Abstract
Closed-loop deep brain stimulation (DBS) systems offer promise in relieving the clinical burden of stimulus parameter selection and improving treatment outcomes. In such a system, a feedback signal is used to adjust automatically stimulation parameters and optimize the efficacy of stimulation. We explored the feasibility of recording electrically evoked compound action potentials (ECAPs) during DBS for use as a feedback control signal. A novel instrumentation system was developed to suppress the stimulus artifact and amplify the small magnitude, short latency ECAP response during DBS with clinically relevant parameters. In vitro testing demonstrated the capabilities to increase the gain by a factor of 1,000x over a conventional amplifier without saturation, reduce distortion of mock ECAP signals, and make high fidelity recordings of mock ECAPs at latencies of only 0.5 ms following DBS pulses of 50 to 100 μs duration. Subsequently, the instrumentation was used to make in vivo recordings of ECAPs during thalamic DBS in cats, without contamination by the stimulus artifact. The signal characteristics were similar across three experiments, suggesting common neural activation patterns. The ECAP recordings enabled with this novel instrumentation may provide insight into the type and spatial extent of neural elements activated during DBS, and could serve as feedback control signals for closed-loop systems.
I. Introduction
Deep brain stimulation (DBS) is a surgical intervention that is effective in treating movement disorders, including essential tremor and Parkinson’s disease.[1],[2] DBS devices currently operate in an open-loop fashion, in which a clinician programs the stimulation parameters and the patient receives invariant stimulation, with periodic re-tuning of parameters as necessary. However, the mechanisms of action of DBS remain unclear [3], and there are a lack of data describing the relationships between stimulation parameters and clinical outcomes.[4] Consequently, the selection of stimulation parameters is an ad hoc, empirical process that requires a great deal of clinical expertise and often results in sub-optimal outcomes. Furthermore, the need for repeated programming sessions is both inconvenient and costly.[5] The prevailing programming methodology, which entails simply increasing stimulation amplitude and frequency if symptom suppression is not satisfactory, can produce side effects, exacerbate symptoms, and cause rapid depletion of device batteries.[6],[7]
A closed-loop DBS system would provide automated tuning of stimulation parameters to respond to patient needs. This approach would improve outcomes as the disease progresses or as the response to DBS changes over time, and could reduce the need for frequent follow-up visits.[8] Recordings of neural activity, obtained from the same electrodes used to deliver stimulation, could serve as the feedback control signal for closed-loop DBS. Previous studies have investigated the use of local field potentials (LFPs), reflecting synchronized neural activity in the recorded brain area, as a feedback signal.[9] However, a direct causal link between LFPs recorded during DBS and the corresponding motor symptoms remains to be demonstrated.[10]
A potential alternative feedback signal is the electrically evoked compound action potential (ECAP). The ECAP signal results from activation of an ensemble of neural elements following each DBS pulse. Transmembrane currents generated during activation of these elements create recordable voltages near the electrode. Both the character and amplitude of the ECAP are expected to vary with the number and type of elements activated by DBS. Since there is a very strong correlation between the number of activated neurons and clinical efficacy during variation in stimulation intensity, we expect to be able to identify ECAP signatures of clinical effectiveness.[11] These signatures would make the ECAP a suitable control signal for closed-loop DBS.
The objective of this work was to determine the feasibility of recording ECAPs during DBS. One of the primary challenges of recording brain activity during stimulation is the presence of large stimulus artifacts that can saturate amplifiers and preclude recording of mV level neural signals. The methods that have been previously developed for artifact removal typically relied on post-hoc signal processing.[9],[12] However, these would not enable high-gain recording of ECAPs without amplifier saturation. We have developed novel instrumentation that uses commercial amplifiers in a custom serial configuration to suppress the artifact and record short latency ECAPs after each DBS pulse. The performance of the instrumentation was validated through in vitro and in vivo testing.
II. Methods
A. Instrumentation for Recording Evoked Potentials
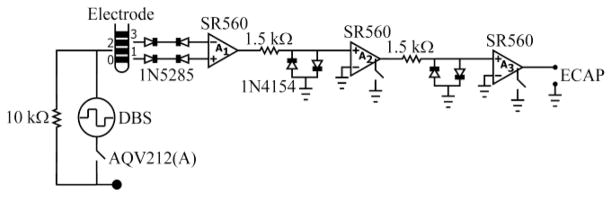
We developed DBS-ECAP instrumentation to suppress the stimulus artifact and enable high fidelity ECAP recordings. The hardware design uses three stages of amplification (SR560, Stanford Research Systems) and a circuit employing anti-parallel diodes and photocouplers to reduce the artifact and simultaneously amplify the ECAP signal (Fig. 1). Differential recordings are made from two non-stimulating contacts on the DBS electrode and serve as inputs to an AC-coupled preamplifier stage (A1), providing gain and preserving high input impedance. Anti-series current-limiting diodes (1N5285) are placed between the DBS leads and each preamplifier input to protect the subject in the event of an electrical transient. The signal is further amplified and filtered (10 Hz to 10 kHz pass-band) using two additional series amplifier stages (A2 and A3). Anti-parallel diodes (1N4154) are placed at the inputs of A2 and A3 to clip selectively the large amplitude stimulus artifact and prevent amplifier saturation. Further, the signal paths in the latter two stages are grounded through an internal opto-isolated CMOS multiplexer, blanking the output for the duration of each stimulus pulse and the subsequent 100 μs. Finally, a PhotoMOS relay (AQV212(A)) disconnects the stimulating electrodes between DBS pulses, preventing capacitive discharge through the stimulator and reducing the duration of the artifact.[13]
Fig 1.
Diagram of the DBS-ECAP instrumentation used for suppression of the artifact and high fidelity ECAP recording.
A custom program was written in LabView (National Instruments) to control simultaneously delivery of DBS pulses, timing of digital outputs to control the photocouplers, and recording of ECAP signals.
B. In Vitro Testing
The performance of the DBS-ECAP instrumentation was first evaluated in vitro. A clinical DBS electrode (Model 3387, Medtronic) was placed in a saline bath and used to deliver monopolar stimulation with a distant counter electrode. The DBS contacts used for recording were symmetrical about the stimulating contact. Each DBS pulse triggered delivery of a mock ECAP through a pair of tungsten microelectrodes, placed in the bath near the DBS electrode. The mock ECAP was synthesized by a waveform generator as a single cycle of sinusoidal current. A distant Ag/AgCl reference electrode was placed in the bath as the recording circuit reference.
The mock ECAP signal was recorded during DBS using two instrumentation systems. Charge-balanced, biphasic DBS was applied at 3 V, 100 Hz, cathodic-phase first polarity, and with pulse widths ranging from 50 to 500 μs/phase (symmetric and asymmetric pulses). The mock ECAP was a 4 kHz sine wave (0.25 ms duration) with 0.1 mA peak-to-peak input amplitude and latencies ranging from 0.1 to 2 ms. The resulting signals were recorded with either the DBS-ECAP instrumentation or a conventional setup using a single SR560 amplifier. This enabled evaluation of the relative performance of the recording systems in suppressing the artifact and recording the ECAP with high fidelity. The raw data sets consisted of 10 s of measurements for every trial, and stimulus-triggered averaging was applied to improve signal-to-noise.
We quantified distortion of the recorded ECAP by comparison to an ideal sinusoidal voltage. The latency and duration of the recorded and ideal ECAPs were matched, and the magnitude of the ideal ECAP was fit using a least-squares approach. After normalizing the magnitudes of the recorded and ideal ECAPs, distortion was calculated as the root-mean square error between the two sinusoids. Finally, this value was divided by the number of samples in the sine waves to get an average value of distortion per sample (DPS). High-fidelity recording was defined as having a DPS value under a cutoff value of 0.5.
C. In Vivo Testing
We also tested the feasibility of recording ECAPs in vivo from three adult cats. The cats were initially anesthetized with ketamine HCl and maintained with alpha-chloralose, and all animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Duke University.
A mini DBS electrode (NuMed) was implanted in the ventrolateral (VL) nucleus of the thalamus. This electrode had a lead body diameter of 0.625 mm, and four contacts of 0.5 mm height with 0.5 mm spacing, and was more appropriate for the size of the cat brain than the clinical electrode. The VL thalamus, which was targeted for implantation, functions as a relay from muscle afferents to the cortex, and was identified using stereotactic technique and single unit recordings of neurons responding to passive contralateral limb movement.[14] Following implantation of the mini DBS electrode through a guide tube, we confirmed accurate implantation by recording evoked responses from the electrode during electrical stimulation of the contralateral sciatic nerve. Further, after completing the protocol, we determined the anatomical location of the electrode using a histological procedure.[15] The brain tissue containing the electrode path was cryosectioned into 50 μm coronal sections and stained with 0.1% cresyl violet to label cell bodies. The location of the electrode was then registered to a stereotactic atlas of the cat brain.[16]
Physiological ECAPs were recorded during DBS using the two instrumentation systems. The DBS stimulation parameters and contact configuration were identical to those tested in vitro, but with the pulse width fixed at 50 μs and with use of both cathodic- and anodic-phase first polarities. We used a retractor in the ipsilateral chest muscle as the counter electrode and a Ag/AgCl cloth pad placed on the back of the neck as the reference electrode. We compared the relative performance of the DBS-ECAP instrumentation and conventional amplifier in making in vivo ECAP recordings. Each ECAP recording trial was conducted for 10 s and separated from the subsequent trial by 10 s. Stimulus-triggered averaging was applied 64 times.
III. Results
A. In Vitro Validation of DBS-ECAP Instrumentation
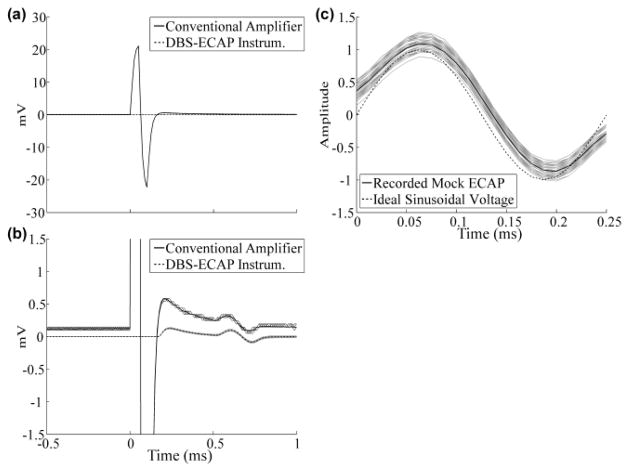
The performance of the DBS-ECAP instrumentation was evaluated, relative to the conventional amplifier, through in vitro testing. The large magnitude stimulus artifact recorded with the conventional amplifier was suppressed with the use of the DBS-ECAP instrumentation (Fig. 2a). As a result, the maximum gain (G) that could be achieved without saturation increased from 100x in the conventional amplifier to 100,000x in the DBS-ECAP instrumentation (three stages with G1 = 100x, G2 = 200x, G3 = 5x). However, to avoid diode clipping of the mock ECAP, the maximum gain was set to 2,500x.
Fig 2.
Stimulus artifact and mock ECAP waveforms recorded in vitro. Both the stimulus-triggered average (black) and single trials (gray) are shown after gain correction. The DBS pulse width used for these data was 50 μs. (a) The large magnitude stimulus artifact recorded with the conventional amplifier was suppressed with the DBS-ECAP instrumentation. (b) The mock ECAP (0.5 ms latency) was recorded with higher fidelity using the DBS-ECAP instrumentation (DPS=0.17) than the conventional amplifier (DPS=1.7). (c) Comparison of the mock ECAP recorded with the DBS-ECAP instrumentation and the ideal sinusoidal voltage after amplitude normalization.
By reducing the stimulus artifact, the DBS-ECAP instrumentation could record short latency mock ECAPs during DBS with high fidelity (Fig. 2b, c). Qualitative analysis reveals that the higher gains enabled by the DBS-ECAP instrumentation improved recording fidelity. Furthermore, decreasing the artifact duration led to a corresponding reduction in the extent of temporal overlap between the artifact and short latency ECAPs. Consequently, the calculated distortion was substantially smaller for the DBS-ECAP instrumentation than the conventional amplifier across stimulation parameters tested.
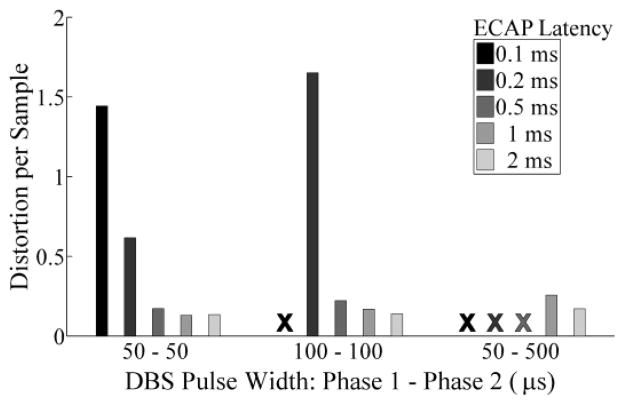
For both recording systems, distortion was reduced with longer ECAP latencies or shorter DBS pulse widths. Fig. 3 shows that the DBS-ECAP instrumentation could achieve high-fidelity recording of ECAPs at latencies of only 0.5 ms after DBS pulses of 50 or 100 μs duration, or latencies of 1 ms for long, asymmetric pulses (50 μs first phase, 500 μs second phase). Conversely, the conventional amplifier did not enable high fidelity recording at any combination of DBS pulse width and ECAP latency tested.
Fig. 3.
Distortion per sample of the mock ECAP recorded with the DBS-ECAP instrumentation across DBS pulse widths and ECAP latencies. The X indicates that the ECAP was completely masked by amplifier blanking and distortion was not calculated.
B. Feasibility of In Vivo ECAP Recording
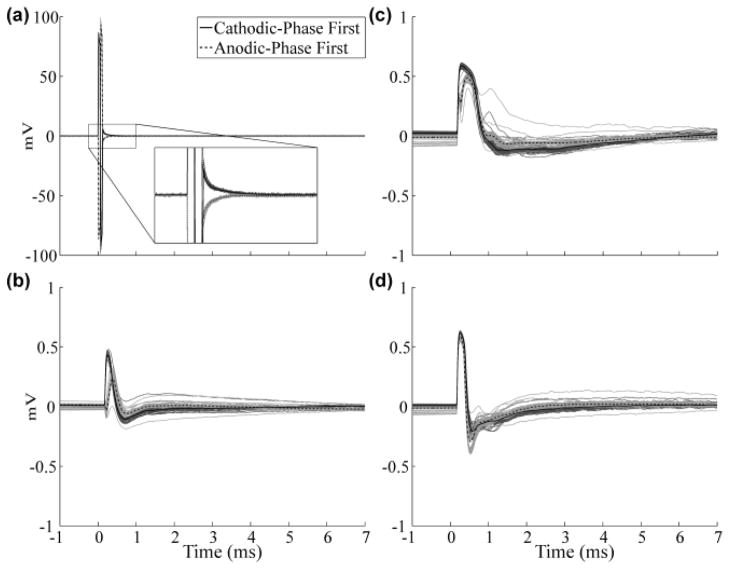
We recorded high fidelity in vivo ECAPs during DBS of the VL thalamus in adult cats. The physiological response was distinguished from the stimulus artifact by comparing signals resulting from cathodic- and anodic-phase first stimulation. For symmetric, biphasic pulses, the neural response is similar for opposite polarities [17], whereas the stimulus artifact is inverted. Using this as a basis for signal analysis, we found that the artifact recorded with the conventional amplifier masked the ECAP response (Fig. 4a). Further, to prevent amplifier saturation, gain was limited to only 50x. On the other hand, the DBS-ECAP instrumentation enabled high-gain recordings of ECAPs, uncontaminated by the artifact, in all three experiments (Fig. 4b–d). With this system, the gain was increased to 5,000x without saturation (G1 = 50x, G2 = 20x, G3 = 5x).
Fig. 4.
Signals observed during in vivo recording in three experiments using (a) the conventional amplifier in cat A, and (b-d) DBS-ECAP instrumentation in cats A, B, and C, respectively. DBS was applied at 3 V, 100 Hz, and 50 μs/phase duration. The stimulus-triggered average waveforms (black) are shown with cathodic- and anodic-phase first DBS polarities. The single trials are also shown for the two polarities in dark and light gray, respectively. The inset in (a) shows a zoomed image of the artifact, which completely masked the ECAP.
The ECAP waveforms recorded in the three experiments were of similar magnitude and were qualitatively similar, with an early positive wave followed by a later negative wave. The peak-to-peak magnitudes were on the order of 1 mV, early wave latencies in the range of 0.25 to 0.5 ms, and maximum duration of approximately 5 ms. These results demonstrate that physiological ECAPs can be recorded during DBS, enabled by the novel instrumentation.
IV. Discussion
We demonstrated that ECAPs can be recorded with high fidelity during DBS with the use of novel instrumentation to suppress the stimulus artifact. The performance of the instrumentation system was validated through both in vitro and in vivo testing.
The DBS-ECAP instrumentation exhibited high performance during in vitro recording of small magnitude, short latency mock ECAPs. Suppression of the stimulus artifact enabled increases in gain by a factor of 1,000x over a conventional biopotential amplifier, and decreased the extent of temporal overlap between the artifact and ECAP. Consequently, we could make high fidelity recordings of mock ECAPs with latencies of only 0.5 ms during DBS applied with clinically-relevant pulse widths of 50 to 100 μs. This was not otherwise feasible with the conventional amplifier. Other techniques to reduce the stimulus artifact have relied on signal processing methods, such as filtering or template subtraction, performed after amplification. These techniques were not suitable for achieving high-gain recordings of ECAPs, and the present results demonstrate the utility of our hardware-based strategy.
We also investigated the feasibility of recording ECAPs in vivo during thalamic DBS in anesthetized cats. The high performance of the DBS-ECAP instrumentation observed in vitro translated to the in vivo experiments, in which physiological ECAPs could be recorded at high gains without contamination by the stimulus artifact. Conversely, the large artifact recorded with the conventional amplifier completely masked the ECAP signal. The similarity in responses recorded with the DBS-ECAP instrumentation for cathodic- and anodic-phase first stimulation corroborates the neural origin of the ECAP response. Further, the ECAP waveform shape and magnitude, latency, and duration characteristics were comparable across the three experiments. The latency and polarity of the ECAP waves may provide insight into the location of neural activation relative to the recording contacts following each DBS pulse, and the magnitude of these waves could indicate the volume of tissue activated. The similarity in ECAP characteristics across the three experiments suggests a common spatiotemporal activation pattern of neural elements during DBS in the VL thalamus.
The capability to make high fidelity recordings of ECAPs using the DBS-ECAP instrumentation provides a potential feedback signal for closed-loop control of DBS. The ECAP signal is expected to depend on the type and spatial extent of neural elements activated during stimulation. Consequently, this signal may provide signatures of clinical effectiveness that can be used for automatic adjustment of stimulation parameters. This could alleviate the clinical burden of DBS programming and improve patient outcomes.
Acknowledgments
This work was supported in part by the National Institutes of Health under grant F31-NS-070460 and the Duke University Department of Biomedical Engineering Robert Plonsey Fellowship.
The authors would like to thank Gilda Mills for animal care and surgical assistance.
Contributor Information
Alexander R. Kent, Email: alexander.kent@duke.edu, Biomedical Engineering Department, Duke University, Durham, NC 27708 USA
Warren M. Grill, Email: warren.grill@duke.edu, Departments of Biomedical Engineering, Neurobiology, and Surgery, Duke University, Durham, NC 27708 USA
References
- 1.Benabid AL, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–6. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 3.Grill WM, McIntyre CC. Extracellular excitation of central neurons: implications for the mechanisms of deep brain stimulation. Thalamus & Related Systems. 2001;1:269–277. [Google Scholar]
- 4.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clinical Neurophysiology. 2004;115:2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Ondo WG, Bronte-Stewart H. The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotactic and Functional Neurosurgery. 2005;83:142–147. doi: 10.1159/000088654. [DOI] [PubMed] [Google Scholar]
- 6.Moro E, et al. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002;59:706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 7.Kuncel AM, et al. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Movement Disorders. 2006;21:1920–1928. doi: 10.1002/mds.21087. [DOI] [PubMed] [Google Scholar]
- 8.Volkmann J, Herzog J, Kopper F, Deuschl G. Introduction to the programming of deep brain stimulators. Movement Disorders. 2002;17:S181–S187. doi: 10.1002/mds.10162. [DOI] [PubMed] [Google Scholar]
- 9.Rossi L, et al. An electronic device for artefact suppression in human local field potential recordings during deep brain stimulation. J Neural Eng. 2007;4:96–106. doi: 10.1088/1741-2560/4/2/010. [DOI] [PubMed] [Google Scholar]
- 10.Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–9. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Kuncel AM, Cooper SE, Wolgamuth BR, Grill WM. Amplitude- and frequency-dependent changes in neuronal regularity parallel changes in tremor with thalamic deep brain stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15:190–197. doi: 10.1109/TNSRE.2007.897004. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGill KC, et al. On the nature and elimination of stimulus artifact in nerve signals evoked and recorded using surface electrodes. IEEE Trans Biomed Eng. 1982;29:129–37. doi: 10.1109/TBME.1982.325019. [DOI] [PubMed] [Google Scholar]
- 14.Berman AL, Jones EG. The thalamus and basal telencephalon of the cat. Madison: The University of Wisconsin Press; 1982. [Google Scholar]
- 15.McConnell GC, et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 16.Snider RS, Niemer WT. A stereotaxic atlas of the cat brain. Chicago: The University of Chicago Press; 1961. [Google Scholar]
- 17.McIntyre CC, Grill WM. Selective microstimulation of central nervous system neurons. Ann Biomed Eng. 2000;28:219–33. doi: 10.1114/1.262. [DOI] [PubMed] [Google Scholar]