Abstract
High-affinity anchoring groups such as isothiocyanate (-N=C=S) are often used to attach organic chromophores (reporter molecules) to colloidal gold nanocrystals for surface-enhanced Raman scattering (SERS), to atomically smooth gold surfaces for tip-enhanced Raman scattering (TERS), and also to scanning tunneling microscopy (STM) probes (nanosized electrodes) for single-molecule conductance measurements. However, it is still unclear how the attached molecules interact electronically with the underlying surface, and how the anchoring group might affect the electronic and optical properties of such nanoscale systems. Here we report systematic surface-enhanced Raman studies of two organic chromophores (malachite green and its isothiocyanate derivative) that have very different functional groups for surface binding, but nearly identical spectroscopic properties. A surprise finding is that under the same experimental conditions, the SERS signal intensities for the isothiocyanate derivative (MGITC) are nearly 500-fold higher than that of the parent dye (MG). Correcting for the intrinsic difference in scattering cross sections of these two dyes, we estimate that the MGITC enhancement factors are approximately 200-fold higher than that for MG. Furthermore, pH-dependent studies reveal that the surface structure of the isothiocyanate derivative is irreversibly stabilized or “locked” in its π-conjugated form and is no longer responsive to pH changes. In contrast, the electronic structure of adsorbed malachite green is still sensitive to pH and can be switched between its localized and delocalized electronic forms. These results indicate that isothiocyanate is indeed an unusual anchoring group that enables strong electronic coupling between gold and the adsorbed dye, leading to more efficient chemical enhancement and higher overall enhancement factors.
The ability to attach organic molecules to nanoparticles such as gold nanocrystals is of major interest in developing advanced nanosystems for use in molecular electronics1–3, chemical sensing4,5, biomedical diagnostics6–8, and solar energy conversion9. Due to their high affinities for gold, thiol-containing molecules are widely used to form self-assembled monolayers on flat surfaces, to coat the surface of nanoparticles, and to connect nanoelectrodes for studying molecular junctions.10–12 Thiolated linkers with conjugated π-electrons are especially interesting because they could allow more efficient electron transfer and molecular orbital overlapping than saturated alkanethiols.13 In addition, conjugated groups such as isothiocyanate (ITC) (-N=C=S, a reactive group for covalent conjugation of organic dyes with biomolecules) have enabled the stable anchoring of reporter molecules to atomically smooth gold surfaces for tip-enhanced Raman scattering (TERS)14,15 and to colloidal gold nanoparticles for surface-enhanced Raman scattering (SERS)16,17. Recent work by Halas and coworkers18 has further shown that the electronic conductance and surface-enhanced Raman spectra of conjugated thiol molecules can be measured simultaneously from nano-sized junctions. However, it is still not clear how the attached chromophores interact electronically with the underlying surface, and how the anchoring group might affect the electronic and optical properties of such nanohybrid systems.
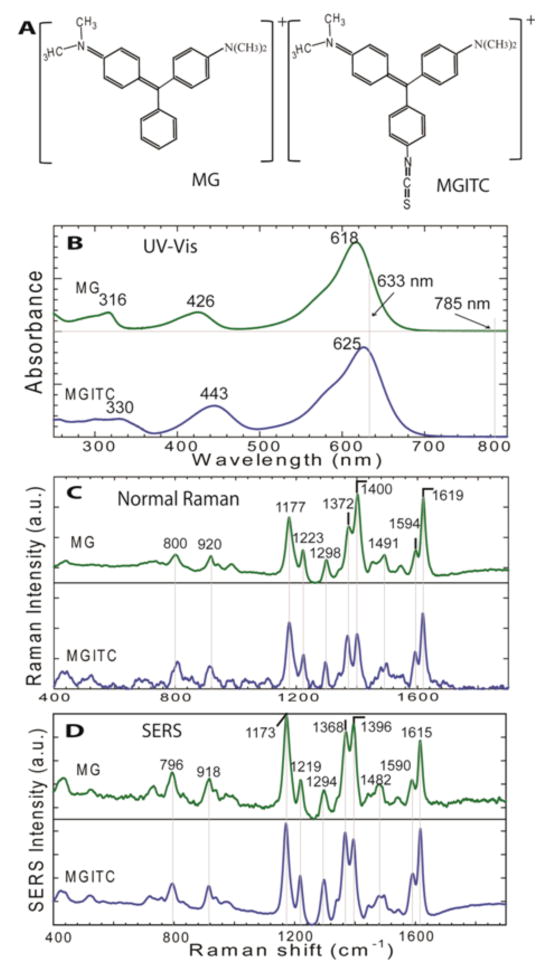
Here we report detailed SERS studies of two organic chromophores (malachite green and its isothiocyanate derivative) in order to examine how the anchoring group might affect the surface enhancement efficiency and the electronic structure of surface-bound molecules. As shown in Figure 1, malachite green and its ITC derivative have nearly identical spectroscopic properties including UV-V is absorption, normal Raman scattering, and SERS (see B to D), but very different chemical groups for surface adsorption. In fact, malachite green is known to adsorb via a single dimethylamino group in a tilted upright configuration, whereas the isothiocyanate derivative is believed to adsorb via a nearly flat configuration involving π–electrons (more discussion later)14,15,19–21. In this context of chemical adsorption and spectroscopic signatures, recent work by Van Duyne, Schatz, and coworkers22,23 have used isotope-substituted organic dyes (deuterated rhodamine 6G and deuterated crystal violet) for competitive adsorption at surface “hot spots” in single-molecule SERS studies. The rationale is that such isotopologues have identical surface adsorption properties, but distinct spectroscopic features that allow identification of each dye from their composite SERS spectra. In this work, malachite green and its ITC derivative are used for the opposite reason - that is, this pair of dyes has nearly identical spectroscopic signatures, but different chemical groups for surface adsorption.
Figure 1.
Malachite green (MG) and its isothiocyanate derivative (MGITC), two organic chromophores that have very different functional groups for attaching to gold surfaces, but nearly identical spectroscopic properties. (A) Schematic chemical structures of MG and MGITC in their delocalized electronic forms (with delocalized π–electrons); (B) Comparison of UV-V is absorption spectra between MG and MGITC; (C) normal Raman spectra and (D) SERS spectra of MG and MGITC. The normal Raman spectra were obtained from MG (2.5 mM) and MGITC (1 mM) in water solution with 2-sec integration time, and were plotted on the same intensity scale. The SERS spectra were obtained from a colloidal gold solution (60-nm particle diameter, 14-pM concentration) mixed with 2.3 μM MG or 0.2 μM MGITC at room temperature, 1-sec integration time. All Raman measurements were acquired by using 785-nm laser excitation, 40-mW laser power. Note that the SERS signal intensities were normalized in order to highlight the spectroscopic features of MG and MGITC.
Both malachite green and its ITC derivative are found to stably adsorb on colloidal gold in a quantitative manner (more than 95% of the added dye is rapidly adsorbed to the gold particles, see Supporting Figure S1). Thus, the number of dye molecules per particle can be calculated within an error of only a few percents. Furthermore, the normal Raman data in Figure 1(C) indicate that malachite green and its ITC derivative have not only similar vibrational modes but also similar scattering cross sections, allowing a direct comparison of their SERS signal intensities and enhancement factors. In fact, at 785 nm nonresonant excitation, the normal Raman scattering intensities are similar for 1 mM MGITC and 2.5 mM MG under otherwise identical experimental conditions. This data indicates that the intrinsic scattering cross sections of the ITC derivative are likely higher than that of the parent dye (MG) by a factor of 2 to 3.
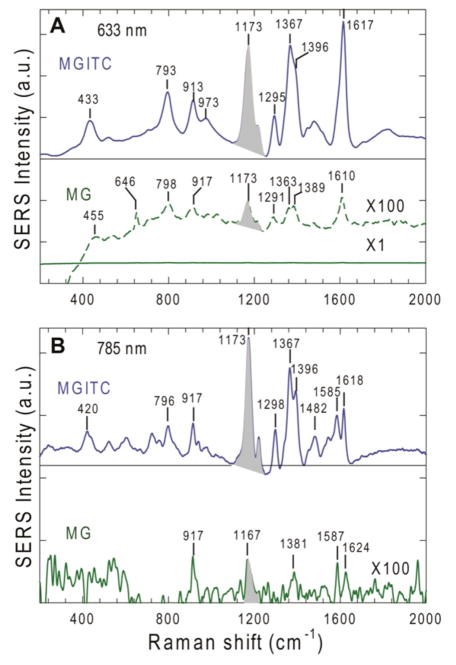
Figure 2 shows SERRS and SERS spectra of MG and MGITC obtained at resonant and nonresonant excitation wavelengths (633 nm and 785 nm, respectively). A surprise finding is that the absolute signal intensities of MGITC are dramatically higher (by approximately 500-fold) than that of MG, even though their spectral patterns (relative intensities) and peak frequencies are similar. Since both MG and MG-ITC have electronic transitions at 633 nm, the observed signals contain a resonance enhancement and is thus surface-enhanced resonance Raman scattering (SERRS). To ascertain whether this resonance enhancement effect could be responsible for the observed difference, we have obtained the SERS spectra of these two dyes at 785-nm excitation, a wavelength that is not in resonance with the electronic transitions of MG or MGITC. The results in Figure 2B show that the MGITC SERS signals are also more intense than that of MG by a large factor under nonresonant excitation conditions. When the SERS intensities are plotted as a function of dye concentration (which is directly related to surface coverage before adsorption saturation), the ITC-derivative data show a fitted slope of 170 (over a roughly linear concentration range), while the MG data show a slope value of only 0.35 (see Supporting Figure S2). The ratio of these slope values is roughly 500, nearly the same as the absolute intensity ratio. This convergence is not a coincidence, but represents a fundamental difference in the surface-enhanced Raman scattering cross sections between MG and MGITC. By correcting for the intrinsic difference in the intrinsic scattering cross sections between MGITC and MG, we estimate that the MGITC enhancement factors are approximately 200-fold higher than that for MG. This dramatic difference is believed to arise from efficient chemical enhancement that is mediated by the ITC anchoring group and strong electronic coupling, as discussed in more details below.
Figure 2.
Surface-enhanced Raman (SERS) and resonance Raman scattering (SERRS) spectra of malachite green (MG) and its ITC derivative (MGITC) obtained under the same experimental conditions at resonant and nonresonant excitation wavelengths. (A) SERRS spectra obtained at 633 nm (in resonance) laser excitation (3 mW, integration time = 1 s). (B) SERS spectra obtained at 785 nm (off resonance) laser excitation (40 mW, integration time = 20 s). The MG and MGITC concentrations were the same (100 nM), and the concentration of the colloidal gold was approximately 14 pM. Under these conditions, there were about 7,000 dye molecules per particle. In both (A) and (B), the MG spectra are expanded by a factor of 100 for spectral details. By using the intensity of the 1173 cm−1 peak (shaded area), the MGTIC signals are calculated to be nearly 500-fold higher than that of MG at both 633 nm and 785 nm excitation wavelengths.
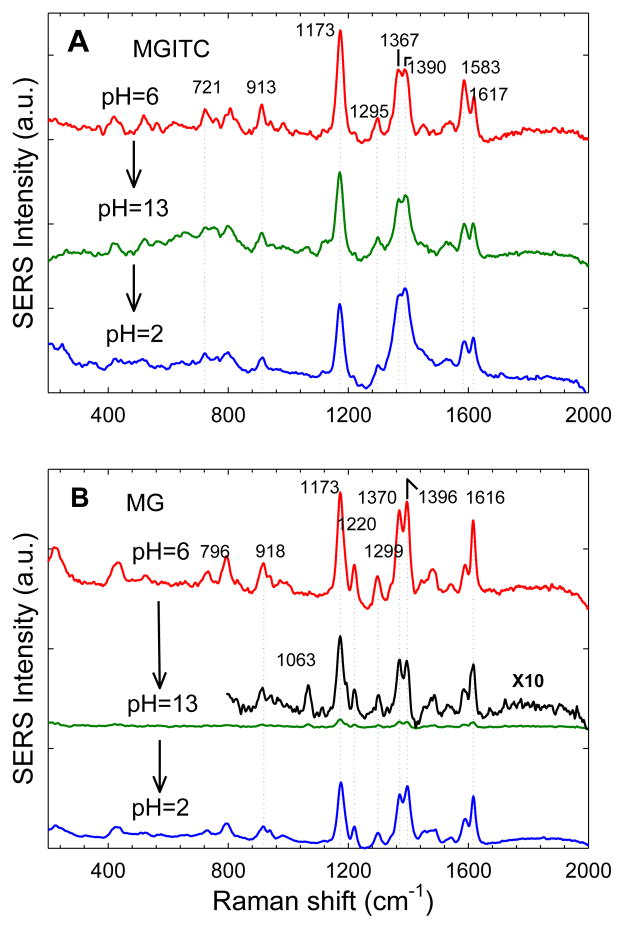
To further investigate how surface absorption could alter the electronic structures of MG and MGITC, we have taken advantage of their pH-responsive properties and have examined how their SERS signals change as a function of pH. Figure 3 shows the SERS spectra of MG and MGITC obtained sequentially at pH 6, 13, and then 2. Similar to other pH indicator dyes,24 both MG and MGITC change from a deeply colored (green) solution (due to delocalized π-electrons) to a nearly colorless solution (due to a change in its electronic structure and the disappearance of optical absorption in the visible spectrum) (Supporting Figure S3). In free solution, these pH-induced structural changes give rise to completely different normal Raman spectra (see Supporting Figure S4). However, for the adsorbed MGITC, similar SERS signal intensities and frequencies are observed under both basic and acidic conditions, indicating a stabilized or locked surface structure that is no longer sensitive to pH. In contrast, adsorbed malachite green is still responsive to pH and its SERS signals largely disappear when the pH is changed from 6 to 13. Interestingly, a trace of the original SERS signals (very weak but reproducible) still remains at pH 13, and the residual SERS spectrum corresponds to adsorbed MG with a delocalized electronic structure (the same structure at neutral and acidic pHs). It is likely that a tiny fraction of the MG molecules is adsorbed at high-affinity sites on faceted gold nanocrystals or at the junction of two particles,25,26 and that its structure is stabilized in the delocalized form, similar to that of MGITC. Also, it is remarkable that the MG SERS signals can be reversibly turned on and off by switching the pH between 2 and 13 (Figure 3B), indicating that the MG molecules are not lost or desorb from the nanoparticle surface at pH 13. In other words, this reversible behavior suggests that the surface coverage is approximately constant at pHs 2 and 13 (note that there are no free dye molecules in solution, so any lost or desorbed MG molecules at pH 13 would not be recovered at pH 2.
Figure 3.
SERS spectra of MGITC (A) and MG (B) obtained with 785 nm laser excitation as a function of pH. As the pH was changed sequentially from 6 to 13 and then to 2, the isothiocyanate dye was found irreversibly stabilized or “locked” in its π-conjugated structure whereas the adsorbed malachite green was still sensitive to pH and could be switched between its localized and delocalized forms. Laser power = 40 mW; integration time = 20 s.
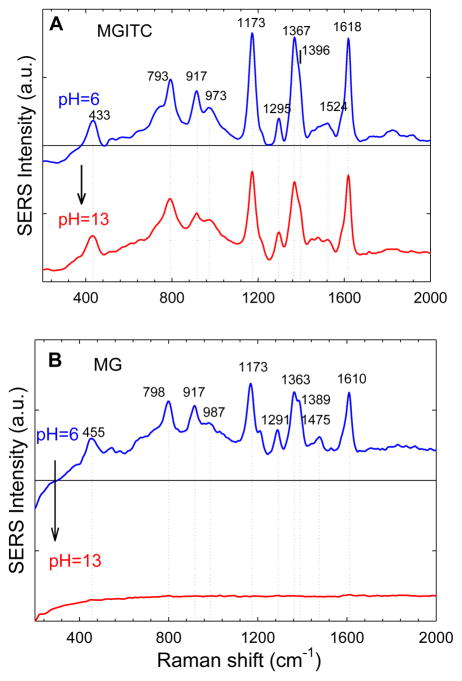
At resonant laser excitation (633 nm), similar pH-dependent behaviors are observed for MGITC and MG (see Figure 4). This data confirm that the electronic structure of MGITC is irreversibly locked on gold nanoparticles, but MG is still responsive to pH and its SERS signals are turned off by changing the pH to 13. These intriguing results cannot be explained by a resonance enhancement effect alone, although resonance enhancement contributes to the SERS signals at 633 nm excitation. Instead, we believe that the observed pH dependence arises largely from a chemical enhancement effect27–30 that is modulated (turned on and off) by pH-induced structural changes and electronic coupling. That is, efficient chemical enhancement is observed for conjugated malachite green at acidic or neutral pH, but this chemical effect is greatly reduced when the dye is converted to its localized electronic structure at pH 13. The electromagnetic field enhancement could be involved,27 but its contributions to the observed pH dependence must be minor because the colloidal nanoparticles do not aggregate and have nearly identical surface plasmon absorption peaks with and without the reporter dyes (see Supporting Figures S5 and S6).
Figure 4.
SERRS spectra of MGITC (A) and MG (B) obtained with 633 nm laser excitation at acidic or basic pHs (pH = 6 or 13). Note that the electronic structure of adsorbed MGITC is locked in its delocalized form and is no longer sensitive to pH, whereas the structure of adsorbed MG is still sensitive to pH and can be switched between its localized and delocalized forms. The laser power was 3 mW, and the integration time was 1 sec.
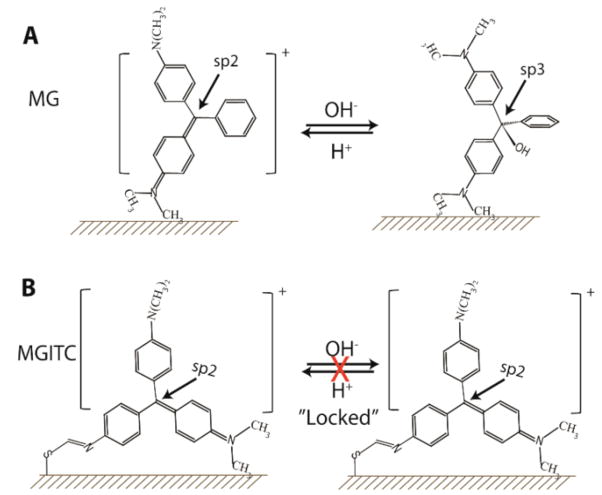
It is believed that triarylmethane dyes such as crystal violet and MG adsorb on gold surfaces via only one nitrogen atom in a tilted upright configuration.19 The observation of strongly enhanced in-plane vibrational modes is consistent with this adsorption geometry.31 Surface binding via a single dimethylamino group does not sufficiently stabilize or lock the dye’s electronic structure. As a result, MG on gold is still responsive to pH and its electronic structure can be reversibly switched by pH changes, similar to pH-induced structural changes in solution. In contrast, surface adsorption via the ITC group irreversibly locks the adsorbed molecule in its delocalized electronic structure. As a result, its SERS and SERRS spectra of MGITC are no longer responsive to pH, as schematically illustrated in Figure 5. Electrical conductance measurements have also shown that stronger headgroup-surface coupling can improve contact conductance, and that isothiocyanate is an effective anchoring group due to its rich π electrons.32,33 Density functional theory (DFT) calculations have further revealed that the isothiocyanate anchoring group binds to gold with a bent angle (∠ Au-S-C) of approximately 103°, in contrast to the nearly linear (standing) geometry for isothiocyanate adsorption on platinum ( that is, ∠ APt-S-C ≈ 180°).34
Figure 5.
(A) Schematic diagram showing that malachite green is adsorbed on gold via a single dimethylamino group in a tilted configuration. In this geometry, the central carbon atom can change from sp2 orbital hybridization (delocalized π–electrons, planner structure) at acidic pHs to sp3 orbital hybridization (localized π–electrons, octahedral structure) at basic pHs. (B) Schematic diagram showing that MGITC is adsorbed on gold via two attachment sites. As a result, its electronic structure is locked in the π-conjugated form and is no longer responsive to pH changes.
We also note that molecules with stronger binding affinities to metal surfaces are known to give larger enhancement in SERS due to both Franck-Condon and Herzberg-Teller (vibronic) mechanisms as predicted by theory.35,36 Our results thus provide further evidence for chemical enhancement in SERS, an elusive and often controversial topic in the literature.
In conclusion, we have used malachite green and its isothiocyanate derivative to examine how the surface-anchoring group affects surface Raman enhancement and the electronic structures of surface-adsorbed dyes. A major finding is that the surface enhancement efficiencies (enhancement factors) are nearly 200-fold higher for the isothiocyanate-derivatized dye than for the parent dye. The results also show that the surface structure of the isothiocyanate derivative is irreversibly stabilized or “locked” in its pi-conjugated form and is no longer sensitive to pH; in contrast, the electronic structure of adsorbed malachite green is still sensitive to pH and can be switched between its localized and delocalized forms. Thus, the use of conjugated anchoring groups such as isothiocyanate can enable strong surface-absorbate electronic coupling, leading to more efficient chemical enhancement and higher overall enhancement factors. These insights are important for the design and development of spectrally encoded SERS nanoparticles with improved signal brightness and stability.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01CA163256-01, RC2CA148265-02, U54CA119338-05, HHSN268201000043C, R01CA133722-04, and P50CA128613-05). We are grateful to Dr. Hong Yi of the Emory Electron Microscopy Core for assistance with TEM studies. S.N. acknowledges the Georgia Cancer Coalition (GCC) for a Distinguished Cancer Scholar award.
Footnotes
Potential Conflict of Interest: One of the authors (S.N.) is a consultant of SpectroPath, Inc., a startup company in Atlanta, GA to develop the use of SERS and other nanoparticle contrast agents for image-guided surgery.
Supporting Information Available: Detailed experimental procedures including preparation of spectrally encoded SERS nanoparticles, normal Raman spectra, and a series of characterization in Supporting Figures S1-S5. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Chen J, Reed MA, Rawlett AM, Tour JM. Science. 1999;286:1550. doi: 10.1126/science.286.5444.1550. [DOI] [PubMed] [Google Scholar]
- 2.Gittins DI, Bethell D, Schiffrin DJ, Nichols RJ. Nature. 2000;408:67. doi: 10.1038/35040518. [DOI] [PubMed] [Google Scholar]
- 3.Xu BQ, Tao NJJ. Science. 2003;301:1221. doi: 10.1126/science.1087481. [DOI] [PubMed] [Google Scholar]
- 4.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Nat Mater. 2008;7:442. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 5.Camden JP, Dieringer JA, Zhao J, Van Duyne RP. Acc Chem Res. 2008;41:1653. doi: 10.1021/ar800041s. [DOI] [PubMed] [Google Scholar]
- 6.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 7.Qian XM, Nie SM. Chem Soc Rev. 2008;37:912. doi: 10.1039/b708839f. [DOI] [PubMed] [Google Scholar]
- 8.Graham D, Thompson DG, Smith WE, Faulds K. Nat Nanotechnol. 2008;3:548. doi: 10.1038/nnano.2008.189. [DOI] [PubMed] [Google Scholar]
- 9.Hagfeldt A, Gratzel M. Acc Chem Res. 2000;33:269. doi: 10.1021/ar980112j. [DOI] [PubMed] [Google Scholar]
- 10.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem Rev. 2005;105:1103. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Li XL, Hihath J, Huang ZF, Tao N. J Am Chem Soc. 2006;128:15874. doi: 10.1021/ja065864k. [DOI] [PubMed] [Google Scholar]
- 12.Xing YJ, Park TH, Venkatramani R, Keinan S, Beratan DN, Therien MJ, Borguet E. J Am Chem Soc. 2010;132:7946. doi: 10.1021/ja909559m. [DOI] [PubMed] [Google Scholar]
- 13.Fu MD, Chen WP, Lu HC, Kuo CT, Tseng WH, Chen CH. J Phys Chem C. 2007;111:11450. [Google Scholar]
- 14.Domke KF, Zhang D, Pettinger B. J Am Chem Soc. 2006;128:14721. doi: 10.1021/ja065820b. [DOI] [PubMed] [Google Scholar]
- 15.Pettinger B, Ren B, Picardi G, Schuster R, Ertl G. Phys Rev Lett. 2004;92:Article 096101. doi: 10.1103/PhysRevLett.92.096101. [DOI] [PubMed] [Google Scholar]
- 16.Doering WE, Nie SM. Anal Chem. 2003;75:6171. doi: 10.1021/ac034672u. [DOI] [PubMed] [Google Scholar]
- 17.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. Nat Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 18.Ward DR, Halas NJ, Ciszek JW, Tour JM, Wu Y, Nordlander P, Natelson D. Nano Lett. 2008;8:919. doi: 10.1021/nl073346h. [DOI] [PubMed] [Google Scholar]
- 19.Fischer D, Caseri WR, Hahner G. J Colloid & Interface Sci. 1998;198:337. [Google Scholar]
- 20.Kikteva T, Star D, Leach GW. J Phys Chem B. 2000;104:2860. doi: 10.1103/PhysRevLett.85.1906. [DOI] [PubMed] [Google Scholar]
- 21.Joo SW. Surface and Interface Analysis. 2006;38:173. doi: 10.1002/sia.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieringer JA, Lettan RB, Scheidt KA, Van Duyne RP. J Am Chem Soc. 2007;129:16249. doi: 10.1021/ja077243c. [DOI] [PubMed] [Google Scholar]
- 23.Kleinman SL, Ringe E, Valley N, Wustholz KL, Phillips E, Scheidt KA, Schatz GC, Van Duyne RP. J Am Chem Soc. 2011;133:4115. doi: 10.1021/ja110964d. [DOI] [PubMed] [Google Scholar]
- 24.Golding PS, King TA, Maddocks L, Drucker DB, Blinkhorn AS. J Photochem and Photobio B-Biology. 1998;47:202. doi: 10.1016/s1011-1344(98)00224-3. [DOI] [PubMed] [Google Scholar]
- 25.Nie SM, Emery SR. Science. 1997;275:1102. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- 26.Michaels AM, Nirmal M, Brus LE. J Am Chem Soc. 1999;121:9932. [Google Scholar]
- 27.Doering WE, Nie SM. J Phys Chem B. 2002;106:311. [Google Scholar]
- 28.Campion A, Kambhampati P. Chem Soc Rev. 1998;27:241. [Google Scholar]
- 29.Otto A, Mrozek I, Grabhorn H, Akemann W. J Phys-Condensed Matter. 1992;4:1143. [Google Scholar]
- 30.Moskovits M. Rev Mod Phys. 1985;57:783. [Google Scholar]
- 31.Lueck HB, Daniel DC, McHale JL. J Raman Spectroscopy. 1993;24:363. [Google Scholar]
- 32.Chen IWP, Fu MD, Tseng WH, Yu JY, Wu SH, Ku CJ, Chen CH, Peng SM. Angew Chem, Int Ed. 2006;45:6244. doi: 10.1002/anie.200600800. [DOI] [PubMed] [Google Scholar]
- 33.Yin CX, Huang GC, Kuo CK, Fu MD, Lu HC, Ke JH, Shih KN, Huang YL, Lee GH, Yeh CY, Chen CH, Peng SM. J Am Chem Soc. 2008;130:10090. doi: 10.1021/ja8016818. [DOI] [PubMed] [Google Scholar]
- 34.Ko CH, Huang MJ, Fu MD, Chen CH. J Am Chem Soc. 2010;132:756. doi: 10.1021/ja9084012. [DOI] [PubMed] [Google Scholar]
- 35.Osawa M, Matsuda N, Yoshii K, Uchida I. Journal of Physical Chemistry. 1994;98:12702. [Google Scholar]
- 36.Canamares MV, Chenal C, Birke RL, Lombardi JR. Journal of Physical Chemistry C. 2008;112:20295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.