Abstract
Background
Glomus tumors are benign painful tumors of the glomus body, a thermoregulatory shunt in the digits. Glomus tumors of the fingers and toes are associated with the monogenic disorder neurofibromatosis type 1 (NF1) and are recently recognized as part of the NF1 phenotype.
Methods and Results
We report our multi-institutional experience with 15 individuals with NF1 and glomus tumors of the fingers or toes. The majority of individuals presented with at least two of the symptoms in the classic triad of localized tenderness, severe paroxysmal pain and sensitivity to cold. Appearance of the nail and finger or toe is often normal. Women are affected more often than men. Multi-focal tumors are common. There is often a delay in diagnosis of many years and clinical suspicion is key to diagnosis, although magnetic resonance imaging may be useful in some scenarios. Surgical extirpation can be curative, however local tumor recurrence and metachronous tumors are common. Three of our patients developed signs and symptoms of the complex regional pain syndrome.
Conclusions
Glomus tumors in NF1 are more common than previously recognized and NF1 patients should be specifically queried about fingertip or toe pain.
Keywords: Neurofibromatosis type 1, Glomus tumor, Glomus body, Fingertip, Complex regional pain syndrome
INTRODUCTION
Neurofibromatosis type 1 (NF1) is a common (~1/3000 birth incidence) disorder of increased tumor predisposition that arises secondary to mutations in the RAS-regulatory gene NF1.[1] Glomus tumors are rare, benign tumors of the glomus body that cause severe paroxysmal pain secondary to changes in temperature or pressure. Glomus tumors of the fingers and toes associated with NF1 [2] arise due to bi-allelic inactivation of the NF1 gene [3] and are only recently recognized as part of the NF1 phenotype. [4–5] They frequently appear as bluish subcutaneous nodules on the trunk and limbs and can be multi-focal. [6–7] The first association of NF1 and glomus tumors was published in 1938. The report described a single 13-year-old girl with features consistent with NF1 and multiple soft, dark blue nodules on her right neck, bilateral lower extremities and left heel. A lesion from the right lower leg had histologic features consistent with a glomus tumor. [8–9]
Glomus bodies are thermoregulatory shunts concentrated in the dermis of the fingertips and other peripheral sites subject to excessive cold and should be distinguished from unrelated adrenal and extra-adrenal paragangliomas, also commonly called “glomus tumors.” [10] Glomus tumors of the fingers consist of a convoluted arterio-venous anastomosis surrounded by a thick layer of modified smooth muscle cells and nerve elements (Figure 1). Glomus tumors are thought to arise from the modified smooth muscle cells of glomus bodies, although they can occur in regions where glomus bodies do not normally occur. [7] Typically, a glomus tumor of the finger presents with a triad of localized tenderness, severe paroxysmal pain (out of proportion to size) and sensitivity to cold. They have a benign clinical course. [7] However, glomus tumors of the fingers are under-recognized. One large series of sporadic glomus tumors of the fingers found that an average of 2.5 physicians (range 0–7), including psychiatrists, were consulted before the correct diagnosis was made. The duration of symptoms averaged 10 years (range 1– 40 years). [11]
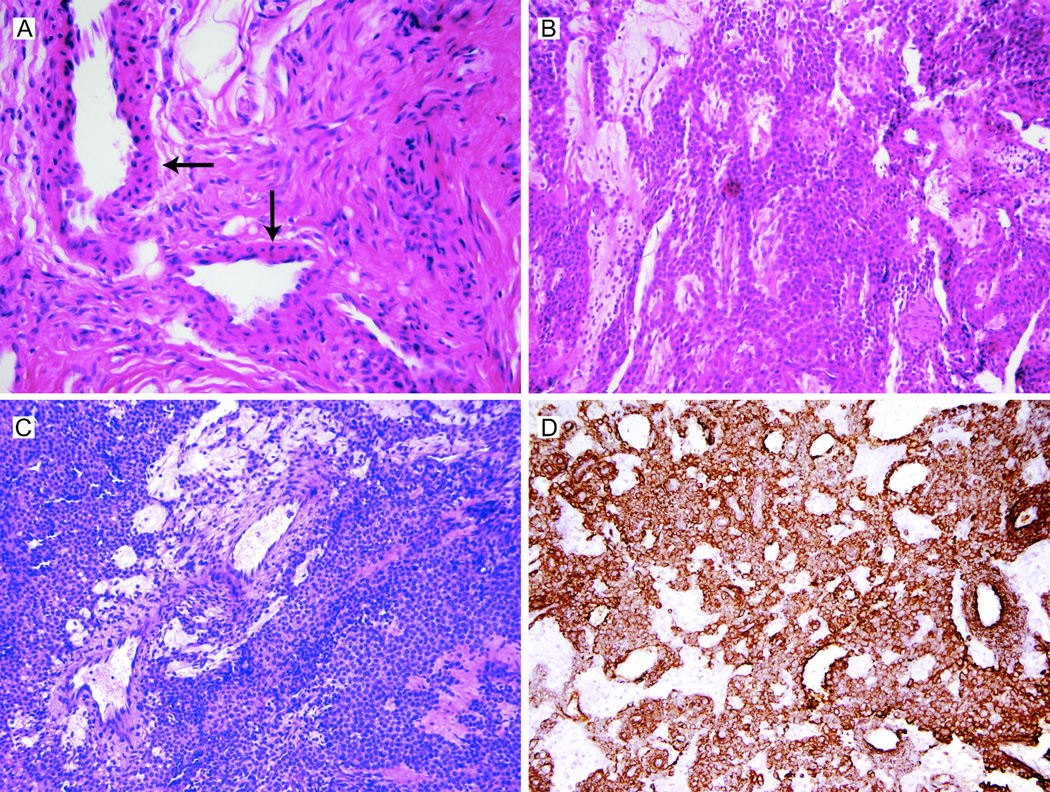
Figure 1.
(A) Normal glomus body in the left F5 of patient NIH-1. Glomus bodies are thermoregulatory shunts that arise from arterio-venus anastomoses and are enveloped by condensed collagenous tissue. The vascular lumens are surrounded by several layers of modified smooth muscle cells (glomus cells; arrows). Heat-induced contraction of the glomus body shunts blood flow through the capillary network, resulting in heat loss. Upon exposure to cold temperatures, relaxation of the glomus body opens the anastomosis, reducing shunted blood flow and conserving body heat (hematoxylin and eosin stain, 40X). (B) and (C): Glomus tumors are highly cellular benign neoplasms thought to arise from glomus cells. Grossly, they form pseudo-capsules (see also Figure S1, panel B). Histologically, the tumors are relatively well-circumscribed nodules with small dilated vascular spaces surrounded by sheets and clusters of glomus cells; the lesional cells have rounded nuclei, moderated amounts of eosinophilic cytoplasm and lack cytologic atypia, necrosis or increased mitotic activity (panel B, left F5 of patient NIH-1, hematoxylin and eosin stain, 40X; panel C, left F4 of patient NIH-2, hematoxylin and eosin stain, 20X). (D) Smooth muscle actin staining of glomus tumor from left F4 of patient NIH-2. SMA-positive staining supports a smooth muscle origin of glomus cells; see also Brems et al 2009 (smooth muscle actin stain, 20X).
Until recently, there were only eight cases of glomus tumors of the fingers and toes in individuals with NF1 in the English language literature (Table 1). [2,12–15] There were no examples of multi-focal tumors in the largest retrospective review of 51 sporadic cases of glomus tumors of the fingers. [11]
Table 1.
Summary of published accounts of glomus tumors of the fingers or toes in neurofibromatosis type 1.
| Reference | Reference patient identifier |
Gender | Age (years) |
No. years of painful fingers/toes |
No. of painful fingers/toes |
No. fingers/toes surgically explored |
No. histologically cofirmed glomus tumors |
Recurrence of tumor | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Klaber 1938 | (Single patient) | F | 13 | N/A | N/A | N/A | N/A | No comment on recurrence | First report of "glomoid tumors" of right neck, left heel and calf in "Recklinghausen's disease" |
| Park et al 1994 | Patient 2 | F | 17 | 3 | 6: Right F2, F3, F4, F5; Left F3; Right hallux | 6 | 6 | No comment on recurrence | First report in English of multiple glomus tumors in fingers in NF1 |
| Okaka et al 1999 | (Single patient) | F | 22 | 3 | 5: Right F4; Left: F2, F3, F4, F5 | 5 | 5 | "There have been no recurrences" | Symptoms resolved after surgery |
| Sawada et al 1995 | Patient 1 | M | 45 | 20 | 5: Right F2, F4, F5; Left hallux, F4 | 5 | 5 | No comment on recurrence | Surgery at 33 and 37 on spine and mediastinum did not reduce pain |
| Patient 2 | F | 28 | Not stated | 5: Right F3, F4, F5; Left F2, F5 | 1 of 5 (Left F5) | 1 | No comment on recurrence | Non-resected tumors less painful; "partial reduction" in tenderness of left F5 | |
| Patient 3 | F | 39 | Not stated | 1: Right F3 | None | (No surgery) | (No surgery) | "Partial control of pain was obtained with a combination of oral carbamazepine (600 mg/day) and a stellate block." | |
| Kim 2000 | (Single patient) | F | 34 | Not stated | 1: Right T2 | 1 | 1 | No comment on recurrence | Letter to the editor. |
| De Smet et al 2002 | Patient 1 | F | 53 | > 1 | 2: Right F4; Left F3 | 2 | 2 | No evidence of recurrence | Symptoms resolved after surgery |
| Patient 2 | M | 35 | Not stated | 2: Right F3 and F4 | 2 | 2 | No comment on recurrence | Symptoms resolved after surgery |
N/A: Not applicable
In this report, we review the published literature and describe our multi-institutional experience (15 patients) with the presentation, diagnosis, imaging, management and complications of glomus tumors in the digits of individuals with NF1.
NIH NF1 AND GLOMUS TUMOR CLINICAL EXPERIENCE
Table 2 and Table S1 summarize clinical findings from our three groups. Patients NIH-1, NIH-2, and Leu-1 through Leu-7 were previously reported in brief tabular form. [3] Leu-2 and Leu-3 were briefly summarized in De Smet et al 2002. [2]
Table 2.
Summary of individuals with neurofibromatosos type 1 and pathologically confirmed glomus tumors. CRPS : complex regional pain syndrome Patients NIH-1, NIH-2 and Leu-1 through Leu-7 previously reported in Brems et al 2009. Patients Leu-2 and Leu-3 previously reported in De Smet et al 2002.
| Patient number | Gender | Age at diagnosis of glomus tumor (yrs) |
Estimated neurofibroma burden at first glomus tumor evaluation |
No. of café-aulait macules (1.5 cm+) |
No. years glomus tumor symptoms at presentation |
No. painful finger(s)/toes |
No. fingers/toes surgically explored (grouped by surgery) |
No. histologically confirmed glomus tumors |
Comments |
|---|---|---|---|---|---|---|---|---|---|
| NIH-1 | F | 35 | 11–50 | 10 | 5 | 6 | 3+4+3 | 4:Left F4 (2 synchronous tumors), F5; Right F3 | 3 surgeries, recurrence in 1 digit, CRPS. See Figure 1. |
| NIH-2 | M | 50 | 1–10 | 17 | 20 | 6 | 3+4+3 | 7: Left F2 (twice), F4 (twice); Right F1 (twice), F4 | 3 surgeries, recurrence in 3 digits, CRPS. See Figure S1. |
| NIH-3 | F | 28 | 101–500 | 16 | 4 | 3 | 3 | 1: Left F3 | CRPS. See Figure S2. |
| NIH-4 | F | 49 | 1–10 | 14 | 40+ | 1 | 1 | 1: Left F3 | See Figure S3. |
| NIH-5 | F | 35 | Unknown | 17 | 18 | 1 | 1 | 1: Left F3 | Surgery at another institution at age 35. No recurrence |
| Leu-1 | F | 42 | 11–50 | Unknown | 1–2 | 2 | 2 | 2: right F4 and right F5 | 1 surgery, no recurrence |
| Leu-2 | M | 35 | 51–100 | Unknown | 2–3 | 2 | 2 | 2: right F3 and right F4 | 1 surgery, no recurrence |
| Leu-3 | F | 52 | 11–50 | Unknown | 1 | 2 +1 | 2+1 | 2: left F3 and right F4; 2: Left F4 | 2 surgeries with 6 year interval, no recurrence in same finger. See Figure 2 |
| Leu-4 | M | 57 | 101–500 | Unknown | 5+ | 1 | 1 | 1: right F4 | 1 surgery, no recurrence. See Figure 2 |
| Leu-5 | F | 41 | 11–50 | Unknown | 4 | 1 | 1 | 1+1: left F3 | 2 surgeries with 13 months interval (recurrence at same location) See Figure 2 |
| Leu-6 | F | 46 | 11–50 | 3 | 1–2 | 1 | 1 | 1: right F3 | 1 surgery, no recurrence, mosaic NF1 |
| Leu-7 | F | 11 | 0 | Unknown | 2–3 | 1 | 1 | 1: left F5 | Mild shortening of left fifth finger, large tumor, 1 surgery no recurrence. See Figure 3. |
| Leu-8 | F | 34 | 100–500 | Unknown | 4 | 1 | 1 | 1: left F4 | 1 surgery, short follow-up. See Figure 2 |
| Hamburg-1 | F | 25 | 20 | 10 | 5 | 2 | 1+1 | 1: left F4 | |
| Hamburg-2 | F | 57 | 450 | 12 | 1 | 1 | 1 | 1: right T1 |
As part of a natural history study of NF1, four individuals were ascertained with severe fingertip pain, a lesion on magnetic resonance imaging (MRI) that correlated with symptoms and at least one histologically proven glomus tumor (Table 2 and Table S1; Figures 1–2 and Figures S1–S3). One woman (NIH–5) underwent resection of a pathologically proven glomus tumor in a single finger at another institution but was evaluated at follow-up at NIH. Repeat surgeries were required in two patients (NIH-1 and NIH-2; three surgeries each), both of whom harbored multi-focal glomus tumors in multiple digits (Table S1). Two individuals (NIH-1 and NIH-2) had local recurrence of tumor after resection or the development of new glomus tumors in other fingers and symptoms consistent with the complex regional pain syndrome (CRPS). At two-year follow-up, one woman (NIH-3) had improvement in her glomus tumor pain after resection but had persistent neuropathic pain in her affected hand consistent with CRPS. Two women, both with single glomus tumors, were pain-free at 18 month (NIH-4) and four-year (NIH-5) follow-up. There was no correlation between café-au-lait macule burden and number of neurofibromas with the development of glomus tumors.
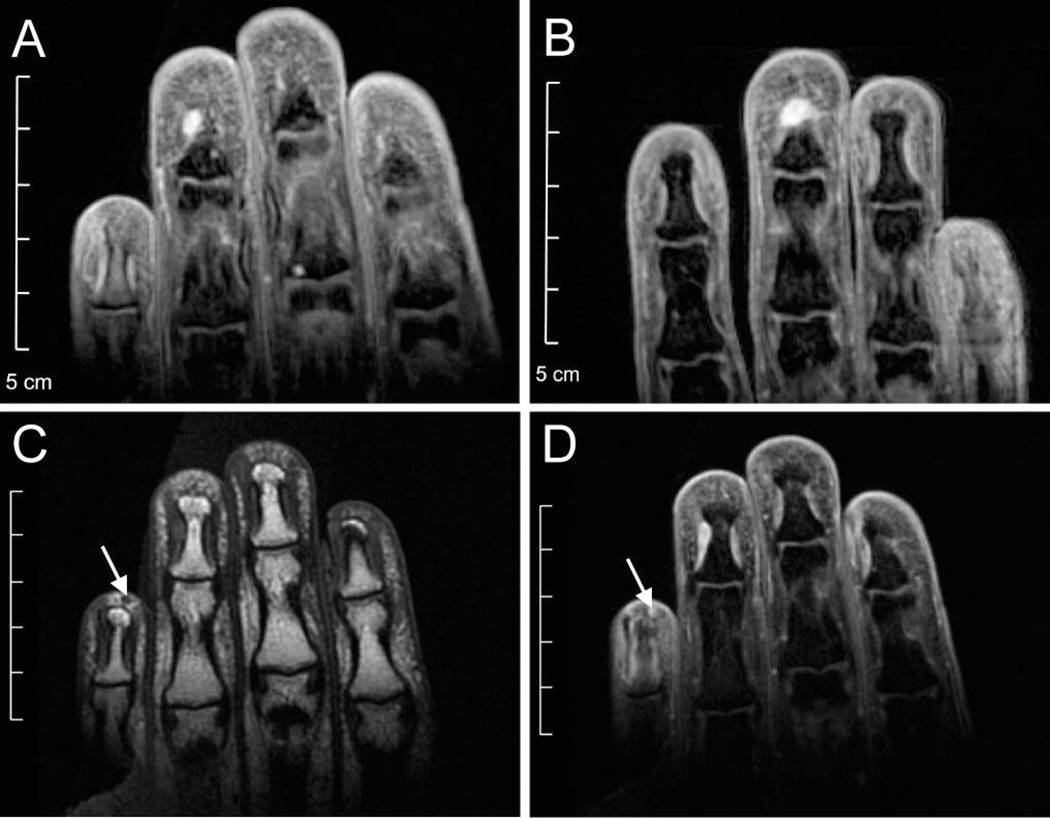
Figure 2.
MRI images from NIH–1. 35 year-old Hispanic woman with NF1 and severe pain in the third, fourth and fifth digits of both hands, exacerbated by cold temperatures, on disability. She also presented with signs and symptoms of the complex regional pain syndrome: allodynia, swelling and vasomotor changes. Over three years, she required multiple surgeries to treat recurrent and metachronous glomus tumors (Table S1). Prior to her first surgery, coronal T2-weighted MRI revealed a ~5 mm lesion in left F4 (A) and ~6 mm lesion in right F3 (B). Coronal T1-weighted pre-(C) and post- (D) gadolinium imaging revealed a ~1.8 mm lesion in left F5. All three lesions were glomus tumors on pathologic examination. No enhancing lesions were visible in other symptomatic fingers.
BELGIAN NF1 AND GLOMUS TUMOR CLINICAL EXPERIENCE
In the last ten years eight patients with NF1 and a glomus tumor of the fingertip were treated. All patients were seen by EL as part of an outpatient clinic for neurofibromatosis and were subsequently seen by EL and LDS in a multidisciplinary clinic for congenital and genetic hand abnormalities at the human genetics outpatient clinic in Leuven, Belgium. All surgeries were performed by LDS and indication for surgery was based on the typical history and clinical examination compatible with glomus tumor of the fingertip. We did not perform systematic ultra-sonography or MRI scanning before surgery. Fourteen pathologically proven glomus tumors were removed from twelve different fingers in 8 patients (Table S1). Only one finger needed a surgical re-intervention 13 months after the first surgery because of recurrence of a glomus tumor at exactly the same position as the first tumor (Leu-5). In this patient, the relapsed tumor was diagnosed clinically and was also visible by ultra-sonography but not by MRI-scan of the finger. None of our eight patients with NF1 were diagnosed with a complex regional pain syndrome after surgical treatment. With the exception of Leu-5, all patients were cured after surgery. In the case of Leu-5, all symptoms were cured after resection of the relapsed tumor. Five of the 12 affected fingers did not show any visible abnormalities at diagnosis. Two fingers showed only a slight hyperemic reddish discoloration of the skin, one finger showed a small localized swelling, one a small localized swelling with a hyperemic reddish discoloration and one a localized swelling of the base of the nail bed with nail dystrophy distal from the position of the localized tenderness (Figure 3). One finger showed a split nail. The only child in the series (Leu-7; 11 years) had a markedly swollen distal phalanx of the right fifth finger with a hyperemic appearance. Radiographs of the fingers showed an impression of the tumor on the distal phalanx with erosion of the distal tuft of the phalanx (Figure 4A). The phalanges of the right fifth finger and metacarpal appeared osteoporotic (Figure 4B and 4C) and the affected fifth finger showed a mild shortening (4.5 mm) suggesting a long-standing severe problem. She was symptomatic for more than two years and protected the finger to prevent touch-induced severe paroxysmal pain.
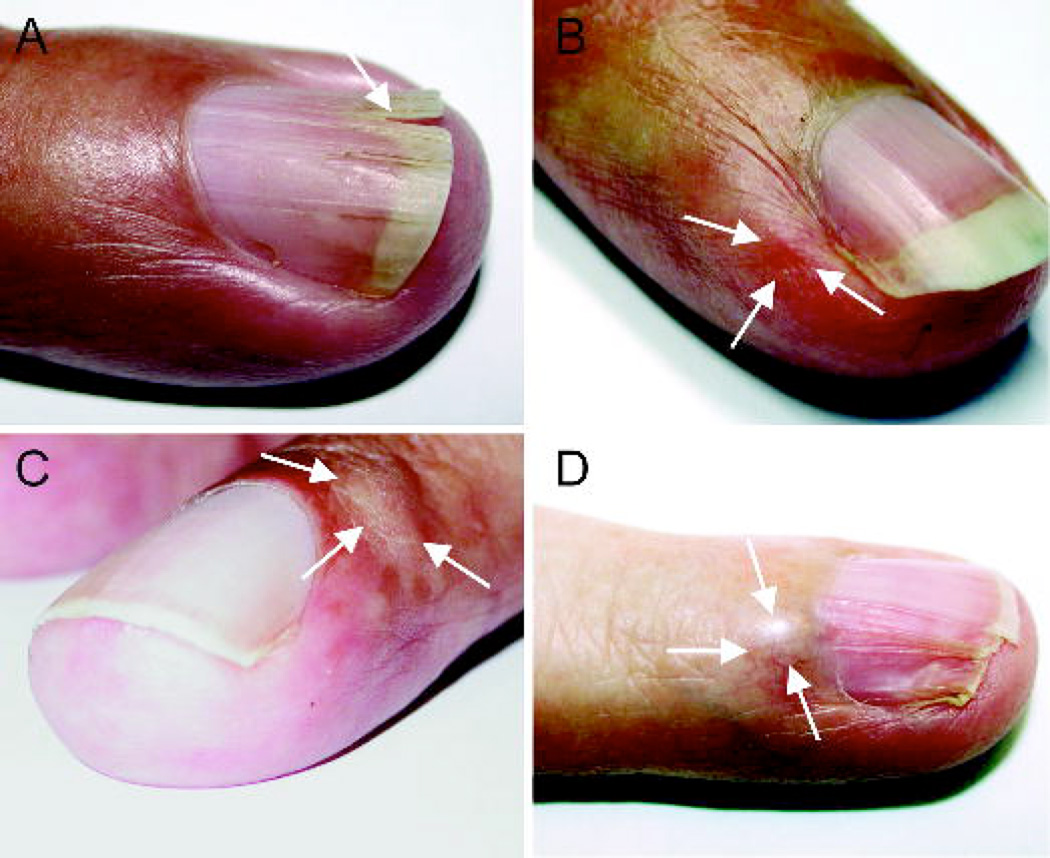
Figure 3.
Fingernail and nailbed abnormalities in four patients with NF1 and glomus tumor. Split nail of left F4 in Leu-3 (A). Reddish discoloration at radial side of left F3 in Leu-5 at the location of the tumor (B). Swelling on radial side of right F4 of Leu-4 (C). Swelling and nail deformation in left F4 in Leu-8 (D).
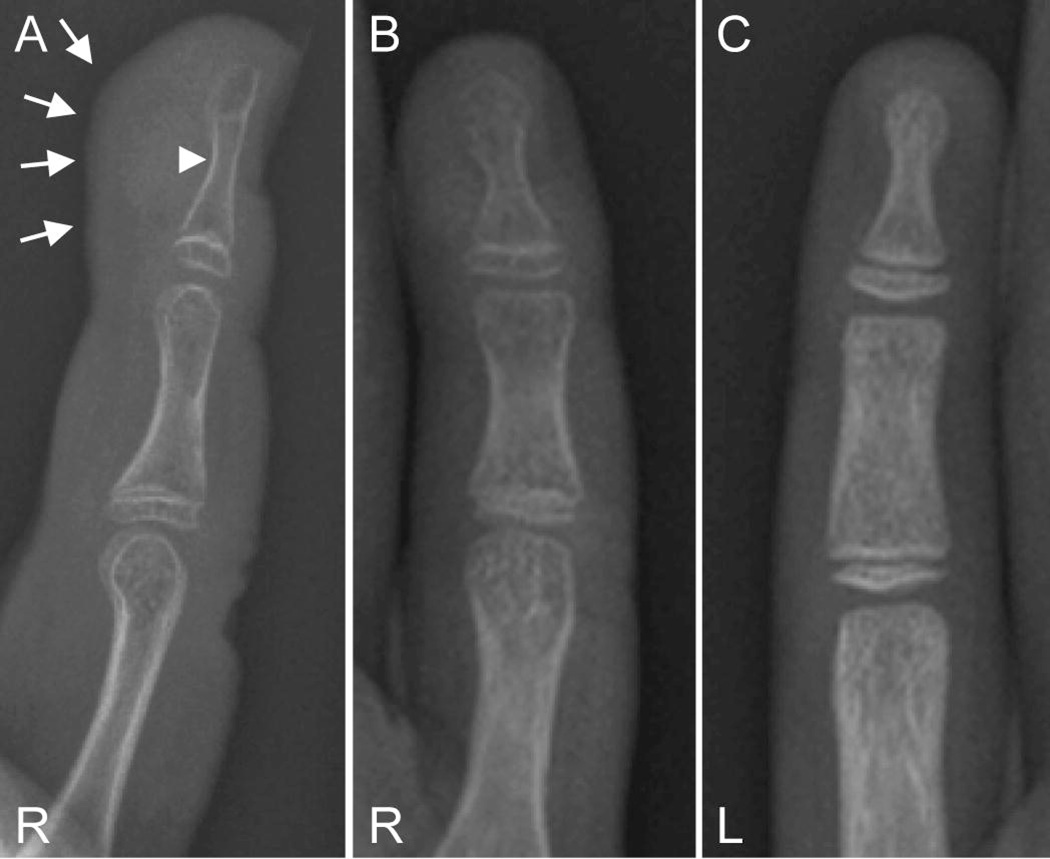
Figure 4.
Right hand radiograph of patient Leu-7 (female, age 11 years) shows soft tissue swelling (solid arrows) and erosion of the distal tuft (arrowhead) of right F5 (A and B). The distal phalanges of F5 appeared osteoporotic (B) when compared to phalanges of F5 of unaffected left hand (C).
PRESENTATION AND DIAGNOSIS
Table 3 summarizes and compares the demographic and physical characteristics of pathologically proven glomus tumors in NF1 in the published literature and in our combined experience. Recurrent tumors were excluded from the summary. We also summarize data from two large recent surveys of sporadic glomus tumors. [11,16] In both sporadic and NF1-associated glomus tumors, the average age of diagnosis and years of symptoms prior to diagnosis are comparable. The tumor affects more women than men (Table 3, “Combined NIH/Belgian/Hamburg Experience” and “Previously Reported NF1-associated Glomus Tumors”: 4 males versus 16 females; P = .006, binomial distribution). There is roughly equal distribution between the right and left hand or foot. Interestingly, the fourth (ring) and third (middle) fingers are the two-most commonly affected digits in both NF1-associated and sporadic glomus tumors. In the combined series (Table 3, “Combined NIH/Belgian/Hamburg Experience” and “Previously Reported NF1-associated Glomus Tumors”) the frequency of affected fingers are not equal (Chi square, P < .014), however only the fourth finger is significantly more frequently affected than expected (binomial distribution, P = .04 after Bonferroni correction for multiple testing). No examples of multi-focal tumors were reported in individuals without NF1.
Table 3.
Summary of demographic and physical features of pathologically-proven sporadic and neurofibromatosis type 1-associated glomus tumors of the fingers and toes. Data on patients Leu-2 and Leu-3 excluded from "Combined NIH/Belgian/Hamburg experience" since they were previously reported[2] and thus accounted in "Previously reported NF1-associated glomus tumors." Includes data on NIH-5. Data on "Previously reported NF1-associated glomus tumors" from references listed in Table 1. Data on sporadic glomus tumors from van Geertruyden et al 1996 and Vasisht et al 2004.
| Feature | Combined NIH/Belgian/Hamburg experience in pathologically proven glomus tumors |
Previously reported NF1- associated glomus tumors (pathologically-proven) |
Sporadic glomus tumors (data combined from van Geertruyden et al 1996 and Vasisht et al 2004) |
|---|---|---|---|
| Male:Female | 2 M (15%); 11 F (85%) | 2 M (29%); 5 F (71%) | 11 M (16%); 59 F (84%) |
| Right:Left (Fingers and toes) | Right: 42%; Left: 58% | Right: 41%; Left: 59% | van Geertruyden: Right: 47%; Left: 53%; Vasisht: Right: 57%, Left: 42% |
| Location of tumor | |||
| Thumb (F1) | 1 (5%) | 2 (10%) | 17 (24%) |
| F2 | 1 (5%) | 3 (14%) | 8 (11%) |
| F3 | 6 (32%) | 5 (24%) | 18 (26%) |
| F4 | 8 (42%) | 7 (33%) | 18 (26%) |
| F5 | 3 (16%) | 4 (19%) | 9 (13%) |
| Finger Totals | 19 (100%) | 21 (100%) | 70 (1005) |
| Toes | 1 | 1 | 0 |
| Patients with multi-focal tumors | 3/15 (20%) | 5/6 (83%) | None |
| Years of symptoms prior to diagnosis | 1 to 40+ years | Up to 20 years | 1 to 40 years |
| Average age of diagnosis (yrs) | 40 (combined, range 11–57); 54 (M, range: 50–57); 38 (F, range 11–57) | 33 (combined, range 17 – 53); 40 years (M, range: 35–45); 31 years (F, range: 17–53) | van Geertruyden: 44 (combined, range 26–83); Vasisht: 43 (combined, range: 14–95); 40 (M, range: 28–72); 43 (F, range: 14–95) |
History
In the eight published descriptions (Table 1) of glomus tumors of the fingers associated with NF1 (hereafter, unless noted, “glomus tumors”), all reported at least one element of the classic glomus tumor triad: localized tenderness, severe paroxysmal pain and sensitivity to cold. In our experience, patients, if asked, will have at least two or more features of the triad. Paradoxically, patients may have lived with the pain for so long (in our series, up to 40 years) that they need to be specifically asked about these symptoms. The tenderness is typically continuous and is exacerbated by exposure to cold (or less commonly, warm) temperatures, which can be as minor as holding a cold drink or reaching into a refrigerator. Patients may wear multiple pairs of gloves or mittens in the cooler months to insulate their fingers. The severe paroxysms of pain can be short-lived (< 1 minute) but are debilitating and frightening and patients can require considerable time (hours) to recover from them. Paroxysms can be triggered by minor everyday vibration (riding a bicycle) and use (typing on a keyboard). Patients are protective of their fingers and may be reluctant to shake hands. The pain is typically non-radiating and localized to a particular (ulnar/radial/central) aspect of a finger. If the pain radiates, it is often limited in extent to the elbow. The distinguishing feature of glomus tumor pain is its severity and location; the quality of the pain is akin to slamming a heavy drawer on a finger. Out of frustration for a lack of diagnosis (and thus effective treatment), we have had patients request amputation of the affected fingers.
Physical exam
On physical exam, the nail and pulp of the affected fingertip is often entirely normal. Patients may guard the affected finger since even a gentle exam is likely to elicit paroxysms of pain. The location of glomus tumors may either be subungual or in the pulp of the fingertip. In a large series of sporadic glomus tumors, a blue discoloration of the nail was observed in 43% of subungual tumors and 10% of tumors of the pulp. [11] In the same series, a nail deformation was noted in 47% of subungual tumors. We observed no examples of bluish discoloration of the nails and three examples (NIH: 1; Belgium: 2) of nail abnormalities in our patients. Tumor nodules are infrequently palpable on exam. However, the location of the tumor can be mapped with some precision by applying gentle pressure from the head of a sterile straight pin (Love’s test). [17] Love’s test has a sensitivity of 100% and 78% accuracy. [18] Other useful physical exam techniques include Hildreth’s test, (application of a tourniquet to the base of an affected finger and repeating Love’s test; pain should be abolished) and the cold-sensitivity test (placing affected hand in cold water). Hildreth’s test has a sensitivity of 71.4%, a specificity of 100% and accuracy of 78% and the cold-sensitivity test has a sensitivity, specificity and accuracy each of 100%. [18]
Imaging
There is an extensive literature on the imaging of glomus tumors. [19–21] In one large series, plain radiography revealed a bony defect in the distal phalanx in 36% of patients, [11] and may be a useful adjunct given sufficient clinical suspicion. Four of our patients (NIH–1, NIH–2, NIH–3, Leu-7) had plain radiographs of the hands. Of the eight fingers in these four patients harboring glomus tumors at the time of radiography, only two (NIH–2, right F1; Leu-7, left F5) had lytic lesions consistent with glomus tumors. We have had little experience with fingertip ultrasound; it has some theoretical advantages and is reported to be useful in locating glomus tumors as small as 3 mm in size, especially if located in the pulp of the fingertip, but is operator dependent. [20] Other modalities (thermography, scintigraphy, arteriography) are no longer indicated.
High-resolution magnetic resonance imaging is capable of detecting normal glomus bodies with a T2-weighted sequence after gadolinium injection. [19] Glomus tumors are proliferations of glomus cells with a relative paucity of vascular lumen. Their signal is thus similar to the surrounding tissue (especially those in the highly vascular nailbed) and can be difficult to detect on T1 sequences. [20] Post-contrast imaging with gadolinium may be helpful.!!Normal glomus bodies and glomus tumors exhibit hyper-intensity on T2 sequences relative to surrounding reticular dermis. Glomus tumors are delimited by a pseudocapsule that forms as a reaction to the surrounding connective tissue. The capsule is best seen on T2-weighted images or on three-dimensional gradient echo images. [20] The NIH imaging protocol includes coronal STIR, axial T1 spin-echo, axial fast spin-echo, T2-weighted and 3D gradient echo post-contrast imaging in axial and coronal planes with a dedicated receive-only wrist coil.
The usefulness of MRI in the diagnosis of glomus tumors is controversial. [21] In one study of 42 individuals with glomus tumors symptoms undergoing hand surgery, 40 had histologically proven glomus tumors. All had MRI imaging pre-operatively. MRI had 90% sensitivity, 50% specificity, a positive predictive value of 97% and a negative predictive value of 20%. The low negative predictive value arose from patients with smaller (2–3 mm) tumors with a lack of clear delineation. [21] The authors note that a glomus tumor of the hand is a clinical diagnosis and that compelling evidence to use pre-operative MRI imaging is lacking.
The NIH experience with the use of MRI to diagnose glomus tumors in NF1 is summarized in Table S1. In classic, uncomplicated presentations of glomus tumor symptoms (e.g. NIH–4 and the initial surgery for NIH–1 and NIH–2), we observed a perfect correlation among clinical impression, MRI imaging and pathology. In these cases, the clinical exam was likely sufficient to diagnose and even plan surgical resection. However, in more complicated scenarios like patients with the complex regional pain syndrome (CRPS; e.g. NIH–3 and second and third surgeries of NIH–1 and NIH–2), the predictive power of the clinical exam declines, and the usefulness of MRI increases. In these cases, we found that MRI correctly predicted the pathological diagnosis of glomus tumor with greater sensitivity than the clinical exam. In patients with CRPS many fingers (indeed the entire hand or limb) hurt, making it difficult for the patient and examiner to distinguish “glomus tumor” pain from “CRPS” pain. In CRPS, where avoidance of additional “pain generators” (like surgical exploration) is paramount, MRI can be useful to avoid unnecessary procedures.
In our experience, glomus tumors on MRI tend to be nodular and exhibit hyperintensity on T2-weighted imaging. A capsule or pseudo-capsule was not typically appreciated on T2 imaging. Lesions tend to be isointense and poorly visualized on non-enhanced T1 imaging. Glomus tumors exhibited a variable degree of enhancement on T1 post-contrast imaging, with most lesions showing mild to moderate enhancement, but some lesions remaining isointense to the surrounding, enhancing nailbed. Potential lesions that exhibit focal hyperintensity only on a STIR or T2 sequence (e.g. NIH–1, left F3, surgery number 2) but without correlative findings on other sequences are more likely to be false positives. In addition, it is important to remember that multiple glomus tumors may be present in one finger (Table S1: NIH–1, left F4, surgery 1, procedure 1; Leu-3, left F4, surgery 2, procedure 1). Evaluation of the post-operative nail bed may be hampered by artifact, and requires careful comparison to prior imaging exams.
MANAGEMENT
For symptomatic glomus tumors, surgical extirpation is the only effective treatment and can be curative. Two surgical approaches are used, depending on the location of the tumor: direct trans-ungual excision (for sub-ungual tumors) or a lateral sub-periosteal approach (for both sub-ungual tumors and tumors of the pulp). [16] The lateral approach may reduce the risk of nail deformity but may afford a more narrow view of the tumor bed, and thus a higher risk of incomplete excision and thus recurrence. [22] Either can be performed under local anesthesia with or without sedation. Although typically small (<5 mm), glomus tumors of the fingers have a pseudocapsule, making excision relatively easy. The tumors are frequently apposed to the bone, necessitating bony curettage to reduce the chance of recurrence. Nail deformities are seen in a small minority of patients especially after use of the transungual approach. [16] Pathologically, glomus tumors are almost always benign but can be misdiagnosed as hemangiomas or venous malformations. [7]
In the Belgian and Hamburg experience, all patients brought to surgery had pathologically proven glomus tumors of the fingers or toes. In the NIH experience, three patients (NIH-1, NIH-2, and NIH-3; Table S1) had, concurrent with successful tumor resection in other digits, fingertip explorations in which no glomus tumor was found. However, all three patients had multiple painful fingertips due to the complex regional pain syndrome (CRPS), lack of imaging of the painful but non-glomus tumor finger, and/or atypical MRI abnormalities (see “Recurrence of pain”, below).
COMPLICATIONS
Recurrence of tumor
In the majority of published cases of glomus tumors in NF1 (Table 1), no comment is made on the recurrence of tumor following surgery. In six large published series (n = 12 to 51 patients) of sporadic glomus tumors of the fingers, the recurrence rate, when noted, ranged from 0% to 33.3%. [16,18,23] Early recurrence may occur within weeks to months of surgery, and presumably reflects inadequate excision. Later recurrence (years) is probably the result of the development of a new tumor. [16] Three of our patients had repeat surgery on five fingers with histologic evidence of tumor recurrence within 13 months of the initial procedure (Table S1: NIH–1: left F4; NIH–2: left F2 and left F4 and right F1; Leu-5: left F3). In four of the five fingers, bony involvement or erosion was noted on initial pre-operative MRI imaging or at time of the first surgery. Recurrence occurred despite bony curettage at the time of initial operation.
Recurrence of pain
In our experience, recrudescence of glomus tumor symptoms may be due to recurrence of tumor (above), growth of a new tumor in a previously unaffected finger (e.g. NIH–2: right F4) or the development of the complex regional pain syndrome (CRPS), which we diagnosed in three patients (NIH–1, NIH–2, NIH–3). Of these three causes, CRPS is the most difficult to treat.
CRPS is a poorly understood chronic pain syndrome affecting one or more limbs. It has a variable course and a heterogeneous pathogenesis. [24] CRPS is classified into type 1 (no known inciting injury; formerly known as reflex sympathetic dystrophy, RSD) and type 2 (known or suspected inciting injury, formerly known as causalgia). We diagnosed three patients (NIH–1, NIH–2, and NIH–3) with CRPS type 2 prior to excision, suggesting that the syndrome arose secondary to the glomus tumors, and not surgery. Patients presented with allodynia, hyperesthesia, dysesthesia, edema, sudomotor and vasomotor changes primarily affecting one limb, a clinical picture not consistent with neurofibromatous neuropathy, an uncommon but wellrecognized neurologic complication in NF1. [25–27] To our knowledge, these three patients are the first reported with CRPS in NF1 and the first with CRPS secondary to glomus tumors of the fingers. One case report described reflex sympathetic dystrophy in a female with a glomus tumor of the leg. [28]. Individuals with NF1 may be at an increased risk to develop chronic pain syndromes like CRPS since neurofibromin (the protein product of NF1) plays a key role in the excitability regulation of nociceptive sensory neurons. [29]
Avoidance or elimination of chronic inciting “pain generators” in CRPS is critical. Specifically in the case of glomus tumors, this is difficult to achieve in practice since the tumors are under-recognized and patients may have symptoms for years or decades before proper diagnosis. Thus, we advocate prompt removal of symptomatic glomus tumors. Similarly, in patients with a previous resection of glomus tumors, MRI of recurrent or newly painful fingers may help to distinguish between true tumor recurrence and the development of CRPS.
Lastly, prevention is the best strategy in managing CRPS. Although the prevalence of glomus tumors in NF1 is not known, it is likely to be higher than previously supposed. To screen for glomus tumors of the fingers, we suggest simply asking all individuals with NF1 about chronic, cold-sensitive fingertip pain and mechanical allodynia.
SUMMARY AND RECOMMENDATIONS
From our experience, we advocate the following:
Glomus tumors of the fingers (and, more rarely, toes) are associated with NF1 and are part of the tumor spectrum of the disorder. Recently published genetic, functional and clinical evidence shows that glomus tumors in NF1 arise secondary to bi-allelic inactivation of NF1. [3]
Glomus tumors in NF1 are more common than previously suspected. Although some of our patients were evaluated following specific referral, many were not, and were diagnosed because we specifically asked about fingertip pain. From our experience, we estimate that glomus tumors of the fingers may affect up to 5 % of the adult NF1 population. Their prevalence in toes is unknown but much lower than that of fingers.
Glomus tumors can cause considerable morbidity and screening efforts should be established. In our experience, glomus tumors profoundly affect quality of life (disability, CRPS) and, in the published literature, can result in unnecessary surgery (see patient 1 from Sawada et al 1995). A simple question (“Do the tips of your fingers ever hurt, especially when cold or bumped?”) should be asked as part of the routine care of adults with NF1.
The natural history of sporadic and NF1-associated glomus tumors is similar (presentation, recurrence of tumor), with some important differences. Specifically, individuals with NF1 can present with multiple tumors in single or multiple fingers. There may be theoretical reasons why individuals with NF1 are at an increased risk to develop CRPS.
Glomus tumors are a clinical diagnosis. In our experience, in uncomplicated cases, MRI correlated highly with physical exam findings. In certain scenarios (recurrence of tumor, CRPS, surgical planning), MRI may be useful.
The establishment of glomus tumors of the fingers and toes as part of the tumor spectrum of NF1 over 100 years after von Recklinghausen’s description exemplifies the need for open-mindedness and creativity, even when evaluating familiar phenotypes. It is our hope that greater awareness of glomus tumors in NF1 leads to improved care.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Supplementary Material
Chronology, clinical impression, MRI imaging, operative findings and pathology results of 14 individuals with NF1-associated glomus tumors.
MRI images from NIH–2. 50 year-old Caucasian man with NF1 and a 20 year history of severe progressive pain in his left arm and hand (F2 and F4), resulting in disuse atrophy. He also had a 5-year history of similar pain in the right thumb. Physical exam showed markedly swollen left hand, brawny skin changes and allodynia, consistent with the complex regional pain syndrome. Over 16 months he required multiple surgeries to resect multiple recurrent and metachronous glomus tumors (Table S1). He died 18 months after the first surgery from a multiforme glioblastoma. Coronal T1 post-gadolinium MRI revealed a lesion in the right thumb (A), matching the location at surgery (B, after nail removal) of an encapsulated pathologically proven glomus tumor. Axial T2-weighted of right F4 prior to third surgery showed a ~3.8 mm lesion (C, arrow).
MRI images from NIH–3. 28-year-old African American female with NF1 and 4-year history of pain, exacerbated by temperature changes and minor trauma, in left F3 and F4 and right F4. There were no abnormalities on physical exam or X-ray. Axial T2-weighted MRI showed ~4 mm lesion in left F4 only; pathologic exam demonstrated glomus tumor. Although her finger pain is improved, she still requires medical management of her complex regional pain syndrome two years after surgery.
MRI images from NIH–4. 49-year-old Hispanic female with NF1 and history of severe, throbbing pain, exacerbated by temperature changes and minor trauma, in left F3 since adolescence. No abnormalities on physical exam. Axial T2-weighted MRI showed a suspected glomus tumors in left F3 (arrow). Pain remains significantly improved 18 months post-operatively.
Acknowledgments
Funding
The work was supported in part by the Division of Intramural Research of the National Human Genome Research Institute (DRS), research grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0096.02, EL), the Interuniversity Attraction Poles granted by the Federal Office for Scientific, Technical and Cultural Affairs, Belgium (2007–2011; P5/25, EL), and by a Concerted Action Grant from the K.U.Leuven (EL, RS).
Footnotes
Statement as required by BMJ Publishing Group
“The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Journal of Medical Genetics and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).”
Competing interests: None
Patient Consent: Obtained
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1.Huson SM. The Neurofibromatoses: Classification, Clinical Features and Genetic Counseling. In: Kaufmann D, editor. Neurofibromatoses. Vol Vol. 16. Basel: Karger; 2008. pp. 1–21. [Google Scholar]
- 2.De Smet L, Sciot R, Legius E. Multifocal glomus tumours of the fingers in two patients with neurofibromatosis type 1. J Med Genet. 2002;39(8):e45. doi: 10.1136/jmg.39.8.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brems H, Park C, Maertens O, et al. Glomus tumors in neurofibromatosis type 1: genetic, functional, and clinical evidence of a novel association. Cancer Res. 2009;69(18):7393–7401. doi: 10.1158/0008-5472.CAN-09-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huson S. Neurofibromatosis: emerging phenotypes, mechanisms and management. Clin Med. 2008;8(6):611–617. doi: 10.7861/clinmedicine.8-6-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons ME, Russo G, Fucich L, et al. Multiple glomus tumors. Int J Dermatol. 1997;36(12):894–900. doi: 10.1046/j.1365-4362.1997.00368.x. [DOI] [PubMed] [Google Scholar]
- 7.Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132(9):1448–1452. doi: 10.5858/2008-132-1448-GT. [DOI] [PubMed] [Google Scholar]
- 8.Klaber R. Morbus Recklinghausen with glomoid tumors. Proc Roy Soc Med. 1938;31(4):347. [PMC free article] [PubMed] [Google Scholar]
- 9.Twiston Davies JH, Hellier FF, Klaber R. The glomus tumor: doubts and difficulties in diagnosis. Br J Dermatol. 1939;51(7):312–318. [Google Scholar]
- 10.Strauchen JA. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;347(11):854–855. doi: 10.1056/NEJM200209123471117. author reply -5. [DOI] [PubMed] [Google Scholar]
- 11.Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg [Br] 1996;21(2):257–260. doi: 10.1016/s0266-7681(96)80110-0. [DOI] [PubMed] [Google Scholar]
- 12.Park YH, Choi SW, Cho BK, et al. Solitary type of glomus tumor developed in multiple sites. Ann Dermatol. 1994;6(2):225–229. [Google Scholar]
- 13.Sawada S, Honda M, Kamide R, et al. Three cases of subungual glomus tumors with von Recklinghausen neurofibromatosis. J Am Acad Dermatol. 1995;32(2 Pt 1):277–278. doi: 10.1016/0190-9622(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 14.Okada O, Demitsu T, Manabe M, et al. A case of multiple subungual glomus tumors associated with neurofibromatosis type 1. J Dermatol. 1999;26(8):535–537. doi: 10.1111/j.1346-8138.1999.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim YC. An additional case of solitary subungual glomus tumor associated with neurofibromatosis 1. J Dermatol. 2000;27(6):418–419. doi: 10.1111/j.1346-8138.2000.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 16.Vasisht B, Watson HK, Joseph E, et al. Digital glomus tumors: a 29-year experience with a lateral subperiosteal approach. Plast Reconstr Surg. 2004;114(6):1486–1489. doi: 10.1097/01.prs.0000138752.36175.d5. [DOI] [PubMed] [Google Scholar]
- 17.Love JG. Glomus tumours: diagnosis and treatment. Mayo Clinic Proceedings. 1944;19:113–116. [Google Scholar]
- 18.Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg [Br] 2002;27(3):229–231. doi: 10.1054/jhsb.2001.0746. [DOI] [PubMed] [Google Scholar]
- 19.Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507–515. doi: 10.1148/radiology.195.2.7724775. [DOI] [PubMed] [Google Scholar]
- 20.Drape JL. Imaging of the tumors of the perionychium. Hand Clin. 2002;18(4):655–670. doi: 10.1016/s0749-0712(02)00046-x. discussion 71. [DOI] [PubMed] [Google Scholar]
- 21.Al-Qattan MM, Al-Namla A, Al-Thunayan A, et al. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg [Br] 2005;30(5):535–540. doi: 10.1016/j.jhsb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 22.McDermott EM, Weiss AP. Glomus tumors. J Hand Surg [Am] 2006;31(8):1397–1400. doi: 10.1016/j.jhsa.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 23.De Maerteleire W, Naetens P, De Smet L. Glomus tumors. Acta Orthop Belg. 2000;66(2):169–173. [PubMed] [Google Scholar]
- 24.de Mos M, Sturkenboom MC, Huygen FJ. Current understandings on complex regional pain syndrome. Pain Pract. 2009;9(2):86–99. doi: 10.1111/j.1533-2500.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 25.Creange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(Pt 3):473–481. doi: 10.1093/brain/122.3.473. [DOI] [PubMed] [Google Scholar]
- 26.Drouet A, Wolkenstein P, Lefaucheur JP, et al. Neurofibromatosis 1-associated neuropathies: a reappraisal. Brain. 2004;127(Pt 9):1993–2009. doi: 10.1093/brain/awh234. [DOI] [PubMed] [Google Scholar]
- 27.Ferner RE, Hughes RA, Hall SM, et al. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1) J Med Genet. 2004;41(11):837–841. doi: 10.1136/jmg.2004.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke ED, Harris J, Fleming CE, et al. Correlation of pain with temperature and blood-flow changes in the lower limb following chemical lumbar sympathectomy in reflex sympathetic dystrophy. A case report. Int Angiol. 1995;14(3):226–228. [PubMed] [Google Scholar]
- 29.Hingtgen CM. Neurofibromatosis: The role of guanosine triphosphatase activating proteins in sensory neuron function. Sheng Li Xue Bao. 2008;60(5):581–583. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chronology, clinical impression, MRI imaging, operative findings and pathology results of 14 individuals with NF1-associated glomus tumors.
MRI images from NIH–2. 50 year-old Caucasian man with NF1 and a 20 year history of severe progressive pain in his left arm and hand (F2 and F4), resulting in disuse atrophy. He also had a 5-year history of similar pain in the right thumb. Physical exam showed markedly swollen left hand, brawny skin changes and allodynia, consistent with the complex regional pain syndrome. Over 16 months he required multiple surgeries to resect multiple recurrent and metachronous glomus tumors (Table S1). He died 18 months after the first surgery from a multiforme glioblastoma. Coronal T1 post-gadolinium MRI revealed a lesion in the right thumb (A), matching the location at surgery (B, after nail removal) of an encapsulated pathologically proven glomus tumor. Axial T2-weighted of right F4 prior to third surgery showed a ~3.8 mm lesion (C, arrow).
MRI images from NIH–3. 28-year-old African American female with NF1 and 4-year history of pain, exacerbated by temperature changes and minor trauma, in left F3 and F4 and right F4. There were no abnormalities on physical exam or X-ray. Axial T2-weighted MRI showed ~4 mm lesion in left F4 only; pathologic exam demonstrated glomus tumor. Although her finger pain is improved, she still requires medical management of her complex regional pain syndrome two years after surgery.
MRI images from NIH–4. 49-year-old Hispanic female with NF1 and history of severe, throbbing pain, exacerbated by temperature changes and minor trauma, in left F3 since adolescence. No abnormalities on physical exam. Axial T2-weighted MRI showed a suspected glomus tumors in left F3 (arrow). Pain remains significantly improved 18 months post-operatively.