Abstract
Though metastasis is considered an inefficient process, over 90% of cancer related deaths are attributed to the formation of secondary tumors. Thus, eliminating circulating cancer cells could lead to improved patient survival. This study was aimed at exploiting the interactions of cancer cells with selectins under flow to selectively kill captured colon cancer cells. Microtubes functionalized with E-selectin and TRAIL were perfused with colon cancer cell line Colo205 either treated with 1 mM aspirin or untreated for 1 or 2 h. Cells were collected from the microtube and analyzed by flow cytometry. Aspirin treatment alone killed only 3% cells in culture. A 95% difference in the number of cells killed between control and TRAIL + ES surfaces was seen when aspirin treated cells were perfused over the functionalized surface for 2 h. We have demonstrated a novel biomimetic method to capture and neutralize cancer cells in flow, thus reducing the chances for the formation of secondary tumors.
Keywords: metastasis, cell adhesion, TRAIL, E-selectin, aspirin, shear flow
Introduction
The formation of metastatic epithelial tumors involves a series of distinct, but related, steps that shift the tumor cells from the primary tumor to a distal location. In the first step of the metastatic cascade, the cancer cell detaches from the primary tumor. Following detachment, they interact with the surrounding tissue and are believed to undergo epithelial-to-mesenchymal transition (EMT) where they may invade a nearby blood or lymph vessel.1−3 Once in the circulation, the cancer cells must evade the immune system and survive the stresses in circulation.4 Cancer cells can interact with the endothelium via selectins, in a manner similar to leukocytes in the inflammatory cascade, and eventually come to rest where they may transmigrate out of the vessel.5−7 Circulating cancer cells may also transmigrate by forming aggregates with polymorphonuclear leukocytes (PMN) either in blood or via secondary recruitment from a previously attached leukocyte.8 Following transmigration, cells are believed to undergo the reverse process of mesenchymal to epithelial transition (MET) to survive in the new environment. To form a secondary tumor all these steps must be successfully completed.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or APO-2L), a member of the tumor necrosis factor (TNF) receptor superfamily, is known to induce apoptosis in transformed cells with no effect on noncancerous cells.9−11 TRAIL is known to bind to five different receptors, two of which are the death receptors (DR4 or TRAIL-R1 and DR5 or TRAIL-R2) and two of which are decoy receptors (DcR1 or TRAIL-R3 and DcR2 or TRAIL-R4), which are highly homologous to the death receptors but are incapable of inducing apoptosis due to the absence of the cytoplasmic death domain or a truncated death domain, respectively.12−14 The fifth receptor, OPG, binds TRAIL with very low affinity.15
Nonsteroidal anti-inflammatory drugs (NSAID) such as aspirin have been implicated in reducing the occurrence of cancer.16 Aspirin pretreatment has been shown to sensitize prostate and colon cancer cells to TRAIL-induced apoptosis by downregulating either Bcl-2 or survivin.17,18
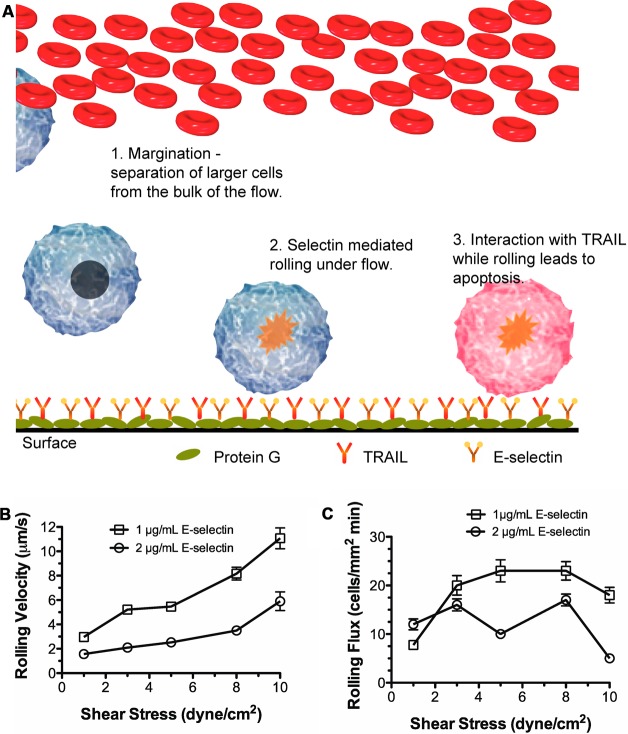
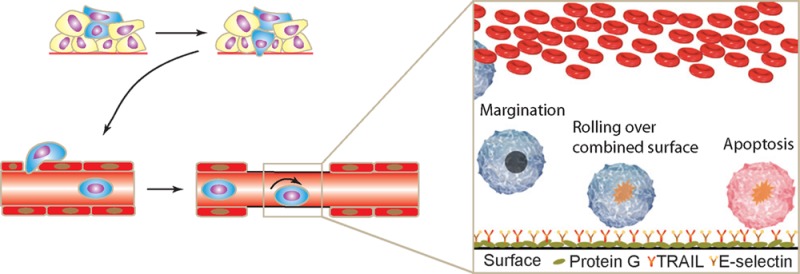
We have previously demonstrated proof-of-concept of a device that can capture leukemic cancer cells from circulation and kill them.19,20 A schematic of the mechanism is shown in Figure 1A. In the current study, we extend the use of the device as an adjunct therapy in which colon carcinoma cells are pretreated with aspirin and then perfused through the microtube device.
Figure 1.
Interaction of Colo205 with microtubes functionalized with E-selectin under physiological shear stress. (A) Schematic of two-receptor delivery system. (B) Rolling velocity and (C) rolling flux as a function of wall shear stress at two incubation concentrations of E-selectin. Each point represents at least 25 cells.
Selectins are believed to be signaling molecules in addition to being adhesive molecules.21 In addition, L-selectin, present on PMNs, is known to interact with E-selectin, causing L-selectin cross-linking and subsequent activation of PMNs.22 Activating PMNs could lead to accumulation of PMNs in the device thereby decreasing the surface available for cancer cells to bind and possibly lead to leukopenia. Alternately, loss of L-selectin could lead to a suppressed immune surveillance. To this end, the ability of primary PMNs to express activated CD11b and loss of L-selectin by rolling over the combined surfaces was also evaluated.
Materials and Methods
Reagents and Antibodies
Human serum albumin (HSA), bovine serum albumin (BSA), Accutase, HEPES, EDTA, aspirin, dimethyl sulfoxide (DMSO) and calcium carbonate were all obtained from Sigma-Aldrich (St. Louis, MO). RPMI 1640 cell culture medium, fetal bovine serum (FBS), Hanks balanced salt solution (HBSS) and phosphate buffered saline (PBS) were all obtained from Invitrogen (Grand Island, NY). His-tagged recombinant human TRAIL (TRAIL), recombinant human E-selectin-IgG chimera (ES), human apoptosis antibody array and TACS Annexin-V FITC Apoptosis Detection Kit were purchased from R&D Systems (Minneapolis, MN). Protein-G and anti-His tag antibody were purchased from EMD Biosciences (San Diego, CA). PBS based enzyme-free cell dissociation media was purchased from Millipore (Billerica, MA).
Cell Lines and Cell Culture
Colon cancer cell line Colo205 (ATCC number CCL-222) was obtained from ATCC (Manassas, VA). These cells were cultured in RPMI 1640 supplemented with 2 mM l-glutamine, 25 mM HEPES, 10% v/v FBS and 100 U/mL PenStrep (complete medium) under humidified conditions at 37 °C and 5% CO2. Cells were maintained so that 80% confluence was not exceeded.
Preparation of Immobilized Protein Surfaces
The microtubes were rinsed with ethanol and washed with 1× PBS. The surface was first incubated with 10 μg/mL protein-G solution for 1.5 h, followed by 2 h incubation with E-selectin chimera (2 μg/mL) or a combination of E-selectin chimera and anti-His tag antibody (2 μg/mL and 10 μg/mL respectively), and then the surface was incubated with 20 μg/mL TRAIL solution. Each incubation step was followed with three washes with PBS. All incubations were at room temperature, and successful immobilization was confirmed by immunofluorescence.
Cell Preparation for Rolling Experiments
Colo205 cells were treated with an enzyme-free cell dissociation medium as per the manufacturer’s instructions. Briefly, 5 mL of enzyme free cell dissociation medium was added to a 10 cm Petri dish and incubated for 5 min. Complete medium (5 mL) was added, and cells were harvested. The cells were washed twice with 1× PBS at 200g in an Allegra X-22 refrigerated centrifuge at 4 °C and resuspended in the flow buffer at a concentration of 2 × 106 cells/mL. The flow buffer consisted of HBSS without Ca2+ and Mg2+ supplemented with 0.5% w/v HSA, 10 mM HEPES and 2 mM Ca2+. For all experiments, at least 90% viability of cells was confirmed by trypan-blue exclusion dye.
Aspirin Treatment
Aspirin (acetylsalicylic acid or ASA) was dissolved in DMSO to a final concentration of 1 M. Cells were cultured for 1 day before treating them with 1 mM ASA in complete medium for 18 h at 37 °C and 5% CO2. After 18 h of exposure to ASA, cells were harvested and washed twice in 1× PBS before being either resuspended in flow buffer for rolling experiments or used for protein array (please see Supporting Information for more details).
Rolling Experiments
Micro-Renathane tubing, 300 μm internal diameter, was obtained from Braintree Scientific (Braintree, MA), cut into 50 cm long segments and secured to the stage of the Olympus IX81 motorized inverted research microscope (Olympus America Inc., Melville, NY) after surface functionalization. The microscope was equipped with a CCD camera (model no: KP-M1AN, Hitachi, Japan) connected to either an S-VHS videocassette recorder (model no: SVO-9500MD2, Sony Electronics, Park Ridge, NJ) or a DVD recorder (model no: DVO-1000MD, Sony Electronics, Park Ridge, NJ) to record video for offline analysis. A syringe pump (KDS 230, IITC Life Science, Woodland Hills, CA) was used to control the flow rate of the cell suspension. Cells were loaded on the surface at a shear stress of 0.5–1 dyn/cm2 for 3 min prior to each flow experiment. Microtube flow experiments were performed at 1.8 dyn/cm2, for a period of 1–2 h. After flow, cells were separated into two fractions, “cells on surface”, that is, cells that were rolling on or remained on the surface at the end of the experiment, and “cells in flow”, that is, cells that were collected into the syringe.
The cells on the surface were harvested using 5 mM EDTA and air embolism at 10 dyn/cm2. These cells were then cultured for 16–18 h and analyzed by Annexin-V assay. E-selectin and His-tag antibody (without TRAIL) functionalized surfaces were used as negative controls.
PMN Extraction
Peripheral blood was collected from healthy adults after informed consent. PMNs were separated using 1-Step Polymorph (Accurate Chemicals) density gradient centrifugation as described earlier.21
Rolling experiments as described earlier were performed. Cells were collected by perfusing Ca2+ free HBSS for analysis. Collected cells were then washed and fixed in 4% paraformaldehyde and labeled for total L-selectin and active CD11b on the cell surface using anti-human CD62L antibody and anti-human CD11b antibody (both from BD Biosciences), respectively. Labeled cells were analyzed by flow cytometry. As positive control, PMNs were activated by treating PMNs with 100 μM interleukin-8 (IL-8).
Data Analysis
“Rolling” cells were defined as those observed to translate in the direction of flow with an average velocity less than 50% of the calculated hydrodynamic free-stream velocity. Rolling flux was determined by counting the number of cells crossing a line drawn in the field of view perpendicular to the flow direction, over a period of 1 min.
Cells were analyzed for death and the mode of death by the Annexin-V apoptosis assay on an Accuri C6 flow cytometer. Manufacturer's instructions were followed to prepare samples for flow cytometry.
Where appropriate, the Student t test and one-way ANOVA with Tukey post test to compare all means were employed at a significance level of α = 0.05. Values plotted are average ± SEM unless noted in the caption. All statistical analyses were performed using GraphPad Prism 5.0c for Mac OS X (GraphPad Software, San Diego, CA USA).
Results
Colon Cancer Cells Exhibit Shear-Dependent Rolling on E-Selectin
The bonding of selectin with its ligand is reversible. While rolling measures the rate of dissociation of the bond, rolling flux gives a measure of the rate of bond formation. In order to estimate the optimal rolling conditions, Colo205 cells were perfused through microtubes functionalized with rhE-selectin at a shear stress range of 1–10 dyn/cm2. The surface density of E-selectin was varied by incubating the tube with different concentrations of E-selectin solution (0.1–10 μg/mL) at room temperature (rt). Figure 1B and Figure 1C summarize the results from rolling experiments. Rolling was observed at incubation concentrations of 1 μg/mL and 2 μg/mL E-selectin. Adhesive interactions were observed only at 1 dyn/cm2 for 0.1 μg/mL E-selectin. At higher concentrations of E-selectin (10 μg/mL and higher) firm arrest was seen with rolling velocities approaching zero even at the higher shear stresses. Extremely slow rolling was observed at 5 μg/mL E-selectin, however, cells recovered had lower viability. The results from these experiments suggest that 2 μg/mL of E-selectin allows stable rolling of Colo205 cells with relatively slow rolling velocities and a maximum rolling flux at a shear stress of 2 dyn/cm2 (Figure 1B,C). With increasing stress (>3 dyn/cm2), a decrease in the number of viable cells postrolling was observed (data not shown).
Microtube Flow Device Coated with TRAIL and E-Selectin Is Able To Capture Colorectal Cancer Cells from Flow and Induce Apoptosis in a Time-Dependent Manner
Colon cancer cells were prepared and rolled over the functionalized surfaces as described in Materials and Methods. Cells collected after flow were cultured in complete medium for 18 h to prevent overestimation due to reversal of apoptosis signal and were later analyzed by the Annexin-V assay as described earlier. Apoptosis signal was not detected immediately after the experiments.
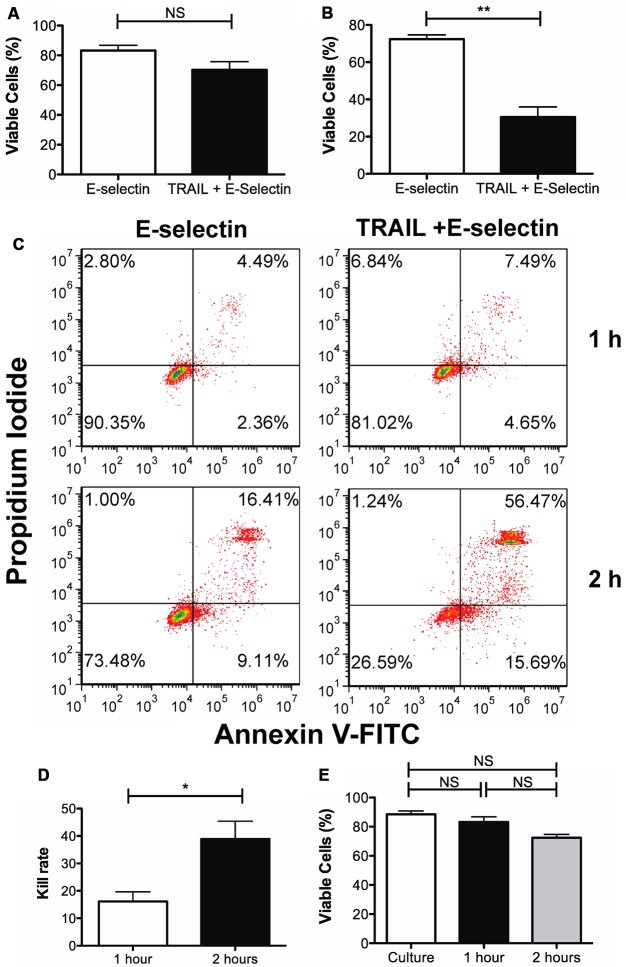
A difference of 13% in viable cells was seen between cells collected from the TRAIL + ES microtube when compared to the control tube functionalized with ES in 1 h (Figure 2A,C). Rolling over the ES surface induced some degree of cell death when compared to cells in culture (Figure 2E).
Figure 2.
Effect of TRAIL and E-selectin functionalized surfaces on viability of rolling Colo205. Percent viable cells after rolling over the functionalized surfaces for (A) 1 h and (B) 2 h as determined by the Annexin-V assay. (C) Representative flow cytometry plots. (D) Rolling delivery is time dependent. When Colo205 cells are perfused over the functionalized surface, an average kill rate of 12.91 ± 6.56 is observed while a kill rate of 41.88 ± 5.91 is observed when perfusing the cells for 2 h. (E) Cell death due to shear force experienced by Colo205 while passing through the microtube device. When compared to cells in culture, a gradual decrease in viability is seen with increased perfusion time. **p < 0.01, *p < 0.05, n = 3.
It was expected that the kill rate could be significantly increased by extending the cell perfusion time to 2 h, still within the realistic contact time achievable under physiological conditions.20,23 To test this hypothesis, cells were prepared and perfused over the functionalized surfaces for 2 h. A 42% difference in the percentage of viable cells between the control surface lacking TRAIL and the two receptor surface of TRAIL + E-selectin was observed (Figure 2B,C). By doubling the interaction time, an approximate 3-fold difference in kill rate was observed (Figure 2D).
Taken together, the device is capable of capturing colon cancer cells from flow and killing them. As seen in Figure 2, the Annexin-V assay confirms that the mode of death is via apoptosis due to interaction of cancer cells with TRAIL.
Rolling on E-Selectin Alone Does Not Activate Integrins on PMNs
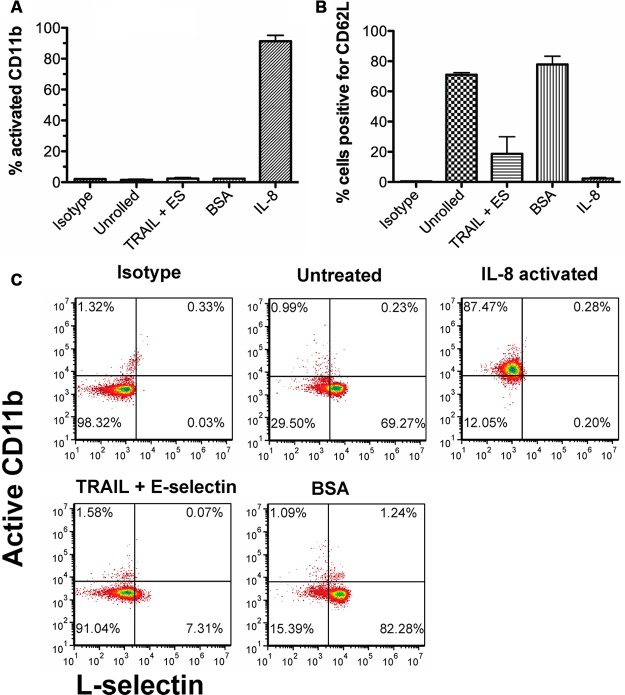
In addition to serving as adhesion molecules, selectins have also been shown to induce intracellular signals. Once activated, the PMNs may adhere to the surface thus saturating it and preventing the interaction of cancer cells. To investigate the activation of PMNs while flowing through the device, PMNs isolated from peripheral blood were isolated as described in Materials and Methods. There was no statistical difference found in the amount of activated CD11b between cells collected from BSA-coated tubes and TRAIL + ES tubes, while over 90% active CD11b was seen with cells treated with IL-8 as a positive control (Figure 3A,C). A decrease in surface expression of L-selectin was observed following rolling (Figure 3B,C).
Figure 3.
Neutrophils shed L-selectin but do not express activated integrins following 2 h of rolling. (A) Percent of total neutrophils expressing active CD11b. (B) Percentage of cells expressing L-selectin. (C) Representative flow cytometry plots of various treatments showing amount of surface bound L-selectin and activated CD11b. n = 3.
Pretreatment with 1 mM Aspirin Increases TRAIL Kill Rate
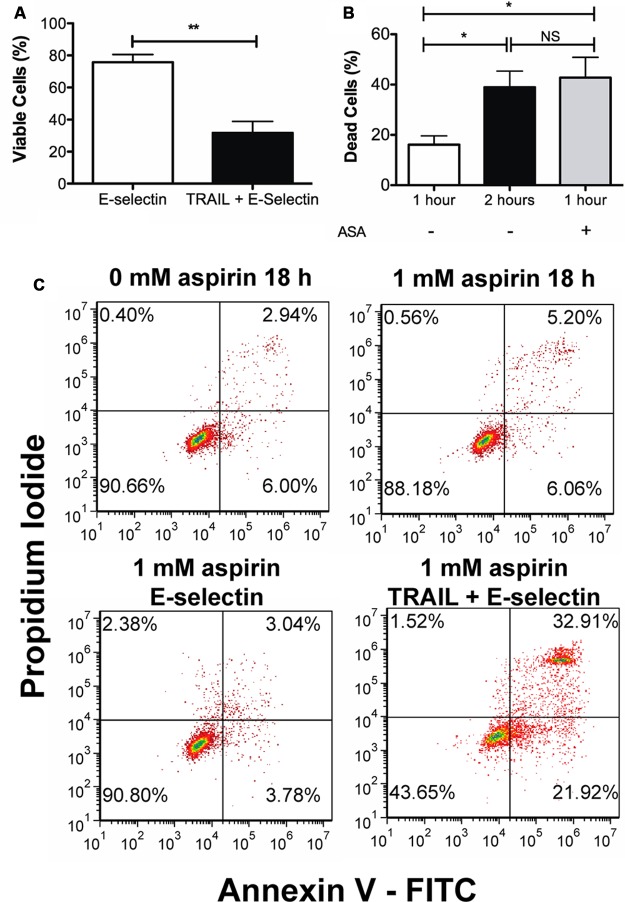
Aspirin has been shown to be effective in reducing the occurrence of colorectal cancer. To test the effect of aspirin-mediated sensitization of colon cancer cells on the TRAIL + ES coated device, Colo205 cells were treated and prepared as described in Materials and Methods. An aliquot of treated cells was taken prior to flow for analysis via the Annexin-V assay. Aspirin treatment alone killed only about 3% of cells when compared to untreated control (Figure 4C top row).
Figure 4.
One hour of Colo205 cell rolling following 1 mM aspirin pretreatment. (A) Percentage of the viable population on the E-selectin and the combined TRAIL and E-selectin surfaces. (B) Comparison of kill rates following perfusion over the combined surfaces with and without aspirin pretreatment. Perfusion over the combined surface for 1 h with aspirin treatment kills a similar proportion of cells that were perfused over the combined surface for 2 h without aspirin treatment. **p < 0.01, *p < 0.05, n = 3. (C) Representative flow cytometry plots showing the results from the Annexin-V assay.
When cells pretreated with 1 mM aspirin were perfused through the microtube flow device for 1 h and analyzed 18 h later for apoptosis by the Annexin-V assay, an average difference in viability of 44.32 ± 8.64% was observed between the control surface of ES and the combined surface of TRAIL + ES (Figure 4A). Kill rate is defined as the difference in the number of viable cells between the control surface and the combined TRAIL + ES surface. The kill rate of cells perfused through the microtube following aspirin pretreatment was found to be similar to perfusion of untreated cells over the combined surface for 2 h (Figure 4B), while perfusing untreated cells for 1 h showed a kill rate of ∼17%.
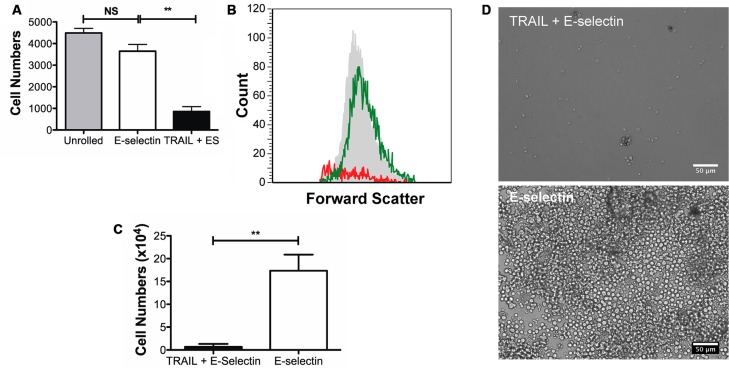
Aspirin treatment followed by 2 h perfusion over the combined surface was expected to further increase the kill rate as was seen with untreated cells. To test this hypothesis, aspirin-treated cells were collected after 2 h perfusion over both the control surface and the combined TRAIL + ES surfaces and cultured for 24 h. Most cells collected from the combined surface had disintegrated in about 18 h and performing the Annexin-V assay was not possible. Consequently, 10,000 ungated events were recorded on the flow cytometer for each sample. Based on the untreated, unrolled Colo205 a gate was set to denote viable cells. This gate was replicated for all samples, and the total number of events within the gate was calculated, yielding the cell count. Figure 5A is a plot of the number of events within the gate for each sample, and representative flow cytometry histograms with counts and representative gating strategy are shown in Figure 5B. An 18% decrease in the cell count was observed between unrolled cells and cells that rolled over the control surface. This decrease in viability may be due to the effects of fluid forces acting on the cells. However, when the control surface with ES is compared to the combined surface, a decrease of ∼76.5% was observed for the number of gated events.
Figure 5.
Effect of pretreatment with 1 mM aspirin and rolling. (A) Cell counts from flow cytometry (B) Representative histograms 18 h after the flow experiments. The gray shaded histogram represents cells that were obtained from culture, green histogram represents the cells obtained from the E-selectin tube while the red histogram represents cells obtained from the TRAIL + ES tube. (C) Cell counts after 3 days in culture; following 2 h of flow device exposure. (D) Representative micrographs after 3 days in culture. Scale bar is 50 μm. **p < 0.01.
In a separate experiment, cells were harvested after the flow experiment and seeded at 100,000 cells/mL in multiwell plates. These cells were allowed to grow in complete medium and culture conditions and counted at day 3 using a hemocytometer. A 96% difference in the number of cells was observed between cells collected from the control surface and cells collected from the combined TRAIL and E-selectin surfaces (Figure 5C). Representative micrographs at day 3 are shown in Figure 5D.
Discussion
In the present study, we build upon our previous study by extending the method of capturing cancer cells on a combined TRAIL + E-selectin surface to include cells of epithelial origin. We have also shown that ASA increases cell death possibly by altering several regulators of apoptosis. Based on measurements of rolling velocities (Figure 1B), sustaining longer rolling contact is possible. It was determined that increasing the contact time from 1 to 2 h increases the kill rate by almost 3-fold. When cells collected from the control surface lacking TRAIL were analyzed for viability, no statistically significant difference in viability was observed between cells from the control surfaces and cells left at culture conditions (Figure 2E). This indicates that death was not due to hypoxia or other nonphysiological conditions. These results are consistent with our prediction that longer duration (potentially with recirculation) would increase the probability of capture and subsequently increase kill rate.20
TRAIL in solution has been shown to exert cytotoxic effects on transformed cells in as little as an hour;24 however, no studies have been carried out using immobilized TRAIL. Though effective, soluble TRAIL is a small protein and thus has a very fast clearance rate (average half-life of ∼30 min).25 Tagged versions of TRAIL, though effective, have been shown to be toxic to nontransformed cells.26,27 On the other hand, a nontagged version of TRAIL has reduced effectiveness.28 In the current study, we use His-tagged TRAIL that is known to have toxicity toward noncancerous cells; however, immobilizing TRAIL we reduce the interaction of TRAIL with noncancerous cells.
An important parameter we have optimized is the contact time between TRAIL and its receptors. By varying either the selectin site density or the applied shear stress, we have controlled the time TRAIL is bound to its receptors. Previous studies have measured the rate kinetics of TRAIL ligand:receptor interactions;15 however, the time required for the receptor to be bound to induce apoptosis is still unknown. Further studies in this area will help achieve precise control over the interaction times and hence further the development of the device.
Cancer cells have been shown to interact with the endothelial lining of the vasculature by a variety of adhesion molecules as initial steps in extravasation.29,30 It is believed that this contact with the endothelium initiates a cascade of activation events, similar to that of PMN recruitment during inflammation, and ultimately leads to the development of a metastatic tumor.31 Our group and others have shown that cancer cells exhibit selectin-dependent rolling under flow.32,33 In particular, E-selectin has been implicated as an important selectin molecule for colorectal cancer interactions with the endothelium.33−35 Similar results highlighting the importance of selectins in metastasis have also been demonstrated in vivo,36−39 thus making E-selectin a natural choice for the biomimetic surface.
Aspirin has been shown to reduce the occurrence of cancer;40,41 however, the dosage that has a cancer preventive action is still debated. In a 5-year followup study in the United States, 22,071 men were randomized to a daily dosage of 325 mg of aspirin or placebo, and researchers reported that the relative risk of developing colorectal cancer in men receiving aspirin every day was lower compared to control.40 A similar cancer preventive effect was seen in patients with breast and prostate cancer.42−44 In recent years, aspirin pretreatment has been shown to sensitize cancer cells to TRAIL-mediated apoptosis. Kim et al. reported that TRAIL resistant LNCaP cells when treated with 1 mM aspirin were sensitized to TRAIL mediated apoptosis by downregulating NF-κB that regulates several antiapoptotic proteins.17 In their study, Kim et al. report that aspirin inhibits the phosphorylation and degradation of the inhibitory subunit IκB. It was then shown that aspirin specifically blocks BCL-2. Similarly, aspirin has been shown to sensitize HeLa cells to TNFα induced apoptosis.45
In another study, Yoo et al. reported that treating LNCaP cells with 5 mM aspirin resulted in a downregulation of survivin leading to TRAIL sensitization.18 Lu et al. showed a similar effect of aspirin mediated downregulation of survivin in breast cancer epithelial cells.46 In their study, Lu et al. demonstrated that when estrogen receptor negative breast cancer cell line (MDA-MB-435) was pretreated with 5 mM aspirin for 48 h, then treated with TRAIL or TRAIL agnostic antibody, synergistic effects were seen. They also reported that aspirin triggered cell arrest in G1 phase while 1 mM did not trigger arrest.
The aforementioned studies used different concentrations of aspirin and found that two different proteins were downregulated; however, no comparison with other antiapoptotic and proapoptotic proteins was made in the prior studies. Consistent with results published by Kim et al., we observed downregulation of BCL-2 when Colo205 cells were pretreated with 1 mM aspirin (Figure S1 in the Supporting Information). However, Kim et al. did not evaluate the effects of aspirin on other proteins involved in apoptosis. Though preliminary, our results suggest the possibility of a complex signaling pathway that is regulated by multiple genes/proteins (Figure S1 in the Supporting Information). Detailed studies are required to elucidate the precise mechanism of aspirin mediated sensitization. Future efforts will be directed toward understanding the effect of aspirin in sensitizing cancer cells to TRAIL. Despite the several advantages of aspirin in sensitizing a variety of cancer cells to apoptosis, clinically aspirin is used as a preventive. Use of aspirin in treating colon cancer should be further evaluated.
The described microtube may be tailored to specific cancer types by functionalizing the surface with small peptides, oligomers or other protein molecules that are more selective at capturing cells or inducing apoptosis. These replacements may be more stable thereby increasing the longevity of the device. In addition, the surface may be customized to capture specific cell types. Cells captured in this manner could then be reprogrammed or neutralized before being released into the circulation. Additionally, the same technology (with modifications) may be utilized to capture and enrich rare cells from peripheral circulation, which may then be used for clinical research.
Acknowledgments
The authors gratefully acknowledge experimental assistance from Jeffery C. Mattison and Sivaprakash Agastin and funding to M.R.K. and C.A.K. from NIH Grant U54CA143876.
Supporting Information Available
Apoptosis array results and death receptor expression. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): M.R.K. is a scientific advisor to CellTraffix, Inc.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Pantel K.; Brakenhoff R. H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4(6), 448–56. [DOI] [PubMed] [Google Scholar]
- Royston D.; Jackson D. G. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J. Pathol. 2009, 217(5), 608–19. [DOI] [PubMed] [Google Scholar]
- Vernon A. E.; LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr. Biol. 2004, 14(17), R719–21. [DOI] [PubMed] [Google Scholar]
- MacDonald I. C.; Groom A. C.; Chambers A. F. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. BioEssays 2002, 24(10), 885–93. [DOI] [PubMed] [Google Scholar]
- Gout S.; Tremblay P.-L.; Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin. Exp. Metastasis 2008, 25(4), 335–44. [DOI] [PubMed] [Google Scholar]
- Krause T.; Turner G. A. Are selectins involved in metastasis?. Clin. Exp. Metastasis 1999, 17(3), 183–92. [DOI] [PubMed] [Google Scholar]
- Lafrenie R. M.; Buchanan M. R.; Orr F. W. Adhesion molecules and their role in cancer metastasis. Cell Biophys. 1993, 23(1–3), 3–89. [DOI] [PubMed] [Google Scholar]
- Geng Y.; Narasipura S.; Seigel G.; King M. Vascular Recruitment of Human Retinoblastoma Cells by Multi-Cellular Adhesive Interactions with Circulating Leukocytes. Cell. Mol. Bioeng. 2010, 3(4), 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A.; Dixit V. M. Death Receptors: Signaling and Modulation. Science 1998, 281(5381), 1305–8. [DOI] [PubMed] [Google Scholar]
- Griffith T. S.; Stokes B.; Kucaba T. A.; Earel J. K. Jr.; VanOosten R. L.; Brincks E. L.; Norian L. A. TRAIL gene therapy: from preclinical development to clinical application. Curr. Gene Ther. 2009, 9(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H.; Miller R. E.; Ariail K.; Gliniak B.; Griffith T. S.; Kubin M.; Chin W.; Jones J.; Woodward A.; Le T.; Smith C.; Smolak P.; Goodwin R. G.; Rauch C. T.; Schuh J. C.; Lynch D. H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5(2), 157–63. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti M. A.; Dougall W. C.; Smolak P. J.; Waugh J. Y.; Smith C. A.; Goodwin R. G. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997, 7(6), 813–20. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti M. A.; Smolak P. J.; Walczak H.; Waugh J.; Huang C. P.; DuBose R. F.; Goodwin R. G.; Smith C. A. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 1997, 186(7), 1165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H.; Degli-Esposti M. A.; Johnson R. S.; Smolak P. J.; Waugh J. Y.; Boiani N.; Timour M. S.; Gerhart M. J.; Schooley K. A.; Smith C. A.; Goodwin R. G.; Rauch C. T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAI. EMBO J. 1997, 16(17), 5386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A.; Sharma S.; Silverman C.; Khandekar S.; Reddy M. P.; Deen K. C.; McLaughlin M. M.; Srinivasula S. M.; Livi G. P.; Marshall L. A.; Alnemri E. S.; Williams W. V.; Doyle M. L. Temperature-sensitive Differential Affinity of TRAIL for Its Receptors. J. Biol. Chem. 2000, 275(30), 23319–25. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. The prevention of colorectal cancer by aspirin use. Biomed. Pharmacother. 1999, 53(7), 303–8. [DOI] [PubMed] [Google Scholar]
- Kim K. M.; Song J. J.; An J. Y.; Kwon Y. T.; Lee Y. J. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J. Biol. Chem. 2005, 280(49), 41047–56. [DOI] [PubMed] [Google Scholar]
- Yoo J.; Lee Y. J. Aspirin enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Mol. Pharmacol. 2007, 72(6), 1586–92. [DOI] [PubMed] [Google Scholar]
- King M. R.; Western L. T.; Rana K.; Liesveld J. L. Biomolecular Surfaces for the Capture and Reprogramming of Circulating Tumor Cells. J. Bionic Eng. 2009, 6(4), 311–7. [Google Scholar]
- Rana K.; Liesveld J. L.; King M. R. Delivery of apoptotic signal to rolling cancer cells: a novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol. Bioeng. 2009, 102(6), 1692–702. [DOI] [PubMed] [Google Scholar]
- Lee D.; Schultz J. B.; Knauf P. A.; King M. R. Mechanical Shedding of L-selectin from the Neutrophil Surface during Rolling on Sialyl Lewis x under Flow. J. Biol. Chem. 2007, 282(7), 4812–20. [DOI] [PubMed] [Google Scholar]
- McEver R. P.; Moore K. L.; Cummings R. D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J. Biol. Chem. 1995, 270(19), 11025–8. [DOI] [PubMed] [Google Scholar]
- Wojciechowski J. C.; Narasipura S. D.; Charles N.; Mickelsen D.; Rana K.; Blair M. L.; King M. R. Capture and enrichment of CD34-positive haematopoietic stem and progenitor cells from blood circulation using P-selectin in an implantable device. Br. J. Haematol. 2008, 140(6), 673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falschlehner C.; Ganten T. M.; Koschny R.; Schaefer U.; Walczak H. TRAIL and Other TRAIL Receptor Agonists as Novel Cancer Therapeutics. Adv. Exp. Med. Biol. 2009, 647, 195–206. [DOI] [PubMed] [Google Scholar]
- Johnstone R. W.; Frew A. J.; Smyth M. J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8(10), 782–98. [DOI] [PubMed] [Google Scholar]
- Jo M.; Kim T. H.; Seol D. W.; Esplen J. E.; Dorko K.; Billiar T. R.; Strom S. C. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 2000, 6(5), 564–7. [DOI] [PubMed] [Google Scholar]
- Leverkus M.; Neumann M.; Mengling T.; Rauch C. T.; Brocker E. B.; Krammer P. H.; Walczak H. Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000, 60(3), 553–9. [PubMed] [Google Scholar]
- Schlosser S. F.; Azzaroli F.; Dao T.; Hingorani R.; Nicholas Crispe I.; Boyer J. L. Induction of murine hepatocyte death by membrane-bound CD95 (Fas/APO-1)-ligand: characterization of an in vitro system. Hepatology 2000, 32(4 Part 1), 779–85. [DOI] [PubMed] [Google Scholar]
- Goetz D. J.; el-Sabban M. E.; Hammer D. A.; Pauli B. U. Lu-ECAM-1-mediated adhesion of melanoma cells to endothelium under conditions of flow. Int. J. Cancer 1996, 65(2), 192–9. [DOI] [PubMed] [Google Scholar]
- Sugino T.; Kawaguchi T.; Suzuki T. Sequential process of blood-borne lung metastases of spontaneous mammary carcinoma in C3H mice. Int. J. Cancer 1993, 55(1), 141–7. [DOI] [PubMed] [Google Scholar]
- Burdick M. M.; McCaffery J. M.; Kim Y. S.; Bochner B. S.; Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. 2003, 284(4), C977–87. [DOI] [PubMed] [Google Scholar]
- Charles N.; Liesveld J. L.; King M. R. Investigating the feasibility of stem cell enrichment mediated by immobilized selectins. Biotechnol. Prog. 2007, 23(6), 1463–72. [DOI] [PubMed] [Google Scholar]
- Tremblay P. L.; Huot J.; Auger F. A. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008, 68(13), 5167–76. [DOI] [PubMed] [Google Scholar]
- Tremblay P. L.; Auger F. A.; Huot J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene 2006, 25(50), 6563–73. [DOI] [PubMed] [Google Scholar]
- Zaifert K.; Cohen M. C. COLO 205 Utilizes E-Selectin to Adhere to Human Endothelium. Clin. Immunol. Immunopathol. 1993, 68(1), 51–6. [DOI] [PubMed] [Google Scholar]
- Gassmann P.; Kang M. L.; Mees S. T.; Haier J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell--endothelial cell interaction. BMC Cancer 2010, 10, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S.; Ullrich S.; Richter U.; Schumacher U. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br. J. Cancer 2010, 102(3), 602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginhoven T. M.; van den Berg J. W.; Dik W. A.; Ijzermans J. N.; de Bruin R. W. Preoperative dietary restriction reduces hepatic tumor load by reduced E-selectin-mediated adhesion in mice. J. Surg. Oncol. 2010, 102(4), 348–53. [DOI] [PubMed] [Google Scholar]
- McDonald B.; Spicer J.; Giannais B.; Fallavollita L.; Brodt P.; Ferri L. E. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int. J. Cancer 2009, 125(6), 1298–305. [DOI] [PubMed] [Google Scholar]
- Gann P. H.; Manson J. E.; Glynn R. J.; Buring J. E.; Hennekens C. H. Low-Dose Aspirin and Incidence of Colorectal Tumors in a Randomized Trial. J. Natl. Cancer Inst. 1993, 85(15), 1220–4. [DOI] [PubMed] [Google Scholar]
- Peto R.; Gray R.; Collins R.; Wheatley K.; Hennekens C.; Jamrozik K.; Warlow C.; Hafner B.; Thompson E.; Norton S.; et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br. Med. J. (Clin. Res. Ed.) 1988, 296(6618), 313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. J.; Rodriguez C.; Mondul A. M.; Connell C. J.; Henley S. J.; Calle E. E.; Thun M. J. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J. Natl. Cancer Inst. 2005, 97(13), 975–80. [DOI] [PubMed] [Google Scholar]
- Ruffin M. T.; Normolle D.; Vaerten M. A.; Peters-Golden M.; Brenner D. E.; Krishnan K.; Rock C. L.; Boland C. R.; Crowell J.; Kelloff G. Suppression of Human Colorectal Mucosal Prostaglandins: Determining the Lowest Effective Aspirin Dose. J. Natl. Cancer Inst. 1997, 89(15), 1152–60. [DOI] [PubMed] [Google Scholar]
- Terry M. B.; Gammon M. D.; Zhang F. F.; Tawfik H.; Teitelbaum S. L.; Britton J. A.; Subbaramaiah K.; Dannenberg A. J.; Neugut A. I. Association of Frequency and Duration of Aspirin Use and Hormone Receptor Status With Breast Cancer Risk. JAMA: J. Am. Med. Assoc. 2004, 291(20), 2433–40. [DOI] [PubMed] [Google Scholar]
- Kutuk O.; Basaga H. Aspirin inhibits TNFalpha- and IL-1-induced NF-kappaB activation and sensitizes HeLa cells to apoptosis. Cytokine 2004, 25(5), 229–37. [DOI] [PubMed] [Google Scholar]
- Lu M.; Strohecker A.; Chen F.; Kwan T.; Bosman J.; Jordan V. C.; Cryns V. L. Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin. Cancer Res. 2008, 14(10), 3168–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.