010). Although genetic and pharmacological evidence supports the hypothesis that CBS or CSE-biosynthesized H2S mediates O2 sensing, many questions remain. It is not known how endogenous H2S at physiological concentrations is oxidized under normoxic conditions, how H2S activates carotid body cells under hypoxic conditions, and to what extend H2S might interact with other proposed O2-sensing mechanisms to coordinate the hypoxic response. Nevertheless, the proposed role of H2S in O2 sensing by mammalian carotid bodies might have an interesting parallel in invertebrates; as described above, hypoxia experience recruits H2S signaling to modulate the suppression of the acute O2-ON behavioral response in C. elegans (Ma et al., 2012). Given that H2S biosynthetic enzymes are evolutionarily conserved (Kimura, 2010; Vozdek et al., 2012) and hypoxia can trigger rapid and large increases of H2S accumulation (Olson, 2011b), acute O2 sensing mediated by H2S might operate in both vertebrates and invertebrates, including C. elegans and D. melanogaster. Furthermore, prolonged or chronic hypoxia modulates the effects of hypoxia on the respiratory motor program via the mammalian EGLN/HIF pathway (Teppema and Dahan, 2010). These findings underscore the importance of the evolutionarily conserved homeostatic EGLN/HIF pathway in mediating the plasticity of acute behavioral responses to O2 level changes in diverse animal species.
Conclusions and future directions
Studies of many organisms have identified molecular mechanisms required for sensing respiratory gases and generating behavioral responses. Some of the gas-sensing mechanisms discovered in C. elegans neurons and D. melanogaster neurons are fundamentally similar and have likely been conserved through evolution (Figure 1). These studies show that gas-sensing neurons of different animals can mediate different behavioral responses to changes in respiratory gases, reflecting adaptation of a fundamental sensory system to different ecological niches.
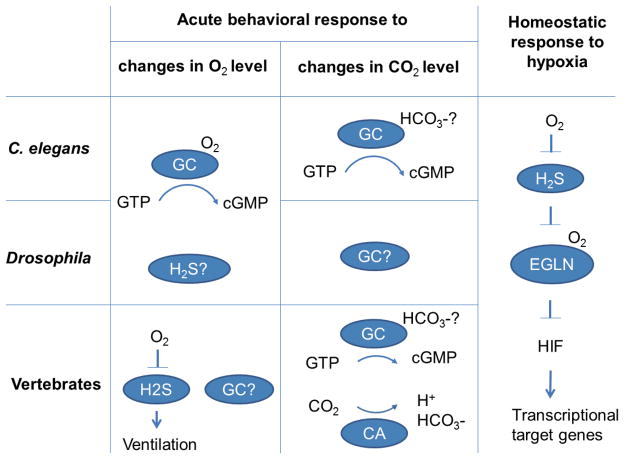
Figure 1. Schematic molecular mechanisms of sensing O2 or CO2 to direct acute behavioral or homeostatic responses in C. elegans, D. melanogaster and vertebrates.
In both C. elegans and Drosophila, changes in O2 levels directs acute behavioral responses mediated by atypical nucleotide guanylate cyclases (GCs). GCs can bind O2, which regulates the enzymatic activity of GCs to convert GTP to cyclic GMP. In vertebrates, sensing reduction in O2 levels directs ventilation responses in the carotid body by altering the H2S level, which increases upon hypoxia. There are also acute behaviors that require mechanisms independently of cyclic nucleotides in C. elegans and it remains unknown whether vertebrates also use GCs to modulate ventilation. Sensing changes in CO2 levels in both C. elegans and vertebrates appears to be mainly mediated by GCs and/or adenylate cyclases; whether this is also the case in Drosophila remains to be seen. The perception of carbonation in mammals uses pH-sensing mechanisms via carbonic anhydrase (CA)-generated protons. In all animal species examined so far, an evolutionarily conserved transcriptional pathway mediates the homeostatic response to hypoxia. The O2-sensing hydroxylase EGLN family proteins is modulated by the antagonizing actions of O2 and H2S to inhibit HIF transcription factors, which ultimately direct adaptive responses to hypoxia by the transcriptional regulation of its numerous target genes.
Cyclic nucleotide signaling and the HIF transcriptional pathway have emerged as major conserved mediators of acute and homeostatic responses, respectively, to modulate diverse animal behaviors (Figure 1). In both C. elegans and D. melanogaster, cyclic nucleotide signaling systems mediate gas-sensing by neurons that drive acute behavioral responses to environmental changes of O2/CO2 rapidly. In vertebrates, soluble guanylate cyclases are well characterized receptors in both non-neuronal cells and neurons for signaling nitric oxide gas (Potter, 2011). A role for cyclic nucleotide signaling in CO2 sensation was suggested by recent studies of the rodent olfactory system (Hu et al., 2007). It remains to be determined whether other modes of gas-sensing by vertebrate neurons are mediated by cyclic nucleotide signals, and whether vertebrate cyclases themselves play a central role as gas receptors as they do in invertebrate model organisms.
In both vertebrates and invertebrates, the HIF transcriptional pathway functions in homeostatic responses to changes in O2. Unlike cyclic nucleotide signaling, HIF mediates transcriptional responses to chronic changes in O2 by regulating gene expression. In the HIF pathway, the sensor for molecular O2 is an evolutionarily conserved HIF hydroxylase, which uses O2 as a substrate to hydroxylate HIF and target it for proteosomal degradation. HIF hydroxylases have low affinities for O2 (Km = 100–250 μM) (Ehrismann et al., 2007) (Ward, 2008) rendering them particularly suitable for sensing ambient O2 levels and driving homeostatic hypoxic adaptation in nearly all metazoans, from humans to the simplest animal Trichoplax adhaerens (Loenarz et al., 2011; Semenza, 2011b). In vertebrates, the HIF pathway is best understood as driving changes to cell metabolism and cell physiology in response to hypoxia. Studies of invertebrate models have revealed roles for HIF in remodeling neuronal circuits and mediating adaptive behavioral responses to chronic hypoxia. Whether HIF similarly functions in the mammalian brain remains to be determined. If so, studies of HIF-mediated remodeling of neural circuits might not only elucidate mechanisms of adaptive behavioral responses to hypoxia but might also serve as powerful and general models for understanding molecular and neural circuit mechanisms that drive behavioral plasticity.
Much progress has been made in understanding the molecular basis of behavioral controls by O2/CO2; many challenges remain. First, studies of invertebrate models have provided evidence for the existence of gas sensors whose molecular identities remain to be determined (Ma et al., 2012; Morton, 2011). Second, we need to understand at both the molecular and neural circuit levels how the initial signals generated by the gas-sensing neurons are translated into motor programs, and how those programs are modulated by experience to generate behavioral plasticity. Genetically tractable model organisms, including C. elegans and Drosophila, will continue to play crucial roles in advancing our understanding of the mechanisms underlying O2/CO2 -sensing and biological responses to them. Finally, since defective or abnormal responses to O2 and CO2 are critically involved in a wide variety of human diseases (Quaegebeur and Carmeliet, 2010; Semenza, 2011b), including blood diseases, behavioral disorders, neurodegeneration and cancer, it is an important and rewarding challenge to translate the knowledge learned from O2 and CO2-related basic biology into clinically useful interventions and medicines to benefit human health.
Acknowledgments
We thank Nikhil Bhatla for discussion and comments. D.K.M. is supported by a Helen Hay Whitney Foundation postdoctoral fellowship. N. R. is supported by the NIGMS (R01-GM098320), the Edward Mallinckrodt Jr. Foundation and the Pew Scholars Program.
Contributor Information
Dengke K. Ma, Email: dkma@mit.edu.
Niels Ringstad, Email: Niels.Ringstad@med.nyu.edu.
References
- Anderson JF, Ultsch GR. Respiratory gas concentrations in the microhabitats of some Florida arthropods. Comparative Biochemistry and Physiology Part A: Physiology. 1987;88:585–588. [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH. Adaptive responses of vertebrate neurons to hypoxia. J Exp Biol. 2002;205:3579–3586. doi: 10.1242/jeb.205.23.3579. [DOI] [PubMed] [Google Scholar]
- Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, Pocock R, Ringstad N. A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PloS One. 2012 doi: 10.1371/journal.pone.0034014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ, Bargmann CI. Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:7321–7326. doi: 10.1073/pnas.0802164105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrismann D, Flashman E, Genn DN, Mathioudakis N, Hewitson KS, Ratcliffe PJ, Schofield CJ. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem J. 2007;401:227–234. doi: 10.1042/BJ20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Guerenstein PG, Hildebrand JG. Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol. 2008;53:161–178. doi: 10.1146/annurev.ento.53.103106.093402. [DOI] [PubMed] [Google Scholar]
- Guillermin ML, Castelletto ML, Hallem EA. Differentiation of carbon dioxide-sensing neurons in Caenorhabditis elegans requires the ETS-5 transcription factor. Genetics. 2011;189:1327–1339. doi: 10.1534/genetics.111.133835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Ratsch G, Miller DM, 3rd, Horvitz HR, Sternberg PW, Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108:254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Huang SH, Rio DC, Marletta MA. Ligand binding and inhibition of an oxygen-sensitive soluble guanylate cyclase, Gyc-88E, from Drosophila. Biochemistry. 2007;46:15115–15122. doi: 10.1021/bi701771r. [DOI] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Lenton TMT. The coupled evolution of life and atmospheric oxygen. Elsevier Science; 2003. [Google Scholar]
- Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- Loenarz C, Coleman ML, Boleininger A, Schierwater B, Holland PW, Ratcliffe PJ, Schofield CJ. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12:63–70. doi: 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Sun L, Hu J. Neural detection of gases--carbon dioxide, oxygen--in vertebrates and invertebrates. Curr Opin Neurobiol. 2009;19:354–361. doi: 10.1016/j.conb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 Interacts with the O2-sensing Hydroxylase EGL-9 to Promote H2S-modulated Hypoxia-induced Behavioral Plasticity in C. elegans. Neuron. 2012 doi: 10.1016/j.neuron.2011.12.037. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina JN. The gas exchangers: structure, function, and evolution of the respiratory processes. Springer; 1998. [Google Scholar]
- McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, Kruglyak L, Bargmann CI. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DB. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J Biol Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- Morton DB. Behavioral responses to hypoxia and hyperoxia in Drosophila larvae: molecular and neuronal sensors. Fly (Austin) 2011;5:119–125. doi: 10.4161/fly.5.2.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR. Hydrogen sulfide is an oxygen sensor in the carotid body. Respir Physiol Neurobiol. 2011a;179:103–110. doi: 10.1016/j.resp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011b;301:R297–312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209:4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- Olson KR, Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal. 2010;12:1219–1234. doi: 10.1089/ars.2009.2921. [DOI] [PubMed] [Google Scholar]
- Padilla PA, Nystul TG, Zager RA, Johnson AC, Roth MB. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol Biol Cell. 2002;13:1473–1483. doi: 10.1091/mbc.01-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A, Gross E, Laurent P, Busch KE, Bretes H, de Bono M. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat Neurosci. 2010;13:610–614. doi: 10.1038/nn.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Coffman JA. Hypoxia signaling and resistance in C. elegans. Trends Endocrinol Metab. 2010;21:435–440. doi: 10.1016/j.tem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Carmeliet P. Oxygen sensing: a common crossroad in cancer and neurodegeneration. Curr Top Microbiol Immunol. 2010;345:71–103. doi: 10.1007/82_2010_83. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Scott K. Out of thin air: sensory detection of oxygen and carbon dioxide. Neuron. 2011;69:194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011a;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011b;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM. To breathe or not to breathe? That is the question. Exp Physiol. 2009;94:1–10. doi: 10.1113/expphysiol.2008.043109. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci U S A. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A, Ainsley JA, Johnson WA, Davies SA, Morton DB. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozdek R, Hnizda A, Krijt J, Kostrouchova M, Kozich V. Novel structural arrangement of nematode cystathionine beta-synthases: characterization of Caenorhabditis elegans CBS-1. Biochem J. 2012 doi: 10.1042/BJ20111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci U S A. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, Marletta MA, Hudson ML, Morton DB, Chronis N, Bargmann CI. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]