Abstract
The aim of this study was to examine prelinguistic vocal development in very young cochlear implant recipients. A prospective longitudinal research design was used to observe the sequence and time-course of vocal development in seven children who were implanted between 10 and 36 months of age. Speech samples were collected twice before implant activation and on a monthly basis thereafter for up to 2 years. Children’s vocalizations were classified according to the levels of the Stark Assessment of Early Vocal Development- Revised (SAEVD-R; Nathani, Ertmer, & Stark, in press). The main findings were (a) six of seven children made advancements in vocal development after implantation, (b) children implanted between 12 and 36 months progressed through SAEVD-R levels in the predicted sequence whereas a child implanted at a younger age showed a different sequence, (c) milestones in vocal development were often achieved with fewer months of hearing experience than observed in typically developing infants and appeared to be influenced by age at implantation, and (d) in general, children implanted at younger ages completed vocal development at younger chronological ages than those implanted later in life. Clinical indicators of benefit from implant use were also identified.
Keywords: COCHLEAR IMPLANTS, CHILDREN, VOCAL DEVELOPMENT, SPEECH PRODUCTION
Cochlear implants (CIs) have been shown to increase hearing sensitivity and improve auditory speech perception, speech production, and language ability in preschool and school-age children (e.g., ASHA, 2004; Blamey, Barry, & Jacq, 2001; Fryhauf-Bertschy, et al., 1997; Svirsky, Robbins, Kirk, Pisoni, & Miyamoto, 2000). Currently, however, little is known about the initial phases of speech development in very young implant recipients. Prelinguistic vocal development (hereafter referred to as “vocal development”) is a process by which infants and toddlers produce increasingly diverse and adult-like utterances before they say words on a regular basis. For very young CI recipients, advancements in vocal development are likely to be among the first observable indications of increased hearing sensitivity and improved speech perception ability. That is, barring motoric, cognitive, or persistent technological problems, infants and toddlers with CIs should make progress in vocal development before words dominate their spoken output.
Typical and Atypical Patterns of Vocal Development
The process of vocal development in typically developing infants and toddlers has been characterized as consisting of overlapping stages during which new vocalization types emerge and become common (See Vihman, 1996 for review). For example, Stark (1980) proposed a five level model in which infants progressed from Reflexive sounds (e.g., cry and discomfort sounds) to Cooing (i.e., the voluntary productions of comfort sounds in the velar area), to Vocal Play (i.e., sounds showing increased control of phonation and articulation), to Reduplicated Babbling (i.e., repeated production of canonical syllables such as [didididi]) to Single Words and Non-Reduplicated Babbling (i.e., productions of phonetically varied, adult-like syllables such as [dibudibu]). Advancements to higher levels of vocal development have been interpreted as signs of progress toward meaningful speech and phonological organization (Stoel-Gammon, 1998; Vihman, Ferguson, & Elbert, 1986). Stark’s model and those of other early researchers (e.g., Koopmans-van Beinum and van der Stelt, 1986; Oller, 1980; Roug, landberg, & Lundberg, 1989; Zlatin, 1975) provided a foundation for research in prelinguistic speech development. This work continues today in the infraphonological approach of Oller (2000), and efforts to refine Stark’s model (Nathani, Ertmer, & Stark, in press), and that of Koopmans-van Beinum and van der Stelt (Gillis, Schauwers, & Govaerts, 2002).
In comparison to typically developing infants and toddlers, those with bilateral, profound hearing losses who use hearing aids (HAs) show substantial delays and deficits in vocal development. Their development is characterized by late onset of and less-frequent production of canonical babbling (Oller & Eilers 1988; Stark, 1983), restricted formant frequency ranges in vowel-like vocalizations (Kent, Osberger, Netsell, & Hustedde, 1987), longer durations of final syllables (Nathani, Oller, & Cobo-Lewis, 2003), comparatively small consonant, vowel, and syllable shape inventories (Stoel-Gammon, 1988), and a lack of jargon and protowords (Stark, 1983). Such deficits are likely to interfere with the efficient acquisition of phonological abilities (Ertmer & Stark, 1995) and to contribute to the low speech intelligibility levels of deaf children.
Vocal Development in Young CI Recipients
Although interest in post-implantation vocal development is increasing, research in this area is currently limited to a few studies with small numbers of participants. One of the first case studies in this area was completed by McCaffrey, Davis, MacNeilage, and von Hapsburg (1999) who documented increased variety of vocalizations and phonetic inventory during the first 9 months of implant use for a child who was implanted at 25 months of age. A second case study (Ertmer & Mellon, 2001), reported that a child implanted at 19 months (“Hannah”) began to produce both canonical syllables and more advanced vocalization types during parent-child interactions within 5 months of implant activation.
More recently, Moore and Bass-Ringdahl (2002) measured the number of tokens of reflexive, precanonical, and canonical utterances, and words produced per minute during an elicitation task with nine children who received cochlear implants between 18 and 20 months of age. On average, the participants began to produce canonical babbling after 6.5 months of CI experience.
A sensory motor approach has also been applied to the study of babbling emergence after implantation. Defining babbling as: “phonation interrupted by two (or more) articulations” Gillis, et al., (2002, p.29), found that seven previously non-babbling children whose implants were activated between 6.4 and 20 months began to babble within 1.6 – 4.0 months afterward. A related study by Schauwers, Gillis, Daemers, De Beuklaer, and Govaerts (2004) found a highly similar time-course for the onset of babbling in children implanted between 6 and 18 months: 1 – 4 months after implant activation. Schauwers and collegues also noted that the four infants implanted before 10 months of age began to babble within the age-range when this behavior typically emerges in infants with normal hearing (6–12 months; see Vihman, 1996 for review).
Although there are differences in methodology and terminology across the cited studies, these findings show that improvements in hearing sensitivity and speech perception are often reflected in advancements in vocal development for young CI recipients. In addition, the amount of hearing experience required for the emergence of canonical productions, such as babbling, appears to be comparable or less than that observed for typically developing infants (Koopmans-van Beinum & van der Stelt, 1986; Oller, 1980; Roug, et al., 1989; Stark, 1980).
Whereas initial studies of post-implantation vocal development have yielded positive findings, there are several reasons to expect that some children might exhibit relatively slow or atypical patterns of vocal development after receiving CIs. First, it must be remembered that, even when using a well-mapped device, children with CIs do not have normal hearing sensitivity. In most cases, they have little or no usable hearing in the non-implanted ear, aided thresholds that are above the normal range in the implanted ear, and they perceive sound through an electronic rather than an acoustic signal. Thus, some children may take longer than normal to make speech gains if auditory input is substantially limited or distorted, or if they experience persistent technological problems. Second, the presence of physical or cognitive disabilities in addition to hearing loss may interfere with the development of speech perception and production skills (Ertmer, Leonard, & Pachuilo, 2002; Pisoni, 2000). It has been estimated that 26 – 30% of deaf children have multiple disabilities (SKI-HI Institute, 1994). Thus, speech development may be affected for a sizable proportion of implant recipients. Third, the quantity and quality of post-implantation intervention services and individual differences in attention and motivation for speech may also affect the rate of vocal development. Finally, the age at which children receive an implant may influence the vocal development process. The findings of Gillis et al., (2002) and Schauwers, et al., (2004) suggest that implantation before 12 months could lead to more normal patterns of vocal development. Further study is needed to determine whether the age at which infants and toddlers receive their implants affects the sequence, time-course, and completeness of prelinguistic speech development.
Theoretical Considerations
As Vihman notes, attempts to explain the underlying processes of vocal development in infants with normal hearing (NH) must consider the interactions between three main factors: “(1) the physiological constraints and perceptual biases of infants, (2) the phonetic profile or ‘affordances’ of the particular language of the child’s environment (‘the ambient language’), and (3) individual patterns of communicative and vocal effort, attention, and integration” (1996, p. 98). Studies of vocal development in young CI recipients can contribute to a better understanding of these factors and the capacity of the implant for overcoming the effects of auditory deprivation.
The physical constraints that Vihman (1996) mentions might influence the rate at which young CI recipients make advancements in vocal development. For example, children who are physically, cognitively, and motorically more mature (e.g., those implanted between 24 and 36 months) might make more rapid progress after implantation than those who are less mature (e.g., implanted before 12 months). If this is the case, it would appear that maturity has stronger influence on the rate of vocal development than amount of hearing experience. Conversely, younger implant recipients might make more rapid progress than older recipients because they begin to hear well-within the age range when vocal development normally takes place (see Vihman for review) and may have fewer maladaptive strategies for speech production to overcome (Higgins, Carney, McCleary, & Rogers, 1996). In this case, it would appear that the time-course of prelinguistic speech development is influenced by early access to auditory information to a greater extent than maturity. Careful study of the effects of age at implantation can help to determine the contributions of auditory experience and maturity to early speech development. Such investigations also afford a unique opportunity to explore the concept of sensitive periods for oral language acquisition (see Bruer, 1999 for review).
Similarly, studies of post-implantation vocal development can be useful in estimating the authenticity with which the electronic CI signal represents the phonetic and suprasegmental characteristics of the ambient language. Infants and toddlers who receive an authentic signal are likely to acquire vocalization patterns that mirror those of adults in their environment. Although failure to produce adult-like vocalizations might result from poor representation of speech features in the implant signal, it may also be due to persistent technical problems, learning difficulties, or a combination of conditions. Assessing the authenticity of the signal will require comprehensive testing of child and device.
Individual differences are often observed during the prelinguistic and early phonological stages of speech development. Typically developing toddlers have been shown to differ in the ages at which canonical babbling emerges (Oller, 1980; Stark, 1980), the amount of vocalizations produced, consonant place and manner features produced, and syllable shape preferences (Vihman, Ferguson, & Elbert, 1986). Comparable differences also appear in the early stages of word production (Stoel-Gammon & Cooper, 1984). Little is known about variability in the speech of very young cochlear implant recipients. Studies in this area could provide insights into the individual traits that allow children to attain adult-like speech competence. For example, differences in the rate at which vocal development milestones are attained might be associated with levels of effort, attention, and motivation (Vihman, 2000), as well as hearing, technological, and intervention factors, among others.
Finally, congenitally deaf children who receive CIs at a very young age provide unique opportunities to examine the role of audition in early speech development. Their pre-implantation samples may reveal speech behaviors that can be acquired with little or no access to auditory models and feedback. Conversely, post-implantation samples may reveal speech behaviors that emerge or become reduced following the introduction of auditory information. Such analyses may provide new insights into the relationship between speech perception and production.
Clinical Applications
Widespread adoption of Newborn Hearing Screening (NHS) in the United States has been accompanied by a pressing need for new ways to measure the speech perception abilities of infants and toddlers who receive hearing aids and cochlear implants (Eisenberg, Martinez, & Boothroyd, 2003). The development of effective tools for very young children is especially challenging because they typically lack the linguistic, social, and cognitive abilities needed to participate in structured listening tasks. Nonetheless, objective measures are essential for determining the adequacy of sensory aid functioning, for intervention planning, and for monitoring progress toward oral communication goals. Currently, the benefits of sensory aid use are most often measured through informal observations of responses to environmental sounds and speech, and through parent reports such as the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAS; Zimmerman-Phillips, Robbins, & Osberger, 1997). Measures of prelinguistic speech development can be useful supplements to these practices. That is, benefit from CI or HA use can be inferred as children’s vocalizations become increasingly diverse in phonetic content and syllable shape and more adult-like in timing and suprasegmental aspects.
Studies of vocal development may also contribute to efforts to identify an optimal age-range for cochlear implant surgery. Although phonological development continues well into childhood (see Vihman, 1996 for review), it seems likely that implant users who acquire age-appropriate skills at relatively young ages would have an advantage for phonological development over those who acquire such skills later in life. This likelihood is supported by data from Schauwers et al., (2004) that suggests that children implanted during the first year of life may be on a more normal trajectory for speech development. Clearly, further studies of communication development in young recipients, along with investigations of the incidence of age-related surgical complications and social and academic progress, are needed before an optimal range of ages for cochlear implantation can be recommended.
Finally, knowledge of the sequence and time-course of post-implantation vocal development can enable clinicians to select intervention targets that are within the child’s zone of proximal development (Vygotsky, 1978), thereby permitting scaffolding of the speech learning process (Ertmer, et al., 2002; Stark, 1989) before children begin to say words on a regular basis. In this way, clinicians can expand the diversity of vocalization types found at the child’s current level of development while stimulating developmentally appropriate targets that have yet to be produced.
A Scale for Assessing Vocal Development
The Stark Assessment of Early Vocal Development-Revised (SAEVD-R; Nathani, Ertmer, & Stark, in press), a hierarchical scale with five ascending levels, was used to assess vocal development in the current study. The SAEVD-R was developed by refining the vocalization types and operational definitions originally used by Stark (1980) and Oller (1980), incorporating aspects of more contemporary research (Mitchell & Kent, 1990), and integrating data from pilot studies (Ertmer & Mellon, 2001; Ertmer, Young et al., 2002; Nathani & Stark, 1996). The SAEVD-R can be used to classify three main types of vocalizations: vegetative sounds (e.g., coughs, burps,), fixed signals (e.g., cry and laughter), and protophones (i.e., vocalizations that are considered precursors to meaningful speech [Oller, 2000]). Because the former types of vocalizations are relatively unchanged across childhood, the present study analyzed the development of protophones only. This analysis replicated the methods used by Nathani, et al. (in press) to study typically developing children.
The first four levels of the SAEVD-R (see Table 1) closely correspond to those identified by Oller (1980; 2000), Koopmans-van Beinum and van der Stelt (1986), and Roug, Landberg, and Lundberg (1989) and those described in the original Stark model (1980). The SAEVD-R differs from earlier models mainly in the vocalization types found in the highest level: Advanced Forms. Advanced Form vocalizations are especially relevant to the current study because they are rarely observed in deaf infants (Stark, 1983; Stoel-Gammon 1988) and may, therefore, reflect improved audition more plainly than protophones from preceding levels.
Table 1.
Protophones identified by the Stark Assessment of Early Vocal Development-Revised (SAEVD-R). Full operational definitions for vocalization types can be found in Nathani et al., (in press).
| SAEVD-R Levels | Vocalization Types |
|---|---|
| 1. Reflexive | Single or multiple productions of Quasi-resonant nuclei 1 |
| 2. Control of Phonation | Single or multiple productions of Closants2, Closant-vocant3 productions, Fully-resonant nuclei4 |
| 3. Expansion | Single or multiple productions of Vocants, Vowel glides5, Ingressive sounds, Squeals, Marginal babbling6 |
| 4. Basic Canonical Syllables | Single CV syllables, Reduplicated and non-reduplicated syllable strings, Whispered vocalizations, Disyllables (CVCV), CV syllable followed by a consonant |
| 5. Advanced Forms | Diphthongs, Jargon7, Complex syllables (VC, VCV, CCV, VCVC, CCVC) |
brief, grunt-like sounds;
consonant-like productions such as clicks or “raspberries;”
vowel-like productions;
longer, grunt-like sounds;
vowels that gradually change in identity;
series of closants and vocants or series of vowel glides in which the transition between closant and vocant is prolonged;
utterances with more than two syllables and at least two different Cs and Vs and changing stress and/or intonation patterns.
The underlying assumption of the SAEVD-R is that relatively simple and non-adult-like productions will be replaced by more complex and adult-like vocalizations as infants and toddlers mature. Thus, profiles of vocal development are expected to show gradual reduction of vocalizations from SAEVD-R Levels 1 – 3 and increasing dominance of those from Levels 4 – 5. Table 1 provides a listing of the protophones found at each SAEVD-R level. A complete description of the SAEVD-R can be found in Nathani et al. (in press).
Nathani and colleagues (in press) used the SAEVD-R to track vocal development during a cross-sectional study of 30 typically developing infants and toddlers, ages 2 weeks – 20 months. Infants were observed at six age intervals: 0 weeks – 2 months, 3 – 5 months, 6 – 8 months, 9 – 12 months, 13 – 15 months, and 16 – 20 months. The main findings across children and intervals were that (a) the mean proportions of Level 1 vocalizations were greatest during the 0 – 2 month interval (33%) before decreasing to approximately 23% during the 3–5 and 6–8 month intervals, and to less than 10% in the remaining intervals, (b) the proportions of Level 2 vocalizations were relatively stable across the first three intervals (M = 26%) before decreasing gradually to less than 10% by the 16–20 month interval, (c) Level 3 vocalizations accounted for 35% of all vocalizations during the first interval and approximately half of all vocalizations from intervals between 3 and 15 months before decreasing to 28% during the final interval, (d) the mean proportion of Level 4 vocalizations was less than 10% for intervals before 9 months, increased to 18 and 20% of the sample during the 9 – 12 and 13–15 month intervals, and peaked at 35% during the 16 – 20 month interval, and (d) Level 5 vocalizations were not observed prior to 6 months of age and the mean proportion of these vocalizations increased gradually from 1% at 6 – 8 months to 8% at 11 – 15 months, before rising to 19% during the 16 – 20 month interval. These findings verify the sensitivity of the SAEVD-R to age-related changes in vocal productions.
The current study employed the SAEVD-R in a prospective longitudinal research design to examine the sequence and time-course of vocal development in seven deaf children who were implanted between 10 and 36 months. Two main questions were explored: (1) Do children implanted by 3;0 (years; months) progress through vocal development in the sequence predicted by the SAEVD-R? and (2) What are the time-courses for establishing adult-like vocalizations and for completing the process of vocal development? Because the participants received their CIs at different ages, the study also afforded an opportunity for a preliminary examination of the influence of age at implantation on vocal development. Findings were also analyzed for clinically useful indicators of benefit from CI use.
Methods
Participants
Four girls and three boys participated in the current study. Individual children will be referenced with a code that indicates their gender and their age at implantation. For example, M-36 received his CI at 36 months. F-30 received hers at 30 months. All children were accepted as appropriate candidates for cochlear implantation through auditory evoked response testing and/or pure tone audiometry at Children’s Memorial Hospital in Chicago, IL or Riley Children’s Hospital in Indianapolis, IN. All participated in hearing-aid trials prior to receiving CIs and received family-centered communication intervention services for 1 – 2 hours per week once their hearing losses were identified. The children ranged in age from 10 to 36 months at the time they received their multi-channel CIs. Table 2 contains audiological and CI information for each child.
Table 2.
Audiometric and cochlear implant information for each participant.
| Child (gender-age at implant in months) | Age Identified (Months) | Device (Processing Strategy) | Implant-aided Pure-tone Averages (all available frequencies; dB HL) | ||||
|---|---|---|---|---|---|---|---|
| Months Post-implantation | |||||||
| 3 | 6 | 12 | 18 | 24 | |||
| M-36 | 32 | Clarion Multi-strategy (CIS) | 27 | 32 | 22 | ||
| F-30 | 12 | Nucleus 24 (ACE) | 26 | 26 | |||
| F-28 | 23 | Clarion Multi-strategy (CIS) | 25 | 34 | 32 | ||
| M-24 | 16 | Clarion Multi-strategy (CIS) | 38 | 31 | 23 | ||
| F-20 | 10 | Clarion Multi-strategy (CIS) | 29 | 28 | |||
| F-18 | 15 | Nucleus 24 (ACE) | 39 | 39 | |||
| M-10 | 2 days | Clarion Multi-strategy (CIS) | 25 | 28 | 22 | ||
The children continued to receive communication intervention after implantation. Four children were enrolled in a full-day oral preschool shortly after their third birthdays (i.e., M-36, F-28, F-20, and M-24). F-30 received 2 hours of individual Auditory-Verbal therapy weekly before and after implantation. F-18 participated in twice weekly, 1 hour family-centered intervention sessions in a university-based program. The youngest recipient (M-10) received weekly family-centered intervention in the home throughout the course of the study. Four families had begun to use manual signs before the start of the study (M-36, F-28, M-24, and F-18); the remainder used oral communication only.
The first author met with each parent-child dyad prior to enrollment in the study and administered the Adaptive Behaviors and Motor Skills subtests of the Battelle Developmental Inventory (BDI; Newborg, et al., 1984) to assess development in areas not directly influenced by hearing status and oral communication ability. All children scored within or above two standard deviations of the mean for their ages on these subtests. The Symbolic Play Test (SPT; Lowe & Costello, 1988) was also administered to determine whether the nonverbal play skills were comparable to chronological ages. Only one child scored below age-level on the SPT: M-36 achieved a score of 25 months when he was 30 months old. The remaining SPT age scores ranged from 0 to 11.9 months above CA. M-10 did not complete the SPT due to scheduling conflicts. Mothers also completed a health and developmental history for their child prior to the start of the study. No additional learning or physical disabilities were reported for any of the children. The functioning of each child’s device was monitored through daily checks and parent and teacher observations as well as regularly scheduled mapping sessions at the implant center.
Two of the participants differed from the others in noteworthy ways. F-30 had the most residual hearing of any participant. Her average threshold for the frequencies .5, 1, 2, and 4 kHz was 92.5 dB HL during unaided sound-field testing, and 50 dB HL with hearing aids. Once implanted, F-30 used her CI alone for approximately 4 months before being fit with a digital hearing aid (HA) for her non-implanted ear. A comparison of F-30’s speech perception ability in the HA + CI condition vs. the CI-alone condition revealed highly similar scores during live voice, auditory-only administration of the Northwestern University Children’s Perception of Speech (NU-Chips; Elliott & Katz, 1980). After 18 months of CI experience (14 months of combined CI and HA use), F-30 identified 76% of stimulus words in the CI + HA condition, and 84% of stimulus words when using her CI alone. These scores were obtained after data for the current study were collected and suggest that her CI was mainly responsible for her speech perception ability. No other children wore HAs during the study.
M-24 showed limited improvement after implantation despite initial testing results and parent reports of typical development. He began to attend an oral preschool program at 3;0 but showed very little progress in developing communication skills during his first year of enrollment. A psychological evaluation was completed to determine the source of his learning difficulties. The report noted patterns of inconsistent attention and behavior, weakness in abstract reasoning and concept formation, and a tendency toward solitary and withdrawn behavior. His limited use and comprehension of manual signs (prior to enrollment in the oral preschool), also suggested the possibility of a language learning disability. As per the recommendations of the psychological evaluation, M-24 subsequently transferred to an educational program that incorporated the simultaneous use of manual signs and speech. All of his speech samples were recorded prior to transfer. M-24’s data are included in the current study to help identify speech behaviors that indicate adequate and inadequate development following CI activation, and to illustrate the impact of secondary learning difficulties on speech development following implantation.
Data Collection
Pre-implant Activation Sessions
Each child was seen for two, half-hour data collection sessions within 2 months before implant activation. With the exception of F4’s first session (recorded in a quiet room at a medical center) all of these sessions took place in the child’s home. During these sessions, mothers were instructed to play with their child in their “usual way” using an assortment of familiar toys. Audio-recordings were made by placing Optimus or Realistic boundary microphones within 3 feet of the child. The microphones were connected to either a preamplifier coupled with a Marantz audio cassette recorder (model PMD 221) or directly to a Sony Handicam 8mm camcorder (model CCD-TRV37). At the start of the study, speech samples were recorded using a pre-amplifier, an audio cassette recorder, and a camcorder. Later on, microphones were connected directly to camcorders so that data collection procedures could be simplified. No difference in the quality of the audio signal was noted because of this change. Video recordings were used to help reliably identify when vocalization occurred and for future analysis of parent-child interactions.
Post-implantation sessions
Audio and video recordings of parent-child interactions were made in the child’s home at monthly intervals beginning one month after implant activation until criteria for completion of vocal development (to be described below) were met. Procedures and equipment for these sessions were the same as those used prior to implantation.
Data Analysis
Audio recordings of the children’s utterances were analyzed in the following ways. A minimum of two judges viewed each half-hour audio-video recording and counted the number of utterances in each 10-minute segment. An utterance was operationally defined as a vocalization or group of vocalizations separated from all others by either audible ingressive breaths or perceived silence of 1 second or longer (Lynch, Oller, & Steffens, 1989). Both non-meaningful utterances and attempts to say words were included in the analysis as well as spontaneous and imitative productions. The vast majority of the utterances were judged to be non-meaningful (98.87%) and spontaneous (99.98%).
Utterances were selected for analysis using the following procedures. The first 65 utterances from the 10-minute segment of each session with the most utterances were digitized using a Kay Elemetrics Computerized Speech Laboratory (Model 4300) and a sampling rate of 20 kHz. This procedure was chosen to gather a representative sample of utterances during a period of time when the child was relatively vocal. Of these digital sound files, the first 50 consecutive utterances that were of adequate quality for auditory perceptual judgments (i.e., sufficient in loudness to be classified and without excessive talk-over or background noise), were classified using protophone definitions from the SAEVD-R (Nathani et al., in press).
Utterances were classified by five graduate students in Speech, Language, and Hearing Sciences at Purdue University. Each judge received training from the first author. Training consisted of an introduction to the operational definitions of the SAEVD-R along with multiple audio examples of each vocalization type. The judges-in-training were then required to identify vocalizations without knowledge of their previously determined types and classifications. They received feedback about their decisions and any error patterns or confusions were discussed. Training was discontinued when the judges were able to identify a full range of vocalizations with at least 80% accuracy. Judges were encouraged to discuss difficult-to-identify tokens with the first author before classifying them after this initial period of training was completed.
Vocalization types were identified primarily through auditory perceptual analysis with supplemental information from wide-band (300 Hz) spectrograms. That is, judges listened to the digitized sound files via headphones to form an initial impression of vocalization type. Spectrograms were consulted when visual information could help to clarify type of vocalization.
Utterances with multiple vocalizations were assigned the highest SAEVD-R level observed within the utterance so that the production of higher level vocalizations would not be obscured by the continued presence of lower-level vocalization types. For example, an utterance containing both a series of vowels (Level 3) and canonical babbling (Level 4) was assigned to level 4--the higher of the two corresponding levels. This procedure was also used by Nathani et al., (in press) to study typically developing children.
A SAEVD-R level was said to be established whenever it accounted for 20% or greater of all vocalizations during two consecutive monthly samples. For example, if Level 4 vocalizations accounted for 26 and 30% of all vocalizations at months 10 and 11, respectively, the child was credited with establishing this level at 10 months. The 20% criterion is based on that used by Oller and Eilers (1988) to determine entry into the Canonical Babbling stage of vocal development. Prior to implantation, children were credited with having established a SAEVD-R level if vocalizations from that level constituted at least 20% of the sample in at least one session. This lowered session criterion was used because fewer speech samples were collected during the pre-implant period. It should also be noted that the current study used more stringent criteria than the Nathani et al., (in press) study of typically developing children. The latter study employed a 10% criterion to indicate the emergence of SAEVD-R levels, and did not require that this criterion be met in consecutive sessions. More stringent criteria were used in the current study to ensure that advancements to higher SAEVD-R levels were stable.
Utterances from post-implantation sessions were analyzed until criteria for the completion of vocal development was met. These criteria were (1) precanonical vocalizations (i.e., those lacking consonants and vowels in combination with adult-like timing; Oller, & Lynch, 1992) constituted the least frequently produced type of vocalization observed in two consecutive sessions. That is, data were analyzed until the proportion of vocalizations from levels 1 – 3 combined was less than that of Level 4 and Level 5 separately, for two months in a row, and (2) the proportions of both Levels 4 and Level 5 were 20% or greater for two consecutive sessions. Meeting these criteria was taken to indicate that the child had completed the process of vocal development by establishing the dominant use of adult-like utterances. Thus, because different rates of progress were observed, the number of analyzed sessions varied across children. A minimum of six post-implantation sessions were analyzed for each child.
Reliability
A grand total of 5,700 utterances was classified during the study. Intra-judge reliability was assessed by having the original judge reclassify 12% of the tokens from each child’s sessions (684 utterances total) and then computing Cohen’s Kappa (Cohen, 1960) for agreement on decisions regarding level of development (i.e., SAEVD-R levels 1 – 5). Intra-judge Kappa coefficients ranged from .78 to .91 (M = .82) across individual children. Inter-judge reliability was assessed by having a second judge reclassify 20% of tokens from each recording session (1,140 utterances total). For this analysis, Kappa coefficients for agreement on SAEVD-R levels ranged from .57 – .84 (M = .73) across individual children. Kappa values of .60 – .75 have been characterized as good agreement and those greater than .75 as excellent agreement by Fleiss (1981).
Results
Figures 1 – 7 display the pre- and post-implantation proportions of vocalizations from SAEVD-R levels for each participant. In these profiles, non-adult-like (precanonical) vocalizations (Level 1 – Level 3) are represented by open diamonds, triangles, and squares, respectively, and adult-like vocalizations (Level 4 and Level 5) are shown with filled diamonds and squares, respectively. The following features of each profile are examined: (a) the proportions of vocalizations from SAEVD-R levels 2 – 5 before and after implant activation (Level 1 vocalizations are not included because they were rarely observed.), (b) the time-course for establishing adult-like utterances (Levels 4 and 5), and (c) the time-course for meeting criteria for completion of vocal development. To simplify discussion, proportions that comprise 60% or greater of each sample will be referred to as “very high,” those from 40 – 59% as “high,” those from 20 – 39% “moderate,” those from 10 – 19% as “low,” and those of ≤9% as “very low.” Profiles are ordered by age at implantation and a summary of findings can be found in Table 3.
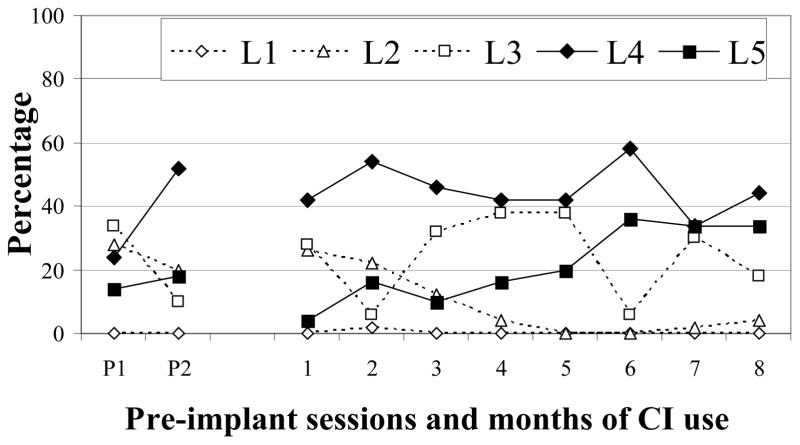
Figure 1.
Proportions of SAEVD-R levels for M-36.
Figure 7.
Proportions of SAEVD-R levels for M-10.
Table 3.
Summary of sequence (as predicted by SAEVD-R vs not predicted) of vocal development and time-courses for establishing adult-like vocalizations and meeting criteria for completion of vocal development.
| Sequence of Vocal Development | Months to Establish Level 4 | Months to Establish Level 5 | Months to Completion of Vocal Development | |
|---|---|---|---|---|
| M-36 | Predicted | Pre-implant | 5 | 7 |
| F-30 | Predicted | Pre-implant | 3 | 4 |
| F-28 | Predicted | 2 | 11 | 12 |
| M-24 | No advancements | Pre-implant | Not established | Not completed |
| F-20 | Predicted | 6 | 7 | 16 |
| F-18 | Predicted | 2 | 11 | 16 |
| M-10 | Not Predicted | 17 | 10 | 20 |
Individual profiles
M-36
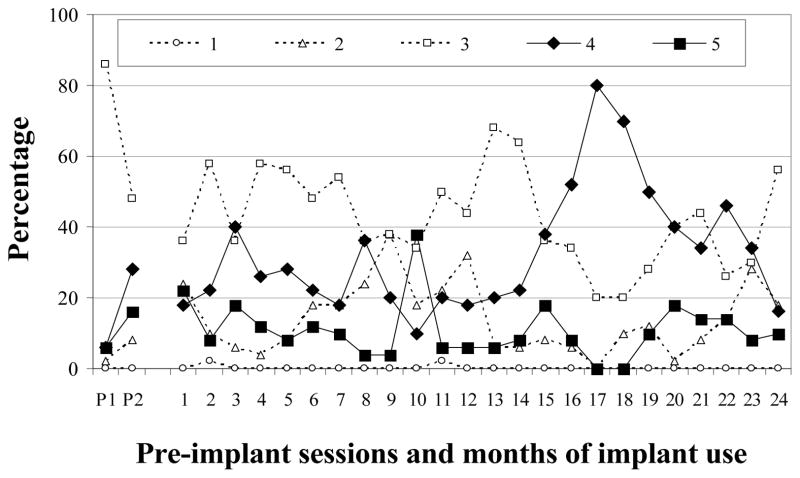
Almost a quarter of M-36’s pre-implantation vocalizations were from Level 2 (24%; Figure 1). Following device activation, the proportions of Level 2 vocalizations remained fairly steady for the first two months before decreasing to very low levels during the 4th month and thereafter. Vocalizations from Level 3 comprised 22% of pre-implantation samples and comparable amounts continued to be produced after CI activation. Vocalizations from Level 4 accounted for 38% of all pre-implantation utterances and continued to be produced in moderate to high proportions (34 – 58%) during all post-implantation months. Level 5 vocalizations accounted for 16% of pre-implantation samples. By post-implantation months 6 – 8, Level 5 had more than doubled from pre-implantation levels (M = 35%). Criteria for completion of vocal development were met in month 7. To summarize, M-36 significantly decreased Level 2 vocalizations within 4 months of implantation. Level 3 remained steady across both phases of the study. Level 4 was established before implantation and Level 5 after 5 months of CI use (see Table 3).
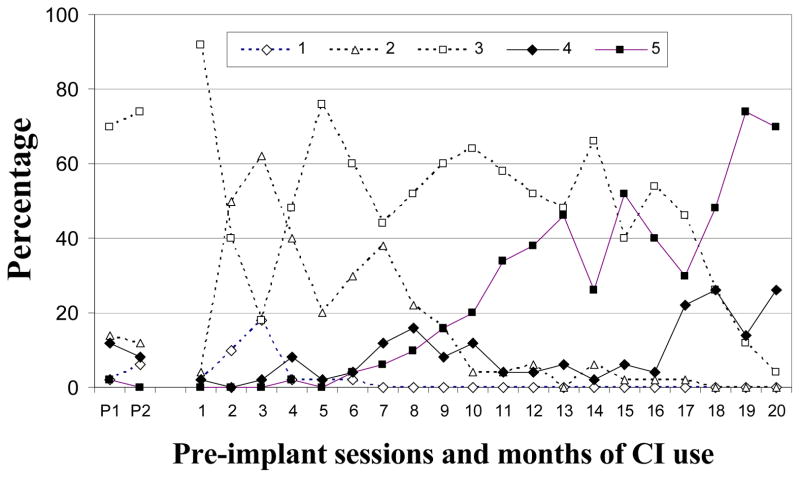
F-30
As figure 2 shows, relatively few Level 2 vocalizations were noted either prior to implantation (M = 6%) or in the months after F-30 was implanted (range: 0 – 12%). Very low proportions of Level 3 vocalizations were observed before implantation (M = 9%), with low to very low proportions across 6 months of CI use (range: 2 – 16%). In contrast, the majority of pre-implantation vocalizations were from Level 4 (M = 77%). Vocalizations from this level were subsequently produced in moderate to high amounts (range: 58–72%) during the first 5 months of CI use before decreasing to 46% in month 6. A different progression was seen for Level 5. These vocalizations were seldom produced prior to implantation (M = 8%) but reached moderate levels after 3 months, and high levels at 6 months. F-30 met criterion for completion of vocal development after 4 months of CI use. In summary, Levels 2 and 3 were rarely observed during the study. Level 4 vocalizations were dominant during both phases of the study. Level 5 was first established after 3 months of CI use (see Table 3).
Figure 2.
Proportions of SAEVD-R levels for F-30.
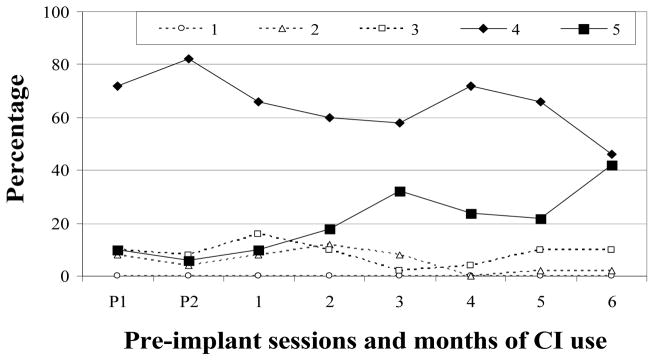
F-28
Level 2 accounted for more than half (59%) of pre-implantation vocalizations (Figure 3) before decreasing to low and very low levels within 2 months after F-28 received her CI. Vocalizations from Level 3 showed a much different pattern. They accounted for a comparatively smaller percentage of pre-implant tokens (M = 22%), and were observed in moderate and very high proportions during the first 10 months of CI use, before decreasing to low and very low levels during months 11–13. Level 4 vocalizations were very infrequent during the two pre-implant sessions (M = 4%). This level was first established 2 months after activation and, with the exception of month 8, accounted for moderate to very high proportions through the remainder of the study. Approximately 8% of F-28’s vocalizations from each pre-implant session were from Level 5. After activation, Level 5 vocalizations were produced in low or very low proportions until becoming established at 11 months. Criterion for completion of vocal development was achieved at 12 months. To summarize, Level 2 vocalizations were dominant prior to implantation but decreased to low and very low levels after implantation. Level 3 and Level 4 vocalizations comprised the majority of vocalizations during the first 10 months of CI use. Increases in Level 5 were accompanied by reductions in Level 3 during months 11 – 13 (see Table 3).
Figure 3.
Proportions of SAEVD-R levels for F-28.
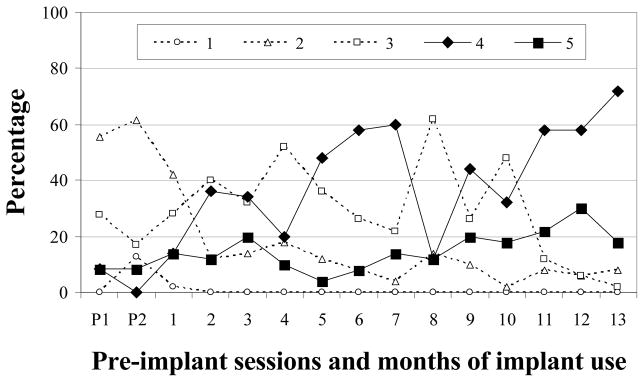
M-24
Level 2 vocalizations were produced in very low proportions prior to implantation but became more frequent in several months after M-24 received his device (Figure 4). Although very low or low proportions of Level 2 vocalizations were noted 18 times, M-24 also produced moderate amounts of Level 2 vocalizations during months 1, 8, 9, 11, 12, and 23. Thus, unlike the previously discussed children, a trend toward the reduction of Level 2 was not apparent. Level 3 vocalizations comprised the majority of M-24’s pre-implantation vocalizations (M = 67%) and continued to be produced in moderate to very high proportions throughout the study. M-24 had established Level 4 vocalizations prior to implantation (criteria met in second pre-implant session). Following implantation, however, considerable variability was noted, with the proportion of Level 4 productions ranging from low to very high. A substantial decrease in the production of Level 4 vocalizations was observed after month 17. M-24 did not establish level 5 or meet criterion for the completion of vocal development during the course of the study. In summary, Levels 3 and 4 comprised the majority of M-24’s vocalizations during both phases of the study. M-24 did not establish level 5 and did not show a consistent reduction in the production of Level 2 vocalizations (see Table 3).
Figure 4.
Proportions of SAEVD-R levels for M-24.
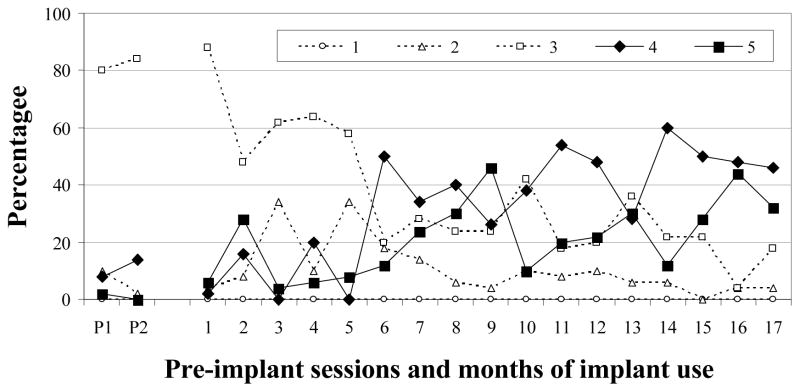
F-20
For F-20, Level 2 vocalizations were infrequent during both pre-implant sessions (M = 6%; Figure 5) but were produced in moderate amounts during months 3 and 5, before settling to low or very low levels during the remaining months. In contrast, an abundance of Level 3 vocalizations was noted during the pre-implant sessions (M = 82%) and for 5 months thereafter. Level 3 decreased to low and very low proportions during the last two months of the study, respectively. Level 4 was not established prior to implantation (M = 11%) and vacillated between 0 and 20% during the first 5 months of CI use. It was first established at 6 months, and moderate to high proportions were noted thereafter. Level 5 vocalizations were very seldom observed prior to implantation. Although a moderate amount (28%) was seen in month 2, Level 5 was first established in month 7. Thereafter, with the exceptions of months 10 and 14, Level 5 vocalizations were produced in moderate to high proportions. F-20 met the criterion for the completion of vocal development after 16 months of CI use. In summary, Level 2 vocalizations increased soon after implantation but decreased to very low levels after month 8. Level 3 was dominant prior to implantation and for the first 5 months of CI use. Decreases in Level 3 were accompanied by increases in Levels 4 and 5.
Figure 5.
Proportions of SAEVD-R levels for F-20.
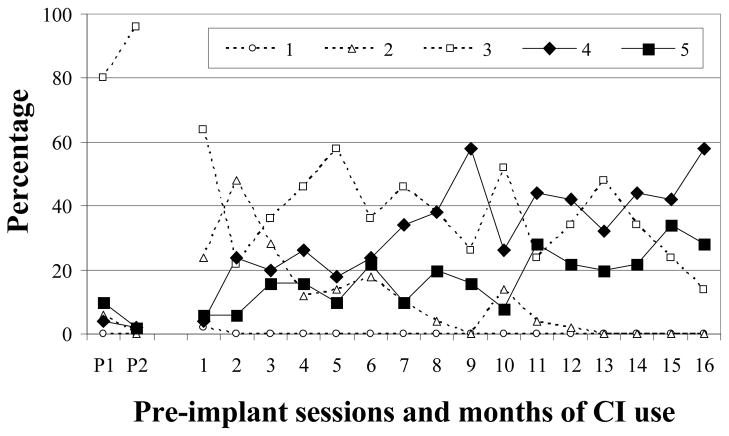
F-18
Level 2 vocalizations accounted for only 3% of F-18’s pre-implantation vocalizations (Figure 6). An increase in Level 2 vocalizations was apparent during the first 7 months of implant experience but (with the exception of month 10), few tokens were noted in the following months. As observed in the two previous children, Level 3 vocalizations were dominant prior to implantation, comprising 88% of F-18’s samples. Vocalizations from this level continued to be produced in moderate to high proportions for sessions 2 – 15 before decreasing to low levels during the last month. Level 4 accounted for only 3% of F-18’s pre-implantation utterances, became established at month 2, and continued to be produced in moderate to high amounts in all remaining months except month 5. A similar, but more gradual increase was noted for Level 5. Approximately 6% of pre-implant vocalizations were from this level and, with two exceptions (months 6 and 8), low and very low proportions of Level 5 vocalizations were noted for 10 months after activation. Level 5 became established at 11 months and moderate proportions were observed thereafter. This child completed vocal development after 16 months of CI experience. In summary, Level 2 vocalizations increased substantially during the first 6 months of CI use before becoming rare after month 10. Level 3 vocalizations were dominant before activation and continued to be plentiful for the first 13 months of the study. Levels 4 and 5 were dominant after 15 months of CI use.
Figure 6.
Proportions of SAEVD-R levels for F-18.
M-10
Low proportions of Level 2 vocalizations were observed during M-10’s pre-implantation sessions (M = 13%; see Figure 7). Level 2 increased substantially during the first 9 months of CI use before decreasing to very low levels (0 – 6%) during the last 10 months of the study. Level 3 vocalizations were dominant prior to implant activation, comprising 72% of all tokens. With the exception of month 3 (18%), moderate to very high proportions of Level 3 vocalizations were noted for the first 18 months of implant use. Level 3 vocalizations steadily decreased after month 17, reaching very low levels in month 20. Vocalizations from Level 4 comprised 10% of the two pre-implantation sessions. Level 4 vocalizations remained at very low or low levels during the first 16 months of implant use before exceeding 20% during 3 of the remaining 4 months. Level 5 vocalizations were rarely observed prior to implant activation (M = 1%), and continued to be rare for the first 5 months of implant experience. Steady increases were made during months 6 – 13, moderate to high proportions were noted during months 14 – 18, and very high proportions during months 19 and 20. Criteria for completion of vocal development were met after 20 months of CI use. In summary, Level 2 vocalizations increased substantially after activation before decreasing to very low levels after 10 months of CI use. Level 3 vocalizations were dominant prior to, and during the first year of CI use before becoming rare after month 19. Level 4 was established at 17 months, approximately 5 months after Level 5 (see Table 3).
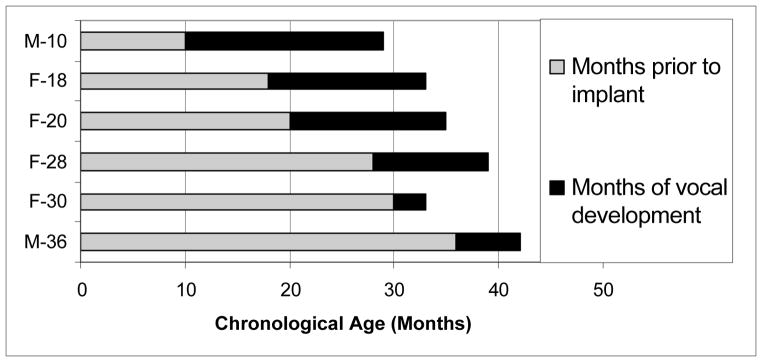
Time-course for the completion of Vocal Development
Progress toward higher SAEVD-R levels has been described in the previous section. Vocal development can also be considered in a more holistic way. Figure 8 illustrates the amount of time needed to reach criterion for completion of vocal development by the six children who established Levels 4 and 5 and reduced their production of vocalizations Levels 1–3 to minority status (all but M-24). These data show a trend toward progressively longer periods of vocal development for younger than older age at implantation children, with F-30 the only exception. They also show that, in most cases, younger CI recipients completed vocal development at younger ages than those implanted later in life. F-30 was the sole exception to the latter trend; completing the process at a younger age than F-28 and F-20.
Figure 8.
Length of deafness and amount time needed to reach criteria for completion of vocal development.
Discussion
Sequence of Vocal Development
Of the six children who made advancements in vocal development after implantation, five followed the hierarchical sequence predicted by the SAEVD-R. M-36 and F-30 established Advanced Forms (Level 5) after having reached the Basic Canonical Syllables level (Level 4) prior to implantation. F-28, F-20, and F-18 established Level 4 and then Level 5 after implant activation. In contrast, the youngest implant recipient, M-10, established Level 5 before Level 4. Although the reasons why he differed from older CI recipients are unclear, at least two possibilities merit consideration.
First, it is possible that the relatively stringent establishment criteria used in the present study masked advancements to Level 4 for the youngest CI recipient. In the Nathani et al. (in press) study, the highest SAEVD-R level that exceeded 10% was designated the child’s most advanced level for a given interval. Figure 7 shows that M-10’s Level 4 vocalizations first exceeded 10% during months 7 and 8. Thus, using this criterion, Level 4 became his most advanced level before Level 5. Applying the 10% criteria reveals that his early advancements in level followed the predicted sequence even though the order in which levels met criteria for establishment in the current study did not.
M-10’s profile might also represent an atypical sequence of development. None of the typically developing infants and toddlers studied by Nathani et al. (in press) established Level 5 before Level 4, and group means at each interval indicated that Level 4 vocalizations were consistently more plentiful than those of Level 5. Thus the findings of Nathani et al. (in press) support the hierarchy of the SAEVD-R scale. Mindful that the cross-sectional data from Nathani et al. (in press) and the longitudinal data of the current study cannot be compared directly, additional longitudinal studies of typically developing children are needed before the sequence followed by M-10 can be judged as typical, atypical, or an artifact of measurement criteria.
Time-course for Establishing Adult-like Vocalizations
Level 4: Basic Canonical Syllables
Four of the five children who had not established Level 4 at the start of the study did so within 17 months of implant activation. There were, however, large differences in the rate of progress (see Table 3). F-28, F-20, and F-18 established Level 4 relatively quickly: in 2, 6, and 2 months, respectively. The time-course for their progress was similar to that observed for “Hannah” a child implanted at 19 months (Ertmer & Mellon, 2001) and some of the children studied by Gillis et al., (2002). In contrast, M-10 required 17 months of hearing experience to establish Level 4. How do these data compare to typically developing infants and toddlers?
Nathani et al., (in press) found that Level 4 first accounted for ≥20% of all sampled vocalizations when infants were between 9 and 12 months old. Thus, F-28, F-20, and F-18 required comparatively fewer months of hearing experience to establish Level 4 than typically developing infants. In contrast, the youngest recipient, M-10, required 5 months of hearing experience beyond the typical range to establish Level 4. Taken at face value, his rate of progress seems to suggest that he had difficulty combining consonants and vowels. However, a closer look at the types of vocalizations that M-10 produced shows that the proportion of canonical syllables (including those from both Levels 4 & 5), first exceeded 20% at 8 months post-activation and was consistently above this level through the end of the study. Thus M-10’s profile does not indicate difficulty producing canonical syllables per se, as much as tendency to produce Advanced Forms more often than Basic Canonical Syllables. Additional research is needed to determine whether such tendencies are related to individual difference or a very young age at implantation.
Establishing Level 5: Advanced Forms
Six of seven children established Level 5 within 11 months after implant activation (see Table 3). The remaining child, M-24, did not establish this level during his first 24 months of CI use. The two oldest recipients (M-36 and F-30) established Level 5 in the least amount of time; 6 and 3 months, respectively. F-20 also reached this level relatively quickly (7 months). The remaining three children established Level 5 in 10 or 11 months. How do these data compare with typically developing children?
Nathani et al (in press) found that approximately 19% of the vocalizations from the typically developing toddlers ages 16 – 20 months (the oldest group studied) were from Level 5. Although this proportion is slightly less than the criteria for establishing a given SAEVD-R level used in the current study, it suggests that the participants in the current study established Level 5 in fewer months of hearing experience than typically developing infants and toddlers and supports the notion that CIs provide an authentic representation of ambient spoken language.
Time-course for Completion of Vocal Development
Three observations can be made regarding time-course for completion of vocal development (see Figure 8). First, in most cases, an older age at implantation was associated with a progressively shorter time-course for completion of vocal development. That is, children implanted at older ages tended to make more rapid progress than younger implant recipients. Second, younger implant recipients often completed the process of vocal development at younger chronological ages than children implanted later in life. Thus, even though the process took longer for younger age at implant participants, most finished it earlier in life than their older age at implantation counterparts. Third, F-30’s data suggest that children who have greater residual hearing prior to implantation (i.e., those with thresholds approximating 90 dB or better) and who have established Level 4 prior to implantation may show very rapid gains after device activation.
Compared to younger age recipients, the older recipients’ greater physical, cognitive, and social maturity, as well as their more extensive experience with speech intervention, appears to have been advantageous for early speech development. That is, older recipients may have been better able to use their improved hearing ability to modify their speech than younger recipients. However, as F-30’s performance points out, inter-subject differences such as amount of residual hearing, age at diagnosis, intelligence, device functioning, and amount of intervention might also have significant impacts on speech development. In addition, it is possible that some of the later-identified children might have had temporary access to sound before identification if their losses were progressive in nature. Large N studies that control for many variables must be completed before the effects of age at implantation can be ascertained for such a heterogeneous population.
Children implanted at younger ages generally completed the process of vocal development at younger chronological ages than those implanted later in life. This finding suggests that early implantation may lead to closer-to-typical trajectories for speech development and, ultimately, better speech intelligibility. It is, however, important to remember that all of the participants without secondary learning problems made substantial progress in vocal development irrespective of age at implantation. Thus, they appeared to receive their devices within a favorable or sensitive period for speech development.
Clinical Indicators of CI Benefit
Several indicators of benefit from CI use were identified. The most consistent of these were the decrease of Level 2 vocalizations to ≤20% by 10 months after implantation, and the establishment of Level 5 by month 11.
With the exception of M-24, each participant produced very few Level 2 vocalizations after 10 months of CI use. This reduction indicates that the non-adult-like sounds (e.g., clicks, lip smacks) and unidentifiable vowel-like productions (e.g., fully resonant nuclei) were minimized with continued hearing experience. Although M-24 produced moderate proportions of Level 2 vocalizations only 3 times after month 10, no other child showed a similar tendency. Moderate amounts of Level 2 vocalizations after 10 months of CI use may be a sign of delayed speech development.
The establishment of Level 5 appears to be a robust indicator of benefit from cochlear implant experience. Three findings support this assertion. First, none of the participants had established Advanced Forms prior to receiving an implant. This finding suggests that routine production of relatively complex vocalizations such as closed syllables and jargon requires access to auditory information; a contention supported by previous research with deaf children who used hearing aids (Stark, 1983; Stoel-Gammon, 1988). Thus, the establishment of Level 5 appears to be a sign of CI benefit. Second, six of seven children established the Level 5 within 11 months after implantation (all but M-24). The fact that this level was established in fewer months than observed for younger children with normal hearing (16 – 20 month interval; Nathani et al., in press) suggests that prelinguistic speech gains can be relatively rapid. Further study is needed, however, to determine whether a rapid pace continues during phonological development. Third, M-24’s failure to establish Level 5 appears to be related to his identified learning difficulties. Thus, the attainment of Level 5 may be influenced by cognitive and behavioral abilities during the early phases of speech and language development (Pisoni, 2000; Vihman, 2000).
As Oller and Eilers (1988) have reported, many deaf children who use hearing aids begin to produce canonical syllables during the second year of life. Our data reconfirmed this finding as three of four children implanted after their second birthdays had established Level 4 before receiving implants. For such children, progress in vocal development was most clearly evidenced by the establishment of Level 5, rather than the production of canonical syllables from Level 4.
The remaining clinical indicator must be considered according to two factors: the age at which an implant was received and whether Level 4 was established prior to implantation. Children who were implanted between 18 and 28 months and who had not established Level 4 prior to implantation usually reached this level quickly: between 2 and 6 months after activation. Thus, the establishment of Level 4 within 6 months of activation appears to be a reasonable expectation for children with similar characteristics. In contrast, the youngest CI recipient (M-10) required 17 months to establish Level 4. Although the difference between these groups suggests an age at implant effect, the fact that M-10 had established Level 5 before Level 4 might have contributed to apparent delays in reaching Level 4. Further study is needed to determine the relationship between age at implantation and the time-course for reaching the Basic Canonical Syllables level after implant activation.
Future directions
The findings of this study are congruent with previous studies of vocal development in typically developing children and young CI recipients. They must, however, be considered preliminary until larger investigations are completed. Such studies will need to account for technological (e.g., device characteristics, hearing aid use, and unilateral vs bilateral implants), social (e.g., socio-economic and family characteristics), intervention (e.g., type and frequency of services), and within-child (e.g., aided hearing levels, speech perception ability, and age at onset of hearing loss) factors if a clearer understanding of post-implantation vocal development is to be gained. In addition, additional aspects of early speech development (i.e., suprasegmental skills, increases in phonetic inventory size, and growth in spoken vocabulary) must be measured to gain a comprehensive picture of the initial phases of speech learning. Such a picture has the potential to advance theories of speech development and inform clinical practices for very young implant recipients as they approach the threshold of spoken language.
Acknowledgments
This research was supported by a grant from the National Institute on Deafness and other Communicative Disorders (1R03DC04226) awarded to the first author. We are especially grateful to the participating children and their parents for their dedicated support of this project. We are also indebted to Kristin Corbett, Kathy Saindon, Jennifer Mellon, and Mary Nallenweg for their assistance in data collection, and to Claire Johnson, Lynnette Strong, Lisa Lachowicz, Christine Miller, and Jennifer Quesenberry for their work in data management and analysis.
Contributor Information
David J. Ertmer, Speech, Language, and Hearing Sciences, Purdue University, West Lafayette, IN
Nancy M. Young, Otology/Neurotolgy and Pediatric Otolaryngology, Children’s Memorial Hospital, Chicago, IL
Suneeti Nathani, Communication Sciences and Special Education, The University of Georgia, Athens, GA.
References
- American Speech-Language and Hearing Association. Technical Report: Cochlear Implants. ASHA Supplement. 2004;24 [Google Scholar]
- Blamey P, Barry JG, Jacq P. Phonetic inventory development in young cochlear implant users. Journal of Speech Language & Hearing Research. 2001;44:73–79. doi: 10.1044/1092-4388(2001/007). [DOI] [PubMed] [Google Scholar]
- Bruer JT. The Myth of the First Three Years. New York: The Free Press; 1999. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Elliott L, Katz D. Development of a new children’s test of speech discrimination. St. Louis, MO: Audiotec; 1980. [Google Scholar]
- Ertmer DJ. Emergence of a vowel system in a young cochlear implant recipient. Journal of Speech-Language and Hearing Research. 2001;44:803–813. doi: 10.1044/1092-4388(2001/063). [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Leonard JS, Pachuilo ML. Communication intervention for children with cochlear implants: Two case studies. Language, Speech & Hearing Services in the Schools. 2002;33(3):205–217. doi: 10.1044/0161-1461(2002/018). [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Young N, Grohne K, Mellon J, Johnson C, Corbett K, Saindon K. Vocal development in young children with cochlear implants: Assessment and implications for intervention. Language, Speech, and Hearing Services in the Schools. 2002;33:185–196. doi: 10.1044/0161-1461(2002/016). [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Mellon JA. Beginning to talk at 20 months: Early vocal development in a young cochlear implant recipient. Journal of Speech-Language and Hearing Research. 2001;44:192–206. doi: 10.1044/1092-4388(2001/017). [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Stark RE. Eliciting prespeech vocalizations in a young child with profound hearing impairment: Usefulness of real-time spectrographic displays. American Journal of Speech-Language Pathology. 1995;4:33–38. [Google Scholar]
- Fleiss JS. Statistical methods for rates and proportions. New York: Wiley; 1981. [Google Scholar]
- Fryhauf-Bertschy H, Tyler R, Kelsay D, Gantz B, Woodworth G. Cochlear implant use by prelingually deafened children: The influence of age at implant and length of device use. Journal of Speech Language & Hearing Research. 1997;40:183–199. doi: 10.1044/jslhr.4001.183. [DOI] [PubMed] [Google Scholar]
- Gillis S, Schauwers K, Govaerts P. Babbling milestones and beyond. In: Schauwers K, Govaerts P, Gillis S, editors. Language Acquisition in Young Children with a Cochlear Implant. Antwerp: University of Antwerp; 2002. pp. 23–40. [Google Scholar]
- Higgins MB, Carney AE, McCleary E, Rogers S. Negative intraoral air pressures of deaf children with cochlear implants. Journal of Speech and Hearing Research. 1996;39:957–967. doi: 10.1044/jshr.3905.957. [DOI] [PubMed] [Google Scholar]
- Kent RD. Articulatory-acoustic perspectives on speech development. In: Stark RE, editor. Language Behavior in Infancy and Early Childhood. New York: Elsevier/North-Holland; 1981. pp. 105–126. [Google Scholar]
- Kent RD. Psychobiology of speech development: Coemergence of language and a movement system. American Journal of Physiology. 1984;246:R888–94. doi: 10.1152/ajpregu.1984.246.6.R888. [DOI] [PubMed] [Google Scholar]
- Kent RD, Osberger MJ, Netsell R, Hustedde lCG. Phonetic development in identical twins differing in auditory function. Journal of Speech and Hearing Disorders. 1987;52:64–75. doi: 10.1044/jshd.5201.64. [DOI] [PubMed] [Google Scholar]
- Koopmans-van Beinum FJ, Van der Stelt JM. Early stages in the development of speech movements. In: Lindbloom B, Zetterstrom R, editors. Precursors of Early Speech. Basingstoke, Hampshire: Macmillan Press; 1986. pp. 37–50. [Google Scholar]
- Lowe M, Costello AJ. The Symbolic Play Test. London: NFER-Nelson; 1988. [Google Scholar]
- Lynch MP, Oller DK, Steffens M. Development of adult-like vocalizations in a child with congenital absence of cochleas: The case of total deafness. Applied Psycholinguistics. 1989;10:315–333. [Google Scholar]
- McCaffrey HA, Davis BL, MacNeilage PF, von Hapsburg D. Multichannel cochlear implantation and the organization of early speech. Volta Review. 1999;101:5–28. [Google Scholar]
- Menyuk P, Menn L, Silber R. Early strategies for the perception and production of sounds. In: Fletcher P, Garman M, editors. Language Acquisition: Studies in First Language Development. 2. Cambridge: Cambridge University Press; 1986. pp. 49–70. [Google Scholar]
- Mitchell PR, Kent RD. Phonetic variation in multisyllabic babbling. Journal of Child Language. 1990;17:247–265. doi: 10.1017/s0305000900013751. [DOI] [PubMed] [Google Scholar]
- Moore JA, Bass-Ringdahl S. Infant vocal development in candidacy for and efficacy of cochlear implantation. Annals of Otology, Rhinology, and Laryngology. 2002;111:52–55. doi: 10.1177/00034894021110s511. [DOI] [PubMed] [Google Scholar]
- Nathani S, Ertmer DJ, Stark RE. Assessing vocal development in infants and toddlers. Clinical Linguistics and Phonetics. doi: 10.1080/02699200500211451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathani S, Oller DK, Cobo-Lewis AB. Final syllable lengthening (FSL) in infant vocalizations. Journal of Child Language. 2003;30:3–25. doi: 10.1017/s0305000902005433. [DOI] [PubMed] [Google Scholar]
- Newborg J, Stock JR, Wnek L. Battelle Developmental Inventory. 2. Itaska, IL: Riverside Publishing; 1984. [Google Scholar]
- Oller DK. The emergence of the sounds of speech in infancy. In: Yeni-Komshian G, Kavanaugh J, Ferguson C, editors. Child Phonology. New York: Academic Press; 1980. pp. 93–112. [Google Scholar]
- Oller KD. The Emergence of the Speech Capacity. Mahwah, NJ: Lawrence Earlbaum Associates; 2000. [Google Scholar]
- Oller DK, Eilers R. The role of audition in infant babbling. Child Development. 1988;59:441–449. [PubMed] [Google Scholar]
- Oller DK, Lynch MP. Infant vocalizations and innovations in infraphonology: Toward a broader theory of development and disorder. In: Ferguson CA, Menn L, Stoel-Gammon C, editors. Phonological Development. Timonium, MD: York Press; 1992. pp. 509–536. [Google Scholar]
- Pisoni DB. Cognitive factors and cochlear implants: Some thoughts on perception, learning, and memory in speech perception. Ear and Hearing. 2000;21:70–78. doi: 10.1097/00003446-200002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roug L, Landberg L, Lundberg LJ. Phonetic development in early infancy: A study of four Swedish children during the first eighteen months of life. Journal of Child Language. 1989;16:19–40. doi: 10.1017/s0305000900013416. [DOI] [PubMed] [Google Scholar]
- Schauwers K, Gillis S, Daemers K, de Beukelaer C, Govaerts PJ. Cochlear implantation between 5 and 20 months of age: The onset of babbling and the audiologic outcome. Otology and Neurotology. 2004;25:263–270. doi: 10.1097/00129492-200405000-00011. [DOI] [PubMed] [Google Scholar]
- SKI-HI Institute. SKI-HI 1992–1993 National Data Report. Logan, UT: Utah State University, SKI-HI Institute; 1994. [Google Scholar]
- Stark RE. Stages of speech development in the first year of life. In: Yeni-Komshian G, Kavanaugh J, Ferguson C, editors. Child Phonology. Vol. 1. New York: Academic Press; 1980. pp. 73–90. [Google Scholar]
- Stark RE. Phonatory development in young normally hearing and hearing-impaired children. In: Hochberg I, Levitt H, Osberger MJ, editors. Speech of the Hearing-Impaired: Research, Training, and Personnel Preparation. Baltimore: University Park; 1983. [Google Scholar]
- Stark RE. Infants and Young Children. 1. 1989. Early intervention: When why, how? pp. 44–53. [Google Scholar]
- Stoel-Gammon C. Prelinguistic vocalizations of hearing-impaired and normally hearing subjects: A comparison of consonantal inventories. Journal of Speech & Hearing Disorders. 1988;53:302–315. doi: 10.1044/jshd.5303.302. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon CS. Role of babbling and phonology in early linguistic development. In: Wetherby AM, Warren SF, Reichle J, editors. Transitions in Prelinguistic Communication. Vol. 7. Baltimore, MD: Brookes; 1998. pp. 87–110. [Google Scholar]
- Stoel-Gammon CS, Cooper JA. Patterns of early lexical and phonological development. Journal of Child Language. 1984;11:247–271. doi: 10.1017/s0305000900005766. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky LS. Mind in society: The development of higher psychological processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- Vihman MM. Later phonological development. In: Bernthal J, Bankson N, editors. Articulation and Phonological Disorders. 5. Boston: Allyn & Bacon; 2004. [Google Scholar]
- Vihman MM. Phonological Development: The Origins of Language in the Child. Cambridge, MA: Blackwell; 1996. [Google Scholar]
- Vihman MM, Ferguson CA, Elbert M. Phonological development from babbling to speech: Common tendencies and individual differences. Applied Psycholinguistics. 1986;7:3–40. [Google Scholar]
- Zimmerman-Phillips S, Osberger MJ, Robbins A. The Infant-Toddler Meaningful Auditory Information Scale. Sylmar, CA: Advanced Bionics, Inc; 1997. [Google Scholar]
- Zlatin M. Final Report, Project No 3-4014, NE-G-00-3-0077. National Institutes of Health; 1975. Preliminary descriptive model of infant vocalization during the first 24 weeks: Primitive syllabification and phonetic exploratory behavior. [Google Scholar]