Abstract
Functional connectivity between brain regions can define large-scale neural networks and provide information about relationships between those networks. We examined how relationships within and across intrinsic connectivity networks were 1) sensitive to individual differences in dopaminergic function, 2) modulated by cognitive state, and 3) associated with executive behavioral traits. We found that regardless of cognitive state, connections between frontal, parietal, and striatal nodes of Task-Positive networks (TPNs) and Task-Negative networks (TNNs) showed higher functional connectivity in 10/10 homozygotes of the dopamine transporter gene, a polymorphism influencing synaptic dopamine, than in 9/10 heterozygotes. However, performance of a working memory task (a state requiring dopamine release) modulated genotype differences selectively, such that cross-network connectivity between TPNs and TNNs was higher in 10/10 than 9/10 subjects during working memory but not during rest. This increased cross-network connectivity was associated with increased self-reported measures of impulsivity and inattention traits. By linking a gene regulating synaptic dopamine to a phenotype characterized by inefficient executive function, these findings validate cross-network connectivity as an endophenotype of executive dysfunction.
Keywords: DAT1, fMRI, functional connectivity, resting state, working memory
Introduction
The functional architecture of the human brain is composed of distinct networks whose regions show correlated activity across time (Bullmore and Sporns 2009). This network organization exists regardless of cognitive state, as the same networks that demonstrate correlated activity during task performance (Esposito et al. 2006; Fransson 2006; Fransson and Marrelec 2008) also show correlated activity at low frequencies (<0.08 Hz) during the task-free “resting state” (Beckmann et al. 2005). Furthermore, the spatial composition of these networks matches patterns of regions that are activated by various tasks (Smith et al. 2009; Gordon, Stollstorff, et al. 2011). For example, correlated network activity is seen both between bilateral auditory cortex and between bilateral visual cortex (Beckmann et al. 2005), regions also activated by auditory and visual tasks, respectively. Furthermore, several networks include regions that are activated by complex cognitive tasks (e.g., working memory), while others include regions that are deactivated during those same tasks; these have been termed “Task-Positive” and “Task-Negative” (or default mode) networks, respectively (Fox et al. 2005). This inverse relationship in activation between Task-Positive networks (TPNs) and Task-Negative networks (TNNs) during task performance is also reflected in the temporal relationship between these networks, as they are anticorrelated during the resting state (Fox et al. 2005). The regional composition and temporal relationships within and between networks (termed functional connectivity) are posited to be established by repeated functional co-activation over a lifetime (Dosenbach et al. 2007).
The strength of functional network connectivity appears to be a key determinant of cognitive abilities. First, the strength of within-network functional connectivity (between nodes of a single network) predicts cognitive performance. Stronger connectivity between major TNN nodes (medial prefrontal cortex and posterior cingulate cortex) is associated with superior working memory performance (Hampson et al. 2006, 2010; Sambataro et al. 2010) as well as superior processing speed, memory, and executive function (Andrews-Hanna et al. 2007). Furthermore, stronger connectivity between major TPN nodes (left and right lateral prefrontal cortex) is associated with superior processing speed and executive function (Gordon, Lee, et al. 2011). Second, the degree of anticorrelation between TPN and TNN impacts cognition, as individuals who have more negative TPN-TNN correlations demonstrated reduced trial-to-trial behavioral variability (Kelly et al. 2008) and superior working memory performance (Hampson et al. 2010). This negative or reduced cross-network connectivity is thought to reflect reduced interference across networks (Kelly et al. 2008). Third, many neuropsychiatric disorders associated with cognitive deficits demonstrate atypical connectivity patterns. Reduced connectivity within the TNN has been observed in attention deficit hyperactivity disorder (ADHD) (Castellanos et al. 2008), autism spectrum disorders (Kennedy et al. 2006), Schizophrenia (Bluhm et al. 2007), and Alzheimer’s disease (Greicius et al. 2004). Elevated or less negative cross-network connectivity between TPN and TNN has been observed in ADHD (Castellanos et al. 2008) and Schizophrenia (Whitfield-Gabrieli et al. 2009). Together, these findings suggest that integrity of cognition depends upon optimal within- and between-network functional connectivity.
What factors may determine the nature of within- and between-network relationships? One likely candidate is the neurotransmitter dopamine (DA), as exogenous manipulations of DA affect functional connectivity. In healthy volunteers, DA depletion reduced resting state connectivity between striatum and the TPN and disrupted the relationship between connectivity and speed of executive task performance (Nagano-Saito et al. 2008). Administration of a DA agonist altered resting state striatal connectivity such that it was increased with TPN and motor networks but reduced with TNN (Kelly et al. 2009). While the behavioral significance of these findings cannot be determined, as subjects were not performing a task, these findings indicate that altered DA levels have widespread effects on temporal relationships of networks. In ADHD, a disorder characterized by DA dysfunction, administration of stimulant medications that enhance DA signaling normalized connectivity between cortical and striatal/cerebellar regions (Rubia et al. 2009), as well as between TPN and TNN (Peterson et al. 2009), such that it was similar to control children. Together, these results suggest that DA function is important for regulating cross-network functional relationships.
Similar to experimental manipulations of exogenous DA, it is possible that endogenous interindividual variation in DA function is associated with functional connectivity differences between individuals. One endogenous source of DA variation is a widely studied genetic polymorphism, the variable number of tandem repeats (VNTR) in the 3′-untranslated region of the DAT1 gene coding for the DA transporter (DAT), a protein that regulates DA signaling by reuptaking DA following its release (Madras et al. 2005). The DAT1 gene’s 2 most common alleles, 9 and 10 repeats, appear to influence the expression of DAT in vitro (Fuke et al. 2001; Mill et al. 2002; VanNess et al. 2005), with greater striatal DAT expression associated with 10-repeat compared with 9-repeat alleles (though in vivo findings have been mixed; Heinz et al. 2000; Jacobsen et al. 2000; Krause et al. 2006). Inheritance of 2 copies of the 10-repeat allele (10/10) has been associated with ADHD (Yang et al. 2007), a disorder defined by reduced executive function (Willcutt et al. 2005). Executive functioning was reduced in healthy adults with a 10/10 genotype, as they showed worse inhibitory performance (Caldú et al. 2007) and reduced benefits of working memory training despite similar baseline performance (Brehmer et al. 2009) relative to 9/10 heterozygotes.
In addition to behavioral effects, differences in DAT expression have also been associated with differences in the functional engagement of brain regions important for executive function. First, higher striatal DAT concentration was associated with less deactivation of TNN regions in healthy adults during visual attention (Tomasi et al. 2009). Second, 10/10 homozygotes had reduced activation compared with 9-repeat carriers in the Task-Positive lateral prefrontal cortex during working memory (Bertolino et al. 2006, 2009, 2008; Caldú et al. 2007; Stollstorff et al. 2010) and response inhibition (Congdon et al. 2009); reduced activation in the Task-Positive striatum during response inhibition (Congdon et al. 2009) and reward processing (Dreher et al. 2009; Forbes et al. 2009); and reduced deactivation in Task-Negative medial prefrontal cortex during working memory (Brown et al. 2011). These findings indicate that putative differences in DAT expression induce individual variations in both behavior and related brain activation. Whether DAT1 influences network connectivity is unknown.
Here, we investigated functional connectivity within and between TPN and TNN during the resting state and during performance of an N-back working memory task in healthy 9/10 and 10/10 carriers. We first identified intrinsic connectivity networks in the resting state, confirmed that network nodes overlapped with activation during the working memory state and then examined effects of DAT1 and cognitive state on functional connectivity between these nodes. We had several goals. First, we examined whether functional connectivity differs by DAT1. As the 10/10 genotype has been associated with ADHD (Yang et al. 2007), we predicted that 10/10 homozygotes would demonstrate connectivity patterns similar to that observed in ADHD—that is, reduced connectivity within TNN and increased (i.e., less negative) connectivity between TPN and TNN (Castellanos et al. 2008). Second, we examined whether subjects’ cognitive state (resting vs. working memory task) modulates connectivity. Working memory demands alter functional connectivity relative to rest (Fransson 2006), both within networks (increases within TPN) and across networks (decreases between TPN and TNN). We expected to replicate these findings. Third, we examined whether DAT1 and cognitive state would interact to modulate functional connectivity. As working memory demands increase DA release (Aalto et al. 2005), differences in DA regulation associated with DAT1 ought to be magnified during working memory relative to rest, yielding a DAT1 × cognitive state interaction on functional connectivity. Finally, as DAT1 has been shown to affect executive control, we examined whether DAT1 × cognitive state interactions in functional connectivity were associated with individual differences in executive control traits.
Materials and Methods
Subjects
Two hundred and ninety-six Georgetown University undergraduates aged 18 to 22 years provided saliva samples that were genotyped for DAT1. Eighty-one subjects were randomly invited from the pool of 10/10 and 9/10 carriers to participate (9/10: n = 37; 10/10: n = 44). Exclusion criteria included self-reports of 1) use of psychotropic medication (e.g., stimulants, SSRIs); 2) overt neurological injury or disease, seizure disorder, psychiatric diagnosis; 3) contraindications for MRI—for example, presence of metal, pregnancy. Four subjects (2 per genotype) were excluded from analysis due to technical problems during scanning. The final sample included thirty-five 9/10 heterozygotes (mean ± standard deviation [SD] age = 20.37 ± 0.96; 14 males) and forty-two 10/10 homozygotes (mean ± SD age = 20.26 ± 1.14; 14 males). Groups did not differ in either age or gender (Ps > 0.4). All subjects gave informed consent in accordance with guidelines of the Georgetown University Institutional Review Board.
Genotyping
DNA was extracted from Oragene saliva kits (DNA Genotek Inc., Ottawa, Ontario, Canada). The 40 bp VNTR polymorphism in the 3′ UTR of DAT1 was genotyped by PCR as previously described (Daly et al. 1999) using the following primers; Forward: 5′-TGTGGTGTAGGGAACGGCCTGAG-3′ Reverse: 5′-CTTCCTGGAGGTCACGGCTCAAGG-3′. PCR was performed using the Accuprime Taq DNA polymerase system (Invitrogen) with the following PCR program: 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 68 °C for 1 min. The PCR products were then run out on a 2% agarose gel stained with ethidium bromide. A 100 bp DNA ladder was then used to identify the various repeat alleles by size: 7-repeat (360 bp), 8-repeat (400 bp), 9-repeat (440 bp), 10-repeat (480 bp), and 11-repeat (520 bp). Genotyping was successful for 286 of 296 subjects in the original sample. Observed genotypic frequencies in the sample were: 10/10–59.1%; 9/10–29.4%; 9/9–8.7%; other, 2.8%.
Behavioral Testing
Subjects completed the Adult ADHD Self-Report Scale v1.0 (Kessler et al. 2005) and the Barratt Impulsiveness Scale version 11 (Patton et al. 1995).
Scanning Procedure
Subjects were scanned during performance of an N-back task and during rest. The N-back task lasted for 6:26 min and consisted of nine 30 s N-back blocks (3 blocks each at 1-, 2-, and 3-back) alternating with eight 15-s blocks of fixation. Each N-back block consisted of 9 serially presented consonants appearing for 500 ms, with an intertrial interval of 2500 ms. The N-back load condition (1-, 2-, or 3-back) varied between task blocks, with condition order pseudorandomized using a modified Latin Square. Each block was preceded by a 3000-ms screen informing the subject of the N-back condition. Subjects were instructed to press a hand-held button with their right hand when the current letter matched the letter n trials ago (e.g., for the 2-back condition, subjects see: R V N W N—button-press for N). Targets were present on 19% of trials; each block contained between 1 and 3 targets with target frequency balanced across conditions. No condition contained sequences of stimuli that were targets in any other condition. Stimuli were presented using E-Prime (Psychology Software Tools Inc., Pittsburgh, PA). The resting scan was always conducted immediately following the conclusion of the N-back task. For the resting run, which lasted 5:04 min, subjects were told to relax with eyes closed and to not think of anything in particular.
fMRI Data Acquisition
Imaging was performed on a Siemens Trio 3-T scanner (Erlangen, Germany). A high-resolution T1-weighted structural scan (magnetization prepared rapid gradient echo [MPRAGE]) was acquired with the parameters: time repetition (TR)/time echo (TE) = 2300/2.94 ms, time to inversion = 900 ms, 90° flip angle, 1 slab, 160 sagittal slices with a 1.0-mm thickness, field of view (FOV) = 256 × 256 mm2, matrix = 256 × 256, resulting in an effective resolution of 1.03-mm isotropic voxels. For the N-back run, 197 whole-brain images were acquired using a gradient echo pulse sequence (34 slices, TR = 2000 ms, TE = 30 ms, 256 × 256 mm FOV, 90° flip angle, voxel dimensions 4 × 4 × 4.2 mm). For the resting run, 152 whole-brain images were acquired using a gradient echo pulse sequence (37 slices, TR = 2000 ms, TE = 30 ms, 192 × 192 mm FOV, 90° flip angle, voxel dimensions 3-mm isotropic). The first 4 images of each functional run were discarded to allow for signal stabilization.
Image Preprocessing
Using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Version 7.10 Mathworks, Inc., Sherborn, MA), images were corrected for translational and rotational motion by realigning to the first image of the session, for each run. All subjects demonstrated less than 2.0 mm of translational motion in any one direction (max translation = 1.25 mm). One subject demonstrated a transient large rotational motion in the first 3 TRs of the N-back run; these TRs were removed from further analyses. Subsequently, all subjects demonstrated less than 2° of rotation around any one axis (max rotation = 1.44°). Two-sample t-tests showed that genotype groups did not differ in maximum motion in any of the 3 translational or 3 rotational directions (all Ps > 0.15). Images were slice-time corrected, normalized to an EPI template, and smoothed using a Gaussian kernel with full-width at half-maximum of 8 mm. For connectivity analyses, a band-pass filter was applied to the resting and working memory data in order to restrict signal variation to frequencies between 0.01 and 0.1 Hz, corresponding to the frequency range established in the literature for fluctuations in resting-state data (Biswal et al. 1995).
Identification of Brain Regions Activated and Deactivated during the N-Back Task
First-level analysis was performed using a general linear model as implemented in SPM8. For each subject, 3 temporal regressors consisting of boxcar time series convolved with a hemodynamic response function were specified: one representing the presence of the Fixation cross, one representing the presence of the N-back task, and one representing the effect of load (constructed by reproducing the N-back regressor and parametrically varying the boxcar height according to the load condition). For each subject, Task > Fixation and Fixation > Task contrasts were specified to delineate regions activated and deactivated during the N-back task after removing contributions of the N-back load condition (this was done for consistency with the connectivity analysis—see below). For group averaging, one-sample t-tests were conducted for both contrasts at P < 0.05, corrected for multiple comparisons using family wise error.
Identification of Functional Networks during Rest
A group-level ICA was performed on the preprocessed filtered resting-state images using the MELODIC toolbox (Beckmann and Smith 2004) implemented within FSL (Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, London, UK). The preprocessed filtered resting data from all subjects were temporally concatenated to create a single time course, and a probabilistic ICA was performed on this time course using the MELODIC toolbox, allowing the program to select the optimal number of components to generate. Within each component, MELODIC generated Z-scores for each voxel by generating a mixture model combining a “noise” Gaussian function with 2 gamma functions modeling “active” voxels and estimating the probability of a given voxel’s intensity fitting the gamma functions rather than the background noise Gaussian function (Beckmann and Smith 2004).
The ICA delineated 20 components in the form of 3D Z-score images. Components in which the areas of maximal covariation were nonneuronal (e.g., white matter, cerebrospinal fluid, brain edge covariation resulting from head motion) were visually identified (see Kiviniemi et al. 2009) and removed from further analysis. The remaining group components were visually identified based on similarities to known brain networks. These components were identified as TPNs or TNNs based on similarity to networks identified by Fox et al. (2005) or as “Task-Neutral” based on a lack of similarity to those networks.
Region of Interest Creation
For each TPN and TNN, the largest clusters of covariation were delineated, and the voxel of peak network connectivity (i.e., with peak Z-score values in the ICA-generated images) within each cluster was identified as a network “node” from which functional connectivity analysis was conducted. To restrict analysis to nodes that were modulated by the N-back task, peak voxels were discarded from further analysis if they did not fall within regions activated or deactivated in the group-level Task > Fixation contrasts. The remaining nodes thus represent regions that were both activated by the working memory task and maximally connected within intrinsic connectivity networks. Spherical regions of interest (ROIs) with radius 6 mm were created centered on each of these node voxels using MARSBAR (Brett et al. 2003) and were labeled based on the general anatomical location of the node voxels (as in Duvernoy 1999); these ROIs were used for all further connectivity analyses.
Removal of Nuisance Signals
To minimize the effects of motion, load (within the N-back run), and physiological noise (such as respiration and heart rate) that would be common to all ROIs, timecourses approximating these signals were regressed out of each voxel. Physiological noise regressors were approximated by obtaining signal timecourses from white matter and CSF segmentations of the MP-RAGE image (Van Dijk et al. 2010). Motion regressors were obtained as the 6 realignment parameter timecourses from the motion correction preprocessing step. For the N-back run, load-effect regressors were obtained by convolving 3 boxcar timecourses (one for each load condition) with a canonical hemodynamic response. The effect of load was regressed out because the manipulation of load in the N-back paradigm was expected to drive substantial and systematic activation differences in many brain regions, including both task-positive regions (Braver et al. 1997; Callicott et al. 1999; Veltman et al. 2003) and task-negative regions (McKiernan et al. 2003). If the load structure of the task was not regressed out, these large activation differences would artificially inflate functional connectivities, such that even regions with no moment-to-moment correlations would appear functionally connected because they were both driven by load effects over the course of the task (for further discussion of this point, see Jones et al. 2010).
The regression of nuisance signals was conducted separately for each run, and the postregression residual voxel timecourses were used for all further analysis.
Functional Connectivity Analysis
For the rest and N-back runs separately, residual voxel timecourses were averaged within each ROI, and mean residual timecourses from all ROIs were then correlated against each other in a pairwise fashion to assess functional connectivity. In the N-back run, analysis was restricted to task performance by excluding the 15 s in each fixation block plus 6 subsequent seconds (to allow for hemodynamic response stabilization). The correlations were thus performed on 148 resting timepoints and 108 N-back timepoints. The resulting Pearson’s r values (from each ROI pair, for each subject in each run) were converted to normally distributed Z-scores using Fisher’s transformation in order to allow further analysis of correlation strengths.
To assess effects of genotype and cognitive state on each functional connection between ROI pairs, a 2 × 2 DAT1 (10/10, 9/10; between subjects) × state (resting state, N-back state; within subjects) analysis of variance (ANOVA) was conducted on the connectivity between each ROI pair using the LinStats software package within Matlab (http://www.mathworks.com/matlabcentral/fileexchange/29876-linstats). For each ANOVA model, an F-test was performed testing the overall fit of the model against a null model (intercept only), and the resulting model fit P-values were tested for significance at P < 0.05 after Bonferroni correction for the number of ANOVA models (corrected alpha = 0.000416). ANOVA models that significantly fit the data were subsequently examined for interaction effects and main effects.
Correlation with Executive Traits
To examine whether connectivity affected by DAT1 and state were also associated with executive control traits, we calculated the state-related change in connectivity (N-back state—resting state) for each subject in each of the functional connections showing a significant DAT1 × state interaction. For each behavioral measure (Inattention and Hyperactivity from the ADHD Self-Report Scale and Impulsivity from the Barratt Impulsiveness Scale) separately, we conducted a stepwise multiple regression to examine whether the calculated changes in connectivity predicted individual differences in the behavioral trait.
Results
Behavior
Rating Scales
On the ADHD Self-Report Scale, Inattention scores were marginally higher in 10/10 than 9/10 subjects (9/10: 13.78 ± 3.74; 10/10: 15.63 ± 5.01; t79 = 1.84, P = 0.069), but scores did not differ on the Hyperactive/Impulsive subscale (9/10: 11.78 ± 4.31; 10/10: 12.23 ± 5.20; P > 0.6). Barratt Impulsiveness Scale ratings were also marginally higher in 10/10 than 9/10 subjects (9/10: 55.89 ± 6.79; 10/10: 59.30 ± 9.88; t79 = 1.77, P = 0.080).
N-back Task Performance
Mean reaction time (RT) for correct N-back target responses and N-back percent accuracy (% hits − % false alarms) were computed for each subject. Genotype groups did not differ in mean RT (9/10: 582 ms ± 172 ms; 10/10: 534 ms ± 145 ms; P = 0.18). Both groups performed near ceiling and did not differ on either accuracy (9/10: 95.4% ± 5.5%, 10/10: 96.0% ± 6.6%; P = 0.66) or on the number of subjects in each group with perfect accuracy (9/10 = 18; 10/10 = 25, P = 0.46).
Identification of Brain Regions Activated and Deactivated by the N-Back Task
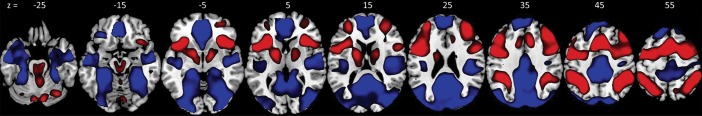
Group averages of activated (N-back > Fixation) and deactivated (Fixation > N-back) regions are shown in Figure 1. Activated regions included bilateral dorsolateral and ventrolateral prefrontal cortex, anterior insula, lateral parietal cortex, medial supplementary motor area, and globus pallidus. Deactivated regions included ventromedial prefrontal cortex and perigenual anterior cingulate, anterior medial prefrontal cortex, and medial parietal cortex (including posterior cingulate and precuneus), as well as bilateral fusiform gyrus, hippocampus/amygdala, posterior insula, anterior middle and superior temporal gyri, and lateral/superior occipital cortex extending into bilateral angular gyrus. These activation/deactivation patterns did not vary by DAT1 genotype (see Supplementary Material I).
Figure 1.
Group average of Task > Fixation (in red) and Fixation > Task (dark blue) contrasts (P < 0.05, FWE-corrected).
Identification of Functional Networks
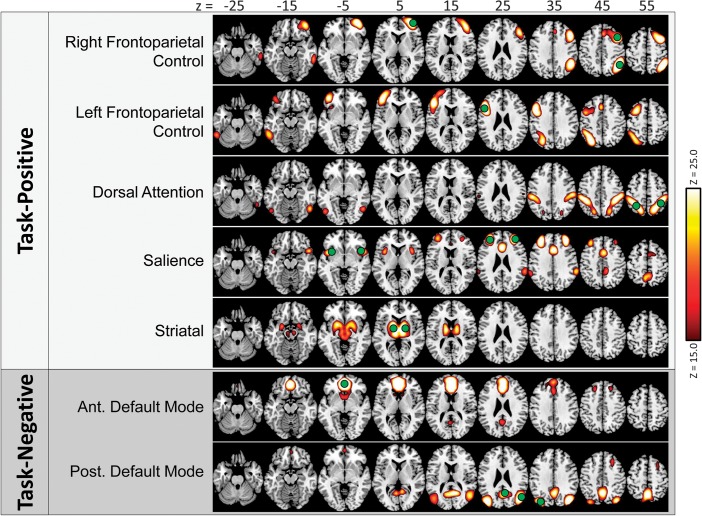
Twenty components were delineated by ICA of the resting-state data. Of these, 7 were visually identified as TPNs or TNNs based on similarity to past reports (Fox et al. 2005), including: a cingulo-opercular salience network, a left-lateralized frontoparietal control (lFPC) network, a right-lateralized frontoparietal control (rFPC) network, a parietal-based bilateral dorsal attention network, and a bilateral striatal network, which were classified as TPN; as well as a posterior default mode network (pDMN) and an anterior default mode network (aDMN), which were classified as TNN (see Fig. 2). Additionally, 6 networks were identified which did not well-match TPN or TNN; these networks, many of which were similar in appearance to previously delineated networks (Kiviniemi et al. 2009), were labeled Task-Neutral (see Supplementary Fig. S1). These included networks with high connectivity in auditory cortex, in primary visual cortex, in sensorimotor cortex, in left-lateralized language regions, in medial posterior and middle cingulate cortex, and in bilateral superior temporal and inferior frontal cortex. As these networks are not relevant to our predictions, they were not included in further analysis. The remaining 7 components were identified as deriving from nonneuronal sources (Kiviniemi et al. 2009) and were thus excluded from analysis: CSF (1), white matter (1), and subject head motion (5).
Figure 2.
Seven networks delineated in the resting-state data by the ICA procedure. Network maps are thresholded for visual purposes at Z = 15.0. Label shadings indicate visual categorization of each network: light gray shading—TPNs; dark gray shading—TNNs. ROIs used in connectivity analyses are overlaid on top in green.
Identification of TPN and TNN nodes (as described in Materials and Methods) resulted in 1–4 node ROIs for each network (Table 1 and Fig. 2, green circles).
Table 1.
Locations of ROIs constructed around peaks of maximal covariation in the resting-state data, within TPNs and TNNs
| Network type | Network | ROI center (Montreal Neurological Institute coordinates) | ROI location | ROI abbreviation |
| Task-Positive | rFPC | 46, 14, 44 | Right posterior dorsolateral PFC | r pdlPFC |
| 34, 58, 4 | Right ventrolateral PFC | r vlPFC | ||
| 46, −54, 48 | Right posterior inferior parietal lobule | r pIPL | ||
| lFPC | −50, 18, 28 | Left posterior dorsolateral PFC | l pdlPFC | |
| Dorsal attention | 42, −38, 52 | Right anterior inferior parietal lobule | r aIPL | |
| −38, −46, 56 | Left anterior inferior parietal lobule | l aIPL | ||
| Salience | 34, 46, 28 | Right anterior dorsolateral PFC | r adlPFC | |
| −30, 46, 28 | Left anterior dorsolateral PFC | l adlPFC | ||
| 46, 14, −8 | Right anterior insula | r aIns | ||
| −42, 10, −4 | Left anterior insula | l aIns | ||
| Striatum | 10, −2, 4 | Right striatum | r Striatum | |
| −10, −6, 8 | Left striatum | l Striatum | ||
| Task-Negative | aDMN | −2, 50,−4 | Ventromedial prefrontal cortex | vmPFC |
| pDMN | −2,−58, 20 | Posterior cingulate | PCCa | |
| 42, −74, 28 | Right angular gyrus | r AG | ||
| −38, −82, 32 | Left angular gyrus | l AG |
Both the pDMN and the aDMN networks contained a node in posterior cingulate cortex (PCC). Because these 2 nodes overlapped, the aDMN PCC node (centered at peak voxel [6, −58, 20]) was discarded from further analysis (as it was not in the anterior portion of the network). There was no other overlap between ROIs.
Functional Connectivity within and between TPN and TNNs
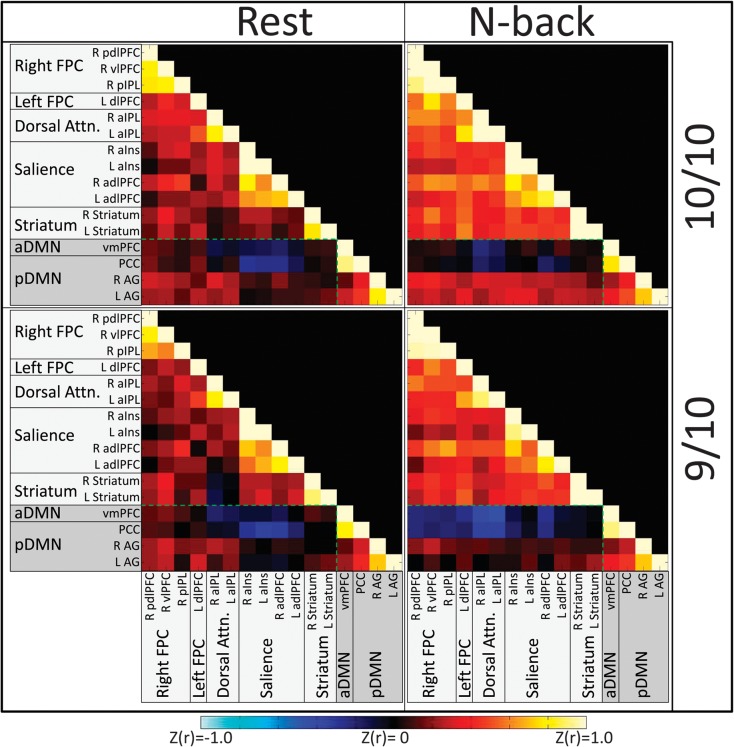
To display the correlational structure of TPN and TNN, we created connectivity matrices by averaging across subjects’ Z-transformed correlation coefficients for each connection, within each genotype group and condition. These matrices are presented in Figure 3. To statistically test for effects of DAT1 and cognitive state, subjects’ Z-transformed correlation coefficients in each pairwise connection were subjected to a DAT1 (9/10, 10/10) × cognitive state (Nback, Rest) mixed ANOVA. Bonferroni correction was conducted at P < 0.05 for the number of ANOVA models. The following significant effects emerged.
Figure 3.
Connectivity matrices indicating Z-transformed r values for each genotype group in each state. Hot colors indicate positive connectivity between ROIs; cool colors indicate negative connectivity. Shadings of ROI labels indicate network categorization: light gray shading—TPNs; dark gray shading—TNNs. The green dotted line demarcates cross-network (Task-Negative to Task-Positive) connections from within-network connections.
Main Effects of Cognitive State
Significant main effects of cognitive state were found in 38 TPN to TPN connections and 13 TPN to TNN connections (Fs1,77 ranged from 16.07 to 71.65). No significant TNN to TNN connections reached significance. In all TPN to TPN connections, as well as in connections between TNN and the TPN salience network, connectivity was higher during the N-back task than during rest. By contrast, in connections between TNN and the TPN FPC/Dorsal Attention networks, connectivity was higher during rest than during the N-back task (see Fig. 4). Thus, as predicted, working memory demands strengthened connectivity between most nodes of TPN. However, contrary to predictions, the hypothesis that working memory would reduce TPN to TNN connectivity was only supported for the FPC and Dorsal Attention TPN and not for the Salience TPN.
Figure 4.
Matrix indicating ROI pairs in which significant main effects of state were observed on connectivity, after correction for multiple comparisons at the model level. Hot colors indicate increased connectivity during the N-back task compared with Rest; cool colors indicate increased connectivity during Rest compared with the N-back task. Shadings of ROI labels indicate network categorization: light gray shading—TPNs; dark gray shading—TNNs. The green dotted line demarcates cross-network (Task-Negative to Task-Positive) connections from within-network connections.
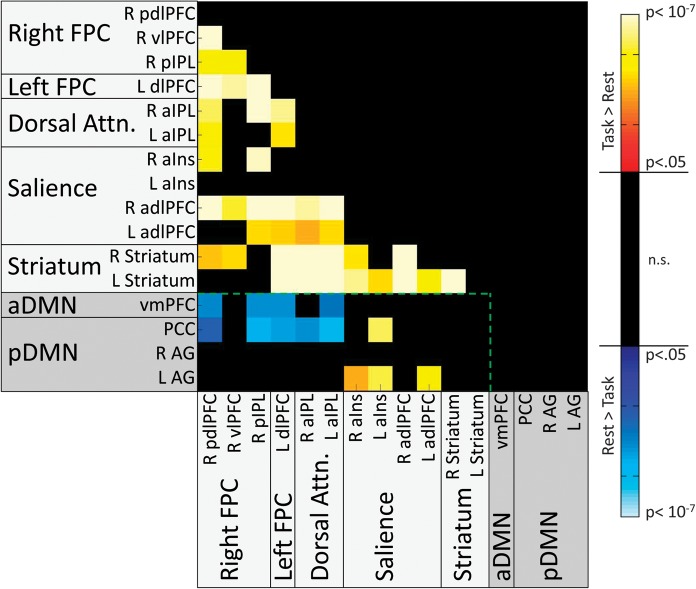
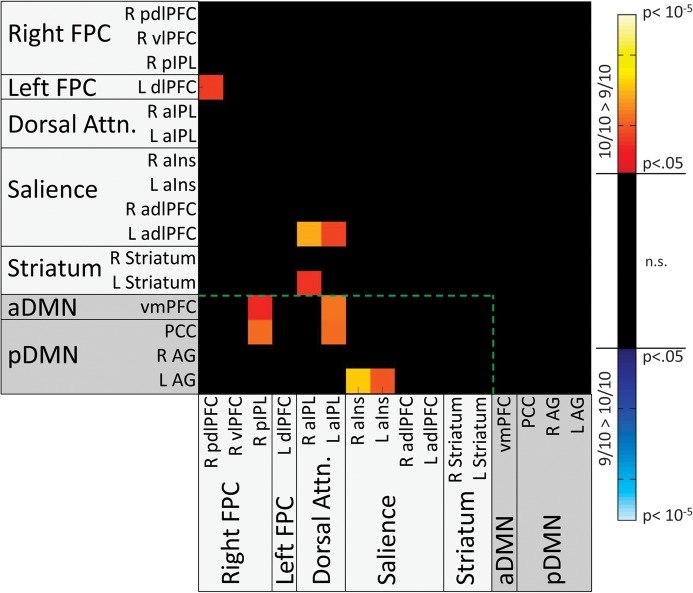
Main Effects of DAT1
Significant main effects of DAT1 were found in 4 TPN to TPN connections and 6 TPN to TNN connections (Fs1,77 ranged from 4.49 to 11.27) but not in any TNN to TNN connections. In all connections showing effects, the 10/10 group exhibited greater connectivity than the 9/10 group (see Fig. 5). The TPN to TPN connections included one connection between bilateral frontal nodes of the FPC networks (L pdlPFC to R pdlPFC), two connections between bilateral parietal nodes of the Dorsal Attention network and a frontal node of the Salience network (L adlPFC to R and L aIPL), and one connection between Dorsal Attention and Striatal networks (L Striatum to R aIPL). The TPN to TNN connections (Fig. 5, within green line) included 2 between DMN and FPC networks (PCC and vmPFC vs. R pIPL), 2 between DMN networks and the Dorsal Attention network (PCC and vmPFC vs. L aIPL), and 2 between the pDMN and bilateral insular nodes of the Salience network (L AG vs. R aIns and L aINS).
Figure 5.
Matrix indicating ROI pairs in which significant main effects of DAT were observed on connectivity, after correction for multiple comparisons at the model level. All significant effects were found to be driven by greater connectivity in 10/10 subjects than in 9/10 subjects (as indicated by hot color shading). Shadings of ROI labels indicate network categorization: light gray shading—TPNs; dark gray shading—TNNs. The green dotted line demarcates cross-network (Task-Negative to Task-Positive) connections from within-network connections.
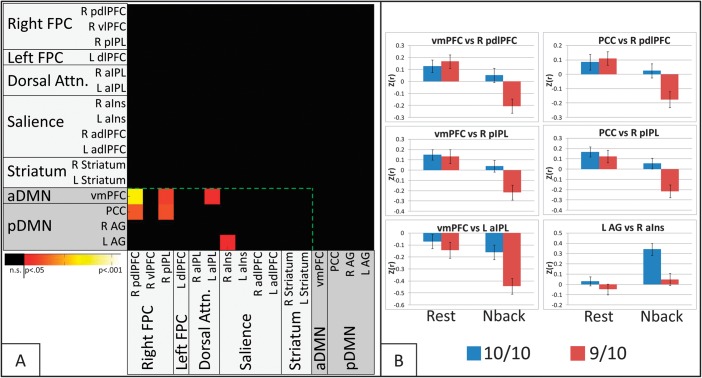
DAT1 × Cognitive State Interaction
Significant interaction between DAT1 and cognitive state (Fig. 6A) was observed in 6 connections, all between TPN and TNN nodes (Fs1,75 ranged from 4.33 to 8.47). Four of these were between TNN and the TPN rFPC network (vmPFC and PCC vs. R pdlPFC; vmPFC and PCC vs. R pIPL). The other 2 interactions were observed between the TNN aDMN and the TPN Dorsal Attention network (vmPFC vs. L aIPL) and between the TNN pDMN and the TPN Salience network (L AG vs. R aINS). No significant interactions were observed in any TPN to TPN or TNN to TNN connections.
Figure 6.
(A) Matrix indicating ROI pairs in which significant DAT1 × state interaction effects were observed on connectivity, after correction for multiple comparisons at the model level. Shadings of ROI labels indicate network categorization: light gray shading—TPNs; dark gray shading—TNNs. The green dotted line demarcates cross-network (Task-Negative to Task-Positive) connections from within-network connections. (B) Connectivity values by DAT and state for each significant ROI pair in A. Error bars represent standard error.
Two-sample t-tests evaluating effects of DAT1 on connectivity in each cognitive state (Fig. 6B) revealed that in each connection, the interaction was due to significantly greater connectivity in 10/10 than in 9/10 subjects during the N-back task (ts75 > 2.72, Ps < 0.008) but not during rest (Ps > 0.25). Paired t-tests evaluating effects of cognitive state in each group separately revealed that state effects on connectivity varied by network. In the 5 connections between TNN and the TPN FPC/Dorsal Attention nodes, 9/10 subjects demonstrated “reduced” connectivity during the N-back task compared with rest, but 10/10 connectivity was unchanged. By contrast, in the connection between the TNN left angular gyrus node and the TPN right anterior insula node, 10/10 subjects demonstrated “increased” connectivity during the N-back task compared with rest, but 9/10 connectivity was unchanged.
In sum, performing the N-back task increased connectivity in several TPN to TPN and TNN to Salience network connections compared with rest but decreased connectivity in several connections between TNN and FPC/Dorsal Attention networks in all subjects. Furthermore, regardless of cognitive state, individuals with the 10/10 genotype showed greater connectivity in various regions of the brain, both within TPN and between TPN and TNN, than individuals with the 9/10 genotype. DAT1 differences depended upon cognitive state only in cross-network TPN to TNN connections, such that connectivity was higher in 10/10 than 9/10 subjects during working memory but not during rest. Overall, these results support 2 posed hypotheses: 1) that 10/10 subjects would demonstrate elevated Task-Positive to Task-Negative connectivity and 2) that these genotype differences in cross-network connectivity would be enhanced during working memory performance.
Association between Functional Connectivity and Executive Traits
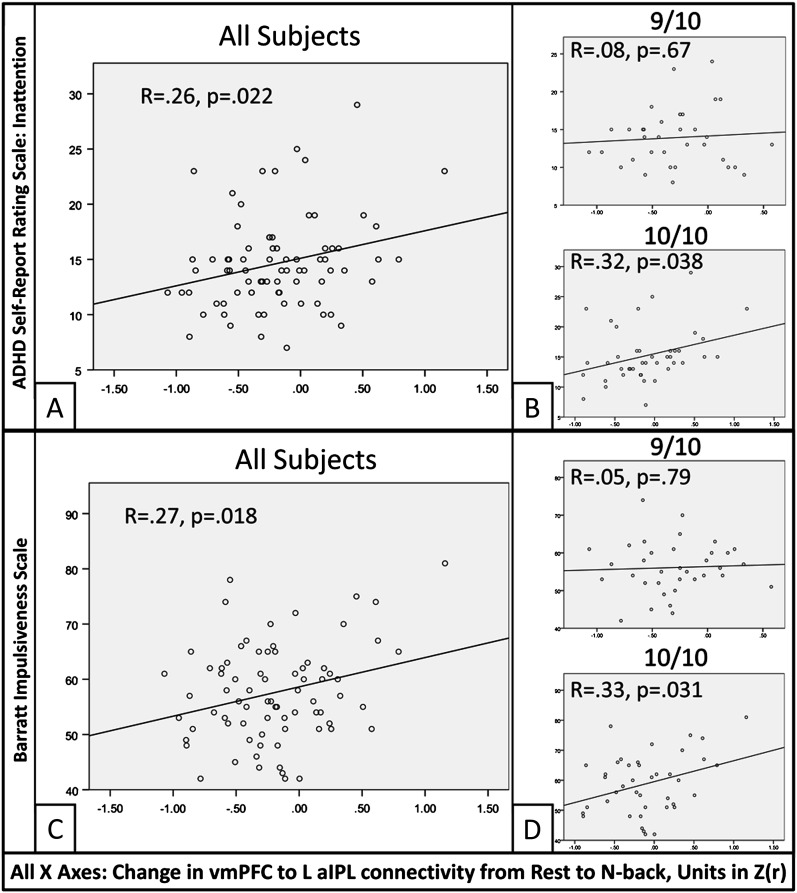
Stepwise multiple regressions were conducted evaluating whether state-related connectivity changes within connections showing DAT1 × state interactions predicted individual differences in behavioral measures of executive traits. These regressions revealed one TPN to TNN connection—between the L aIPL node of the Dorsal Attention network and the vmPFC node of the aDMN—which significantly predicted both Inattention from the ADHD Self-Report Scale (F1,75 = 5.45, model R = 0.26, P = 0.022, Fig. 7A) and Impulsivity from the Barratt Impulsiveness Scale (F1,75 = 5.84, model R = 0.27, P = 0.018, Fig. 7C), such that increased connectivity was associated with increased Inattention and Impulsivity. Hyperactivity scores were not predicted by connectivity changes within any connection (Ps > 0.4).
Figure 7.
Associations between behavioral traits of executive control and the change in vmPFC to L aIPL connectivity from the Rest scan to the N-back task. (A) Significant correlation between Inattention, as measured by the ADHD Self-Report Rating Scale, and the change in connectivity. (B) The correlation between connectivity and Inattention was nonsignificant in 9/10 subjects (top) but significant in 10/10 subjects (bottom). (C) Significant correlation between Impulsivity, as measured by the Barratt Impulsiveness Scale, and the change in connectivity. (D) The correlation between connectivity and Impulsivity was nonsignificant in 9/10 subjects (top) but significant in 10/10 subjects (bottom).
To determine the extent to which these associations differed by DAT1 genotype, we examined these relationships for each genotype group separately. Correlations were statistically significant in the 10/10 group for both Inattention (R = 0.32, P = 0.038) and Impulsivity (R = 0.33, P = 0.031) but not in the 9/10 group (Ps > 0.65) (Fig. 7B,D).
Thus, the degree to which the N-back task induced increases in cross-network connectivity between vmPFC and L aIPL was associated with self-reported inattention and impulsivity, and these associations were strongest in the 10/10 genotype group.
Discussion
The primary novel finding from this study was that a polymorphism of the dopamine transporter gene, which regulates synaptic dopamine, influenced cross-network functional connectivity, which in turn was associated with behavioral traits associated with executive dysfunction. Regardless of cognitive state, connections between frontal, parietal, and striatal nodes of TPNs and TNNs showed higher functional connectivity in 10/10 homozygotes than in 9/10 heterozygotes. However, performance of a working memory task modulated genotype differences selectively, such that connectivity between TPN and TNN was higher in 10/10 than 9/10 subjects during working memory but not during rest. Such elevated cross-network connectivity has been thought to signify cross-network interference, suggesting inefficient cognition. Indeed, the magnitude of elevated cross-network connectivity was positively correlated with self-reported inattention and impulsivity in the present study. This association was primarily driven by the 10/10 homozygotes, who also had marginally higher scores on those measures than the 9/10 heterozygotes, despite both groups performing equally well, with high accuracy, on the working memory task. Furthermore, we also replicated a previously reported finding that engagement in working memory strengthened connectivity within TPN nodes and reduced some cross-network TPN to TNN connectivities. By linking a gene regulating synaptic dopamine to a phenotype characterized by inefficient executive function, our primary findings validate cross-network connectivity as an endophenotype of executive dysfunction.
Two methodological considerations are important for interpreting these results. First, our N-back paradigm varied working memory load by including 1-back, 2-back, and 3-back blocks. As our primary hypotheses concerned effects of DAT1 and cognitive state, these load effects were regressed out, thereby ensuring that observed genotype differences were not driven by differential response to load. Thus, the results represent effects on connectivity during working memory performance, without including effects due to variability associated with changing demands between the 1-back, 2-back, and 3-back conditions. Importantly, analyses conducted without regressing out load resulted in a similar pattern of DAT1 effects on connectivity, with DAT1 effects observed primarily in cross-network connections (Supplementary Figs S3 and S4), suggesting that reported DAT1 differences were not driven by the load manipulation. (The main effect of state was affected by load—see next section).
Second, we interpret all results from the present study in relative terms (more or less negative connectivity in different groups/states) rather than in absolute terms (negative or positive connectivity), as interpreting the negative connectivity sometimes observed in cross-network connections is ambiguous. While negative cross-network correlations may reflect competitive or mutually antagonistic network relationships (Fox et al. 2005), recent work has shown that the emergence of negative connectivity strongly depends on the processing steps used, as regression of the global signal can introduce widespread and (arguably) artifactual negative connectivity (Chang and Glover 2009; Fox et al. 2009; Murphy et al. 2009). Therefore, we used alternate processing steps, including regression of motion parameters and signal from white matter/cerebrospinal fluid, which have been proposed as a middle ground (Van Dijk et al. 2010). While these steps are known to reduce the appearance of negative connectivity, it is not possible to determine definitively whether remaining negative connectivity actually represents antagonistic relationships (Chang and Glover 2009).
Effect of Cognitive State on Connectivity
The present examination of the effect of working memory on functional connectivity partially replicates past findings. In support of the hypothesis that working memory would increase connectivity within TPN compared with rest, we found that connectivity indeed increased within TPN. However, the hypothesis that working memory would decrease connectivity between TPN and TNN was only partially supported, as we found connectivity decreases between TNN and the TPN Frontoparietal Control/Dorsal Attention networks but connectivity “increases” between TNN and the TPN Salience network.
Previous work has shown that, when compared with a resting state, performance of lower level sensory/motor tasks increased connectivity within the regions engaged by the task, whether that task was auditory (e.g., listening to speech, Arfanakis et al. 2000), visual (e.g., watching a flashing checkerboard, Arfanakis et al. 2000; Hampson et al. 2004; Nir et al. 2006), or motor (e.g., finger tapping, Arfanakis et al. 2000; Jiang et al. 2004). These findings suggest a general principle that connectivity during task-evoked states specifically increases within activated regions. Similarly, performance of a higher level cognitive task such as the N-back working memory task increased connectivity within the same Task-Positive regions (Fransson 2006) that are nominally activated during working memory (Owen et al. 2005). This finding was replicated in the present study. However, Fransson (2006) also found that working memory decreased connectivity between TPN and TNN regions, which in the present study was found to be true only for the Frontoparietal Control and Dorsal Attention TPN but not for the Salience TPN. The most likely explanation for this discrepancy is that we removed effects of load, which was not done by Fransson (2006). Increasing working memory load is known to parametrically activate TPN regions (Braver et al. 1997; Veltman et al. 2003) and deactivate TNN regions (McKiernan et al. 2003). If not removed from consideration in the functional connectivity analysis, these activation changes could artificially drive the connectivity analysis, such that decreased connectivity might reflect opposing effects of task condition on activation on a timeframe the length of a condition block rather than decreased temporal synchronization on a second-to-second timeframe (Jones et al. 2010). This could reduce the ability to detect positive association between TPN and TNN. Indeed, examination of effects of cognitive state without removal of load (Supplementary Material II) showed results similar to Fransson (2006): connectivity within TPN increased, while connectivity between TNN and the TPN Frontoparietal Control networks decreased, and the connectivity increases between TNN and the TPN Salience network almost completely disappeared (Supplementary Fig. S2).
Effect of DAT1 Genotype on Connectivity
Our study is the first to demonstrate effects of the DAT1 genotype on functional connectivity either during a task or during the resting state. As hypothesized, DAT1 genotype affected connectivity, and those effects also depended upon cognitive state. Three main findings emerged from this examination of DAT1 effects. First, regardless of cognitive state, subjects with the 10/10 genotype demonstrated greater connectivity than 9/10 subjects in connections within TPN, as well as in connections between TPN and TNN. Second, cognitive state selectively modulated DAT1 differences in connections between TPN and TNN, such that higher connectivity in 10/10 than 9/10 groups was observed during working memory engagement but not during the resting state. Third, within one cross-network, TPN to TNN connection between ventromedial prefrontal cortex and lateral parietal cortex, the state-related connectivity increases predicted self-reported inattention and impulsivity in everyday behavior, especially in 10/10 subjects.
DAT1 differences in connectivity emerged regardless of cognitive state in connections between various TPN (such as between right and left Frontoparietal Control networks, between Salience and Dorsal Attention networks, and between Striatal and Dorsal Attention networks), as well as in connections between TPN and TNN (such as between Default Mode and right Frontoparietal Control, Dorsal Attention, and Salience networks). In all of these connections, 10/10 subjects had higher functional connectivity than 9/10 subjects across resting and N-back scans. In order to gain insight into these overall genotype differences, we examined patterns of connectivity effects in both cognitive states to determine whether they were true main effects or whether they suggested interactive effects of DAT1 and state (Supplementary Material III). Notably, the pattern of mean connectivities in the cross-network TPN to TNN connections resembled interactive effects similar to the significant DAT1 × state interactions discussed below. Thus, these effects may be interpreted as weak interactions that may require a higher sample size to reach significance. By contrast, TPN to TPN connections appeared to exhibit true main effects, with higher connectivity in 10/10 than 9/10 subjects during both the task and rest states. Overall differences by DAT1 in connectivity in bilateral 5 frontal, frontal–parietal, and striatal–parietal connections within TPN suggest baseline differences in the communication of information across these regions that is sensitive to dopaminergic differences. It remains to be seen whether these overall connectivity differences are replicated in future DAT1 investigations.
Performance of a working memory task was found to modulate the effect of DAT1 genotype on connectivity only in cross-network connections between TPN and TNN. These included connections between the Default Mode network and the right Frontoparietal Control, Dorsal Attention, and Salience networks. Such elevated connectivity between TPN and TNN has been interpreted as indicating more interference or reduced segregation of the networks (Kelly et al. 2008); thus, in the present study, carriers of the 10/10 DAT1 genotype demonstrated reduced segregation of TPN and TNN, particularly during a cognitive state that is associated with increased dopamine release. There is growing evidence suggesting that such reduced TPN-TNN segregation is associated with inefficient cognition. Specifically, higher connectivity between TPN and TNN has been linked to increased trial-to-trial response variability (Kelly et al. 2008) and reduced working memory performance (Hampson et al. 2010). Increased interference from TNN has also been linked to task-irrelevant thought (Buckner et al. 2008), attention lapses (Weissman et al. 2006), and mind wandering (Mason et al. 2007). Such behaviors are known consequences of reduced executive function. These behavioral effects (Castellanos et al. 2005; Willcutt et al. 2005; Klein et al. 2006) as well as elevated cross-network TPN to TNN connectivity (Castellanos et al. 2008) also characterize ADHD, a disorder defined by symptoms of inattention and impulsivity. Therefore, elevated cross-network connectivity is believed to reflect increased interference between networks that may induce task-irrelevant thoughts, resulting in inattention and impulsivity, which in turn yields inefficient cognitive processing (Sonuga-Barke and Castellanos 2007). Indeed, in the present study, greater working memory-related increases in cross-network TPN to TNN connectivity predicted increased self-reported behaviors of inattention and impulsivity in everyday life, and this relationship was stronger (and significant) in 10/10 subjects. The 10/10 genotype, which has been associated with ADHD (Yang et al. 2007), has also been associated with inefficient executive function, even in populations without a diagnosis of ADHD. Worse performance was observed on tasks of inhibitory control in healthy 10/10 adults (Caldú et al. 2007) and children (Loo et al. 2003; Cornish et al. 2005) relative to their 9/10 peers. Furthermore, hyperactivity, a defining behavior of childhood ADHD, was higher in 10/10 than 9/10 children (Mill et al. 2005). Similarly, in the present study, inattentiveness and impulsivity, which are associated with adult ADHD, tended to be higher in 10/10 than 9/10 subjects. Thus, our findings in healthy subjects demonstrate that cross-network connectivity increases are both associated with 10/10 homozygosity—a genotype linked to ADHD—and predict ADHD-like behaviors in that group. These findings validate the proposal of elevated cross-network connectivity as an endophenotype of ADHD (Sonuga-Barke and Castellanos 2007; Castellanos et al. 2008) and further contribute to an emerging theme in the literature that functional connectivity serves as an endophenotype for a variety of gene-behavior relationships (Esslinger et al. 2009; Meyer-Lindenberg 2009; Woodward et al. 2009; Smit et al. 2010; Walter et al. 2011).
For connections between TPN and TNN, the nature of DAT1 effects on connectivity varied by network. In frontal–parietal and parietal–parietal connections between TNN and the TPN Frontoparietal Control/Dorsal Attention networks, the 9/10 group demonstrated reduced connectivity during working memory compared with rest, but minimal change was observed in the 10/10 group. By contrast, in a parietal–insular connection between TNN and the TPN Salience network, the 10/10 group demonstrated elevated connectivity during working memory compared with rest, but minimal change was observed in the 9/10 group. Thus, it appears that during working memory, 10/10 subjects demonstrate an increase in cross-network interference of the Salience network, along with a failure to reduce cross-network interference of the Frontoparietal Control and Dorsal Attention networks. In light of past findings, the observed pattern of results suggests that differences in segregation of TPN during working memory are associated with DAT1 genotype. The Dorsal Attention network, which primarily includes regions along the intraparietal sulcus, has been argued to control voluntary, top-down orienting of attention, and selection of behavior (Corbetta and Shulman 2002) and specifically to be involved in rehearsal during working memory (Corbetta et al. 2002), while the Frontoparietal Control networks, which include lateral frontal and parietal regions, are believed to initiate and adjust executive control processes (Dosenbach et al. 2007, 2008; Seeley et al. 2007; Vincent et al. 2008). During working memory, these functions, which are primarily relevant to immediate task goals, are likely to be strongly segregated from the TNN, which process task-irrelevant thought (Fox et al. 2005; Spreng et al. 2010). By contrast, the Salience network, which includes anterior insula, anterior middle frontal gyrus, and dorsal anterior cingulate, is believed to maintain longer term task goals and process stimuli salient to those goals (Dosenbach et al. 2007, 2008; Seeley et al. 2007). Based on the involvement in this canonical network of the dorsal anterior cingulate, which is known to perform monitoring processes (Carter and Van Veen 2007), as well as the involvement of the anterior insula, which is believed to mediate dynamic interactions between brain networks (Menon and Uddin 2010), we speculate that increased TNN to Salience connectivity during working memory may reflect an increased need to detect TNN-based cross-network interference and segregate the networks appropriately. In this context, the failure to reduce connectivity between TNN and Frontoparietal Control/Dorsal Attention networks in 10/10 subjects reflects a lack of segregation between networks, and those subjects’ increased connectivity between TNN and Salience networks reflects increased effort needed to prevent this lack of segregation from interfering with task performance.
The mechanism by which DAT1 may influence differences in connectivity is not clear. DAT1 genotype affects expression of dopamine transporter (DAT), as higher expression is associated with the 10-repeat allele (Fuke et al. 2001; Mill et al. 2002; VanNess et al. 2005), likely leading to genotype differences in DA signaling (Madras et al. 2005). In light of observations of greater phasic DA release during working memory than during rest (Aalto et al. 2005), DAT1-related differences may be enhanced during working memory, as more DA would be available in the synapse to be reuptaken at differential rates (depending on genotype); this likely explains why the effects of DAT1 on cross-network connections emerged most strongly during working memory. DAT concentrations are highest in striatum (Hall et al. 1999; Madras et al. 2005), moderate in parietal cortex (Lewis et al. 2001), and relatively low in prefrontal cortex (Karoum et al. 1994). Therefore, DAT1 effects on brain function should be strong within striatum and reduced within prefrontal and parietal cortex—yet the present study, and past functional magnetic resonance imaging (fMRI) studies (Bertolino et al. 2006, 2009; Caldú et al. 2007; Stollstorff et al. 2010), found effects of DAT1 in prefrontal and parietal cortex. This is consistent with positron emission tomography studies, which have found that the degree of DAT expression (Tomasi et al. 2009) and dopamine synthesis (Braskie et al. 2011) within the striatum predicts cortical activation. We speculate that these DAT1 effects on corticocortical connectivity might be driven by the degree to which the striatum “gates” communication between different networks; as suggested by Braskie et al. (2011), this gating is likely enabled through the striatal–palladal–thalamiccortico loops which innervate both prefrontal and parietal cortex (Alexander et al. 1986; Schmahmann and Pandya 2006). Both neurocomputational models (Hazy et al. 2007) and imaging evidence (van Schouwenburg et al. 2010) suggest that the striatum plays a causal role in allowing or preventing (“gating”) information transfer between cortical regions. Thus, increased cross-network TPN to TNN interference observed in 10/10 DAT1 subjects could be due to lower striatal DA function reducing the ability of the striatum to gate information transfer between networks. While DAT1 effects were observed on connectivity between striatum and the parietal Dorsal Attention network, these effects were insensitive to state, suggesting that this connection is unlikely to be a gating signal mediating the DAT1 × cognitive state interaction effects on cross-network connectivity. We speculate that the gating effect may be causally “upstream” of network connectivity effects and so undetectable using connectivity assessed via pairwise correlations. Future investigations using more complex connectivity measures such as dynamic causal modeling might profitably investigate this question.
Limitations
While associations between cross-network connectivity and trait-level measures of executive function were successfully observed, this study was notably limited in its inability to investigate relationships between DAT1, connectivity, and behavioral performance on the N-back task, as overall N-back accuracy was very close to ceiling (mean ± SD = 96% ± 5.8%), and likely as a result, the genotype groups did not differ in accuracy in any condition (Ps > 0.15). This lack of difference is not unusual: while deficits in 10/10 subjects have been observed on the N-back task in children (Stollstorff et al. 2010), such deficits have not been found in adults (Bertolino et al. 2006, 2009, 2008; Caldú et al. 2007; Blanchard et al. 2011).
Associations with connectivity were observed with Barratt Impulsiveness Scale scores but not with the Hyperactive/Impulsive scores of the ADHD Self-report Rating Scale. This is likely because the ADHD Scale is designed to assess the presence of ADHD in adults with a limited number of questions, and therefore may not be sensitive to individual variation in hyperactivity/impulsivity within a nonclinical population. By contrast, the Barratt Scale is designed to assess impulsivity in nonclinical populations within several domains. By this interpretation, the fact that connectivity did correlate with Inattention on the same clinically oriented ADHD Scale suggests a particularly strong relationship between connectivity and inattentiveness.
We regressed out the effect of the N-back load condition to avoid contaminating the N-back connectivity analysis with load-related coactivations. However, using a block design prevented us from being able to model and remove effects of coactivation in individual trials; nor could we model and remove error trials (though errors were sparse in both genotype groups). The extent to which the inclusion of these trial-by-trial coactivations and errors may be altering our functional connectivity results is unknown.
Conclusions
The present results, which show that cross-network connectivity is sensitive to a genetic polymorphism important for regulating synaptic dopamine, provide an endophenotype for inefficient executive function, as reflected in higher impulsivity and inattention. This finding has important implications for cognitive disorders associated with dopamine dysregulation, such as schizophrenia and, especially, ADHD. Future studies are required to address the mechanisms by which dopamine transporter expression may lead to elevated cross-network connectivity between networks engaged and suppressed during externally oriented cognitive engagement.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health (R03MH86709 to C.J.V., F31MH088066 to E.M.G., R01NS029525 to J.M.D., and R24HD050846-06 and UL1RR031988 to Children’s National Medical Center); Canadian Institutes for Health Research grant to M.S.
Acknowledgments
We would like to thank Lindsay Anderson for assistance with subject recruitment and behavioral testing and Rusan Chen for statistical guidance. Conflict of Interest : None declared.
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25(10):2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18(8):921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J. Neurosci. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, Sambataro F, Caforio G, Sinibaldi L, Latorre V, Rampino A, Taurisano P, Fazio L, et al. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biol Psychiatry. 2008;64(3):226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J Neurosci. 2009;29(4):1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Chamberlain SR, Roiser J, Robbins TW, Müller U. Effects of two dopamine-modulating genes ( DAT1 9/10 and COMT Val/Met) on n-back working memory performance in healthy volunteers. Psychol Med. 2011;41(3):611–618. doi: 10.1017/S003329171000098X. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld R, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O’Neil JP, Baker SL, Madison CM, Jagust WJ. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp. 2011;32(6):947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bellander M, Fürth D, Karlsson S, Bäckman L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett. 2009;467(2):117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2003;16(Suppl) (CD-ROM) [abstract] [Google Scholar]
- Brown AB, Biederman J, Valera E, Makris N, Doyle A, Whitfield-Gabrieli S, Mick E, Spencer T, Faraone S, Seidman L. Relationship of DAT1 and adult ADHD to task-positive and task-negative working memory networks. Psychiatry Res: Neuroimaging. 2011;193(1):7–16. doi: 10.1016/j.pscychresns.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MÁ, Serra-Grabulosa JM, Junqué C. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37(4):1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly AMC, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal BB, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47(4):1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol Psychol. 2009;81(3):144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4(2):192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 2nd ed. New York: Springer-Verlag; 1999. [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F. Independent component model of the default-mode brain function: assessing the impact of active thinking. Brain Res Bull. 2006;70(4–6):263–269. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science (New York, N.Y.) 2009;324(5927):605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1(2):152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Lee PS, Maisog JM, Foss-Feig J, Billington ME, VanMeter J, Vaidya CJ. Strength of default mode resting state connectivity relates to white matter integrity in children. Dev Sci. 2011;14(4):738–751. doi: 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Stollstorff M, Vaidya CJ. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21306. Advance Access published July 14, 2011, doi: 10.1002/hbm.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J-C, Farde L, Sedvall G. Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage. 1999;9(1):108–116. doi: 10.1006/nimg.1998.0366. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28(8):1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung H-C, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15(8):1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157(10):1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22(1):63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29(22):7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, Veijola J, Moilanen I, Isohanni M, Zang Y-F, et al. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30(12):3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Krause J, Dresel SH, Krause K-H, La Fougère C, Zill P, Ackenheil M. Striatal dopamine transporter availability and DAT-1 gene in adults with ADHD: no higher DAT availability in patients with homozygosity for the 10-repeat allele. World J Biol Psychiatry. 2006;7(3):152–157. doi: 10.1080/15622970500518444. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42(8):986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain struct funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: brain networks under genetic control. Hum Brain Mapp. 2009;30(7):1938–1946. doi: 10.1002/hbm.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3’ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114(8):975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mill J, Xu X, Ronald A, Curran S, Price T, Knight J, Craig I, Sham P, Plomin R, Asherson P. Quantitative trait locus analysis of candidate gene alleles associated with attention deficit hyperactivity disorder (ADHD) in five genes: dRD4, DAT1, DRD5, SNAP-25, and 5HT1B. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):68–73. doi: 10.1002/ajmg.b.30107. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30(4):1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An fMRI study of the effects of psychostimulants on default-mode processing during stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7–8):640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan H-Y, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJA, Boersma M, van Beijsterveldt CEM, Posthuma D, Boomsma DI, Stam CJ, de Geus EJC. Endophenotypes in a dynamically connected brain. Behav Genet. 2010;40(2):167–177. doi: 10.1007/s10519-009-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollstorff M, Foss-Feig J, Cook EH, Stein MA, Gaillard WD, Vaidya CJ. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53(3):970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang G-J, Chang L, Ernst T, Fowler JS, Rustichini A. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4(6):e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HEM, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30(29):9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SARB, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C, Mier D, Schmitgen MM, Rietschel M, Witt SH, et al. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry. 2011;16(4):462–470. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109(1–3):182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Yang B, Chan RCK, Jing J, Li T, Sham P, Chen RYL. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3’-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]