Abstract
Background
The development of specific biomarkers to aid in diagnosis and prognosis of neuronal injury is of paramount importance in cardiac surgery. Alpha II-spectrin is a structural protein abundant in neurons of the central nervous system and cleaved into signature fragments by proteases involved in necrotic and apoptotic cell death. We measured cerebrospinal fluid (CSF) alpha II-spectrin breakdown products (αII-SBDP’s) in a canine model of hypothermic circulatory arrest (HCA) and cardiopulmonary bypass (CPB).
Methods
Canine subjects were exposed to either 1 hour of HCA (n=8, mean lowest tympanic temperature 18.0 ± 1.2 °C), or standard CPB (n=7). CSF samples were collected prior to treatment and 8 and 24 hours post-treatment. Using polyacrylamide gel electrophoresis and immunoblotting, SBDP’s were isolated and compared between groups using computer-assisted densitometric scanning. Necrotic versus apoptotic cell death was indexed by measuring calpain and caspase-3 cleaved αII-SBDP’s (SBDP 145+150 and SBDP 120, respectively).
Results
Animals undergoing HCA demonstrated mild patterns of histological cellular injury and clinically detectable neurologic dysfunction. Calpain-produced αII-SBDP (150kDa+145kDa bands-necrosis) 8 hours post HCA, were significantly increased (p=0.02) as compared to levels prior to HCA and remained elevated at 24 hours post HCA. In contrast caspase-3 αII-SBDP (120kDa band-apoptosis) were not significantly increased. Animals receiving CPB did not demonstrate clinical or histological evidence of injury, with no increases in necrotic or apoptotic cellular markers.
Conclusions
We report the use of αII-SBDP’s as markers of neurological injury following cardiac surgery. Our analysis demonstrates that Calpain and Caspase produced αII-SBDP’s may be an important and novel marker of neurologic injury following HCA.
Keywords: Brain Injury, cardiac surgery, neuroprotection, hypothermic circulatory arrest, biomarkers
Introduction
Neurological injury following cardiac surgery is a devastating complication. In addition to stroke with an incidence of 1%-6%, subtle alterations in neurocognition frequently occur, leading to stress for both patients and families (1, 2). Brain injury additionally results in increases in hospital length of stays, costs, and admissions to rehabilitation facilities.
A technique of importance for understanding brain injury after cardiac surgery is hypothermic circulatory arrest (HCA). HCA remains a widely used form of neuroprotection for complex cardiac cases involving aortic arch abnormalities and congenital malformations. Despite its utility, patients who receive HCA are at particularly high risk for neurologic dysfunction such as stroke, seizures, developmental delays, and neurocognitive decline (3, 4).
In clinical practice, a reliable method of detecting brain injury following cardiac surgery does not currently exist. Brain injury is unfortunately, often diagnosed several hours or even days post surgery when it becomes clear that a patient is not progressing as expected. Given the substantial mortality, morbidity, and cost resulting from brain injury, developing readily obtainable biomarkers for both diagnosing the injury early and predicting the magnitude of injury is of paramount importance for patients, physicians, and families.
To this end, several potential serum biomarkers have been devised for acute brain injury with modest success. Specifically, S-100B (5), neuron-specific enolase (NSE) (6), glial fibrillary associated protein (GFAP), and myelin basic protein (MBP) (7) are among some widely studied. No biomarker for neurological injury has yet proven ideal. For example, S100B and NSE are non-specific for brain injury and do not predict outcomes with high sensitivity (5). Furthermore, no biomarker exists specific for brain injury resulting from cardiopulmonary bypass (CPB) or HCA. The identification of a reliable specific serum biomarker for neurological injury following cardiac surgery would be very beneficial.
Recent work has focused on the use of disease-specific proteolysis of αII-spectrin as a biochemical marker of brain injury (8, 9). Intact αII-spectrin is a major structural component of the cortical membrane cytoskeleton abundant in axons and presynaptic terminals (10, 11). Importantly, αII-spectrin is a major substrate for cysteine proteases involved in necrotic (calpain) and apoptotic (caspase-3) cell death (12). There is considerable evidence that αII-spectrin is processed to cleavage products also known as spectrin break down products (SBDP), of molecular weight 150 kDa (SBDP150) and 145 kDa (SBDP145) by calpain. In addition, it is cleaved to a major cleavage product of molecular weight 120 kDa (SBDP120) by caspase-3 (Figure 1). There is evidence for the detectable presence of SBDP’s in in vitro neuronal cell culture models of injury (13), in vivo studies of mice (14) and in humans studies of traumatic brain injury (15, 16).
Figure 1.
Alpha II spectrin is vulnerable to specific calpain and caspase mediated cleavage, generating fragments SBDP150 and SBDP120. This schematic presents the breakdown pathways for alpha II spectrin illustrating that necrotic cell death is calpain mediated whereas apoptosis is caspase mediated.
Given the potential utility of αII-SBDP’s as biomarkers for neuronal injury, we sought to apply this concept to neurologic injury resulting from cardiac surgery. We therefore tested for the presence of αII-SBDP’s in an established canine model of HCA and CPB alone. We hypothesize that for animals subjected to HCA for a clinically relevant duration, αII-SBDP’s will be present and identifiable in the cerebral spinal fluid (CSF) and serve as indicators of neuronal injury following HCA. We further hypothesize that αII-SBDP’s will be present, but to a lesser extent, in animals subjected to CPB alone. Identification of these proteins is the CSF is a first step towards the identification of a biomarker It is our hope that in future experiments we will identify αII-SBDP’s in serum and that they may therefore serve as a clinically applicable biomarkers for diagnosis of post-cardiac surgery neurologic injury, even when that injury is subtle on clinical exam.
Material and Methods
Animals
For all experiments, we utilized a well established and clinically relevant canine model of HCA and CPB used in our laboratory for over 15 years (17-20). Conditioned, heartworm negative, 6-12 month old, male, class A dogs weighing approximately 30kg were used for all experiments (Marshal Bioresources, 5800 Lake Bluff Rd, North Rose, NY). All experiments were approved by The Johns Hopkins University School of Medicine Animal Care and Use Committee and have complied with the “Guide for the Care and Use of Laboratory Animals” (1996, U.S. National Institutes of Health).
Experimental Design
Canine subjects were exposed to either 1 hour of HCA (1H HCA) (n=8), or standard CPB (n=7) and survived to either 8 hours (n=4 for 1H HCA and n=3 for CPB) or 24 hours (n=4 for 1H HCA and n=4 for CPB) following treatment. CSF samples were collected immediately prior to treatment, 8 hrs, and 24 hrs (when available) post-treatment. For animals survived to 24 hours after treatment, clinical neurological scoring (performed independently by 2 study team members) using the Pittsburgh Veterinary Scoring System (higher scores indicate worse neurological function) (21) was performed prior to giving any sedation or narcotic pain medication. The score utilized includes 22 specific clinical questions relating to level of consciousness, respiration, cranial nerve function, reflexes, behavior, and motor and sensory function. All animals utilized had no levels of neurological impairment prior to experimentation and no additional sedation is given within 12 hours of neurological assessment.
At the conclusion of the experiment, all canine subjects were sacrificed by exsanguination. During the sacrifice, selective perfusion of the head with cold saline (4°C) was performed and brains were harvested for histological analysis.
αII-SBDP’s, which isolated from CSF samples, were compared between groups using computer-assisted densitometric scanning. Necrotic versus apoptotic cell death was indexed by measuring calpain and caspase-3 cleaved αII-SBDP’s (SBDP 145+150 and SBDP 120, respectively).
Historical controls
For purposes of comparison of neurological outcomes, two sets of historical controls were used (data not published elsewhere). The first set (2H HCA-24s) (n=5) were subjected to 2 hours of HCA and sacrificed at least 24 hours after the treatment. A second set of controls (2H HCA-8s) (n=8) were subjected to 2 hours of HCA and sacrificed at 8 hours post HCA. The historical control experiments were performed between 2/20/2006 and 10/23/2006. With the exception of HCA duration and lack of CSF samples, no differences in experimental protocol, setup, or equipment existed for these animals relative to the study subjects. These additional groups of animals are included for comparison of neurological outcomes with our experimental groups of 1 hour HCA and CPB alone.
Detailed Procedures
Surgical HCA Procedure
Our experimental model has been described previously (17, 19, 20). In brief, canine subjects are induced with sodium thiopental (25mg/kg IV) endotracheally intubated and maintained on isoflurane inhalational anesthesia (0.5-2%), 100% inspired oxygen, and IV fentanyl at 150-200 μg/dose during invasive procedures. Tympanic membrane probes (which correlate closely with cerebral temperature), nasopharyngeal probes, and rectal probes are placed to monitor temperatures throughout the experiment. A left femoral artery cannula is placed via an open surgical cut-down technique prior to the initiation of CPB, for monitoring of blood pressures and sampling of arterial blood gases (ABGs).
The cardiopulmonary bypass (CPB) circuit consists of a Cobe membrane oxygenator with 40 μm arterial filter (Cobe Laboratories, Inc, Lakewood, CO), and Sarns Roller pump system (Sarns, Inc, Ann Arbor, MI). The circuit is primed with lactated Ringer’s solution with potassium chloride (20 mEq). After heparinization (300 U/kg IV), the right femoral artery is cannulated (12F to 14F), and the cannula is advanced into the descending thoracic aorta. Venous cannulae (18F to 20F) are advanced to the right atrium from the right femoral and right external jugular veins. All arteries and veins are accessed via an open cutdown technique. Closed-chest CPB is initiated, and the animals are cooled until tympanic membrane temperature reaches 18°C (approximately 30 minutes). Pump flows of 60 to 100 ml/kg/min are required to maintain a mean arterial pressure of 50 to 60 mmHg. Once tympanic temperatures reach 18°C the pump is stopped and blood is drained by gravity into the reservoir.
The animals undergo 1 hour of HCA with standard hemodilution and alpha-stat regulation of ABGs. Once HCA is finished, CPB is restarted and the animals are re-warmed to a core temperature of 37°C over the course of 2 hours. If sinus rhythm does not return spontaneously, the heart is defibrillated at 32°C. At 37°C the animal is weaned from CPB, the cannulae are removed, vessels are ligated, wounds are appropriately closed, and heparin is reversed with protamine (3mg/kg IV).
The animals then recover from anesthesia while intubated, with frequent monitoring of vital signs, ABGs, and urine output. Once hemodynamically and clinically stable they are extubated and transferred to their crate for recovery.
Cardiopulmonary Bypass (CPB)
After induction and cannulation, animals undergo 2.5 hours of CPB with no HCA. Animals are cooled to 32°C, similar to routine clinical cardiopulmonary bypass. The heart continues to beat during this operation. The animals are recovered from anesthesia as described above.
CSF and Serum Collection
For both baseline and subsequent CSF collection, under sedation and in a routine sterile fashion, the spinal canal is entered with a 22 gauge needle via the cisterna magnum (at the base of the skull posteriorly). Samples are immediately frozen in a −80°C freezer. Blood samples are obtained through previously placed peripheral intravenous catheters, cold centrifuged to collect serum, and frozen at −80°C.
Sacrifice and Histological Evaluation
Animals are sacrificed by exsanguination. They are sedated, intubated, and maintained on inhalational anesthesia. The dogs then undergo median sternotomy and cannulation of the ascending aorta using a 22-French cannula. CPB is initiated after clamping the descending aorta to ensure the brains are perfused with 12L of cold saline (4°C) at 60 mmHg. The right atrial appendage is transected and the venous return is allowed to drain into a reservoir. The brains are harvested with the right hemibrain fixed in 10% formalin and embedded in paraffin. Left hemibrains are stored in saline for future biochemical work (−80°C). Paraffin embedded tissues are sectioned into 8 μm slices and are stained with hematoxylin and eosin stains for histological evaluation in a blinded manner by a single neuropathologist (JCT).
Eleven distinct regions of the canine brain are evaluated for the presence of apoptosis and necrosis. These regions include midfrontal cortex, superior parietal cortex, basal ganglia, hippocampus (dentate gyrus and pyramidal gyrus), entorhinal cortex, amygdala, cerebellum (molecular layer, Purkinje layer and granule layer), and brainstem. A semiquantitative scale is used to assess the degree of necrosis and apoptosis in each area. The scale ranges from a minimum value of 0 (no damage) to a maximum of 99 (severe neuronal damage).
CSF analyses for proteolytic fragments of αII-spectrin
All CSF analyses for the presence of SBDP’s were performed by colleagues at Baynan Biomarkers, Inc. (Alachua, FL). Protein concentrations of CSF were determined by DC-Protein assays (BioRad, San Diego, CA USA) with albumin standards. Protein balanced samples (7μl of CSF samples) were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in two-fold loading buffer containing 0.25 M Tris (pH 6.8), 0.2 M DTT, 8% SDS, 0.02% bromophenol blue, and 20% glycerol in distilled H2O. Twenty micrograms (20μg) of protein per lane were resolved by SDS-PAGE on 6.5% Tris/glycine gels for 2 h at 200 V. After electrophoresis, separated proteins were laterally transferred to polyvinylidene fluoride (PVDF) membranes in a transfer buffer containing 0.500 M glycine and 0.025 M Tris-HCl (pH 8.3) 10% methanol at a constant voltage of 20 V for 2 h at 4°C in a semi-dry transfer unit (Bio-Rad).
Immunoblot analyses
After electrotransfer, membranes were blocked for 1 hour at ambient temperature in 5% non-fat milk in TBS and 0.05% Tween-2 (TBST), then incubated in primary monoclonal αII-spectrin, antibody (Affinity Res. Prod. Nottingham, UK #FG6090) in TBST with 5% milk at 4°C overnight, followed by four washes with TBST and a 2-hour incubation at ambient temperature with either a secondary antibody linked to horseradish peroxidase (enhanced chemiluminescence (ECL) method; for CSF samples), or biotinylated secondary antibody followed by a 30 min incubation with strepavidin-conjugated alkaline phosphatase (colorimetric method; for tissue lysate). ECL reagents were used to visualize the immunolabeling on x-ray film. Molecular weights of intact proteins and their potential breakdown products were assessed by running along side rainbow colored molecular weight standards. Semiquantitative evaluation of protein and the breakdown product levels were performed via computer-assisted densitometric scanning (Epson XL3500 high resolution flatbed scanner) and image analysis with Image J software (NIH). The results were expressed in arbitrary densitometric units.
Statistical Analysis
All data are presented as means ± standard deviation. Comparisons of both neurological and histological scores among groups was performed by one way analysis of variance (ANOVA). For all subjects, the paired comparisons t-test was examined differences in levels of SBDP from baseline to 8 hours after treatment. For those animals survived to 24 hours after treatment, repeated measures (RM) ANOVA was used to account for the repeated CSF samples from within the same canine subject over time. For both one way and RM ANOVA, post hoc pairwise comparisons between specific treatments time points were conducted via the Tukey-Kramer method. Statistical analysis was performed with the aid of STATA software (version 9.2, StataCorp LP, College Station, TX).
Results
Subjects
A total of 8 dogs underwent 1 hour of HCA. The mean lowest tympanic temperature during HCA was 18.0 ± 1.2 °C in this group. Mean time to HCA was 26.3 ± 5.8 minutes with a mean time from HCA to 37.0°C of 105 ± 14.1 minutes. Mean hemoglobin (Hb) levels during and following HCA were 8.5 ± 1.6 mg/dL and 10.7 ± 1.3 mg/dL (p=0.01), respectively. Four of these animals were sacrificed at 8 hours with the remainder (n=4) sacrificed at 24 hours. CSF samples were obtained for 6 of the 8 dogs (two of the 8 hour sacrifice animals did not yield CSF for technical reasons). 7 dogs underwent CPB alone. Mean lowest tympanic temperature in this group was 30.6 ± 0.8 °C. Additionally, mean Hb levels during and following CPB were 7.8 ± 1.1 mg/dL and 8.0 ± 1.2 mg/dL (p=0.7), respectively. Similar to the HCA group, 3 were sacrificed at 8 hours with 4 sacrificed at 24 hours. One of these animals did not yield usable CSF. In addition, for the remaining 6 animals at two time points (8 hours in one animal, and 24 hours in another), technical difficulties prevented CSF samples from being obtained. Thus, in the CPB group there were 5 CSF samples from 8 hours after CPB and 3 samples at 24 hours following CPB. There were no additional operative or technical complications in any group. Animals that did not yield CSF were not excluded as data was still used for neurological and histological evaluation.
Neurological Scores
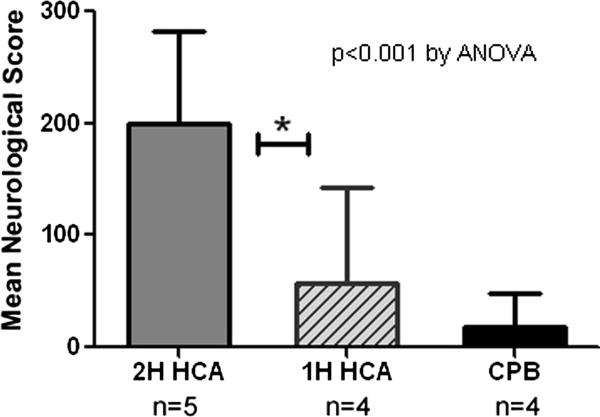
The canine scoring system ranges from 0-480 with higher scores indicating worse neurological function (21). For animals undergoing one hour of HCA, mean neurological scores at 24 hours post HCA ranged from 10 to 105.5 points with a mean score of 57.6 (median 57.5, sd 53.5). This was in sharp contrast to scores from 2 hour HCA historical control animals whose mean neurological scores were 171 ± 17.8 (range 149.5-186.6 ) (p<0.05) (Figure 2). In contrast to 2 dogs in the 1 hour HCA group who were effectively normal with neurological scores <20, no dog receiving 2 hours of HCA had a neurological score less than 140 – all were severely impaired neurologically. Animals undergoing CPB alone had only mild decreases in cognitive function with mean scores at 24 hours of 6.25 ± 4.8 (range 0-10). No dog in the CPB group appeared to be cognitively impaired at 24 hours indicating at most mild neurological injury.
Figure 2.
Mean neurological scores (based on two observers) for animals 24 hours after undergoing 2 hours of HCA (historical controls), 1 hour of HCA, or CPB alone. Based on University of Pittsburg Canine Neurological Score (0-100). Higher score indicates worse neurological function. *indicates p<0.05 by Tukey-Kramer method.
Histology
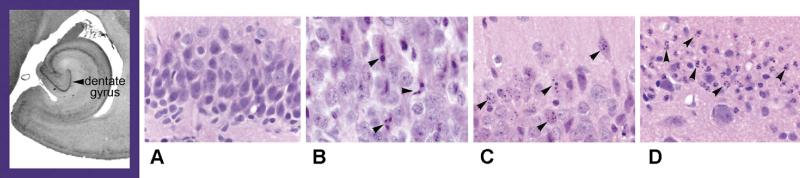
We examined specimens for histological analysis from animals sacrificed 8 hours after either 2 hour HCA, 1 hour HCA, and CPB alone. Dogs subjected to 2 hours of HCA, had the highest levels of apoptosis on histological examination (11% apoptosis, mean histological score of 2.7 ± 1.6) (Table 1). This level was statistically different from both 1 hour HCA and CPB alone. Both 1 hour HCA and 2 hour HCA animals had damage in the parietal cortex, hippocampus, basal ganglia and cerebellum. Notably, the hippocampus and basal ganglia appeared to be the most severely affected regions. Histological damage was noticeably minimal for animals undergoing CPB alone. Provided is a representative sample obtained from the dentate gyrus (hippocampus) for normal dogs (similar to CPB only), dogs undergoing 2 hours of HCA, and dogs undergoing 1 hour of HCA (Figure 3). Animals undergoing 2 hours of HCA had increased overall cell death (Figure 3, panels C and D) from both apoptosis and necrosis.
Table 1.
Mean histological scores (both total and stratified by neurologic region) for animals who underwent either 2 hour HCA, 1 hour HCA, or CPB alone.
| Brain Region | Potential Score for region |
2 Hour HCA (n=7) (historical controls) |
1 Hour HCA (n=3) |
CPB alone (n=3) |
P value* |
|---|---|---|---|---|---|
|
Necrosis
Total |
55 | 2.7 (±1.6) (5% necrosis) |
3 (±2) (5.5% necrosis) |
0.33 (±0.6) (0.75% necrosis) |
0.1 |
|
Apoptosis
Total |
44 | 4.7 (±1.7) †,§ (11% apoptosis) |
1.7 (±1.6) (4% apoptosis) |
0.33 (±0.6) (0.75% apoptosis) |
0.002 |
|
| |||||
| Notable Regions | |||||
|
| |||||
|
Parietal
Cortex |
|||||
| Necrosis | 5 | 0.1 (±0.4) (2% necrosis) |
0.3 (±0.6) (6% necrosis) |
0 (±0) (0% necrosis) |
0.6 |
| Apoptosis | 4 | 0.3 (±0.5) (7.5% apoptosis) |
0.3 (±0.6) (7.5% apoptosis) |
0 (±0) (0% apoptosis) |
0.6 |
|
| |||||
|
Basal
Ganglia |
|||||
| Necrosis | 5 | 1 (±0) † (20% necrosis) |
0.7 (±0.6) † (14% necrosis) |
0 (±0) (0% necrosis) |
<0.001 |
| Apoptosis | 4 | 0.2 (±0.4) (5% apoptosis) |
0 (±0) (0% apoptosis) |
0 (±0) (0% apoptosis) |
0.65 |
|
| |||||
| Hippocampus | |||||
| Necrosis | 10 | 0.3 (±0.5) (3% necrosis) |
0.7 (±0.6) (7% necrosis) |
0.3 (±0.6) (3% necrosis) |
0.6 |
| Apoptosis | 8 | 2.7 (±0.5) †,§ (34% apoptosis) |
1.3 (±0.6) (16% apoptosis) |
0.3 (±0.6) (4% apoptosis) |
<0.001 |
|
| |||||
| Cerebellum | |||||
| Necrosis | 15 | 0.4 (±1.6) (3% necrosis) |
0.5 (±1.5) (5.5% necrosis) |
0 (±0) (0% necrosis) |
0.6 |
| Apoptosis | 12 | 0.3 (±0.5) (3% apoptosis) |
0.3 (±0.6) (4% apoptosis) |
0.3 (±0.6) (4% apoptosis) |
0.4 |
P value corresponds to results from one way ANOVA.
significantly different from reference (CPB) on post hoc pairwise comparisons using Tukey Kramer method
significantly different from 1H HCA on post hoc pairwise comparisons using Tukey Kramer method
Figure 3.
Histology of dentate gyrus (hippocampus) showing mild injury among dogs receiving 1 hour of HCA and sacrificed at 8 hours post HCA (B) when compared to normal animals (A). Note the increased overall cell death in animals receiving 2 hours of HCA (C and D) and progressively increased apoptosis at 24 hours post HCA. Arrows show apoptotic cells
Cerebral Spinal Fluid Analysis
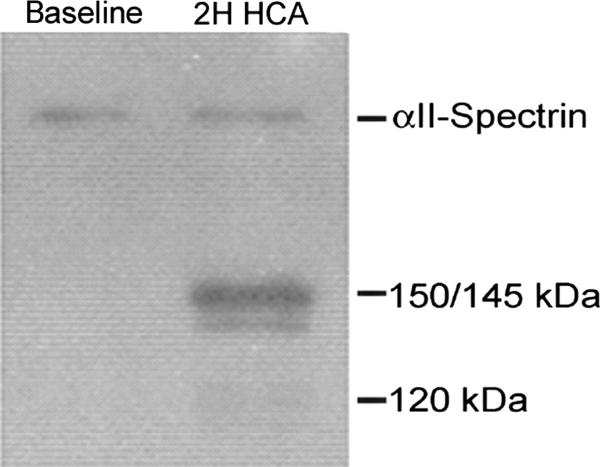
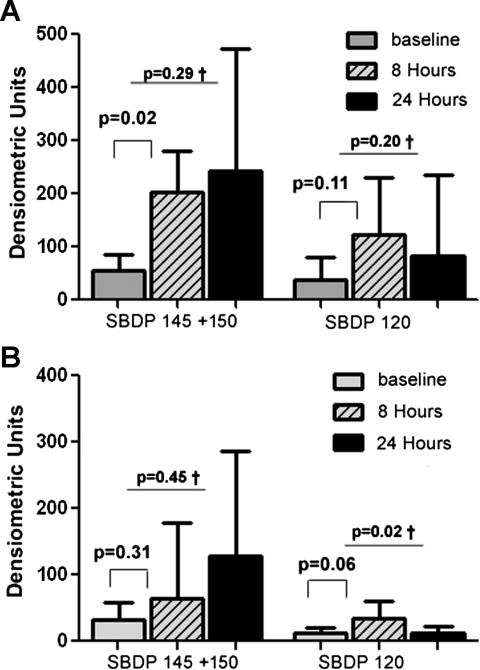
Following HCA, accumulation of full-length αII-spectrin (280 kDa) as well as 150 +145, and 120 kDa αII-SBDP were visible on immunoblots. Note that these fragments were not present at baseline (Figure 4). For animals that underwent 1 hour of HCA, the levels of the necrotic marker (SBDP 145+150) rose significantly from baseline at 8 hours following HCA (55 ± 48 to 203 ± 128 DU, p=0.02). The apoptotic marker (SBDP 120) also rose from 37 ± 16 to 121 ± 42, but this change was not statistically significant (p=0.11). In the series of animals followed and sacrificed at 24 hours following HCA, both SBDP 145+150 and SBDP 120 continued to remain above baseline (242 ± 296 for SBDP 145+150 and 82 ± 96 for SBDP 120) (Figure 5). Animals that underwent CPB alone, had similar baseline levels to HCA dogs and did not show significant increases in SBDP’s. There was a trend towards increased SBDP 120 at 8 hours post treatment in the CPB group (p=0.06).
Figure 4.
Full-length αII-spectrin (280 kDa) and 150/145, and 120 kDa αII-SBDP in the CSF are 8 hours post injury (2H HCA) treatment (Fig. 2). Note the absence of the relevant bands in the baseline sample on the left.
Figure 5.
Changes in necrotic (SBDP 145+150) and apoptotic (SBDP 120) markers for animals receiving (A) 1 hour of HCA and (B) CPB alone, at 3 time points (pretreatment, 8 hours post treatment, and 24 hours post treatment), n=6 for CSF samples at 8 hours post treatment, and n=4 for CSF samples 24 hours post treatment. † value corresponds to results from RM ANOVA.
Comment
In this study we examined canine subjects who underwent either 1 hour HCA or CPB alone to identify the presence αII-SBDP’s in the CSF. Dogs undergoing shortened HCA had a significant increase in the level of the necrosis marker SBDP 145+150 when compared to baseline. In addition, the apoptotic marker SBPD 120 also increased from baseline although this result was not statistically significant.
Importantly, the presence of biomarkers were associated with subtle changes on both histologic and neurologic exam (when compared to dogs undergoing prolonged HCA for 2 hours). This result indicates that SBDP’s may be a useful indicator when neurological injury is mild or difficult to detect. Importantly, for animals undergoing CPB alone, there was no significant increase in either SBDP 145+150 or SBDP 120, indicating that the breakdown products appeared to be consistent with neurological dysfunction and not from the common insult of having been on CPB.
The fact that the necrosis marker increased in the 1 hour HCA group correlated well with the high levels of necrosis seen on histological examination at 8 hours in the 1 hour HCA dogs (5.5%). Although a surprising finding, given the high functional level of the animals, SBDP’s did correlate with histological evidence of injury. When SBDP levels at 24 hours post treatment were examined, the necrotic markers continued to rise while apoptotic markers plateaued. The significance of this trend is unclear, but may indicate a difference in time course of generation of the 145+150 fragment and 120 fragment of αII-spectrin.
We chose to use 1 hour of HCA in this model, because that is the approximate duration of HCA used clinically. Our goal was to create subtle neurological injury which would lend itself to the use of biomarkers for diagnosis. Historical 2 hour HCA controls were used to verify that this subtle injury pattern had been achieved. We are confident that 1 hour of HCA produces a more clinically relevant neurologic injury than the traditional model of severe injury produced with 2 hours of HCA. The group of dogs undergoing CPB alone were included both to serve as controls and to determine if SBDP’s would increase in the presence of CPB alone. Although definitive injury was not observed with CPB alone via either histology or clinical exam, it is noteworthy that there was a trend towards increasing apoptotic markers in dogs receiving CPB alone. This raises the possibility that CPB can lead to subtle neurologic injury in these animals. Additional work is needed however, to see if these changes in biomarkers translate into documented neurologic injury or changes in neurocognition.
αII-Spectrin Breakdown Products as Brain Injury Biomarkers
The use of αII-SBDP’s as biomarkers offers numerous advantages over existing biomarkers. Although αII-spectrin is not specific to the CNS, it is present in high levels in neurons. Furthermore, its presence in glial cells is minimal making it highly specific for neuronal damage. αII-spectrin is not found in erythrocytes. This important property makes blood contamination unlikely to affect results of sample collection (9). Importantly, studies of TBI have documented increases in calpain and caspase-3 proteases following traumatic brain injury translating into increases in αII-SBDP’s released in the CSF (8). These properties make αII-SBDP’s attractive candidates for biomarkers despite the relative lack of brain specificity. That there are two fragments, specific for necrosis (calpain mediated 145/150 kDa fragment) and apoptosis (caspase mediated 120 kDa fragment) further reinforces it’s potential utility. Necrosis and apoptosis represent the two main cell death pathways in the CNS. Different injuries confer distinct patterns of necrosis and apoptosis, and as a result, differential levels of the fragments may yield important insights into injury mechanism. Studies in humans have further shown temporal changes in calpain and caspase related fragments over time (16). These unique properties underscore the potential importance of αII-SBDP’s for monitoring of brain injury from a variety of etiologies including those that occur from CPB and HCA.
Prior work
A significant amount of basic research has now confirmed the importance of αII SBDP’s as potential biomarkers in brain injury (8, 9, 22). Specifically there have been in vitro neuronal cell culture models of mechanical stretch injury (23), as well as excitotoxicity (24) and glucose oxygen deprivation (25) which confirm the potential importance of these biomarker in human injury following cardiac surgery. In vivo work in mice has suggested that the presence of SBDP’s correlates with permanent neurodegeneration in a model of hippocampal traumatic injury (14).
Cardali and colleagues have recently demonstrated the presence of SBDP’s in the CSF of human trauma patients (15). In a case control study, patients with Glasgow coma scores of <8 were examined for the presence of both caspase and calpain derived αII-SBDP’s. Both were substantially higher in cases than controls and those patients with improved neurological scores 6 months after injury has lower levels of SBDP’s. These findings demonstrate that levels of αII-SBDP’s may correlate with long term prognosis after brain injury. A similar study was subsequently performed with Pineda and colleagues with similar results (16).
Further important work relating to cardiovascular surgery has been performed by Simon and colleagues at the University of Pennsylvania School of Medicine. Using an approach whereby they identified proteins released from cultured neurons (26, 27), the group identified several important proteins for neurological degeneration. Those specifically identified include several fragments from αII-spectrin cleaved by calpain, plus a deubiquitinating enzyme UCH-L1. The group recently published an important study demonstrating the up regulation of several of these SBDP’s and UCH-L1 in 19 surgical cases of aortic arch repair including 7 performed with HCA (28).
Taken together, the aforementioned results clearly point to a potential role for examination of αII-SBDP’s in the setting of neurological injury from HCA or CPB. Further work with these biomarkers will help to establish the time course and patterns of protein expression in cases of neurological injury following cardiac surgery.
Limitations
Our study is limited by small sample size and limited information on the temporal pattern of biomarker expression. In this initial study, our goal was to confirm the presence of SBDP in our established canine model, evaluate the effect of CPB, and focus on short term changes. We acknowledge that the current study provides little information on long term changes in this biomarker. The study is further limited by the use of CSF for detection. Clearly, cannulation of the spinal canal is not possible in the post cardiac surgery setting when patients are coagulopathic. In cases where preoperative spinal drains are place, CSF samples may be more readily obtainable. We are in the process of developing the ability to reliably measure SBDP’s in the serum. As of yet, we do not have such a method. The use of CSF however provides initial proof of concept that αII-SBDP’s are increased with HCA in our canine model. Our experimental design is further limited by a lack of animals receiving anesthesia alone. In this experiment all animals received inhalational anesthetic agent, and we do not know the effects of that treatment on spectrin breakdown product levels. Finally, no control group received CBP alone (without HCA) at very low temperatures (18°C) and we therefore do not know the incremental effect of arrest beyond hypothermia on brain injury in these animals.
Conclusions
Brain injury resulting from various etiologies is a significant international health concern. Unlike other organ-based diseases where biomarkers exist for diagnosis and guidance of treatment, no such definitive tests exist for brain injury. Our analysis demonstrates that Calpain and Caspase produced αII-SBDP’s represent an important and novel marker of neurologic injury following HCA which may be useful for identifying subtle injuries and guiding prognosis.
Acknowledgments
This study was supported by the Dana and Albert Broccoli Center for Aortic Diseases, the Mildred and Carmont Blitz Cardiac Research Fund, and the National Institutes of Health (NIH R37NS31238-10 WAB, and NIH 2T32DK007713-12, ESW). Dr. Weiss is the Irene Piccinini Investigator in Cardiac Surgery and Drs Allen and Nwakanma are Hugh R. Sharp Cardiac Surgery Research Fellows. The authors wish to thank Mr. Jeffrey Brawn, Mrs. Melissa Jones, and Ms. Tamara Treat, for their outstanding technical assistance. This project could not have been completed without their participation.
The authors would also like to acknowledge support of Department of Defense grants DAMMED-03-1-0066 and NIH grant R01 NS049175-01-A1. Drs. Kevin Wang and Ronald Hayes own stock, receive royalties from and are executive officers of Banyan Biomarkers Inc. as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
Abbreviations
- HCA
Hypothermic circulatory arrest
- αII-SBDP
Alpha II spectrin breakdown product
- NSE
neuron-specific enolase
- GFAP
glial fibrillary associated protein
- MBP
myelin basic protein
- CPB
Cardiopulmonary bypass
- CSF
Cerebral spinal fluid
- ANOVA
Analysis of variance
- ABG
Arterial blood gas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 55th annual meeting of the Southern Thoracic Surgical Association, November 5-8, 2008, Austin, TX
References
- 1.Redmond JM, Greene PS, Goldsborough MA, Cameron DE, Stuart RS, Sussman MS, et al. Neurologic injury in cardiac surgical patients with a history of stroke. The Annals of thoracic surgery. 1996 Jan;61(1):42–7. doi: 10.1016/0003-4975(95)00903-5. [DOI] [PubMed] [Google Scholar]
- 2.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. The New England journal of medicine. 1996 Dec 19;335(25):1857–63. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. The New England journal of medicine. 1995 Mar 2;332(9):549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 4.Hickey PR. Neurologic sequelae associated with deep hypothermic circulatory arrest. The Annals of thoracic surgery. 1998 Jun;65(6 Suppl):S65–9. doi: 10.1016/s0003-4975(98)00334-8. discussion S9-70, S4-6. [DOI] [PubMed] [Google Scholar]
- 5.Unden J, Astrand R, Waterloo K, Ingebrigtsen T, Bellner J, Reinstrup P, et al. Clinical significance of serum S100B levels in neurointensive care. Neurocritical care. 2007;6(2):94–9. doi: 10.1007/s12028-007-0005-0. [DOI] [PubMed] [Google Scholar]
- 6.Muley T, Ebert W, Stieber P, Raith H, Holdenrieder S, Nagel D, et al. Technical performance and diagnostic utility of the new Elecsys neuron-specific enolase enzyme immunoassay. Clin Chem Lab Med. 2003 Jan;41(1):95–103. doi: 10.1515/CCLM.2003.017. [DOI] [PubMed] [Google Scholar]
- 7.Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, et al. Identification of novel brain biomarkers. Clinical chemistry. 2006 Sep;52(9):1713–21. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 8.Pike BR, Flint J, Dave JR, Lu XC, Wang KK, Tortella FC, et al. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004 Jan;24(1):98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- 9.Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. Journal of neurochemistry. 2001 Sep;78(6):1297–306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain research bulletin. 1995;36(6):593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 11.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. The Journal of cell biology. 1986 Jun;102(6):2088–97. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, et al. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. The Journal of biological chemistry. 1998 Aug 28;273(35):22490–7. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- 13.Beer R, Franz G, Srinivasan A, Hayes RL, Pike BR, Newcomb JK, et al. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. Journal of neurochemistry. 2000 Sep;75(3):1264–73. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. Journal of neurotrauma. 2005 Feb;22(2):252–65. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- 15.Cardali S, Maugeri R. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. Journal of neurosurgical sciences. 2006 Jun;50(2):25–31. [PubMed] [Google Scholar]
- 16.Pineda JA, Lewis SB, Valadka AB, Papa L, Hannay HJ, Heaton SC, et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. Journal of neurotrauma. 2007 Feb;24(2):354–66. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 17.Barreiro CJ, Williams JA, Fitton TP, Lange MS, Blue ME, Kratz L, et al. Noninvasive assessment of brain injury in a canine model of hypothermic circulatory arrest using magnetic resonance spectroscopy. The Annals of thoracic surgery. 2006 May;81(5):1593–8. doi: 10.1016/j.athoracsur.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Redmond JM, Gillinov AM, Zehr KJ, Blue ME, Troncoso JC, Reitz BA, et al. Glutamate excitotoxicity: a mechanism of neurologic injury associated with hypothermic circulatory arrest. The Journal of thoracic and cardiovascular surgery. 1994 Mar;107(3):776–86. discussion 86-7. [PubMed] [Google Scholar]
- 19.Williams JA, Barreiro CJ, Nwakanma LU, Lange MS, Kratz LE, Blue ME, et al. Valproic acid prevents brain injury in a canine model of hypothermic circulatory arrest: a promising new approach to neuroprotection during cardiac surgery. The Annals of thoracic surgery. 2006 Jun;81(6):2235–41. doi: 10.1016/j.athoracsur.2005.12.060. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 20.Shake JG, Peck EA, Marban E, Gott VL, Johnston MV, Troncoso JC, et al. Pharmacologically induced preconditioning with diazoxide: a novel approach to brain protection. The Annals of thoracic surgery. 2001 Dec;72(6):1849–54. doi: 10.1016/s0003-4975(01)03192-7. [DOI] [PubMed] [Google Scholar]
- 21.Tisherman SA, Safar P, Radovsky A, Peitzman A, Marrone G, Kuboyama K, et al. Profound hypothermia (less than 10 degrees C) compared with deep hypothermia (15 degrees C) improves neurologic outcome in dogs after two hours’ circulatory arrest induced to enable resuscitative surgery. The Journal of trauma. 1991 Aug;31(8):1051–61. discussion 61-2. [PubMed] [Google Scholar]
- 22.Ringger NC, O’Steen BE, Brabham JG, Silver X, Pineda J, Wang KK, et al. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. Journal of neurotrauma. 2004 Oct;21(10):1443–56. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- 23.Pike BR, Zhao X, Newcomb JK, Glenn CC, Anderson DK, Hayes RL. Stretch injury causes calpain and caspase-3 activation and necrotic and apoptotic cell death in septo-hippocampal cell cultures. Journal of neurotrauma. 2000 Apr;17(4):283–98. doi: 10.1089/neu.2000.17.283. [DOI] [PubMed] [Google Scholar]
- 24.Dutta S, Chiu YC, Probert AW, Wang KK. Selective release of calpain produced alphalI-spectrin (alpha-fodrin) breakdown products by acute neuronal cell death. Biological chemistry. 2002 May;383(5):785–91. doi: 10.1515/BC.2002.082. [DOI] [PubMed] [Google Scholar]
- 25.Nath R, Probert A, Jr., McGinnis KM, Wang KK. Evidence for activation of caspase-3-like protease in excitotoxin- and hypoxia/hypoglycemia-injured neurons. Journal of neurochemistry. 1998 Jul;71(1):186–95. doi: 10.1046/j.1471-4159.1998.71010186.x. [DOI] [PubMed] [Google Scholar]
- 26.Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiology of disease. 2004 Jul;16(2):311–20. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Siman R, Zhang C, Roberts VL, Pitts-Kiefer A, Neumar RW. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels corrrelate with severity of ischemic neurodegeneration in the rat. J Cereb Blood Flow Metab. 2005 Nov;25(11):1433–44. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- 28.Siman R, Roberts VL, McNeil E, Dang A, Bavaria JE, Ramchandren S, et al. Biomarker evidence for mild central nervous system injury after surgically-induced circulation arrest. Brain research. 2008 Jun 5;1213:1–11. doi: 10.1016/j.brainres.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]