Abstract
The neurons of the locus coeruleus (LC) fire in response to novelty, and LC activation coupled with hippocampal afferent stimulation leads to long-term depression (LTD). The encoding of novel spatial information also involves activation of dopamine D1/D5 receptors. It is unclear if, or how, the noradrenergic and dopaminergic systems interact mechanistically in processing novelty. Novel spatial exploration when coupled with Schaffer collateral (SC) test-pulse stimulation results in short-term depression at SC-CA1 synapses, which is not observed in the absence of afferent stimulation. However, activation of D1/D5 receptors under these conditions without concomitant afferent stimulation enables slow-onset depression. LTD (>24 h) is facilitated when novel exploration occurs concurrently with low-frequency stimulation of CA1. Effects are not improved by a D1/D5 agonist. Facilitation of LTD (>4 h) by coupling LC stimulation with CA1 test-pulse stimulation was blocked by a D1/D5 antagonist, however, as was habituation to the holeboard environment. Novel spatial learning during LC stimulation did not enhance LTD further, whereas D1/D5 agonist treatment enabled LTD to persist for over 24 h. These data suggest that the regulation of hippocampal LTD by the LC is supported by D1/D5 receptors and that their contribution to information storage becomes important when the thresholds for persistent LTD have not been reached.
Keywords: CA1, dopamine, hippocampus, in vivo, learning-facilitated synaptic plasticity, LTD, noradrenaline, novelty
Introduction
The ability to store novel spatial information in memory has adaptive value. Changes in an animal's spatial environment may be associated with changes in the position of scarce resources necessary for survival. An animal's ability to acquire these resources may depend on its ability to update representations of its environment in memory. The hippocampus is integral to the formation of spatial and episodic memory (O'Keefe 1978; Morris et al. 1982; Eichenbaum 1996). Persistent changes in synaptic strength at Schaffer collateral (SC)-CA1 synapses are implicated in the formation of spatial and inhibitory avoidance learning (Heynen et al. 1996; Manahan-Vaughan and Braunewell 1999; Sacchetti et al. 2002; Li et al. 2003; Kemp and Manahan-Vaughan 2004; Whitlock et al. 2006). Neuromodulatory systems may initiate or enhance the encoding of novel information by persistently modifying hippocampal synaptic strength. Noradrenaline (NA) and dopamine are 2 neuromodulators that may act synergistically to provide this learning signal (Harley 2004). Locus coeruleus (LC) activation and the resulting diffuse release of NA in the brain are associated with the experience of novel sensory events (Aston-Jones and Bloom 1981; Sara et al. 1994; Vankov et al. 1995; Kitchigina et al. 1997) and changes in reward contingencies (Sara and Segal 1991; Wittmann et al. 2007). Dopaminergic neurons, in addition to being activated by novelty (Bunzeck and Duzel 2006), are also activated by unpredicted reward and their firing suppressed by the absence of predicted reward (Hollerman and Schultz 1998).
The predominant physiological model for learning and memory is synaptic plasticity. Neurons are believed to form memory engrams through the strengthening and weakening of their synaptic connections. Long-term potentiation (LTP) and long-term depression (LTD) in the CA1 region in vivo are evoked through patterned afferent stimulation in the form of brief high-frequency (100 Hz) or prolonged low-frequency (1 Hz) periods of electrical stimulation, respectively. It is difficult to assess how closely these protocols mimic naturalistic synaptic encoding events (Albensi et al. 2007). However, we have previously shown that novel spatial exploration in the absence of patterned stimulation (but in the presence of test-pulse afferent stimulation) leads to short-term depression (STD) in the hippocampal CA1 region (Manahan-Vaughan and Braunewell 1999) and that learning-facilitated plasticity in the hippocampus is regulated by both beta-adrenoreceptors (Kemp and Manahan-Vaughan 2008b) and dopamine D1/D5 receptors (Lemon and Manahan-Vaughan 2006). Furthermore, activation of the LC facilitates hippocampal LTD (Lemon et al. 2009).
Hippocampal LTP and LTD are associated with different aspects of novel spatial information acquisition: SC-CA1 late-LTP (L-LTP) is facilitated through the exploration of an empty novel environment, whereas late-LTD (L-LTD) is facilitated through the exploration of novel objects or familiar objects in novel spatial configurations (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007; Lemon and Manahan-Vaughan 2006; Hagena and Manahan-Vaughan 2011). We refer to this phenomenon as “learning-facilitated plasticity.” It describes the ability of hippocampal synapses to respond with long-lasting synaptic plasticity to the coupling of afferent stimulation with a spatial learning experience. Different types of learning-facilitated plasticity have been observed at multiple hippocampal synapses (Kemp and Manahan-Vaughan 2008b; Clarke et al. 2010; Hagena and Manahan-Vaughan 2011).
How is the synaptic transmission of information in hippocampal neurons affected by novelty and reward signaling in a way that encodes that information into memory? To answer this question, we recorded from the SC-CA1 synapses of the hippocampus in freely moving rats. These synapses were chosen for their proposed role in the detection of spatial novelty (Lisman and Otmakhova 2001). CA1 pyramidal cells receive both direct sensory information from the cortex and sequential phase-precession information from the dentate gyrus (DG)-CA3 recurrent network. CA1 pyramidal cells may thus detect mismatches between predictions from the DG-CA3 network relayed through the SCs with actual sensory input from the cortex (Lisman and Otmakhova 2001). Activation of the LC facilitates hippocampal LTD (Lemon et al. 2009), whereas dopamine D1/D5 receptors strongly regulate learning-facilitated plasticity (Lemon and Manahan-Vaughan 2006). In the present study, we activated the noradrenergic system through stimulation of the LC and explored whether the LC interacts with the dopaminergic system in mediating hippocampal synaptic plasticity. Specifically, we examined whether dopamine D1/D5 receptors influence synaptic plasticity in the hippocampus that is facilitated by novel spatial exploration and LC stimulation.
Materials and Methods
Male Wistar rats (Charles River, Sulzfeld, Germany), 7–8 weeks old at the time of surgery, underwent implantation of electrodes and a guide cannula under anesthesia (52 mg/kg pentobarbital), as described previously (Manahan-Vaughan 1997; Lemon et al. 2009). Briefly, under sodium pentobarbitone (Synopharm, Germany) anesthesia (“Nembutal,” 52 mg/kg, intraperitoneally), animals underwent implantation of a monopolar recording and a bipolar stimulating electrode (made from 0.1 mm diameter Teflon-coated stainless-steel wire [Biomedical Instruments, Zöllnitz, Germany] attached to cardboard) into the CA1 region and a stimulating electrode into the LC. A drill hole (1 mm diameter) was made for the recording electrode, and 2 further drill holes (1 mm diameter) were made for the stimulation electrodes. On the contralateral side, 2 holes were drilled (1.2 mm in diameter) into which anchor screws were inserted. The anchor screws also served as reference or ground electrodes. The coordinates for CA1 recordings comprised 2.8 mm posterior to bregma and 1.8 mm lateral to the midline for the recording electrode and 3.1 mm posterior to bregma and 3.1 mm lateral to the midline for the stimulation electrode. The dura was pierced through both holes, and the recording and stimulating electrodes were lowered into the CA1 stratum radiatum and the SCs, respectively, for CA1 recordings. The correct location of the electrodes was established by means of the electrophysiological characteristics of the field potentials evoked. The coordinates for the LC stimulation electrode were 3.1 mm posterior and 1.3 mm lateral to lambda. This bipolar stimulation electrode was implanted in the LC (8.4 mm ventral to dura mater, entering at a 15° angle to the plane of the skull). The entire assembly was sealed and fixed to the skull with dental acrylic (Paladur, Heraeus Kulzer GmbH, Hanau, Germany).
Measurement of Evoked Potentials
After surgery, the animals were housed in single cages under a 12 h light/12 h dark light cycle and were allowed 7–10 days of recovery before the experiments began. Recordings were obtained in the CA1 stratum radiatum by stimulation of SCs. To determine the stimulus intensity that evoked field excitatory postsynaptic potentials (fEPSPs), which were 40% of the maximum, every experiment commenced with the recording of an input–output curve over a range of 10–100 μA. Test fEPSPs were evoked at a frequency of 0.025 Hz. Each time point was the average of 5 consecutive stimulations. The first 6 data points recorded served as baseline, and all data were expressed as a mean percentage ± standard error of the mean of the average baseline value. To ensure that there was no drift in the response to stimulation, control experiments were conducted in which basal synaptic transmission was evoked by test pulses and followed for the same duration as plasticity experiments. In this study, we defined LTD as a depression that endured for >4 h. Statistical evaluations were performed by using analysis of variance (ANOVA) with repeated measures, and post hoc t-tests were used to assess differences among individual time points. The level of significance was set to P < 0.05.
LC Stimulation
The current level for stimulation of the LC of each animal was chosen through a preliminary input/output assessment (referring current injection to behavioral response) performed 1 week prior to experimental recording. Initial experiments established that 200 μA of current applied within our 100 Hz stimulation protocol consistently resulted in behavioral stress to the animal as displayed by freezing and the production of fecal boli and at times a slight head twitch. By reducing the amount of current to between 60 and 100 μA, no observable behavioral stress was induced and it did not affect the amount of time spent by the animal exploring an open field, subsequent to stimulation. LC stimulation consisted of 2 trains of 100 pulses at 100 Hz with each train lasting 1 s with a 20-s intertrain interval. Stimulus strength was 60–100 μA with single biphasic square wave pulses of 0.1-ms duration per half wave.
Histology
At the end of the study, brains were removed for histological verification of electrode and cannula localization. Brain sections (16 μm) were embedded in paraffin, stained according to the Nissl method using 1% toluidine blue, and then examined using a light microscope. Brains in which incorrect electrode localization was found were discarded from the study.
Compounds and Drug Treatment
The D1/D5 receptor agonist chloro-PB (Sigma-Aldrich, St Louis, MO) and the D1/D5 receptor antagonist SCH23390 (Sigma-Aldrich) were dissolved in doubled distilled water. A volume of 5 μL of drug solution, or vehicle, was injected over a period of 5 min via a cannula implanted in the lateral cerebral ventricle. Injections were administered following 30 min of baseline recording and 30 min before holeboard exposure. The same concentration of chloro-PB has previously been shown to facilitate electrically induced STD into LTD and electrically induced STP into LTP, while the same concentration of SCH23390 has been shown to block electrically induced LTD and LTP in vivo (Lemon and Manahan-Vaughan 2006), whereby, the terms “electrically induced” plasticity and “learning-facilitated” plasticity are used to distinguish between synaptic plasticity induced exclusively by electrical stimulation and plasticity that is facilitated by the combination of novel spatial exploration with mild electrical stimulation (that would normally not induce long-lasting plasticity).
Behavioral Paradigm for Novel Spatial Exploration
To enable acclimatization, animals were always placed in the room where experiments were performed on the day before experimentation and remained in the chamber for at least 12 h before experimentation began. The recording chamber measured 40 × 40 × 40 cm. It was constructed of gray Perspex, except for the removable front wall, which was made of clear Perspex. The boxes were open at the top. The implanted electrodes were connected by a flexible cable and swivel connector to the stimulation and recording equipment; thus, the animal could move around freely in the recording chamber. Each animal was assigned one recording chamber where all experiments were conducted. Plasticity experiments in the absence of a holeboard were always performed a minimum of 8 days before holeboard experiments. In subsequent experiments, a holeboard (39.8 × 39.8 cm, washable gray plastic) was inserted into the floor of the recording chamber. This was done after baseline recordings. The holeboard was removed after 10 min of exposure. Each corner of the holeboard corner contained a hole, 5.5 cm in diameter and 5 cm deep. Objects were placed in 3 of the 4 holes. The objects differed from each other in appearance and size and easily fitted within the holes. All animals were naïve to both the holeboard and the objects.
Results
The Dopamine D1/D5 Ligands Do Not Affect Basal Synaptic Transmission at the Concentrations Tested
SC-CA1 synaptic transmission was not significantly affected by intracerebral ventricular injection of either vehicle (n = 8), the selective D1/D5 agonist chloro-PB (42 μg); 2-way ANOVA: P = 0.2455; n = 8 or the selective D1/D5 antagonist SCH23390 (30 μg); 2-way ANOVA: P = 0.4303; n = 10 (Fig. 1).
Figure 1.
The dopamine D1/D5 ligands do not affect basal synaptic transmission at the concentrations tested. SC-CA1 synaptic transmission was stable in vehicle-injected animals throughout the recording period. Basal synaptic transmission was not significantly affected by intracerebroventricular injection of a selective D1/D5 agonist (42 μg of chloro-PB) or a selective D1/D5 antagonist (30 μg of SCH23390).
Exposure to the Novel Holeboard in the Presence of Test-Pulse Stimulation of CA1 Afferents Enables STD
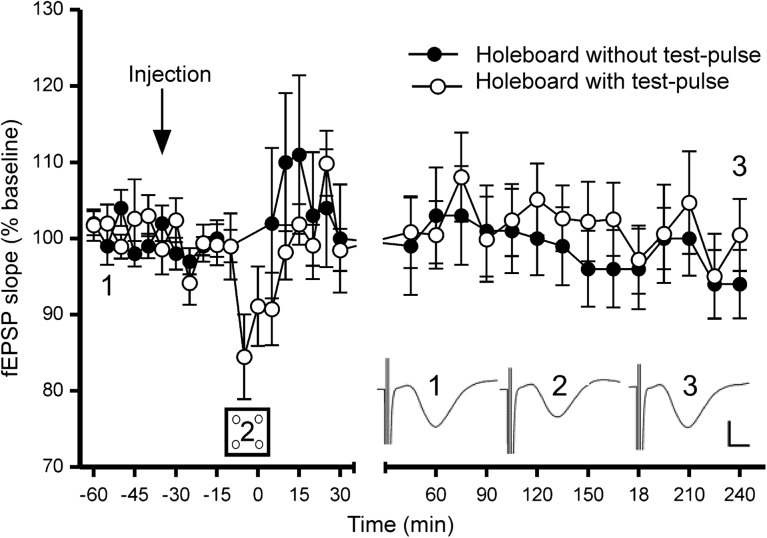
In accordance with previous reports (Manahan-Vaughan and Braunewell 1999), we observed STD (∼2 h) at SC-CA1 synapses when test-pulse stimulation was coupled with novel holeboard exploration (Fig. 2). The effect of the holeboard exploration on SC-CA1 synaptic transmission was tested on 2 groups of vehicle-treated animals. Vehicle was applied 30 min prior to exposure to the holeboard. In 1 group (n = 14), baseline recordings were taken followed by 10 min of holeboard exploration in which no test-pulse stimulation was given during exploration. In a second group (n = 9), the same recording procedure was used with the exception that during the 10 min of holeboard exploration, test-pulse stimulation was administered. Exploration of a holeboard containing novel objects for 10 min in the absence of test-pulse stimulation resulted in no significant change in SC-CA1 synaptic strength after the holeboard was removed (n = 14) (ANOVA; Fisher's least significant difference LSD; P = 0.684). However, in the group that underwent test-pulse recording during holeboard exploration, a significant depression was observed in the first 15 min of exploration (Fig. 2) (n = 9) (Fisher's LSD; P = 0.0025). This suggests that a coincidence of afferent stimulation and novel spatial exploration results in short-term synaptic depression.
Figure 2.
Exposure to the novel holeboard in the presence of test-pulse stimulation of CA1 afferents enables STD. SC-CA1 synaptic strength was not significantly altered after 10 min of holeboard exploration in vehicle-treated animals. Vehicle was applied 30 min prior to exposure to the holeboard (signified by arrow). Test pulses delivered during holeboard exploration detected STD at SC-CA1 synapses during the first 5 min of exploration. In the absence of test-pulse stimulation, an STD occurred following spatial exploration. Insets: Analog traces representing SC-CA1 field potentials during 1) baseline, 2) holeboard exploration, and 3) 4 h after holeboard exploration. Calibration: vertical bar, 1 mV; horizontal bar, 3 ms.
Holeboard Habituation Is Prevented by Antagonism of D1/D5 Receptors
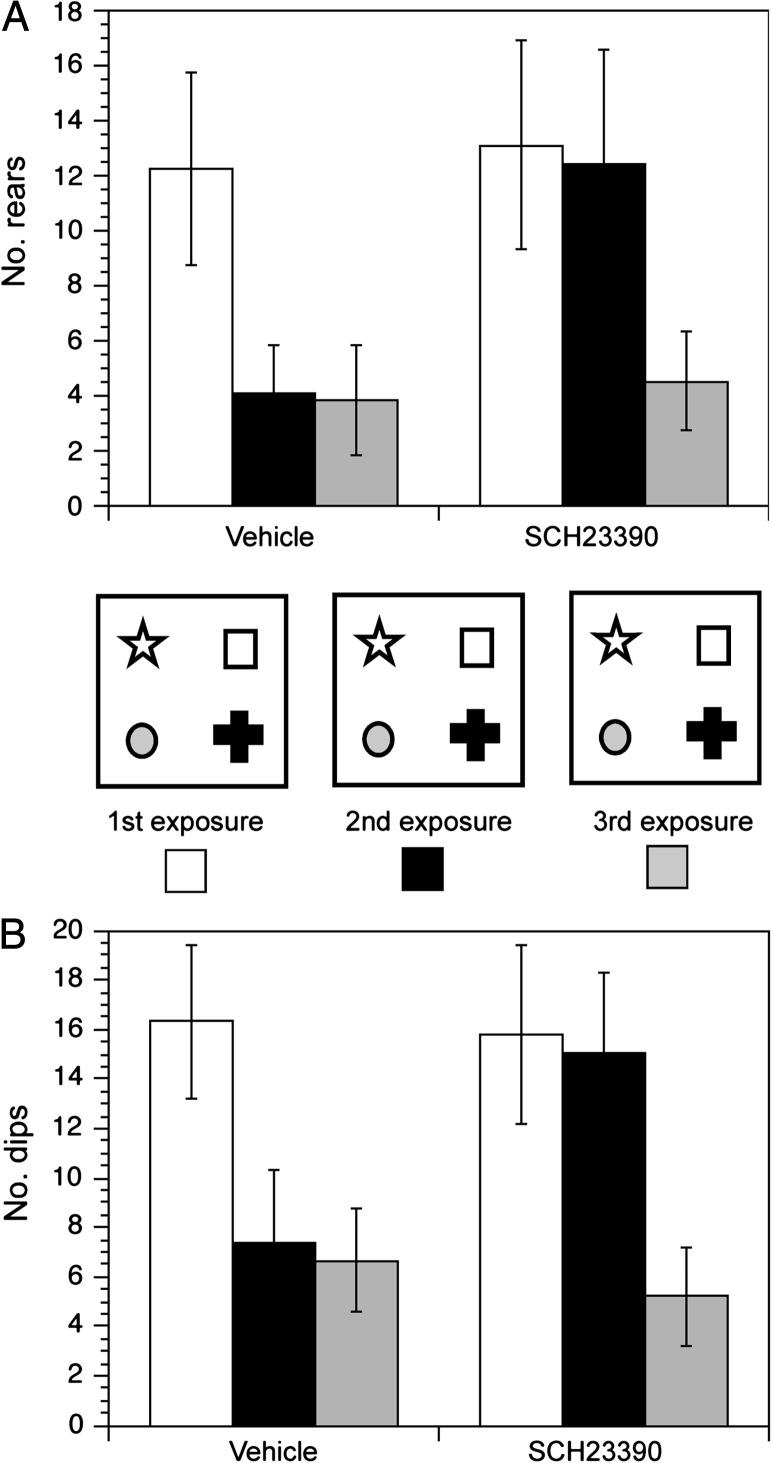
We previously showed that inhibition of either beta-adrenoceptors or dopamine D1/D5 receptors by a specific antagonist prevents learning of object–place configurations (Lemon and Manahan-Vaughan 2006; Lemon et al. 2009). Here, we explored whether antagonism of D1/D5 receptors influenced learning of the novel object constellations in the current holeboard paradigm (Fig. 3). We compared rearing (Fig. 3A) and dipping (Fig. 3B) behavior in the holeboard during the first exposure with the object-containing holeboard in vehicle (n = 6) and SCH23390-treated animals (30 μg, n = 6) and evaluated behavior again upon reexposure to the holeboard. In previous studies, we showed that a marked habituation effect is evident in controls upon reexposure to the environment (Manahan-Vaughan and Braunewell 1999; Popkirov and Manahan-Vaughan 2011). Here, in line with this, the first exposure to the environment was associated with marked exploratory activity in both treatment groups. Reexposure to the environment (ca. 7 days after the first exposure) was coupled with habituation in the control group (P < 0.01 compared with first exposure), whereas the D1/D5 antagonist-treated animals explored the objects with equivalent interest to their first exposure. Upon a third exposure to the environment, a further 7 days later, both animal groups showed habituation to the environment (P < 0.01 compared with first exposure for both groups). This suggests that the D1/D5 antagonist prevented learning of the first exposure to the environment and that learning of the environment first happened when the animals saw the environment for the second time (when the antagonist was no longer systemically present).
Figure 3.
Holeboard habituation is prevented by antagonism of D1/D5 receptors. Analysis of rearing (A) and dipping (B) behavior during exposure to the novel and familiar object–place configurations demonstrated that whereas vehicle-treated animals (n = 6) exhibited habituation to the object–place configuration (second exposure, 7 days after first exposure), animals treated with the D1/D5 antagonist SCH23390 (30 μg, n = 6) did not habituate. A third exposure to the same object–place configuration, 7 days after the second exposure, revealed habituation behavior in both the control and the antagonist-treated groups.
Treatment with a D1/D5 Agonist in the Presence of a Novel Holeboard Elicits Slow-Onset Depression. Learning-Facilitated LTD Is Unaffected
Novel holeboard exploration in the absence of test-pulse stimulation elicited no significant effect on subsequently evoked SC-CA1 responses (n = 14) (Fig. 4A), in line with previous reports (Manahan-Vaughan and Braunewell 1999). Intracerebral treatment with a D1/D5 agonist (chloro-PB, 42 μg, n = 8) 30 min prior to holeboard exploration in the absence of test-pulse stimulation of SC-CA1 synapses resulted, however, in the induction of a slow-onset depression that lasted for over 4 h (Fig. 4A). Effects were significant: ANOVA: F1,484 = 79.97; P < 0.0001. Chloro-PB, in the same concentration, has no effect on basal synaptic transmission (Fig. 1).
Figure 4.
Treatment with a D1/D5 agonist in the presence of a novel holeboard elicits slow-onset depression. Learning-facilitated LTD is unaffected. (A) SC-CA1 synaptic strength was not significantly altered following holeboard exploration in the absence of test-pulse stimulation. In contrast, holeboard exploration in the absence of test-pulse stimulation but in the presence of a selective D1/D5 agonist (42 μg of chloro-PB), resulted in slow-onset depression lasting >4 h. Insets: Analog traces represent SC-CA1 field potentials during baseline, 15 min, and 4 h after holeboard exploration. Calibration: vertical bar, 1 mV; horizontal bar, 3 ms. (B) LFS of SCs (LFS, 1 Hz, 600 pulses) resulted in STD that lasted for approximately 2 h. When LFS was given concurrently with novel exploration of the object-containing holeboard, STD was facilitated into LTD that lasted for over 24 h. Application of the D1/D5 agonist (chloro-PB, 42 μg, n = 8) had no effect on the profile of learning-facilitated LTD elicited.
We then explored whether pharmacological activation of D1/D5 receptors influences learning-facilitated plasticity that occurs when novel object–place configurations are explored during low-frequency afferent stimulation of the CA1 region (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2008a; Popkirov and Manahan-Vaughan 2011). As reported previously, low-frequency stimulation (LFS) of SCs (1 Hz, 600 pulses) resulted in STD that lasted for approximately 2 h (Fig. 4B). When LFS was given concurrently with novel exploration of the object–place configurations in the holeboard, STD was facilitated into LTD that lasted for over 24 h (n = 8, Fig. 4B). Effects were significant: ANOVA: F1,24 = 54.2; P < 0.0001. Application of the D1/D5 agonist (chloro-PB, 42 μg, n = 8) had no effect on the profile of learning-facilitated LTD seen (Fig. 4B).
LTD Mediated by Costimulation of the LC and CA1 Region Is Prevented by a D1/D5 Antagonist
Previous studies have shown that LC stimulation results in an activity-dependent β-adrenergic– and NMDA receptor–dependent form of LTD at SC-CA1 synapses of freely behaving adult rats (Lemon et al. 2009). Here, in line with these findings, LC stimulation (100 Hz, 2 trains of 100 pulses) resulted in a persistent synaptic depression in CA1, lasting >4 h (Fig. 5A, n = 29, F1,24 = 115.33; P < 0.0001), which had recovered 24 h later to prestimulation basal levels (F1,4 = 1.3; P = 0.255). LC stimulation-induced CA1 LTD was blocked by intercerebral application of the dopamine D1/D5 receptor antagonist SCH23390 (30 μg) (Fig. 5A; F1,24 = 45.6; P < 0.0001).
Figure 5.
LTD mediated by costimulation of the LC and CA1 region is prevented by a D1/D5 antagonist. LC stimulation coupled with novel spatial exploration results in CA1 LTD that lasts for approximately 4 h and is extended >24 h by treatment with a D1/D5 agonist. (A) LC burst stimulation (100 Hz) coupled with test-pulse stimulation of SC-CA1 synapses induces CA1 LTD that lasts for over 4 h. The D1/D5 receptor antagonist, SCH23390 (30 μg), blocks LC stimulation–induced SC-CA1 LTD. Analog traces represent SC-CA1 field potentials during baseline, 15 min, and 4 h after holeboard exploration. Calibration: vertical bar, 1 mV; horizontal bar, 3 ms. (B) LC activation (100 Hz) followed by test-pulse stimulation of CA1 afferents results in LTD that lasts for >4 h (see Fig. 5). Combining novel spatial exploration with LC stimulation did not change the profile of LTD elicited in the CA1 region (n = 8). However, application of the D1/D5 agonist chloro-PB (42 μg) resulted in LTD that lasts for >24 h (n = 8).
LC Stimulation Coupled with Novel Spatial Exploration Results in CA1 LTD That Last for approximately 4 h. Treatment with a D1/D5 Agonist Extends LTD to >24 h
LC activation in the presence of CA1 test-pulse stimulation results in LTD that lasts for >4 h (Fig. 5A). We explored whether LC stimulation during novel spatial exploration and test-pulse CA1 stimulation has cumulative effects (n = 4). However, we observed no change in the profile of LTD when LC stimulation was combined with novel spatial exploration (compared with only LC activation during test-pulse CA1 stimulation, n = 4) (Fig. 5B). Here, LTD was expressed that lasted >4 h.
Given the fact that agonist activation of D1/D5 receptors has no effect on baseline (Fig. 1) but enables slow-onset depression, when given prior to novel spatial exploration (Fig. 4A), we investigated if the D1/D5 agonist influenced synaptic depression under the above-mentioned conditions (n = 4). Here, we observed that in the presence of chloro-PB (42 μg), LC activation coupled with test-pulse CA1 stimulation during novel spatial exploration resulted in LTD that lasts for >24 h (Fig. 5B). Effects were significant: ANOVA: F1,24 = 49.2; P < 0.0001. The late phase of LTD (>3 h) in the presence of chloro-PB showed a greater depression than the first 3 h after LFS. This may reflect a cumulative effect of the slow-onset depression mediated by chloro-PB and the facilitation of LTD by novel spatial exploration during LC stimulation.
Discussion
In the present study, we show that exploration of a novel environment results in intrinsic STD at SC-CA1 synapses of freely behaving adult rats, if test-pulse stimulation is given to SC-CA1 synapses during exploration. LTD results if LFS (that normally elicits STD) is coupled with novel spatial exploration. Novel exploration in the absence of afferent stimulation has no effect on synaptic strength. However, pharmacological activation of dopamine D1/D5 receptors during novel spatial exploration in the absence of SC-CA1 stimulation results in slow-onset depression of SC-CA1 synapses. This suggests that D1/D5 receptors may lower the threshold for information storage by LTD in hippocampal synapses. In line with this possibility, we also show that LTD that lasts for 4 h that is elicited by costimulation of the LC and CA1 synapses, is converted into LTD that lasts for over 24 h if coupled with agonist activation of D1/D5 receptors. Learning-facilitated LTD (enabled by LFS) that lasts for over 24 h is not affected by agonism of D1/D5 receptors.
Furthermore, LC-mediated CA1 LTD is abolished by administration of a D1/D5 receptor antagonist. The D1/D5 antagonist also prevents learning of the novel spatial environment. These data suggest that the regulation of hippocampal LTD by the LC is supported by D1/D5 receptors and that the contribution of D1/D5 receptors to hippocampal information storage becomes important when the thresholds for persistent (>24 h) LTD have not been reached. The D1/D5 receptors therefore contribute to the regulation of hippocampal synaptic strength by the LC. This permissive role of the D1/D5 receptors in LC regulation of hippocampal plasticity is likely to relate to the role of the noradrenergic and dopaminergic systems in processing novelty and is probably mediated by mutual axonal projections and hippocampal neurotransmitter release.
LTD is a persistent activity-dependent and protein synthesis–dependent reduction in synaptic efficacy, which is typically induced experimentally through LFS at 1 Hz (Manahan-Vaughan et al. 2000). However, persistent expression of LTD is also facilitated by the exploration of novel contexts (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2008b; Hagena and Manahan-Vaughan 2011; Popkirov and Manahan-Vaughan 2011). This form of LTD is regulated by both noradrenergic (β) and dopaminergic (D1/D5) receptors (Lemon and Manahan-Vaughan 2006; Kemp and Manahan-Vaughan 2008a; Lemon et al. 2009). We reported in the past that novel exploration coupled with test-pulse stimulation of SC-CA1 synapses results in STD in the absence of any other kind of stimulation (such as LFS at 1 Hz)(Manahan-Vaughan and Braunewell 1999). Here, we report that in the “absence” of test-pulse stimulation, persistent changes in synaptic weight do not occur. These findings are in line with previous reports (Manahan-Vaughan and Braunewell 1999). However, even in the absence of test-pulse stimulation, novel exploration leads to persistent LTD, if exploration is coupled with agonist activation of dopamine D1/D5 receptors. This suggests that the neuromodulatory influence of dopamine is quite important for the successful facilitation of LTD by spatial learning. Of course, it cannot be presumed that agonist activation of dopamine D1/D5 receptors leads to the kind of input-specific synaptic plasticity that is believed to be a prerequisite for learning (Hebb 1949). But the potency of the facilitation of LTD in the presence of the novel spatial environment suggests that activation of D1/D5 receptors may comprise an important component of learning-facilitated LTD.
In previous studies, we reported that exploration of the holeboard environment used in the present study comprises learning (Manahan-Vaughan and Braunewell 1999; Lemon and Manahan-Vaughan 2006; Popkirov and Manahan-Vaughan 2011) and that this effect is regulated by both dopamine D1/D5 receptors (Lemon and Manahan-Vaughan 2006) and beta-adrenoreceptors (Kemp and Manahan-Vaughan 2008a). Here, we showed that antagonism of D1/D5 receptors prevented habituation to the spatial environment. Thus, the facilitatory effects of the novel object-containing holeboard that we observed in the present study are more likely to have arisen due to spatial learning than merely due to exploration. This is supported by other studies, where it was shown that first exposure to the novel holeboard facilitates LTD, whereas reexposure to the same environment does not (Kemp and Manahan-Vaughan 2004). Furthermore, passive viewing of novel object constellations also facilitate LTD (Kemp and Manahan-Vaughan 2011). These observations also provide a very tight link between the spatial learning event and the facilitation of LTD.
In the past, we reported that LC stimulation lowers the threshold for SC-CA1 LTD induction. Combining LC stimulation with test-pulse stimulation of SC-CA1 synapses results in persistent hippocampal LTD (in the absence of LFS: this study and see Lemon et al. 2009). This effect is prevented by treatment with a β-adrenoreceptor antagonist and is associated with elevations of NA in the hippocampus. The LC projects throughout the brain and is believed to undergo both a feedforward and a feedback regulation by dopaminergic structures such as the ventral tegmental area (VTA) (Sara 2009). Electrical stimulation studies indicate that NA-mediated excitatory modulation of VTA DA neurons may occur through single-pulse LC stimulation (Grenhoff et al. 1993). Both LC (Vankov et al. 1995) and VTA (Schultz 2007) neurons are activated by novel unexpected events and have been proposed to act synergistically as learning signals (Harley 2004). We therefore explored whether dopamine D1/D5 receptors may be involved in the facilitation of SC-CA1 LTD by LC stimulation. We found that pharmacological blockade of D1/D5 receptors using a selective antagonist (Billard et al. 1984; Beckstead 1988; Hjorth and Carlsson 1988) prevents LC-mediated hippocampal LTD. On the other hand, LC-mediated CA1 LTD that typically lasts for 4 h is facilitated into LTD that lasts for over 24 h if a D1/D5 agonist is applied. In contrast, learning-facilitated LTD that lasts for over 24 h, which is triggered by the coupling of LFS with spatial learning, is not enhanced further by a D1/D5 agonist. This finding suggests that the D1/D5 receptor system may be important in situations where increased arousal or novel perception are important factors in information storage: If the information is salient enough in its own right to meet the threshold required for persistent storage, additional activation of the dopaminergic system becomes redundant.
Others have reported that hippocampal dopamine receptors play an essential role in spatial learning and the storage of unpredicted information/novelty detection (Besheer et al. 2001; Lisman and Otmakhova 2001; Bevins et al. 2002; Li et al. 2003; Lemon and Manahan-Vaughan 2006). Substantial data also supports the importance of D1/D5 dopamine receptor activation for synaptic plasticity in the hippocampus (Frey et al. 1991; Otmakhova and Lisman 1996; Li et al. 2003; Sajikumar and Frey 2004; Lemon and Manahan-Vaughan 2006) and dopamine-mediated changes in hippocampal CA1 synaptic plasticity may gate the entry of novel information into long-term memory (Lisman and Grace 2005). Activation of D1 receptors can affect hippocampal basal synaptic transmission in vitro (Navakkode et al. 2007). Previous dose–response studies (Kulla and Manahan-Vaughan 2000) and preliminary evaluations (Lemon and Manahan-Vaughan 2006) established that the drug concentrations used in the present study were the minimum concentrations of chloro-PB and SCH23390 needed to affect synaptic plasticity without affecting basal synaptic transmission. We also confirmed a lack of effect of the ligands on basal synaptic transmission at the concentrations used here. The effect of the agonist, chloro-PB, is very likely to have been mediated by D1/D5 receptor activation as the KI value of chloro-PB for D1 receptors is 2.2 nM compared with over 1000 nM for D2 receptors (Andersen and Jansen 1990). Furthermore, activation of D2 receptors has previously been shown to inhibit depotentiation (Manahan-Vaughan and Kulla 2003). Thus, a subthreshold activation of D1/D5 receptors may serve to prime hippocampal synapses to be particularly receptive to a learning event.
Concluding Comments
The SOCRATIC model of Lisman and Otmakhova (2001) suggests that dopaminergic input to the hippocampal cortex during novelty exposure may selectively enhance CA3-CA1 transmission while blocking interfering sensory input from the entorhinal cortex. This would reinforce the encoding of freshly acquired novel information into memory. The firing of noradrenergic and dopaminergic cells is temporally associated with environmental change and unpredicted reinforcers, but fire only transiently (Harley 2004). Persistent elevations of catecholamine levels in the temporal lobe are observed, however, when an animal learns that a novel context is associated with an unconditioned stimulus (McIntyre et al. 2002). Recent research on humans suggests that dopaminergic processing of novelty might be important in driving exploration of new environments (Wittmann et al. 2007). We show here, for the first time that a combination of D1/D5 receptor activation and novelty signaling (either through LC stimulation or novel spatial exploration) persistently depresses CA3-CA1 transmission. The putative ability of SC-CA1 synapses to encode salient novel information in association with LTD therefore requires, at least in specific cases, both LC and D1/D5 receptor activation. The contribution of D1/D5 receptors to hippocampal information storage becomes important when the thresholds for persistent LTD have not been reached. This may reflect the conferral of quality and context to newly experienced information as measured by the differing degrees of arousal and novelty it evokes.
Funding
German Research Foundation (Deutsche Forschungsgemeinschaft) (grant Ma1843 to D.M.V.).
Acknowledgments
We gratefully acknowledge the technical assistance of Beate Krenzek and Jens Klausnitzer and thank Nadine Gomell for animal care. We sincerely thank Dr Oxana Eschenko for advice and guidance with regard to the LC electrophysiology.Conflict of Interest : None declared.
References
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM. Association of dopamine D1 and D2 receptors with specific cellular elements in the basal ganglia of the cat: the uneven topography of dopamine receptors in the striatum is determined by intrinsic striatal cells, not nigrostriatal axons. Neuroscience. 1988;27:851–863. doi: 10.1016/0306-4522(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Short KR, Bevins RA. Dopaminergic and cholinergic antagonism in a novel-object detection task with rats. Behav Brain Res. 2001;126:211–217. doi: 10.1016/s0166-4328(01)00245-5. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129:41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Billard W, Ruperto V, Crosby G, Iorio LC, Barnett A. Characterization of the binding of 3H-SCH 23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984;35:1885–1893. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Is the rodent hippocampus just for ‘place’? Curr Opin Neurobiol. 1996;6:187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann KG. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 2011;21:2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behaviour: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A. In vivo receptor binding, neurochemical and functional studies with the dopamine D-1 receptor antagonist SCH23390. J Neural Transm. 1988;72:83–97. doi: 10.1007/BF01250232. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008a;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008b;19:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb Cortex. Forthcoming 2011 doi: 10.1093/cercor/bhr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara S J. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D. Depotentiation in the dentate gyrus of freely moving rats is modulated by D1/D5 dopamine receptors. Cereb Cortex. 2000;10:614–620. doi: 10.1093/cercor/10.6.614. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on {beta}-adrenergic receptor activation. Cereb Cortex. 2009;19:2827–2837. doi: 10.1093/cercor/bhp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. The hippocampus as a cognitive map. Oxford: Clarendon; 1978. [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–519. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur J Neurosci. 2002;15:143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioural dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Duzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]