Abstract
Objective
To investigate secretor gene fucosyltransferase2 (FUT2) polymorphism and secretor phenotype in relation to outcomes of prematurity.
Study design
Study infants were ≤32 weeks gestational age. Secretor genotype was determined from salivary DNA. Secretor phenotype was measured by H antigen, the carbohydrate produced by secretor gene enzymes, in saliva samples collected on day 9±5. The optimal predictive cut-point in salivary H values was identified by Classification and Regression Tree analysis. Study outcomes were death, necrotizing enterocolitis (NEC, Bell’s stage II/III), and confirmed sepsis.
Results
There were 410 study infants, 26 deaths, 30 cases of NEC, and 96 cases of sepsis. Analyzed by genotype, 13% of 95 non-secretors, 5% of 203 heterozygotes, and 2% of 96 infants who were secretor dominant died (p=0.01). Analyzed by phenotype, 15% of 135 infants with low secretor phenotype died, compared with 2% of 248 infants with high secretor phenotype (predictive value=76%, p<0.001). Low secretor phenotype was associated (P<.05) with NEC, and non-secretor genotype was associated (P=.05) with gram negative sepsis. Secretor status remained significant after controlling for multiple clinical factors.
Conclusions
Secretor genotype and phenotype may provide strong predictive biomarkers of adverse outcomes in premature infants.
In the United States, infants with very low birthweight (VLBW, ≤1500 g) who are ≤32 weeks gestational age account for only 2% of births but at least one-third of infant deaths.1 Approximately 15% of these infants die before they are discharged from the hospital.2 The most common causes of deaths among VLBW infants in the first postnatal week are respiratory complications and congenital malformations, and necrotizing enterocolitis (NEC) and infection are the most common causes of death after the first postnatal week. A study of 8608 infants with a birthweight ≤1000g found that prediction of mortality was 85% by using 15 prenatal and birth-related clinical variables. After the first postnatal week, however, mortality was more difficult to predict; twice as many clinical variables were needed to achieve 79% prediction.3 To date, neither severity scores, clinical judgment, nor potential biomarkers such as peripheral cytokines, have improved upon statistical models that include only clinical covariates to predict outcomes of prematurity.4–6 Novel biomarkers are needed to provide new insight into disease pathogenesis and better predict and prevent the adverse outcomes that too often occur in premature infants.2
Polymorphism in the secretor fucosyltransferase 2 (FUT2) gene and variable expression of H antigen, the carbohydrate produced by enzymes of the secretor gene, provide promising candidate biomarkers to predict outcomes of prematurity that may be related to infection or inflammation, including death, NEC, and sepsis. Individuals with an active FUT2 allele, known as secretors, richly express H antigen on mucosal surfaces, in saliva and other secretions, including fetal tissues and secretions.7–9 Due to a major polymorphism in the secretor gene, over 20% of individuals are non-secretors who do not express H antigen on mucosal surfaces, in saliva and other secretions. The differing carbohydrate phenotype of secretors and non-secretors modifies their susceptibility to various pathogens.10–16 In mouse pups, secretor gene expression increases with normal postnatal maturation and intestinal microbial colonization.17, 18 In premature infants, lack of maturity and aberrant microbial colonization contribute to adverse outcomes.19–21 We thus hypothesized that lack of early postnatal secretor gene expression may indicate poor prognosis, and examined early postnatal H antigen expression and secretor genotype as a predictive biomarker for risk of NEC, sepsis, and death in premature infants.
Methods
Ontogeny studies
We first examined the normal ontogeny of H antigen expression in two studies of infants with VLBS born ≤32 weeks gestational age (GA) who survived until discharge from hospital. In the first study, matched week 1 and 2 saliva samples were prospectively collected from 17 infants. The second study involved analysis of 28 banked tracheal aspirate samples previously collected from 10 infants between postnatal days 1 and 7, with closed suction as part of the care of infants with mechanical ventilation. H antigen was analyzed in these samples using methods described below. Institutional review board approval was obtained for each study.
Biomarker study
We then analyzed data and samples from a unique extant cohort study that was originally conducted to examine salivary epidermal growth factor in relation to NEC.21 Data and saliva samples were available for study from 430 infants with VLBW who were ≤32 weeks gestational age. Infants were enrolled from April 2000 through December 2004 at three hospitals in the Cincinnati region with level III neonatal intensive care units. All study infants were participants in the National Institute of Child Health and Human Development Neonatal Research Network (NRN) database. As part of the NRN system, infants were enrolled immediately after delivery and observed until discharge or 120 days postpartum, with extensive clinical data collected by research nurses (Table I). Saliva samples were collected between 5:00 and 10:00 a.m. before feeding. Sterile cotton-tipped swabs were placed in the infant’s mouth, saturated with saliva, and stored at −80 °C until tested.
Table 1.
Characteristics of the 410 very preterm study infants+
| Variable Description | Description | Value |
|---|---|---|
| Maternal and Prenatal Information | ||
| Prenatal steroids given (any) | No. (%) | 364 (89) |
| Non-Hispanic black | No. (%) | 117 (29) |
| Mother had hypertension/eclampsia | No. (%) | 100 (24) |
| Mother had prepartum hemorrhage | No. (%) | 75 (18) |
| Mother’s age (years) | Median (range) | 26 (14, 45) |
| Pregnancy history, gravida | Median (range) | 2 (1, 10) |
| Pregnancy history, parity | Median (range) | 2 (1, 10) |
| Prenatal care (≥ 1 prenatal care visit) | No. (%) | 395 (96) |
| Multiple birth | No. (%) | 127 (31) |
| Mother was married | No. (%) | 234 (57) |
| Labor and Delivery Information | ||
| Presence of labor | No. (%) | 222 (54) |
| Tocolytic agent used | No. (%) | 235 (57) |
| Infant birth weight (grams) | Median (range) | 1039 (424,1500) |
| Gestational age (weeks) | Median (range) | 28 (23,32) |
| Postnatal Infant Information | ||
| Infant antibiotic use ≥ 5 days | No. (%) | 151 (37) |
| 5-min Apgar score of <3 | No. (%) | 19 (5) |
| 5-min Apgar score of 3–6 | No. (%) | 76 (19) |
| Male | No. (%) | 183 (45) |
| Clinical features of respiratory distress syndrome | No. (%) | 366 (89) |
| Indomethacin given within 24 hours | No. (%) | 118 (29) |
| Number of days with conventional ventilation at day 7 | Median (range) | 1 (0,7) |
| Number of days with high-frequency ventilation at day 7 | Median (range) | 0 (0,7) |
| Breast milk given within in 24 hours prior to sample collection+ | No. (%) | 254 (70) |
| Hospital site | ||
| Hospital A NICU* | No. (%) | 273 (67) |
The clinical factors reported are those previously identified by Ambalavanan et al 3 as predictors of death that had complete data for analysis in this study. We also included history of feeding breast milk to the study infant within 24 hours prior to sample collection.
The hospital NICU site was included in the model as the largest hospital vs. the other two sites combined.
Salivary H antigen was measured in week 2 samples with enzyme immunoassay using a monoclonal antibody that detects H antigen (Accurate Chemical and Scientific Corp, Westbury, NY).9 Selection of week 2 sample for analysis of H antigen was based on the findings of the ontogeny studies and a methodological study of 190 infants, which indicated that week 2 values were more sensitive and specific than week 1 values for prediction of outcomes. To evaluate H antigen as a predictor, study outcomes (death, NEC and sepsis), were limited to those occurring in week 2 and later. NEC was defined using modified Bell’s stage II or III criteria.22 Sepsis cases were culture-confirmed. NEC deaths and sepsis deaths were defined as deaths that occurred in cases prior to discharge.
DNA was extracted from remaining banked samples using silica-coated magnetic beads (Promega Corp., Madison, WI). The DNA was washed twice and eluted. Secretor genotype was determined at a single null mutation FUT2 428G>A, Trp149 ->STOP, rs601338 by the TaqMan 5′ nuclease assay (Applied Biosystems, Foster City, CA) and the 7900HT Fast Real-Time PCR System. This single nucleotide polymorphism is the predominant coding non-synonymous FUT2 variant in the European and African-American populations that comprised our cohort. The phenotyping and genotyping laboratories were independent and blinded to infant history. Secretors were defined as having one or more active FUT2 alleles or detection of salivary H antigen.
Statistical methods
Salivary H optical density (O.D.) values were plotted and analyzed by Wilcoxon rank sum test to compare groups. The predictive cut-point in salivary H in relation to risk of death was determined by Classification and Regression Tree (CART) analysis (DTREG software version 4.5, Phillip H. Sherrod), an empirical method of recursive binary partitioning that identifies mutually exclusive risk groups using an impurity criterion to minimize the residual sum of squares. Outcomes were compared in risk groups with Kaplan-Meier curves and the Fisher’s exact test. Significance was set at p≤0.05. A comprehensive set of clinical predictors of mortality identified from an earlier national study3 (Table I) were entered with secretor status into multiple logistic regression models to control for confounding. The predictive value of the models was determined by area under the receiver operating characteristic (ROC) curve. The best model was determined by inclusion of all variables, then backward elimination of one variable at a time on the basis of the highest p-value, maintaining any with p-values ≤0.05. To clarify the role of secretor phenotype and genotype in relation to NEC and sepsis cases per se, we conducted a case-control analysis that calculated ORs and 95% CIs, to compare cases with control subjects defined as NEC-free survivors, and sepsis-free survivors, respectively, Analyses were conducted using SAS software (Cary, NC).
Results
Ontogeny studies
Our ontogeny studies found that that H antigen typically increased in the first week of life in saliva (paired t-test, p=0.022) and tracheal aspirates (generalized estimating equation analysis, p<0.001) of VLBW infants, confirming increasing postnatal expression of the secretor gene, as hypothesized.
Biomarker study
Of the 430 infants in the original study, 425 survived week 1. Of these, 410 infants were characterized for either secretor phenotype, genotype, or both, and were thus included for analysis: 383 (93%) had a week 2 (postnatal day 9 ± 5) saliva sample analyzed for H antigen, 394 (96%) had secretor genotype determined, and 367 (90%) were characterized both phenotypically and genotypically. Of the 410 study infants, 26 deaths, 30 NEC cases, and 96 sepsis cases occurred after postnatal week 1 and were analyzed as study outcomes.
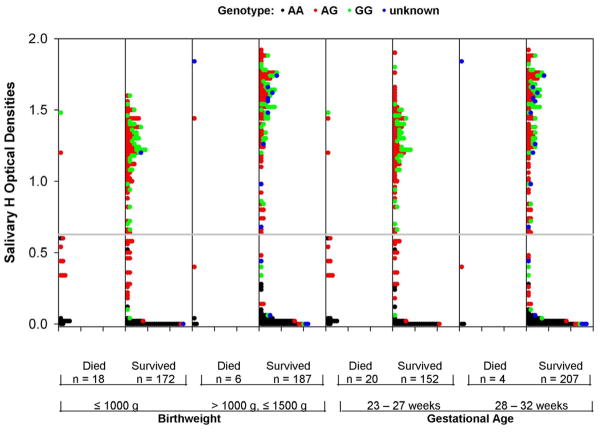
The clinical characteristics of study infants (Table I) were thoroughly evaluated. Maternal hypertension/eclampsia, hospital, birthweight and prenatal care were the only factors associated (p<0.05) with secretor status, but they did not confound the relationship between secretor status and study outcomes. Figure 1 indicates the salivary H distribution in all study infants by birthweight, gestational age and survival status. Salivary H values ranged from 0 to 1.9 O.D. units, with a bimodal distribution that reflected the null values of genetic non-secretors and a mode within the secretors. Salivary H values were significantly lower in those who died than in infants who survived (rank sum test, p<0.001). With CART identified a cut-point in salivary H antigen was identified at O.D. ≤0.627 that strongly differentiated risk of death; 135 (35%) infants had a low salivary H value, and 248 (65%) had a high salivary H value (O.D. >0.627). Bootstrap re-sampling found the cut-point to be precise and reliable. The cutoff point was confirmed with idenpendent receiver operating characteristics analysis.
Figure 1.
Dot plots of the salivary H optical density (O.D.) values in the 383 study infants with phenotype data by survivorship status, birthweight and gestational age. Genotype is indicated for each infant (black dots represent non-secretor (AA) genotype; red dots – heterozygote secretor (AG) genotype; green dots – homozygote secretor (GG) genotype; and blue dots – unknown genotype). The distribution of H is bimodal in survivors, reflecting the genetic non-secretor and secretor infants. The dotted line indicates the CART cut-point for optimizing differences between groups in death outcome. The number of infants with values above and below the cut-point is indicated for each sub-group. The H antigen O.D. values of non-secretors approach zero, consistent with expectation. The H antigen values of GG secretors is significantly higher (p<0.001, rank sum test) than AG secretors. The H antigen values are significantly lower in deaths than survivors (p<0.001). Differences in salivary H distribution between death and survivor groups are not due to confounding by gestational age or birthweight.
Secretor genotype was consistent with expected frequencies: 95 infants (24%) were non-secretors (AA); 203 infants (52%) were heterozygotes (AG); and 96 infants (24%) were secretor dominant (GG). Secretor genotype corresponded closely to the salivary H antigen values higher and lower than the CART cut-point. All genetic non-secretors (AA) were appropriately classified in the phenotype analysis as low H phenotype, and 93% of the genetic dominant secretors (GG) had a high H phenotype. Of the heterozygote secretors (AG), 17% had low salivary H values and 83% had high salivary H values, suggesting epigenetic determinants of salivary H antigen.
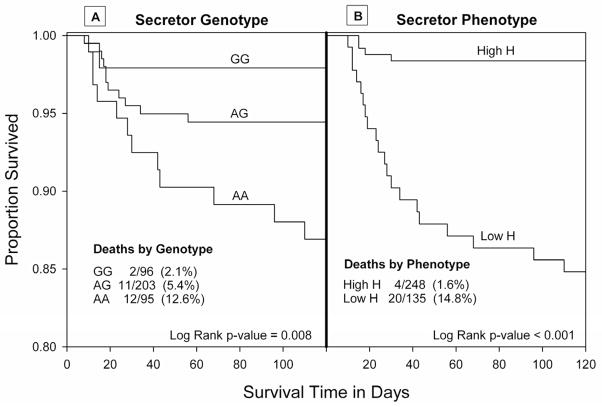
As shown by the Kaplan-Meier curves (Figure 2), mortality differed significantly by secretor genotype and phenotype. Twelve (13%) deaths occurred in the 95 genetic non-secretors, 11 (5%) deaths occurred in the 203 heterozygotes, and only two (2%) deaths occurred in the 96 homozygote secretors (panel A, log rank test, p=0.008). Similarly, 15% of infants in the low salivary H group died compared with only 2% in the high salivary H group (panel B, log rank test, p<0.001). Mortality in the non-secretor (AA) and secretor dominant (GG) genotypes resembled the low and high salivary H phenotypes, and the genetic heterozygotes (AG) had an intermediate level of risk explained by two distinct phenotypic subgroups. There were 32 heterozygotes with low H phenotype, 25% of whom died. In contrast, there were 157 heterozygotes with high H phenotype, only 1% of whom died (p<0.001). The survival curves differentiated with increasing postnatal age. After the first month of life, all deaths occurred in infants of low H phenotype. In the 26 infants who died, the median age at death was significantly later in the low salivary H group (26 days) compared with the high salivary H group (15 days, ranksum test, p=0.017).
Figure 2.
Kaplan-Meier survival curves: Secretor genotype (panel A) and secretor phenotype (panel B) are each significantly associated with risk of death. Non- secretor genotype (AA) was at high risk of death, dominant secretor (GG) was at low risk of death, and risk in heterozygotes (AG) was intermediate (panel A). Secretor phenotype was characterized as low salivary H (high risk of mortality) and high salivary H (low risk of mortality).
The primary causes of death in study infants were NEC (11 deaths) and sepsis or infection (10 deaths). Low salivary H phenotype was associated (Table II) with 10-fold increased odds of NEC deaths (p<0.001), 18-fold increased odds of sepsis deaths (p<0.001), 4-fold increase in surgical NEC (p=0.02), and 3-fold increase in NEC per se (p=0.02). Similar to low H phenotype, non-secretor genotype was associated with significantly increased odds of NEC deaths (OR, 4.1, 95% CI, 1.01-17.4; p= 0.0240) and sepsis deaths (OR, 13.4, 95% CI, 2.6-848.0; p<0.001). However, non-secretor genotype was not significantly associated with surgical NEC (OR, 2.3, 95% CI; 0.6-7.4) or NEC (OR, 1.6; 95% CI, 0.7-3.8). Further, no association was observed between secretor phenotype or genotype and risk of sepsis per se. Of the 96 sepsis cases, 18 were gram negative (Psedomonas aeruginosa, Klebsiella, Serratia, Enterobacter, and E coli species), 75 were gram positive (Staphylococcus, Group B Streptococcus, and Bacillus species), and 3 were due to candida species. Of the 18 gram negative cases, 44% were non-secretor genotype, while 23% of the 294 infants who survived to hospital discharge free of sepsis were non-secretor genotype (Fisher exact P = .05). Neither gram positive nor overall sepsis was associated with secretor status.
Table 2.
Case-control analysis of NEC and sepsis outcomes in relation to secretor phenotype
| Disease or control status | Secretor Phenotype | ||||
|---|---|---|---|---|---|
| Cases and controls | n* | No. Low H | No. High H | Odds Ratio (95% CI) | P value** |
| Necrotizing enterocolitis (NEC) | |||||
| NEC deaths | 11 | 9 | 2 | 9.6 (1.9, 92) | 0.001 |
| Surgical NEC | 14 | 9 | 5 | 3.8 (1.1, 15) | 0.018 |
| NEC cases | 27 | 15 | 12 | 2.7 (1.1, 6.4) | 0.019 |
| Controls (NEC-free survivors) | 363 | 112 | 251 | 1.0 | |
| Sepsis | |||||
| Sepsis deaths | 10 | 9 | 1 | 17.9 (2.4, 789) | <0.001 |
| Sepsis cases | 91 | 31 | 60 | 1.03 (0.6, 1.7) | 1.00 |
| Controls (Sepsis-free survivors) | 272 | 91 | 181 | 1.0 | |
Salivary H phenotype was not determined for 27 infants missing a week 2 saliva sample, including 5 sepsis cases and 3 NEC cases.
The p-value was determined by 2-sided Fisher’s exact test comparing cases to controls.
Predictive modeling
Low versus high salivary H was a better predictor of death than the salivary H antigen OD value or secretor genotype (area under the ROC=76% vs 70% vs 66%, respectively). Multiple logistic regression models of death first included low salivary H in relation to all clinical variables in table 1; low salivary H remained independently associated with death (p<0.001). A sensitivity analysis was conducted to evaluate the impact of missing salivary phenotype data for 27 infants. Using genotype to impute missing salivary phenotype, the three genetic non-secretors were classified as low H and the 24 genetic secretors were classified as high H. Whether infants with imputed values were included or excluded form analysis, the results were similar, with no change in significance or interpretation. Breast milk feeding in week 2 was not a confounder, effect modifier, or significant independent risk factor, though there was a trend consistent with breast milk protection (OR=0.4, p=0.08). The final model thus included all subjects and five significant predictors of death: low salivary H, mother’s marital status, infant birthweight, number of days of conventional ventilation at day 7, and high-frequency ventilation at day 7 (area under the ROC of the model =88%, indicating very good predictive value). Similarly, secretor genotype remained significant in multivariable models and contributed independently to predictive value.
Discussion
Secretor (H) antigen increased significantly in the saliva and tracheal aspirate samples of premature neonates in the first weeks of life, indicating increased expression of the secretor gene. Consistent with our hypothesis, low or non-secretor status strongly predicted risk of adverse outcomes that occurred after the first postnatal week, including overall mortality and death in sepsis and NEC cases. Non-secretor genotype was associated with gram negative sepsis, but not overall sepsis. Low H phenotype was also associated with 4-fold increased odds of surgical NEC and 3-fold increased odds of NEC per se. Low H phenotype predicted death whether analyzed as the H antigen O.D. value or as a dichotomous variable generated with CART or receiver operating characteristic analysis, particularly deaths occurring at older postnatal ages. The mortality rate in heterozygote secretors with low H phenotype was similar to the genetic non-secretors, and mortality in heterozygotes with high H phenotype was similar to the dominant secretors. The results are unlikely by bias, because several laboratories were involved, blinded to infant clinical status. Clinical data collection followed a nationally standardized protocol and preceded the laboratory analyses. The results are also unlikely caused by chance: The outcomes were robustly associated with secretor status, significant associations persisted in multivariable models, and risk of mortality increased in a dose-dependent fashion across the non-secretor, heterozygote, and secretor dominant groups.
The secretor gene appears important in early ontogeny. The secretor gene is known to be expressed in fetal epithelial tissues and saliva.9 Uncolonized gut contains limited amounts of H antigen. During the first postnatal weeks, concurrent with bacterial colonization, the intestinal epithelium shows a pronounced increase in fut2-mRNA and fucosyltransferase activity that can be ablated with intensive antimicrobial administration.17, 18 Amniotic fluid and human milk contain secretor antigens that bathe the fetal and neonatal mucosa.23, 24 In this study, we found that secretor (H) antigen increases postnatally in the saliva and tracheal aspirates of infants born live. Although the mechanisms explaining absent or low H antigen as a biomarker of adverse outcomes in prematurity remains to be determined, potential explanations include correlation with mucosal immaturity or aberrant colonization.
The immature immune system of premature infants increases susceptibility to pathogens that can cause NEC or sepsis.25, 26 Individual risk of infection may vary with the availability of preferred antigen for pathogen attachment to mucosal surfaces. Most noroviruses, many E. coli, campylobacter and Streptococcus pneumoniae prefer binding to secretor antigen and thus, may infect secretors more frequently.11–16, 23 However, other pathogens preferentially bind to non-secretor antigens.7, 10, 12 A potential explanation for the findings of our study is increased virulence of pathogens causing NEC or sepsis in non- or low-secretors. However, sepsis case fatality was higher in non- and low-secretors regardless of whether the organism causing sepsis was gram negative or gram positive.
Alternatively, low expression of secretor antigen may contribute to the hyper-inflammatory response that is the hallmark of preterm infants. Non-secretors are reported to have a greater inflammatory response to infection with H. pylori and renal scarring in acute pyelonephritis compared with secretors.27, 28 Although functional studies of fucosylation are lacking, the regulation of inflammation may be impaired in premature infants in the absence of secretor antigen. Acute phase response results in defucosylation of plasma proteins, including alpha-1 acid glycoprotein, which is involved in regulation of inflammation.29, 30 In the third trimester, the alpha-1 acid glycoprotein in amniotic fluid is highly fucosylated, and contains substantial secretor antigen.24
Secretor phenotype and genotype provide powerful information that cannot be inferred through clinical factors and may help target more effective surveillance and interventions. Measuring these biomarkers could provide a novel approach to prediction of NEC and sepsis outcomes. While the explanation of our findings remains to be determined, a clue may have been provided by the recent report that non-secretor status is associated with Crohn’s Disease, a condition thought to result from an abnormal immune response to intestinal microflora. We thus speculate that non-secretor status may contribute to an aberrant colonization and immune response in some adverse circumstances, such as premature birth. In this initial study, we used extant data and samples. A prospective study has been initiated to confirm these novel biomarkers in relation to neonatal outcomes. Increased understanding of the impact of the secretor gene in prematurity should provide important insights into the pathogenesis of disease progression and infant vulnerability. Greater attention to glycosylation in innate immunity is warranted.
Acknowledgments
Supported in part by grants from the National Institute of Child Health and Human Development (P01 HD 13021, R01 HD 059140, U10 HD 027853), the National Institute of Diabetes and Digestive and Kidney Diseases (R03 DK61596 and P30 DK078392), and the Translational Research Initiative of Cincinnati Children’s Research Foundation.
We gratefully acknowledge the research team responsible for the original sample and data collection and the nursing staff at Good Samaritan Hospital, Cincinnati Children’s Hospital Medical Center, and University Hospital for assistance in specimen procurement; Dr. Xiujing Sun for preliminary phenotyping of this cohort; Ms. Patricia Herbers for biostatistical support; and Ms. Donna Wuest for manuscript preparation. We also thank Drs. Thomas Boat, Guillermo Ruiz-Palacios, Jeffrey Whitsett, and James Greenberg for their thoughtful review of this manuscript.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Carlo WA, Bobashev G, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–1373. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Carlo WA, D’Angio CT, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain F, Gilshenan K, Gray PH. Does lactate level in the first 12 hours of life predict mortality in extremely premature infants? J Paediatr Child Health. 2009;45(5):263–267. doi: 10.1111/j.1440-1754.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 6.Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for Infants at Risk for Necrotizing Enterocolitis: Clues to Prevention? Pediatr Res. 2009 doi: 10.1203/PDR.0b013e31819dba7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer-Admetlla A, Sikora M, Laayouni H, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26(9):1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 8.Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108(1):1–28. doi: 10.1034/j.1600-0463.2000.d01-1.x. [DOI] [PubMed] [Google Scholar]
- 9.Szulman AE, Marcus DM. The histologic distribution of the blood group substances in man as disclosed by immunofluorescence. VI. The Le and Le antigens during fetal development. Lab Invest. 1973;28(5):565–574. [PubMed] [Google Scholar]
- 10.Ahmed T, Lundgren A, Arifuzzaman M, Qadri F, Teneberg S, Svennerholm AM. Children with Lewis a+b- blood group have increased susceptibility to diarrhea caused by enterotoxigenic Escherichia coli expressing colonization factor I-group fimbriae. Infect Immun. 2009 doi: 10.1128/IAI.01571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang P, Farkas T, Zhong W, et al. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79(11):6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Pendu J, Ruvoen-Clouet N, Kindberg E, Svensson L. Mendelian resistance to human norovirus infections. Semin Immunol. 2006;18(6):375–386. doi: 10.1016/j.smim.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linden S, Mahdavi J, Semino-Mora C, et al. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 2008;4(1):e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran AP. Relevance of fucosylation and Lewis antigen expression in the bacterial gastroduodenal pathogen Helicobacter pylori. Carbohydr Res. 2008;343(12):1952–1965. doi: 10.1016/j.carres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278(16):14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 16.Higgins MA, Abbott DW, Boulanger MJ, Boraston AB. Blood Group Antigen Recognition by a Solute-Binding Protein from a Serotype 3 Strain of Streptococcus pneumoniae. J Mol Biol. 2009 doi: 10.1016/j.jmb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng D, Newburg DS, Young C, et al. Bacterial symbionts induce a FUT2-dependent fucosylated niche on colonic epithelium via ERK and JNK signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G780–87. doi: 10.1152/ajpgi.00010.2007. [DOI] [PubMed] [Google Scholar]
- 18.Nanthakumar NN, Dai D, Newburg DS, Walker WA. The role of indigenous microflora in the development of murine intestinal fucosyl– and sialyltransferases. FASEB J. 2003;17(1):44–46. doi: 10.1096/fj.02-0031fje. [DOI] [PubMed] [Google Scholar]
- 19.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 125(4):777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 20.van Saene HK, Taylor N, Donnell SC, et al. Gut overgrowth with abnormal flora: the missing link in parenteral nutrition-related sepsis in surgical neonates. Eur J Clin Nutr. 2003;57(4):548–553. doi: 10.1038/sj.ejcn.1601578. [DOI] [PubMed] [Google Scholar]
- 21.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007;150(4):358–363. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr. 2005;135(5):1304–1307. doi: 10.1093/jn/135.5.1304. [DOI] [PubMed] [Google Scholar]
- 24.Orczyk-Pawilowicz M, Hirnle L, Katnik-Prastowska I. The expression of fucose isoforms of amniotic and plasma alpha-1-acid glycoprotein derived from 2nd and 3rd trimester normal pregnancies. Clin Biochem. 2009 doi: 10.1016/j.clinbiochem.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11(5):369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Turcios-Ruiz RM, Axelrod P, St John K, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008;153(3):339–344. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomberg H, Hellstrom M, Jodal U, Svanborg Eden C. Secretor state and renal scarring in girls with recurrent pyelonephritis. FEMS Microbiol Immunol. 1989;1(6–7):371–375. doi: 10.1111/j.1574-6968.1989.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 28.Anderson N, Pollacchi A, Hayes P, et al. A preliminary evaluation of the differences in the glycosylation of alpha-1-acid glycoprotein between individual liver diseases. Biomed Chromatogr. 2002;16(6):365–372. doi: 10.1002/bmc.167. [DOI] [PubMed] [Google Scholar]
- 29.Chavan MM, Kawle PD, Mehta NG. Increased sialylation and defucosylation of plasma proteins are early events in the acute phase response. Glycobiology. 2005;15(9):838–848. doi: 10.1093/glycob/cwi067. [DOI] [PubMed] [Google Scholar]
- 30.McGovern DP, Jones MR, Taylor KD, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum Mol Genet. 2010 Sep 1;19(17):3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]