Abstract
Famous people and artifacts are referred to as “unique entities” (UEs) due to the unique nature of the knowledge we have about them. Past imaging and lesion experiments have indicated that the anterior temporal lobes (ATLs) as having a special role in the processing of UEs. It has remained unclear which attributes of UEs were responsible for the observed effects in imaging experiments. In this study, we investigated what factors of UEs influence brain activity. In a training paradigm, we systematically varied the uniqueness of semantic associations, the presence/absence of a proper name, and the number of semantic associations to determine factors modulating activity in regions subserving the processing of UEs. We found that a conjunction of unique semantic information and proper names modulated activity within a section of the left ATL. Overall, the processing of UEs involved a wider left-hemispheric cortical network. Within these regions, brain activity was significantly affected by the unique semantic attributes especially in the presence of a proper name, but we could not find evidence for an effect of the number of semantic associations. Findings are discussed in regard to current models of ATL function, the neurophysiology of semantics, and social cognitive processing.
Keywords: anterior temporal lobe, concepts, face perception, semantic memory, social neuroscience
Introduction
The faces of members of our own species are one of the most salient stimuli for human beings because they carry a wealth of information critical for social interaction. When a face is familiar, our behavior is not only governed by perception of facial features, expressions, and speech articulation but also, and most importantly, what we know about that person and his/her role and status in our social environment. This knowledge determines appropriate behavior to a specific family member, friend, colleague, or the person working at the cash register of our local store. The faces of famous people represent a special class within this category. Famous people differ widely between cultures and subcultures and reflect their particular norms and values. Famous people are usually highly familiar to us and often a great deal is known about them without ever having directly interacted with them. The specific information about their attributes, accomplishments, and status in society is often unique to them.
It is these attributes that put famous people in the domain of so-called “semantically unique items” or “unique entities” (UEs), which is a term from the field of semantic memory denoting an entity (here a person) with unique conceptual and lexical associations (Damasio et al. 1996; Gorno-Tempini and Price 2001). These unique semantic associations put famous individuals into a semantic category with no other members (Grabowski et al. 2001). Semantic uniqueness therefore represents an extreme on the continuum of semantic specificity spanning from unique (Barack Obama) over less specific (politician) to nonspecific or general (human). UEs are often denoted by a proper name and include famous buildings, places, or landmarks, such as the Empire State Building, famous animals, such as “Lassie,” and notable objects, such as The Book of Kells.
Damasio and colleagues reported in the 1990s that focal anterior temporal lobe (ATL) damage led to deficits in identifying and retrieving information about UEs, most frequently observed for face stimuli but in some cases also observed for landmarks (Damasio 1989; Damasio et al. 1996; Tranel, Damasio, et al. 1997; Tranel, Logan, et al. 1997). In their model for brain processes underlying lexical retrieval, Damasio (1989) and Damasio et al. (1996) suggested that UEs may have a special role regarding their neural representation in the brain and that the retrieval of lexical information is mediated not only by perisylvian structures as the classical aphasia literature suggested but also by intermediary regions called “convergence zones.” Convergence zones serve as mediators between conceptual knowledge and lexical representations but do not contain the conceptual representations or lexical or phonetic information themselves. Within this framework, UEs such as famous faces are thought to require the highest disambiguation of perceptual features and the highest recall of pertinent contextual knowledge (Damasio et al. 2004). The authors proposed that the convergence zone for UEs lies within the demarcations of the right temporal pole, whereas the left temporal pole contains convergence zones that mediate between distributed structures supporting conceptual knowledge located in left perisylvian language areas supporting the implementation of word forms in vocalization.
Right-lateralized ATL damage usually causes a loss of feelings of familiarity and access to specific semantic information about the UE, while the left-lateralized ATL damage more often causes severe proper naming deficits (reviewed by Gainotti 2007). Further evidence for this functional lateralization of proper naming and semantic recall is found in a training study where participants learned the names and occupations of people depicted on photographs (Tsukiura et al. 2006). The authors found evidence that the left ATL mediates between person semantic information and proper names, while the right ATL links visual face information and person semantic information.
Neuroimaging studies have also reported that the ATL is sensitive to the naming or retrieval of information about famous people and landmarks (Gorno-Tempini and Price 2001; Grabowski et al. 2001). In a positron emission tomography (PET) study involving famous and nonfamous faces and buildings, Gorno-Tempini and Price (2001) found that the left anterior middle temporal gyrus (MTG) was equally active for famous faces and famous buildings. The equivalent location in the right MTG showed a small effect for famous faces but not for famous landmarks. They speculated that operations on famous faces and UEs in general elicit more activation in areas associated with the retrieval of semantic features. They hypothesized that recovery of unique semantic features place specific demands on neural processes, translating into an increased blood oxygenation level–dependent (BOLD) response. They point out, however, that the observed effects may be due to the unintended retrieval of lexical (proper names) and not unique semantic information.
A PET imaging study by Grabowski et al. (2001) confirmed the special role of UEs by asking participants to name famous faces and landmarks. They found that the left temporal polar region was significantly activated when subjects named unique people and landmarks. They attributed this effect to processes associated with the naming of UEs but not the retrieval of other semantic properties of the stimuli. This notion received further support by a lesion overlap study by Tranel (2006) who showed that patients with left temporal polar lesions performed inferior in a person- and landmark-naming task than patients with right temporal polar lesions.
An important caveat in the interpretation of the neuroimaging studies of UEs is that their task design could not distinguish between processes underlying access to the presumed unique semantic associations of the stimuli and the retrieval of their proper names (Gorno-Tempini and Price 2001; Grabowski et al. 2001). This is a general problem that arises when known entities such as famous people are used as stimulus material: the knowledge about them is assumed but not actually controlled. The activations associated with famous/UEs could therefore be due to a variety of variables including their different (unique) semantic associations and their association with a proper name. A further possible confound in studies using known stimulus materials is that participants simply know more about famous people/places than ones that they have never encountered, thus the amount of associated semantic information differs. Finally, the degree of their familiarity and distinct visual features may vary. All of these variables are difficult to control using stimulus material consisting of well-known famous people and places.
The primary goal of this study was to investigate whether brain regions known to be involved in the processing of knowledge about people and landmarks are modulated by the nature of their semantic attributes. More specifically, we tested whether the uniqueness of semantic associations, the association with a proper name, and the number of semantic associations (richness) would differentially engage the ATLs while controlling for stimulus familiarity.
Our approach was to avoid the use of stimulus materials already known to participants (famous places and landmarks) by training participants to associate new knowledge with people and places for which they had no prior knowledge. Training occurred for several days prior to scanning. We systematically varied the associations between faces/landmarks with a proper name and the amount of semantic labels associated with them. We provided semantic attributes for each person and place that either rendered them famous and/or unique (“invented television”) or attributes that are shared among many people or places (“worked in television”). We also varied the association with a proper name and the number of attributes associated with each item. In the scan session, we employed a functional localizer task involving well-known famous people and landmarks (e.g., Barack Obama/Eiffel Tower) to identify brain regions that are involved in processing famous faces and landmarks in conjunction. In the main experimental runs, participants were presented with the trained visual stimuli and performed a simple recall task on the learned material.
We reasoned that if the quality of semantic information (uniqueness) is primarily responsible for the joint activations of famous people and landmarks in the ATL shown in previous research, then items with unique semantic associations should engage shared neural substrate between people and landmarks more than nonunique items. If in past studies, the ATLs were activated because of their association with a proper name (although not always explicitly recalled as suggested by Grabowski et al. 2001), then items labeled with a proper name should drive activity in these areas. If the number of semantic associations is primarily responsible for shared neural activations of famous/UEs, then activity in these regions should be correlated with the number of semantic attributes associated with the people and landmarks. Finally, various interactions between variables are explored statistically using general linear models.
Materials and Methods
Participants
Sixteen participants without a history of neurological, psychiatric, and psychopathological disorders or present or past psychotropic medication intake based on self-report volunteered for this study. Participants' structural data were inspected for abnormalities by a clinical neuroradiologist. The data of the first participant were excluded from further analysis because pulse sequence parameters were changed in all subsequent scans; 3 participants were excluded because they did not reach the minimum performance criterion and one could not be scanned because of technical difficulties. Eleven participants were included in the final analysis (7 females; mean age: 23). All participants were right handed native English speakers with normal or corrected-to-normal vision. Informed consent was obtained according to the guidelines of the Institutional Review Board of the Temple University Philadelphia. Every participant received monetary compensation for participation or academic course credits. All participants were naïve in respect to the purpose of the experiment and were debriefed after completion of the experiment.
Stimuli: Main Experiment
Stimuli that were presented in the training consisted of standardized gray scale photographs of 32 people and 32 buildings/landmarks. People and places were selected to have a low likelihood of being known by the average American undergraduate or graduate student but had distinct attributes that rendered them unique and famous in a European ethnogeographic region.
Two classes of information were taken from the histories of these people and places. First, “unique” information consisting of information that rendered the person a unique and outstanding member of society was taken. These items could therefore be considered unique and famous. This consisted of unique occupations (e.g., first German chancellor after the Second World War WWII) or accomplishments (e.g., invented television). Similarly, unique attributes of famous landmarks/buildings were collected (e.g., largest Roman gate north of the Alps). Second, nonunique information about people and landmarks was collected. This was defined as information that was shared among many items in the respective semantic class (e.g., had 3 children; built with marble). The completed list of stimuli consisted of 64 pictures of people and buildings/landmarks with 5–7 associated unique and nonunique facts and the respective proper names.
Stimuli: Functional Magnetic Resonance Imaging Localizer Task
To localize neural regions that have previously been associated with famous face and landmark processing (Gorno-Tempini and Price 2001; Grabowski et al. 2001), 1 run was devoted to a fame-localizer task consisting of famous people and places. In order to assure that the images of famous people and landmarks were well known to our study cohort, the stimuli were pilot tested by presenting 9 paid volunteers with a total of 549 pictures of famous people and landmarks. From this set, we chose the 20 most highly recognized faces and the 20 most recognized landmarks. These items were correctly identified by all participants. This was compared with a control condition consisting of 20 nonfamous faces and 20 nonfamous landmarks that were taken from publically available sources on the internet. One-half of these were familiar, having been seen by the participants during their training session but had no semantic or proper name associations. An additional baseline control condition consisted of 10 people and 10 landmarks that were rendered unrecognizable using a combination of high- and low-pass filters in Adobe Photoshop.
Training
Before training, we assessed whether participants were indeed naïve about the stimulus material by presenting them in a slide presentation on a computer. Subsequently, all picture stimuli were presented with their respective semantic associations. Participants were instructed to indicate whether the information rendered them unique and/or famous to assure that they were aware of the semantic distinction between famous and nonfamous stimuli. Ten pictures of faces and landmarks, respectively, were presented without any associations and were later used in the localizer task as nonfamous but visually familiar images.
During training, participants viewed slides containing either a person or a place, with or without associated information. The information consisted of short factual statements as in the examples above. Three factors were varied by manipulating this information: 1) uniqueness, 2) presence or absence of a name, and 3) semantic richness (defined as the amount of semantic information; in this case, 1 or 4 factual statements). The word count was controlled between corresponding unique and nonunique conditions.
Participants were instructed to learn the information that was presented with each item. When no information was presented, they were asked to look at the image on the slide. Each slide was viewed for 10 s, and participants indicated on a sheet whether the associated information rendered the person or place famous and/or unique. This manipulation was introduced to assess whether the information selected for each item was suitable to produce unique and nonunique stimuli. At the end of the first session, the full stimulus set was presented twice in a slide presentation.
Participants were subjected to a 3-day training regime. On the evening of the first day, participants completed another 15-min training session. The following training sessions consisted of a recall test (recall session) where participants were presented with the images for 10 s without the associated information. During these 10 s, they recalled the learned information and afterward received a feedback slide containing the complete information (10 s) during which they had the opportunity to learn the information that was not recalled correctly. Participants completed 4 recall sessions during the following 2 days that were at least 6 h apart. On the day before the scan, participants were invited into the lab for a test session in which their recall performance was assessed. Participants who recalled less than 70% of the proper names or items were excluded from the study. The remaining participants were asked to complete another short training session right before scanning.
Functional Magnetic Resonance Imaging Design
Experimental Design: Functional Localizer
The scanner protocol consisted of 1 functional localizer run and 4 main runs. During the functional localizer, 3 types of stimuli were presented: famous, nonfamous, or baseline. During a given block, 2 stimuli were presented in succession (0-s interstimulus interval), for the duration of 4 s for each stimulus. Each blocked presentation was preceded by a 2-s prompt with a brief reminder of the instruction and followed by a 2-s response prompt. Blocks consisted of pairs of pictures that were both famous (10) (the numbers in brackets correspond to the number of blocks of each condition presented during 1 localizer run), both nonfamous (10), mixed (famous and nonfamous) (3), face baseline (5), and landmark baseline (5) totaling 53 blocks and a duration of 10 min and 46 s. Participants were instructed to press a button with their left index finger when they saw either 2 famous or 2 nonfamous or 2 scrambled images in succession. They were told to press with the right index finger when it was a mixed condition. Responses were executed during a 2-s response period.
Experimental Design: Runs 1–4 (Main Runs)
The 4 “main” runs consisted of successive pictures of people and landmarks. This full factorial design had the conditions of stimulus class (faces, landmarks), uniqueness (unique, nonunique), name (proper name, no proper name), and richness (1 fact, 4 facts) resulting in 16 stimulus combinations. The assignment of the stimulus combinations to the blocks was arranged so that individual blocks (except mixed blocks) contained stimuli of the same stimulus combination. Within each run, a block either contained 2 famous/unique (16), 2 nonfamous/unique (16), or mixed blocks (2). Four blocks contained scrambled images of faces and 4 contained images of landmarks. Participants were asked to determine whether the stimuli presented in each individual block were either both famous or both not famous (left index finger) or mixed (right index finger). This decision was to be made on the basis of the information they learned about the stimuli during training. Each of the 4 runs consisted of a total of 42 blocks taking 9 min and 8 s to complete.
Imaging Procedure
Neuroimaging sessions were conducted at the Imaging Center of the Temple University Hospital on a 3.0-T Siemens Verio scanner (Erlangen, Germany) using a 12-channel Siemens head coil. Functional T2*-weighted images sensitive to BOLD contrasts were acquired using a gradient echo echo-planar pulse sequence (repetition time [TR], 2 s; echo time [TE], 19 ms; field of view [FOV] = 240 × 240; voxel size, 3 × 3 × 3 mm; matrix size, 80 × 80; flip angle = 90°) and automatic shimming. This pulse sequence was optimized for ATL coverage based on functional and anatomical data from a pilot scan where various combinations of voxel size and TE were tested (see Ross and Olson 2010) and visually inspected for signal coverage. Visual inspection of the coregistered functional image confirmed signal coverage in the ATLs in all participants. However, signal coverage was weaker in the surface gray matter of the parahippocampal gyrus (PHG) near the amygdala, the orbitofrontal cortex, and the region of the temporal lobes near the ear canals (please see the temporal signal-to-noise ratio map in the Supplementary Figure). Thirty-eight interleaved axial slices with 3 mm thickness were acquired to cover the temporal lobes. On the basis of the anatomical information of the structural scan, the lowest slice was individually fitted to cover the most ventral aspect of the inferior temporal lobes. Full brain coverage was attained in all participants.
The 5 functional runs were preceded by a high-resolution structural scan. The T1-weighted images were acquired using a 3D magnetization-prepared rapid acquisition gradient echo pulse sequence (TR, 2s. TE, 3 ms; FOV = 201 × 230 mm; inversion time, 900 ms; voxel size, 1 × 0.9 × 0.9 mm; matrix size, 256 × 256 × 256; flip angle = 15°, 160 contiguous slices of 0.9 mm thickness). Visual stimuli were shown though goggles purchased from Resonance Technologies, California. Responses were recorded using a 4-button fiber optic response pad system. The stimulus delivery was controlled by E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA) on a windows laptop located in the scanner control room.
Image Analysis
Functional magnetic resonance imaging (fMRI) data were preprocessed and analyzed using BrainVoyager software (Goebel et al. 1998). The preprocessing of the functional data included a correction for head motion (trilinear/sinc interpolation), the removal of linear trends, and frequency temporal filtering. The data were coregistered with their respective anatomical data and transformed into Talairach space (Talairach and Tournoux 1988). The resulting volumetric time course data of the localizer run were smoothed using an 8-mm Gaussian kernel, whereas the volume time courses of the main runs were smoothed at 4 mm to retain special accuracy.
It was assumed that brain processes of interest would be elicited by the task regardless of the task decision of the subject. Therefore, all blocks were included in the analysis except “mixed blocks.” A canonical hemodynamic response function was modeled for spanning the period in each block in which the face and landmark stimuli were presented.
Statistical Analysis Strategy
The first step in our analysis was performed within the demarcations of the ATLs. We defined a left- and right-hemispheric anatomical gray matter region of interest (ROI) similar to procedures laid out in Grabowski et al. (2001). In a Talairach transformed brain, we defined an axis extending midway between the ventral and dorsal (sylvian fissure) aspect of the temporal lobes and a perpendicular plane originating at the junction of the ascending ramus and the anterior horizontal ramus of the sylvian fissure. We included all voxels anterior to this plane, which included the temporal poles and the anterior sections of the superior, medial, and inferior temporal gyrus.
We identified brain regions that are involved in the processing of famous people and famous landmarks by performing a conjunction analysis (conjunction null, Nichols et al. 2005) of 2 contrasts isolating areas involved in people and landmark fame [(fFaces vs. nfFaces) ∩ (fLandm vs. nfLandm)] at a false discovery rate (FDR)–corrected threshold of (q = 0.05). We used a voxelwise FDR approach, which has been common practice in imaging research since its introduction in 2002 (Genovese et al. 2002). It should be noted that this method was recently criticized because of its inability to account for the topologically smooth distribution of the signal and the resulting interdependence of voxelwise activations that can lead to an underestimation of type I error (Chumbley and Friston 2009). This analysis was performed on voxels within the cortical gray matter of the whole brain using a gray matter mask that was created using gray/white matter segmentation procedures available in BrainVoyager. A Fixed-Effects generalized linear model (GLM) was employed because the generalization of the identified brain regions served the subsequent analysis in the same group of subjects rendering the possibility of the generalization of the results to the general population unnecessary. We also intended to keep the probability of type II errors low in order to avoid missing brain regions that have been identified in similar tasks in the past. Peak voxels within regions that survived an FDR-corrected threshold of (q = 0.05) were identified by iteratively increasing the statistical threshold. Activated voxels within a maximal cluster spread range of 10 voxels in the normalized anatomical image were selected as ROIs.
In the second step, we performed a Random-Effects GLM of our data attained in the main runs (within the regions identified by the previous conjunction analysis) of our experiment with stimulus class (2), uniqueness (2), name (2), and richness (2) as predictors. The resulting beta weights were extracted and entered in a repeated measures analysis of variance (RM-ANOVA) where the above predictors served as factors and determined main effects and interactions. In the last step, the analysis described above was performed on the whole-brain volume to explore brain regions engaged in the processing of UEs that were not identified in previous experiments.
Results
Behavior
As outlined in the Materials and Methods, we presented participants with all new face and landmark stimuli with the associated semantic information and proper names before training began. We first assessed whether participants subjectively judged items with unique semantic information as unique/famous. On average, people labeled 93.33% of items with unique descriptors as unique/famous, however, some (an average of 19.36%) of the nonunique items were labeled as unique and/or famous. This occurred most frequently with items that only had one semantic association and for labels that are often associated with famous people and landmarks but do not strictly render the items unique or famous.
Before the scan participants' recall performance was assessed. On average, 92.85% (standard deviation [SD]: 5.31%) of all semantic associations (excluding names) and an average of 90.91% (SD: 9.63%) of proper names were recalled. Recall of semantic information did not differ between the stimulus classes of faces (91.6%, SD: 6.46%) and landmarks (94.1%, SD: 4.15%) (t10 = −1.19, P = 0.26). Importantly, unique items (92.61%, SD: 4.56%) were recalled at approximately the same rate as nonunique ones (93.06%, SD: 4.38%) as evidenced by the result of a paired sample t-test (t10 = −0.468, P = 0.65). Proper names were recalled at a higher rate for landmarks (94.87%, SD: 7.82) than for faces (86.93%, SD: 7.82) (t10 = −3.13, P = 0.01). There was a small but reliable difference in the failure to recall unique items (5.97%, SD: 6.62) than nonunique items (3.13%, SD: 3.42), (t10 = 2.35, P = 0.04). However, it should be emphasized that we were only concerned with the assurance that the recall was overall high which was clearly shown.
During the localizer scan, participants identified homogeneous trials containing famous faces (93% correct, 2% omission, and 5% incorrect) and nonfamous faces (86% correct, 1% omission, and 13% incorrect), famous landmarks (84% correct, 1% omission, and 15% incorrect) and nonfamous landmarks (73% correct, 11% omission, and 16% incorrect). Mixed trials (distractors) were identified correctly in 73% (13% omissions and 14% errors) of the cases and the task on the baseline stimuli was correctly performed in 94% of all cases (2% omissions and 4% incorrect). One participant was excluded from this analysis because he/she misunderstood the instructions. Please also note that the term “correct” is relative here because some items that were in the nonfamous category may have been experienced by the subject as familiar and therefore judged as famous. On the other hand, some famous items may not have been known to participants or were depicted in an unfamiliar pose. Overall, participants were less familiar with landmarks than they were with famous people, which explains the observed difference between face and landmark trials.
Localizer Task: Activations to Faces and Landmarks
In this first step, we determined whether our data conformed to previous findings reporting a typical pattern of differences between cortical activations to faces and landmarks. For that, we performed a random effect GLM using the conditions of our localizer task (fFaces, nfFaces, fLandm, and nfLandm) as predictors of interest. We performed a contrast between all faces and landmarks [(fFaces + nfFaces) vs. (fLandm + nfLandm)] at an FDR-corrected threshold [q(FDR) < 0.05, P < 0.006] for the whole brain. The inspection of the resulting t-map (Fig. 1, Panel 1) revealed that faces relative to landmarks activated regions previously shown to be engaged in face processing, such as the medial prefrontal cortex (mPfc) of the left and right hemisphere, sections of the right ATL, the fusiform face area, the right and left posterior superior temporal sulcus (STS), and the left and right angular gyri. Landmarks engaged a typical pattern of dorsal visual stream, retrosplenial cortex, and posterior parahippocampal gyri. A similar analysis of faces and landmarks of our learned stimulus material in the main runs yielded a comparable pattern of activation (not depicted). (A fixed effects analysis GLM on the gray matter of the whole brain was performed on the main runs after a random effects GLM at q(FDR) = 0.05 failed to reveal significant differences.)
Figure 1.
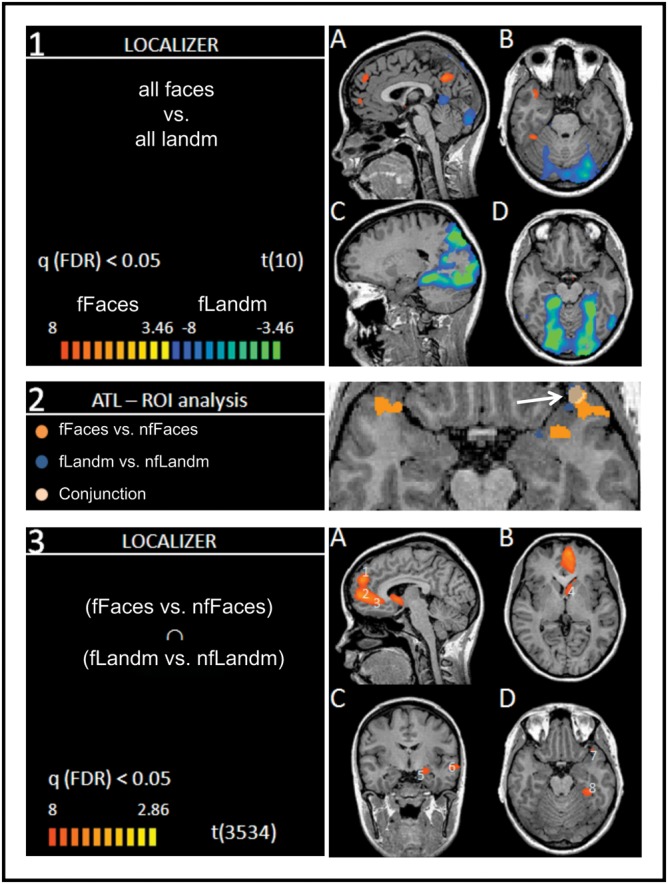
Left and right in radiological convention. Panel 1: t-map (P < 0.003) of the contrast (A,B) [(famous faces + nonfamous faces) vs. (famous landmarks + nonfamous landmarks)] of the localizer task; orange: famous faces > famous landmarks; (C,D) blue: famous landmarks > famous faces. Panel 2: t-maps within the anatomical demarcations of the ATLs. Orange: famous faces versus nonfamous faces; blue: famous landmarks versus nonfamous landmarks; off-white: conjunction [(famous faces vs. nonfamous faces) ∩ (famous landmarks vs. nonfamous landmarks)]. Panel 3: (A–D): Whole-brain conjunction analysis (t-map (P < 0.006)) from the localizer task; “fame effect” shown [(famous faces vs. nonfamous faces) ∩ (famous landmarks vs. nonfamous landmarks)]. Numbers in light blue (1–8) mark locations of ROIs used in the subsequent analysis of the main run and correspond to numbers 1–8 in the Talairach table in the Supplementary Material. Locations with significantly larger activations in nonfamous conditions are not included.
Separate contrasts for famous versus nonfamous faces and landmarks of the localizer task within the anatomical demarcations of the left and the right ATL revealed that famous faces activated the ATLs bilaterally (see Fig. 1, Panel 2). This was more extensive in the left ATL than in the right ATL. Activations due to famous landmarks were evident in a small section of the left ATL, at the polar tip. The conjunction analysis between the contrasts of famous versus nonfamous faces and famous versus nonfamous landmarks showed a significant activation in the polar aspect of the left ATL. We used this conjunction analysis to define the “conjunction” ROI for UEs.
ROI Analysis: Left and Right ATL
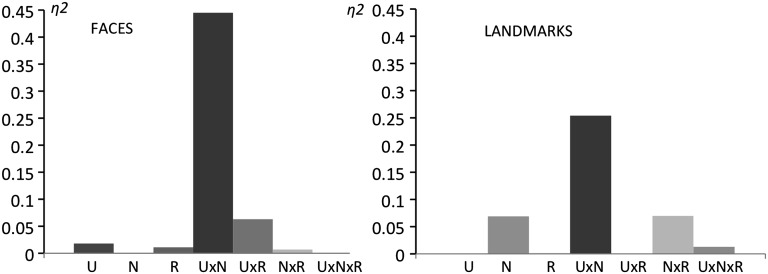
Turning to the main experimental data from the trained faces and places, a RM-ANOVA found a 3-way interaction between the factors of stimulus class, uniqueness, and naming in the left ATL conjunction ROI (F1,10 = 13.41, P = 0.004).
When separate analyses were carried out for faces and landmarks, the interaction between uniqueness and names was significant for faces (F1,10 = 8.03, P = 0.018) and approached significance for landmarks (F1,10 = 3.4, P = 0.095). For a summary of the observed effect sizes, please refer to Figure 2. Any other main effects and interactions remained insignificant in the left ATL.
Figure 2.
Effect sizes (eta squared: η2) for main effects and interactions of predictors within the conjunction ROI in the left ATL. U, uniqueness; N, names; R, richness for faces (left bar graph) and landmarks (right bar graph).
We further explored whether the left and right ATL as a whole was more responsive to faces than to landmarks. For that, we directly compared BOLD signal to faces and landmarks in the localizer task and the main runs (paired sample t-test) within the predefined demarcations of the left and right ATL. In the localizer task, famous and nonfamous faces engaged the left ATL significantly more than famous (t10 = 3.42, P = 0.006) and nonfamous landmarks (t10 = 2.66, P = 0.022). However, BOLD signal between these stimulus categories was not significantly different in the right ATL for famous (t10 = 1.148, P = 0.278) and nonfamous stimuli (t10 = 0.869, P = 0.405). The statistical comparison between faces and landmarks for the trained stimuli in the main runs was nonsignificant when performed for all faces and landmarks in the left ATL (t10 = −0.349, P = 0.735) and the right ATL (t10 = 1.004, P = 0.339).
Using the entire ATLs as a ROI for this comparison may be too insensitive, in that, one stimulus class may engage a small subsection of the left or right ATL. We therefore computed a statistical t-map (P < 0.01, uncorrected, cluster threshold = 5 voxels) of the comparison between faces and landmarks from the main runs within the left and right ATL. The t-map remained without significant differences.
Exploratory Whole-Brain Analysis: People and Landmark Fame
We reasoned that cortical areas other than the ATLs may be involved in the processing of UEs. We therefore performed a whole-brain conjunction analysis (conjunction null, Nichols et al. 2005) of the face and landmark contrast (famous vs. nonfamous). ROIs were defined within areas with significant activation for famous faces and landmarks, and the factors of our “main runs” (stimulus class, uniqueness, names, and semantic richness) were tested within those regions with an RM-ANOVA.
The conjunction analysis between the respective contrasts of famous and nonfamous faces (fFaces vs. nfFaces) and landmarks (fLandm vs. nfLandm) of the localizer task revealed a network of regions (see Fig. 1, Panel 3 and Supplementary Table). The activations for famous items showed a left-hemispheric dominance including large sections in mPfc including the superior frontal gyrus (sFG) and middle frontal gyrus (mFG) and the adjacent anterior cingulate (aC) gyrus. Further activations included the caudate head (CH), the amygdala (Amyg), and the left polar ATL as identified in our previous anatomical ROI analysis, the STS, and the PHG all within the left hemisphere. For a comprehensive list of brain regions engaged to famous and nonfamous items, please refer to the Talairach table in the Supplementary Material. As laid out in the Materials and Methods, we defined ROIs around peak voxels within regions identified in this conjunction analysis.
ROI Analysis: Uniqueness, Naming, and Richness
The result of the conjunction analysis of our localizer task did not indicate the ATLs as an exclusive site for the processing of UEs but revealed a large network of brain areas. We conducted random effects analyses GLM and a subsequent RM-ANOVAs with the factors stimulus class × uniqueness × name and semantic richness within the individual ROIs that were determined in the preceding conjunction analysis. The results of the separate RM-ANOVAs are detailed in Table 1.
Table 1.
Results of RM-ANOVAs within ROIs identified in the preceding whole-brain conjunction analysis
| Locations | lSFG | lMFG | lAC | lCH | lAmyg | lSTS | lATL | lPHG |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Main effects | ||||||||
| Class | 0.02 | 3.07 | 0.16 | 0.02 | 2.47 | 0.15 | 2.32 | 0 |
| Uniqueness | 0.18 | 14.78** | 8.22* | 1.23 | 0.27 | 4.23 | 0.09 | 0.39 |
| Name | 0.76 | 0.51 | 0.04 | 1.07 | 0.01 | 4.56 | 0.19 | 0 |
| Richness | 0.2 | 0 | 0.3 | 1.79 | 1.41 | 0.16 | 0.02 | 0.42 |
| Interactions | ||||||||
| C × U | 0.08 | 0.92 | 1.67 | 1.14 | 0.45 | 4.66 | 0.75 | 0.57 |
| C × N | 1.59 | 0.1 | 2.08 | 0.33 | 1.94 | 18.73** | 1.22 | 0.3 |
| U × N | 5.87* | 3 | 37.47*** | 0.01 | 19.97*** | 5.49* | 0 | 2.79 |
| C × U × N | 2.78 | 0.78 | 2.04 | 0.04 | 6.75* | 0.23 | 8.37* | 0.27 |
| C × R | 1.96 | 0.34 | 0.09 | 0.56 | 0.3 | 0 | 0.03 | 1.85 |
| U × R | 0.19 | 0.51 | 0 | 0.88 | 0 | 0.28 | 0 | 0.03 |
| C × U × R | 0.57 | 0.47 | 0.06 | 0.86 | 0.08 | 0.14 | 0.31 | 0.14 |
| N × R | 1.62 | 1.49 | 0.43 | 2.97 | 1.4 | 1.21 | 0.06 | 0.66 |
| C × N × R | 0.49 | 0.63 | 0.04 | 0 | 1.19 | 0.16 | 0.28 | 3.31 |
| U × N × R | 1 | 0.12 | 0.01 | 3.86 | 0.86 | 1.95 | 0.09 | 0.01 |
| C × U × N × R | 0.02 | 0.75 | 0.52 | 0.16 | 1 | 0.65 | 0.04 | 0.47 |
Note: C, stimulus class; U, uniqueness; N, proper name; R, richness; l, left hemisphere; CH, caudate head.
F values, *P < 0.05, **P < 0.01, ***P < 0.001.
The results suggest that the brain regions under investigation contribute to the processing of different aspects of UEs. We found a significant main effect of semantic “uniqueness” in the left MFG (F1,10 = 14.78, P < 0.05) and the left AC (F1,10 = 8.22, P < 0.05). In both cortical locations, there were no significant interactions with “stimulus class” suggesting that these regions have a general role in processing unique semantic information. Significant interactions between “uniqueness” and “name” were found in the left STS (F1,10 = 5.49, P < 0.05), left SFG (F1,10 = 5.87, P < 0.05), left AC (F1,10 = 37.47, P < 0.001), and the left amygdala (F1,10 = 19.77, P < 0.01). Interestingly, the factor “name” had no main effect on any of the cortical regions identified in the conjunction analysis unless this factor was combined with unique semantic information.
Further interactions were found in the left STS and the left amygdala. The existence of a name engaged the left STS, but this effect was dependent on stimulus class (F1,10 = 18.73, P < 0.01) with greater activations observed to famous faces (t10 = 2.87, P = 0.017).
Discussion
Our knowledge about people and objects is crucial in guiding our social interactions and behavior. There is a vast amount of research on brain processes underlying the perception and cognition of people and objects; however, most of it has focused on their perceptual rather than semantic properties. The general motivation for the experiment reported here was to investigate brain processes underlying the representation of knowledge about people and objects using fMRI. The more specific goal of this study was to test the UE account in which it has been proposed that the ATLs process unique semantic associations. In addition, we assessed other proposals about the function of the ATLs—that it is sensitive to proper name retrieval and/or the amount or richness of retrieved semantic information.
The Unique Entity Account is based on findings showing that focal ATL lesions cause diminished recall of knowledge about known people and places such as proper names and person-specific information (e.g., Damasio et al. 2004; Tranel 2006). Neuroimaging studies have shown greater ATL activations to the retrieval of names of famous than nonfamous people and landmarks (Gorno-Tempini et al. 1998; Gorno-Tempini and Price 2001; Grabowski et al. 2001). A problem associated with the use of previously well-known stimulus material is that it is difficult to control for the amount and quality of associated knowledge, and it therefore remained unclear which ATL process gave rise to the observed differences in BOLD response. To better understand this, we trained participants to associate different kinds and amounts of semantic information with newly learned faces and landmarks. The type of semantic information (uniqueness), the association with a proper name, and the amount of semantic information was varied.
In the following paragraphs, we will briefly summarize the main findings of this experiment and then discuss how our findings relate to well-known models of ATL function and semantic memory. Finally, we will discuss the findings of our whole-brain analysis and put ATL processes into the context of a wider cortical network.
Summary of Findings
The first step in our analysis was to determine whether we could replicate findings of differences between faces and landmarks reported in the literature (Gorno-Tempini and Price 2001; Grabowski et al. 2001). We found a typical pattern of differences in activation between faces and landmarks in the localizer run. These differences were also present in the main runs but were of much smaller magnitude. This provided assurance that stimulus material and design were appropriate for this experiment.
In the localizer run, truly famous faces and landmarks significantly engaged the same region within the left ATL in line with previous evidence showing ATL involvement in the processing of UEs such as known people and landmarks (Nakamura et al. 2000; Gorno-Tempini and Price 2001; Allen et al. 2006). In the subsequent analyses of the training task, we found that the association of faces or places with unique semantic information and proper names significantly modulated BOLD activity within the left ATL. Overall, faces affected left ATL activity more than landmarks, but this was only the case in the localizer task and not the trained stimulus material. There was no evidence for an effect of semantic richness on activity in this region.
The left ATL was not the only location in the brain that was involved in the processing of famous people and landmarks. Rather, it was one part of a larger left hemispheric network. This network is in large parts similar to the one often shown in imaging experiments using social cognitive tasks especially those involving face stimuli (e.g., Gobbini and Haxby 2007). Within this network, many regions were modulated by the presence of unique semantic information in the training stimuli. Most prominently, locations in the left medial frontal gyrus and the left anterior cingulate responded to retrieved unique semantic information, regardless of stimulus class. The left AC was particularly engaged when the stimulus material was unique and associated with a proper name. This combination of attributes activated the left amygdala, the left sFG, and the left STS.
Processes within the ATLs
The convergence zone account (Grabowski et al. 2001; Tranel 2001; Damasio et al. 2004; Tranel et al. 2005; Tranel 2006) and the HUB account (e.g., Patterson et al. 2007 ) are the most prominent theories of ATL function. They belong to a class of theories of semantic memory that propose that the neural representations underlying semantic knowledge are widely distributed throughout the brain. The modality-specific features of objects as well as their associated motor actions are represented in their respective modality-specific regions. According to these distributed models, this requires a “binding” mechanism that can simultaneously access modality-specific features when required by a task.
UEs and the Convergence Zone Account
Results from comprehensive lesion overlap studies motivated Damasio and colleagues to suggest several binding or convergence sites at various locations throughout the cortex. Convergence regions are thought to lie close to the brain regions they link, and according to this model, there are different convergence regions for unique and non-UEs. Both classes of items are thought to differ in their representational complexity with a more anterior distribution of cortical structures underlying the representation of UEs (Damasio et al. 1996). This elaboration of the convergence zone model proposes that there is some topographical specificity regarding classes of objects because of a hierarchical organization (Barsalou et al. 2003). More posterior cortical regions contain convergence zones that bind features of a specific type, whereas convergence zones in the more anterior regions combine features into complex configurations. This gradient provides the basis for making distinctions between objects on different levels of specificity. A similar idea was expressed earlier with the proposal of a topographic gradient in the representation of concepts along the rostrocaudal axis of the inferior temporal lobes (Martin and Chao 2001).
Damasio and colleagues proposed that one convergence zone is located within the left ATL, the function being to mediate between stored conceptual knowledge and the recall of proper names for UEs (Gorno-Tempini and Price 2001; Grabowski et al. 2001; Allen et al. 2006) but is not otherwise involved in general semantic knowledge (Grabowski et al. 2001; Tranel 2001; Damasio et al. 2004; Tranel et al. 2005; Tranel 2006). Furthermore, they proposed that the left ATL is specifically engaged when the task demands the recall—either explicitly or covertly—of proper names for classes of UEs, and the main reason why this region activates to UEs in imaging studies is because these, unlike nonunique objects, are denoted by a proper name.
Our results speak to this model in several ways: 1) we showed that UEs such as famous faces and landmarks activate the left ATL; 2) however, we found that explicit name recall is not required to elicit activity in the left ATL; and 3) activation within the left ATL to UEs is modulated by the interaction of unique semantic associations and proper names. This later finding supports previous lesion findings by Tranel and Damasio and suggests that this region is preferentially sensitive to unique semantic information in the context of a name. However, it may not be exclusively engaged in the retrieval of proper names.
The HUB Account and Stimulus Specificity
An alternate account for the preferential activation in the ATLs to UEs is offered by the Hub Account of semantic memory (Rogers, Hocking, et al. 2006). With evidence derived from lesion data of patients with semantic dementia with their broad amodal semantic impairments but otherwise intact cognitive function, the HUB model locates a single domain general–binding mechanism within the ATLs of both hemispheres. Like convergence zones described above, the putative hub links modality-specific representations of object attributes (McClelland and Rogers 2003). The hub differs from convergence zones in that only one region of convergence exists with only one functional specialization: the linking of attributes of all objects within the ATL. A major appeal of this account is its comprehensiveness providing a computational model on how the formation of concepts is instantiated, predicting performance patterns in neurotypical individuals and in patients with semantic dementia (Rogers et al. 2004).
The Hub Model states that the ATLs store similarity relationships between objects, allowing one to generalize across different instances and modalities. One key assumption is that the ATLs are more engaged when the task requires the differentiation of very similar specific-level concepts as compared with more general concepts that share many properties. Tasks requiring judgments about UEs would necessitate specific-level judgments (Rogers, Hocking, et al. 2006), thus explaining the pattern of findings described by Tranel and Damasio (Grabowski et al. 2001; Tranel 2001; Damasio et al. 2004; Tranel et al. 2005; Tranel 2006). Patterson et al. (2007) explain that a specific-level task such as naming the bird species in response to a picture of a robin requires the ATLs to instantiate the robin representation in a very precise manner. In contrast, naming the same item at a basic level (e.g., “bird”) only requires the hub to instantiate a gist-like bird representation. It is assumed that a stronger metabolic response occurs in tasks that require the differentiation of highly overlapping representations. This notion finds support in neuroimaging studies that show that the ATL is more sensitive to specific-level semantic comparisons as compared with more general semantic comparisons (Gauthier et al. 1997; Tyler et al. 2004; Rogers, Hocking, et al. 2006).
According to the Hub Account, activity in the ATLs to UEs is not due to the nature of the objects as belonging to certain stimulus classes but rather to the specificity of the semantic operations performed on them (Rogers, Hocking, et al. 2006). For example, identifying or naming Barack Obama would presumably elicit a stronger BOLD response in the ATLs than classifying him on a basic level (politician). Our task required participants to make a somewhat general decision as to whether stimuli belonged to the class of famous stimuli; no specific-level decisions were required. Therefore, according to the specificity explanation, our task was not suitable to elicit enhanced BOLD responses in the ATLs. Thus, it seems unlikely that the observed differences between unique and nonunique conditions were due to differences in the specificity of semantic operations.
The Hub Account, despite its comprehensive nature and ability to explain a wide variety of symptoms associated with SD has not remained without contradictory evidence. One critical assumption of the model is that the hub in the ATLs is responsible for linking features for all stimulus or concept classes (domain generality) and should not show a differential response to certain stimulus classes when attributes such as stimulus specificity are held constant. Testing this particular property of the HUB Account, a recent study showed domain-specific effects when stimulus specificity was held constant. Interestingly, enhanced responses in the ATLs were only found when study participants encoded facts about people (Simmons et al. 2009).
Our findings do not contradict the existence of a semantic hub, but they do indicate that there may be more complexity and heterogeneity to the ATLs than is widely appreciated (Martin 2009). Zahn et al. (2007) suggested that there may be a topographical dorsal–ventral gradient in the representation for multisensory versus abstract social conceptual knowledge. Indeed, activations to social stimuli in one of our previous studies (Ross and Olson 2010) and the studies by Zahn et al. (2007) and Simmons et al. (2009) were found in more dorsal sections of the ATLs, whereas domain-general semantic specificity effects have been located in ventral posterior section of the ATLs (Tyler et al. 2004; Rogers, Ivanoiu, et al. 2006; Visser et al. 2010) in a region that is most commonly referred to as perirhinal cortex. A recent study by Brambati et al. (2010) showed that task specificity engaged the left ATL in a semantic task involving known faces.
Domain generality as proposed by the Hub Account is also called into question by findings showing that there is sensory specificity within the ATL: visual stimuli tend to activate the ventral ATL, auditory stimuli activate the dorsal ATL, and multisensory audiovisual stimuli activate the polar tip (Skipper et al. 2011).
The Wider Neural Network Underlying the Processing of UEs
In the whole-brain analysis, we identified a left hemispheric network of cortical regions that were modulated by famousness in faces and places. Within these regions, the quality of the semantic information (uniqueness) was the variable most reliably affecting BOLD response. Interestingly, a region in the left anterior STS was close to the one reported by Gorno-Tempini and Price (2001) who found equivalent activation for famous faces and landmarks within this region and speculated that this region is involved in the analysis of unique semantic attributes. They point out, however, that the retrieval of semantic and lexical information may have occurred together automatically, and it is therefore possible that this region is involved in linking semantic and lexical information.
In our experiment, the activity in this region was modulated by the association with a proper name in unique items, especially people. This does not answer the question of whether this region is primarily involved in naming or other semantic processes. However, this region is unlikely to support general lexical access since we found no main effects for naming but an interaction between naming and uniqueness. It remains unclear why this region is sensitive to this interaction for faces but not places (a class by name interaction).
In our study robust modulations were observed when unique-level semantic information was associated with a proper name. This interesting interaction was observed for both faces and landmarks in the left anterior cingulate cortex (lAC), the sFG, the left amygdala, and the left STS. Proper names, even without their explicit retrieval, and associated semantic information are crucial elements in the identification process of persons and other UEs such as landmarks. They distinguish entities from one another and specify and disambiguate proper responses toward them. While our data do not speak to the question of whether networks involved in semantic and proper name recall are distinct from one another, we can say that the brain regions identified here respond preferentially to their co-occurrence, most prominently in the regions of the amygdala and the anterior cingulate. Both regions have been implicated in fear conditioning and extinction (see review by Sehlmeyer et al. 2009), reward processing (see review by Haber and Knutson 2010), the processing of affective information of faces (Killgore and Yurgelun-Todd 2004) and show abnormal functional coupling in anxiety disorders (review by Damsa et al. 2009), bipolar disorder (review by Cerullo et al. 2009), and antisocial behavior (review by Raine and Yang 2006) with functional coupling between both regions (Pezawas et al. 2005; Kienast et al. 2008). This diversity in processes engaging these brain regions raises the question of whether these regions support different functions or whether they share a common function that may underlie the effects observed in the present experiment.
A possible answer to this question comes from Adolphs (2010) who recently suggested that the amygdala is responsive to such a wide range of social and emotional stimuli because it processes “salience” or “relevance” rather than threat. This view of the amygdala and closely connected structures explains why we found that these regions are responsive to UEs with proper names. These are exceptionally salient stimuli that are relevant for social behavior and typically associated with unique identifiers like proper names. It is therefore likely that stimulus salience as delivered by the unique semantic information and proper names is responsible for the enhanced activation within the network shared by famous people and landmarks. This also explains why known famous people and landmarks used in the localizer block (which one can assume are more salient than the trained stimuli) led to a much stronger BOLD response than the stimuli used in the training stimuli.
It is possible that the engagement of the amygdala to highly salient stimuli has consequences for BOLD activation in a wider cortical network serving a modulatory role. In turn, other research has established the anterior cingulate as having modulatory influences on the amygdala (Bissiere et al. 2008). It is reasonable to assume that the identity and importance of salient stimuli is established through an intricate interplay of these regions with a wider cortical network coding stimulus and task-relevant functions. Stimulus salience as coded by these regions may therefore be an important and overlooked variable in the research concerned with social cognitive processing, particularly involving known famous entities.
This study was motivated by the question about which attributes of famous stimuli result in the activations in cortical areas that are usually reported in experiments comparing famous with nonfamous stimuli. It should be pointed out that this list of variables possibly accounting for observed differences is not exhaustive. Some of the activations in the network reported here may have been associated with differences in memory processes due to the fact that famous faces and landmarks and nonfamous items were differentially mediated through hippocampal binding. Medial temporal lobe structures such as the hippocampus play a general role in relational memory (reviewed by Davachi 2006). The hippocampus is thought to play a role in the both encoding and retrieval of associations between names and faces, for instance (Sperling et al. 2001; Kirwan and Stark 2004; Tsukiura et al. 2008) as well as between other types of associations. While there were no BOLD differences in the hippocampus evident in our analysis, we did find significant differences in BOLD response between famous and nonfamous faces and landmarks in a section of the PHG. We did not find effects within this region in our training data, and its precise role in processes underlying our task and stimuli therefore remains undetermined. However, famous and nonfamous items in our localizer task differed in regard to their episodic associations. Personal knowledge about truly famous faces and landmarks is acquired throughout life, namely conversations, media, school, and travel and is rich in episodic detail. In this regard, our trained stimuli were relatively impoverished.
UEs as Social Stimuli
While it is intuitively evident that people and the knowledge associated with them should engage social brain structures, it is less obvious why famous places and landmarks should be considered as belonging to the class of items relevant to social behavior. We mention this because famous landmarks engaged many of the core “social brain” regions that were engaged by famous faces. A closer look reveals that famous landmarks are important social artifacts in that they serve purposes relevant to social interaction in a wide spectrum of domains such as religion (churches), politics (White House), sports (Yankee Stadium), science (observatory), and art and architecture (Eiffel Tower). Furthermore, they are the subject of social interaction, receiving their salient status through social consensus.
Our research group (Olson et al. 2007; Ross et al. 2010; Ross and Olson 2010; Skipper et al. 2011), as well as other groups (Moll et al. 2005; Zahn et al. 2007; Simmons and Martin 2009; Simmons et al. 2009; Zahn et al. 2009), have proposed that the ATL is involved in the processing of social knowledge. The motivation for suggesting a social function of, at least sections of the ATLs was motivated by findings of domain-specific effects within the ATL (Zahn et al. 2007; Simmons and Martin 2009; Simmons et al. 2009) and the numerous reports of ATL involvement in imaging experiments involving social cognitive tasks. The precise nature of this social semantic function, however, warrants further investigation as it is not clear whether the role of the ATLs can be understood as a “store” of social semantic representations or whether they are instrumental in linking social semantic knowledge with the respective features located in sensory-specific areas in the form of a “person identity node” (e.g., Bruce and Young 1986; Gainotti et al. 2010). Although testing the social semantic hypothesis was not the primary goal of the experiment reported here, the data presented are generally in agreement with the idea that the ATLs have some function in the representation of social knowledge. However, this interpretation alone does not explain why the quality of the semantic information in association with a name differentially impacts left ATL social semantic processes.
A related but alternative explanation that we have alluded to above more readily explains the pattern of effects observed here. BOLD effects in the left ATL and possibly other nodes of the network identified may not directly be determined by the social nature of unique and famous stimuli or their semantic specificity but rather their intrinsic salience delivered by semantic associations. It is possible that activity in domain-general semantic mechanisms as proposed by hub and convergence models are modulated by input from the amygdala coding for stimulus salience (Adolphs 2010; Pessoa and Adolphs 2010). This is supported by a recent finding (Simmons et al. 2009) showing that sections of the ATL showing domain-specific activation to person knowledge are functionally connected to the wider social brain network, namely the amygdala, the mPFC, the posterior STS, and the fusiform gyrus.
The precise purpose of amygdala processing in coding stimulus salience and its timing within the information processing hierarchy are currently under debate. The fact that the amygdala widely projects to other brain regions suggests a broad modulatory role, affecting many cognitive functions such as attention, orientation, and memory (Adolphs 2010). In our experiment, the activation in the amygdala and other structures was significantly affected by the unique semantic attributes, especially in the presence of a unique label—a proper name. In our study, salience may have been established through “higher order” evaluation of semantic labels rather than automatic response to basic reward, threat, or emotional stimuli. This suggests that the presumed coding for salience is established by a complex interaction with brain regions serving the cognitive evaluation and storage of semantic memory like the ATL.
Caveats
It remains possible that we would have observed greater ATL activations to proper names alone, had we used an explicit naming task (Grabowski et al. 2001; Tsukiura et al. 2009; Ross et al. 2010) although it has been suggested that the mere association with a proper name may engage ATL structures automatically (Grabowski et al. 2001). On the other hand, it is possible that in prior studies of proper naming, the ATL was activated because of uncontrolled unique-level semantic information. It should be pointed out here that the ATLs may not necessarily contain the lexical–phonological representations of names themselves but may contain links between semantics and word form (Burton et al. 1990; Burton and Bruce 1992, 1993; Galdo Alvarez et al. 2009; Semenza 2009), which would be more consistent with a lack of lexical–phonological deficits in patients with ATL lesions (Snowden et al. 2004). The task that participants performed in the scanner was designed to elicit the retrieval of learned semantic attributes without explicit name retrieval. It should be noted as a possible caveat that the task may have somehow biased our results toward effects of differences between unique and nonunique associations.
Conclusions
This study was motivated by the question about the nature of semantic attributes of famous people and landmarks that affect neural activity in brain regions, such as the ATL, that have previously been associated with the processing of UEs. We found that activity within the left ATL was significantly modulated by the unique semantic attributes of visual stimuli when associated with a proper name. In addition, UEs engaged a wider left hemispheric cortical network consisting of regions widely implicated in social processing, such as the amygdala and mPfc. Activity within these regions was modulated by unique semantic attributes especially in combination with a proper name for both faces and landmarks. We propose that semantic associations rendering persons and objects salient representatives of their class are an important factor that modulates neural activity in social brain regions, including the ATL.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by a National Institute of Health (grant RO1 MH091113 to I.R.O). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Supplementary Material
Acknowledgments
We would like to thank Anjan Chatterjee's lab for helpful advice. Conflict of Interest : None declared.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28(4):457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Kyle Simmons W, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends Cogn Sci. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63(9):821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Benoit S, Monetta L, Belleville S, Joubert S. The role of the left anterior temporal lobe in the semantic processing of faces. Neuroimage. 2010;53:674–681. doi: 10.1016/j.neuroimage.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77(Pt 3):305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Burton AM, Bruce V. I recognize your face but I can't remember your name: a simple explanation? Br J Psychol. 1992;83(Pt 1):45–60. doi: 10.1111/j.2044-8295.1992.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Burton AM, Bruce V. Naming faces and naming names: exploring an interactive activation model of person recognition. Memory. 1993;1(4):457–480. doi: 10.1080/09658219308258248. [DOI] [PubMed] [Google Scholar]
- Burton AM, Bruce V, Johnston RA. Understanding face recognition with an interactive activation model. Br J Psychol. 1990;81(Pt 3):361–380. doi: 10.1111/j.2044-8295.1990.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Cerullo MA, Adler CM, Delbello MP, Strakowski SM. The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 2009;21(4):314–322. doi: 10.1080/09540260902962107. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2009;22(1):96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia. 2007;45(8):1591–1607. doi: 10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Ferraccioli M, Marra C. The relation between person identity nodes, familiarity judgment and biographical information. Evidence from two patients with right and left anterior temporal atrophy. Brain Res. 2010;1307:103–114. doi: 10.1016/j.brainres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Galdo Alvarez S, Lindin Novo M, Diaz Fernandez F. Naming faces: a multidisciplinary and integrated review. Psicothema. 2009;21(4):521–527. [PubMed] [Google Scholar]
- Gauthier I, Anderson AW, Tarr MJ, Skudlarski P, Gore JC. Levels of categorization in visual recognition studied using functional magnetic resonance imaging. Curr Biol. 1997;7(9):645–651. doi: 10.1016/s0960-9822(06)00291-0. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W. The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci. 1998;10(5):1563–1573. doi: 10.1046/j.1460-9568.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RSJ. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13(4):199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Grunder G, Cumming P, Kumakura Y, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11(12):1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21(4):1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14(7l):919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Circuits in mind: the neural foundations of object concepts. In: Gazzaniga MS, editor. The cognitive neurosciences. 4th ed. Cambridge (MA): MIT Press; 2009. pp. 1031–1045. [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4(4):310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nat Rev Neurosci. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, et al. Functional delineation of the human occipito-temporal areas related to face and scene processing A PET study. Brain. 2000;123(Pt 9):1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci. 2006;1(3):203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price CJ. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6(3):201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer's disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20(3):319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Wolk DA, Coslett HB, Olson IR. Improved proper name recall by electrical stimulation of the anterior temporal lobes. Neuropsychologia. 2010;48(12):1356–1364. doi: 10.1016/j.neuropsychologia.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza C. The neuropsychology of proper names. Mind Lang. 2009;24:347–369. [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc. 2009;15(5):645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2009;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper L, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. Forthcoming 2011 doi: 10.1016/j.neuropsychologia.2011.07.033. doi:10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127(Pt 4):860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart (Germany): Thieme Verlag; 1988. [Google Scholar]
- Tranel D. Combs, ducks, and the brain. Lancet. 2001;357(9271):1818–1819. doi: 10.1016/S0140-6736(00)05010-8. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20(1):1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Grabowski TJ, Lyon J, Damasio H. Naming the same entities from visual or from auditory stimulation engages similar regions of left inferotemporal cortices. J Cogn Neurosci. 2005;17(8):1293–1305. doi: 10.1162/0898929055002508. [DOI] [PubMed] [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR. Explaining category-related effects in the retrieval of conceptual and lexical knowledge for concrete entities: operationalization and analysis of factors. Neuropsychologia. 1997;35(10):1329–1339. doi: 10.1016/s0028-3932(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Mano Y, Sekiguchi A, Yomogida Y, Hoshi K, Kambara T, Takeuchi H, Sugiura M, Kawashima R. Dissociable roles of the anterior temporal regions in successful encoding of memory for person identity information. J Cogn Neurosci. 2009;30(2):617–626. doi: 10.1162/jocn.2009.21349. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Mochizuki-Kawai H, Fujii T. Dissociable roles of the bilateral anterior temporal lobe in face-name associations: an event-related fMRI study. Neuroimage. 2006;30(2):617–626. doi: 10.1016/j.neuroimage.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Suzuki C, Shigemune Y, Mochizuki-Kawai H. Differential contributions of the anterior temporal and medial temporal lobe to the retrieval of memory for person identity information. Hum Brain Mapp. 2008;29(12):1343–1354. doi: 10.1002/hbm.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. Processing objects at different levels of specificity. J Cogn Neurosci. 2004;16(3):351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The inferior, anterior temporal lobes and semantic memory clarified: novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48(6):1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132(Pt 3):604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.