Abstract
Type III procollagen (amino-terminal propeptide of procollagen type III) and type IV collagen are considered to be reliable serum markers for monitoring the progression of liver fibrosis. The peritoneal dissemination of gastric cancer is also characterised by abundant collagen deposition in the peritoneum. The present study was performed to investigate the potential of serum type III procollagen and IV collagen as biomarkers for peritoneal dissemination in gastric cancer. The study population consisted of 117 patients with gastric cancer: 32 patients had peritoneal dissemination which was pathologically diagnosed by laparotomy or laparoscopic examination, while 85 patients (45/40, early/advanced gastric cancer) had no peritoneal dissemination. We measured the serum levels of type III procollagen and type IV collagen in comparison to the commonly accepted tumor markers carcinoembryonic (CEA), carbohydrate antigen (CA)19-9 and CA125. The median type III procollagen levels showed no significant differences between the two groups, whereas the median type IV collagen levels were significantly (201 ng/ml) higher in patients with than in those without peritoneal dissemination (early/advanced gastric cancer, 124/136 ng/ml) (P<0.05). In receiver operating characteristic (ROC) curve analysis, type IV collagen had the largest area under the curve (0.83), followed by CA125 (0.72), CA19-9 (0.64), CEA (0.59) and type III procollagen (0.48). Type IV collagen was an independent marker (P<0.0001, odds ratio 15.7) for predicting peritoneal dissemination along with CA125 (P=0.0086, odds ratio 9.4) based on multivariate logistic regression. In conclusion, serum type IV collagen levels may be significant in the early detection and management of patients with peritoneal dissemination of gastric cancer.
Keywords: type IV collagen, gastric cancer, peritoneal dissemination
Introduction
Gastric cancer is the fourth most common type of cancer and the second leading cause of cancer-related death worldwide (1). Peritoneal dissemination is a common finding and the most frequent site of recurrence in gastric cancer; it is associated with a poor prognosis even after curative resection (2–4). In recent years, certain reports have focused on the efficacy of chemotherapy for peritoneal dissemination (5–9). Staging laparoscopy is currently the most sensitive method for detecting peritoneal dissemination and minimising the possibility of unnecessary laparotomy (10,11). However, it is often extremely difficult to make a decision regarding indications and timing in performing laparoscopy in preoperative diagnosis and during postoperative monitoring. Although computed tomography (CT) is a diagnostic modality for the detection of peritoneal metastasis in conventional imaging study, CT has a limited capacity for revealing peritoneal metastasis unless the disease has progressed sufficiently to cause obstruction of the intestinal, biliary or urinary tract (12–14). High-speed spiral CT showed a sensitivity of only 47.4% in patients with peritoneal dissemination of abdominal malignancies (15).
Therefore, it is necessary to identify an ideal molecular marker with which to determine the optimum time for staging laparoscopy so as not to retard chemotherapy due to the inevitable delay in detection. Such a marker is expected to be less invasive and easily monitored, for example by a simple blood test. Carbohydrate antigen (CA) 125 has been investigated for putative diagnostic value, but its utility as a screening marker is limited due to its low sensitivity (16).
Peritoneal dissemination in gastric cancer is characterised by abundant collagen deposition in the peritoneum (17), resulting in various severe complications such as ileus, hydronephrosis and obstructive jaundice. The mechanism proposed for the formation of this desmoplastic stroma is the apparent increase caused by the accumulation of the preexisting matrix or synthesis by neoplastic cells themselves. In the liver, extracellular matrix components, the amino-terminal propeptide of procollagen type III (type III procollagen) and type IV collagen are considered to be reliable serum markers of active fibrogenesis and are used clinically to assess the progression of fibrosis (18–20).
The present study was performed to investigate whether the progression of peritoneal metastasis in gastric cancer is accompanied by such changes in collagen-and basement membrane-related metabolites in serum, as well as whether these metabolites are useful in the diagnosis of disease progression in clinical practice. The serum levels of type III procollagen and type IV collagen were examined in gastric cancer patients with and without peritoneal dissemination. Additionally type III procollagen and IV collagen were compared to commonly accepted tumor markers, such as carcinoembryonic antigen (CEA), CA19-9 and CA125. Findings of this study showed that type IV collagen can be used as a serum marker for peritoneal dissemination in gastric cancer.
Materials and methods
Patients and serum samples
Serum was obtained at the time of diagnosis with informed consent according to our Institutional Review Board-approved guidelines. A total of 117 serum samples were obtained from patients with pathologically confirmed gastric adenocarcinoma, all of whom underwent surgical treatment or laparoscopic examination at the Department of Gastroenterological Surgery of Kanazawa University Hospital between 2004 and 2008.
Inclusion criteria for the study involved patients with a confirmed diagnosis of gastric adenocarcinoma and the provision of written informed consent. Exclusion criteria included inability to provide informed consent, patients with chronic hepatitis or liver cirrhosis, to eliminate false-positive results, and patients with other malignancies diagnosed or treated within the last 5 years.
Of the 117 patients included in this study, 32 (27.3%) had peritoneal dissemination (17 male, 15 female; mean age 57 years, range 28–80). All of the patients underwent laparotomy (n=7) or laparoscopic examination (n=25) to pathologically confirm the peritoneal dissemination of gastric cancer. Based on the laparotomy findings, 85 of the 117 patients (72.6%) had no peritoneal dissemination. These patients were divided into two groups based on pathological findings: 40 patients (34.1%) in the advanced gastric cancer group (23 male, 17 female; mean age 68 years, range 47–78) and 45 patients (38.6%) in the early gastric cancer group (22 male, 23 female; mean age 67 years, range 50–75). Staging was carried out according to the classification of the Japanese Endoscopic Society, in which the distribution of early gastric cancer was defined as a T1 tumor (tumor invasion of mucosa or submucosa) (21).
Samples were centrifuged after collection and stored at −70°C until the assays were performed. The samples were labelled with a unique identifier to protect the confidentiality of the patients. None of the samples were thawed more than twice before analysis.
Biochemical assays
CEA and CA19-9 levels were measured by a counting immunoassay using a Ranream CEA kit (TOA Medical Electronics Co., Kobe, Japan) and a Ranream CA19-9 kit (Toray-Fuji Bionics, Tokyo, Japan). CA125 analysis was performed using a Cobas Core CA125 enzyme-immunoassay analysis kit (Roche, Basel, Switzerland). The serum type III procollagen level was determined using a commercial RIA kit (Behringwerke AG, Marburg, Germany), and type IV collagen concentration was determined by ELISA using a commercial kit (Fuji Chemical Ind., Tokyo, Japan). Each sample was assessed in triplicate. The cut-off values of CEA, CA19-9 and CA125 were set according to the manufacturer’s instructions (6.5 ng/ml, 37 U/ml and 35 U/ml, respectively).
Statistical methods
A comparison between the quantitative variables was performed using the Mann-Whitney U test. The diagnostic accuracy of each of the candidate biomarkers was evaluated using receiver operating characteristic (ROC) curve analysis, which correlates true and false-positive rates [sensitivity and (1 - specificity)]. In addition, the differences in the area under the curve (AUC) values were determined. The optimal cut-off point for type IV collagen was selected based on the ROC curve analysis. Sensitivity, specificity, positive and negative predictive values were calculated using a 2 × 2 table of the collected data. Multivariate logistic regression for the odds ratio was used to assess the simultaneous contribution of each covariate in the multivariate analysis. In all analyses, P<0.05 was considered to be statistically significant. Statistical analyses were carried out using SPSS® v12.0 software.
Results
Serum levels of type III procollagen and type IV collagen
The median serum type III procollagen levels in the early and advanced gastric cancer groups were 0.55 and 0.62 U/ml, respectively. The corresponding value in patients with peritoneal dissemination was 0.68 U/ml. No significant differences were noted in the median serum type III procollagen levels among the three groups (Fig. 1).
Figure 1.
Individual serum levels of type III procollagen in early and advanced gastric cancer patients and patients with peritoneal dissemination. No significant differences were found in the median serum type III procollagen levels among the three groups. N.S., not significant.
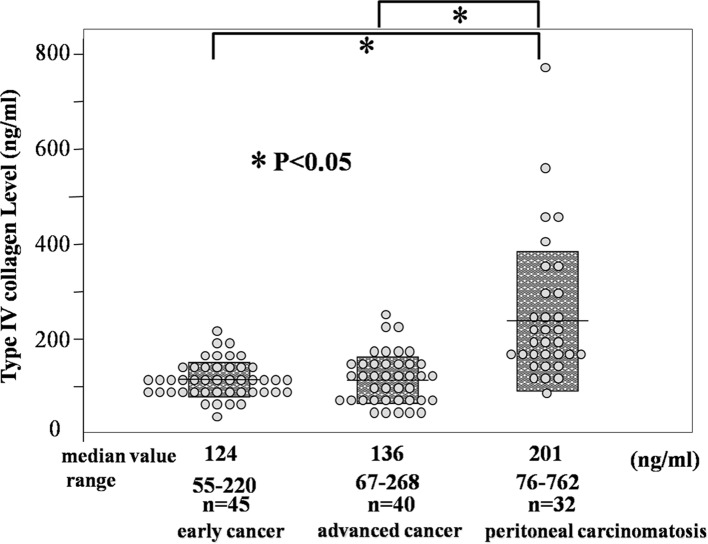
In contrast, the median serum type IV collagen level was significantly higher in patients with peritoneal dissemination (201 ng/ml) in comparison with the early gastric cancer patients (124 ng/ml) and advanced gastric cancer patients (136 ng/ml) (P<0.05) (Fig. 2).
Figure 2.
Comparison of serum levels of type IV collagen among the three groups. The median type IV collagen level was significantly higher in patients with peritoneal dissemination in comparison with the levels for early and advanced gastric cancer patients (p<0.05).
ROC curve for the diagnosis of peritoneal dissemination
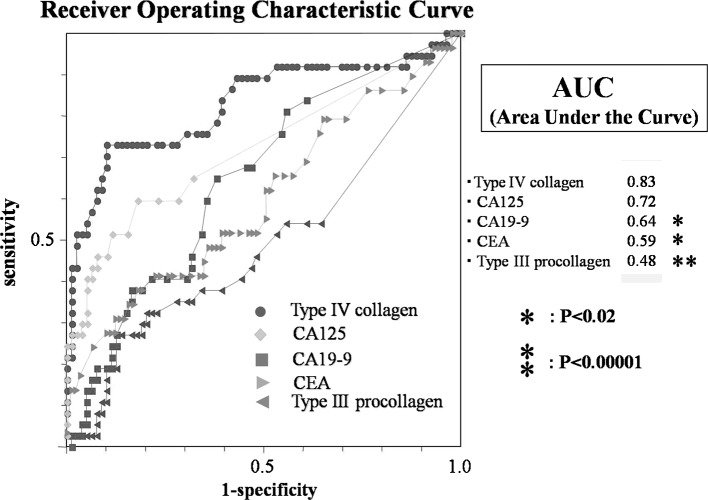
To evaluate the performance of type III procollagen and IV collagen as a diagnostic test, ROC curves were generated as shown in Fig. 3. The diagnostic accuracy of the test is expressed by the AUC. Among the individual markers, type IV collagen had the largest AUC (0.83), followed by CA125 (0.72), CA19-9 (0.64), CEA (0.59) and type III procollagen (0.48). Although no significant difference was found between the AUC values of type IV collagen and CA125 (P=0.15), the AUC value of type IV collagen was significantly higher than those of CA19-9, CEA and type III procollagen (P<0.02–0.00001). These observations suggested that type IV collagen is a more useful marker for predicting peritoneal dissemination than type III procollagen.
Figure 3.
Receiver operating characteristic (ROC) curves of type IV collagen, type III procollagen, CEA, CA19-9 and CA125 for the diagnosis of peritoneal dissemination in gastric cancer. ROC curves were derived by plotting the relationship between the specificity and sensitivity at various cut-off levels. The diagnostic accuracy of the test is expressed by the area under the curve (AUC). Among the individual markers, type IV collagen had the largest AUC (0.83), followed by CA125 (0.72), CA19-9 (0.64), CEA (0.59) and type III procollagen (0.48).
The correlation between the levels of type IV collagen and CA125 was investigated. The data showed no correlation between these markers with a coefficient (R2) of 0.06 (Fig. 4). This result suggested that serum type IV collagen is useful as a serum marker for peritoneal dissemination involving a different mechanism than that responsible for the elevation of CA125.
Figure 4.
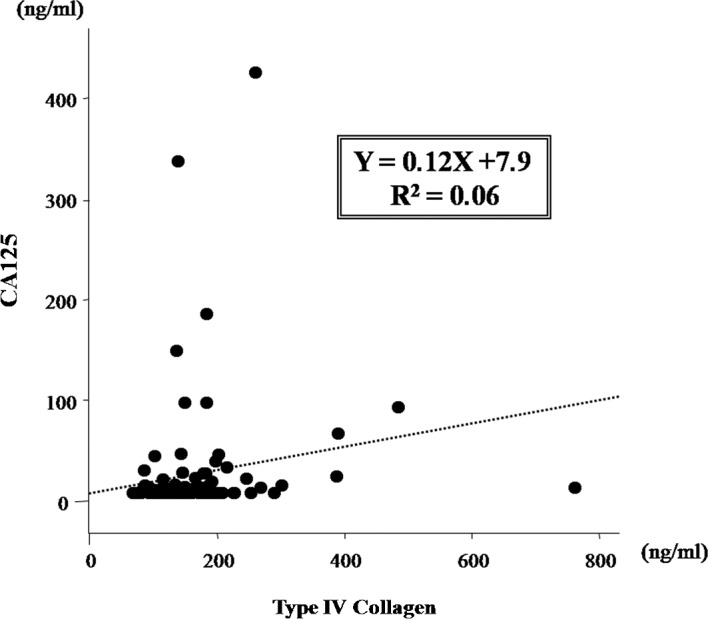
Scatter plot for serum type IV collagen and serum CA125. No correlation was noted between the serum type IV collagen and serum CA125 levels (coefficient, R2=0.06). Serum type IV collagen may behave as a serum marker for peritoneal dissemination in gastric cancer involving a different mechanism than that of CA125.
The correlations between the levels of other markers were also investigated, but no significant correlations were observed (data not shown).
With regard to type IV collagen, the best compromise between true and false positives was achieved by a threshold of ~170 ng/ml according to the ROC curve analysis.
Comparison of biomarkers for the diagnosis of peritoneal dissemination
Table I shows the performance of the biomarkers for the diagnosis of peritoneal dissemination in gastric cancer. For type IV collagen, a cut-off value of 170 ng/ml was used in this investigation. The sensitivity of type IV collagen was much higher than that of the other markers. The specificity and positive predictive values of CA125 were higher than those of type IV collagen. The highest negative predictive value was observed for type IV collagen. Type IV collagen, CA19-9 and CA125 significantly predicted peritoneal dissemination in the univariate analyses. Based on multivariate logistic regression, type IV collagen and CA125 independently predicted peritoneal dissemination. The odds ratios for type IV collagen and CA125 were 15.667 (95% CI, 5.534–44.312) and 9.435 (95% CI, 1.765–50.459), respectively (Table II).
Table I.
Comparison of the diagnostic ability of serum biomarkers for the diagnosis of peritoneal dissemination.
| Biomarker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
| CEA | 27.03 | 87.34 | 50.00 | 71.88 | 2.56 | 0.98–6.69 | 0.05600 |
| CA19-9 | 21.62 | 92.41 | 57.14 | 71.57 | 3.36 | 1.11–10.06 | 0.03100 |
| CA125 | 29.73 | 94.94 | 73.33 | 74.26 | 7.93 | 3.74–9.21 | 0.00050 |
| Type IV collagen | 70.27 | 86.08 | 70.27 | 86.08 | 14.616 | 2.80–9.07 | <0.00001 |
PPV, positive predictive value; NPV, negative predictive value; OR, odds ratio; CI, confidence interval; CAE, carcinoembryonic antigen; CA, carbohydrate antigen.
Table II.
Multivariable logistic regression analyses to predict peritoneal dissemination.
| Biomarker | SD | P-value | OR | 95% CI |
|---|---|---|---|---|
| CA19-9 | 0.7805 | 0.8300 | 0.84 | 0.18–3.89 |
| CA125 | 0.8553 | 0.0086 | 9.43 | 1.76–50.45 |
| Type IV collagen | 0.5306 | <0.0001 | 15.66 | 5.53–44.31 |
SD, standard error; OR, odds ratio; CI, confidence interval; CA, carbohydrate antigen.
Association between type IV collagen and the size of the primary tumor
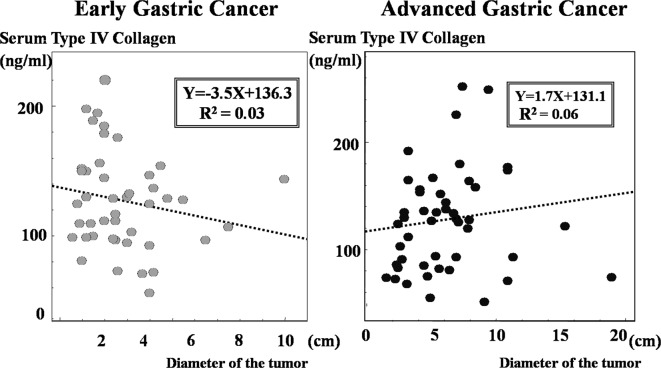
The correlation between the size of the primary tumor and serum type IV collagen levels in patients with early and advanced gastric cancer was analysed. As shown in Fig. 5, no correlations were found between the size of the primary tumor and serum type IV collagen levels with coefficients (R2) of 0.03 in early gastric cancer and 0.06 in advanced gastric cancer.
Figure 5.
Scatter plots showing the correlation between size of the primary tumor and serum type IV collagen levels. No correlations were noted between size of the primary tumor and serum type IV collagen levels with coefficients (R2) of 0.03 in early gastric cancer and 0.06 in advanced gastric cancer.
Type IV collagen in patients with scirrhous gastric cancer
The serum type IV collagen levels were measured in patients with scirrhous gastric carcinoma, which is characterised by increased fibrous stroma in the primary tumor (n=3). As shown in Fig. 6, the median serum type IV collagen level in patients with peritoneal dissemination (238 ng/ml, 142–600 ng/ml) was significantly higher than in patients without peritoneal dissemination (140 ng/ml, 67–268 ng/ml). The results suggested that serum type IV collagen levels are closely related to the process of peritoneal dissemination.
Figure 6.
Serum levels of type IV collagen in patients with scirrhous gastric cancer, characterized by increased fibrous stroma in the primary tumor. The median type IV collagen level in patients with peritoneal dissemination was significantly higher than that in patients without peritoneal dissemination (p<0.05).
Discussion
Extracellular matrix components, particularly type III procollagen and IV collagen, have been used as indirect fibrogenesis markers in various chronic liver diseases, such as haemochromatosis, viral hepatitis and alcoholic liver disease. These components are reported to be useful indicators of collagen matrix turnover in other diseases, such as interstitial pneumonia, cardiomyopathy, diabetic nephropathy and systemic sclerosis which are characterised by the accumulation of collagen in the organs (22–25). Furthermore, elevation of the extracellular matrix components in body fluid was confirmed in patients with carcinomas, including stomach, lung, liver, colon and breast (26–28). Akazawa et al reported that the serum levels of type III procollagen in scirrhous gastric cancer patients were elevated above normal values (29). Korenaga et al also reported that type IV collagen levels in peritoneal lavage in patients with peritoneal dissemination of gastric cancer were significantly higher than patients without peritoneal dissemination (30). In the present study, the serum levels of type IV collagen were significantly higher in patients with than in those without peritoneal dissemination. No significant differences were found in the serum type III procollagen levels between the two groups of patients.
The most significant criterion for tumor markers is the sensitivity/specificity diagram ROC curve (31). The area under the ROC curve indicates the clinical usefulness of a tumor marker, and a larger AUC corresponds to a more favorable tumor marker. In this study, type IV collagen had the largest AUC of the individual markers. We used a cut-off point of 170 ng/ml for type IV collagen in the ROC analysis. The sensitivity and negative predictive values of type IV collagen were much higher than those of other conventional markers, but were lower than those of CA125. Multivariate analysis indicated that the serum type IV collagen level was an independent predictive factor for peritoneal dissemination. To the best of our knowledge, this is the first study of serum type IV collagen elevation in gastric cancer patients with peritoneal dissemination.
The present study showed that CA125 is more useful than the conventional biomarkers CEA and CA19-9. Since no significant correlation was noted between the serum levels of type IV collagen and CA125 and these markers were independent predictive factors in multivariate analysis, it is possible that a combination assay with type IV collagen and CA125 may be more useful in the improvement of diagnostic parameters for the detection of peritoneal dissemination.
Subsequently, we investigated whether serum type IV collagen levels were affected by fibrosis in the primary tumor. No correlation was found between the size of the primary tumor and serum type IV collagen levels. Moreover type IV collagen levels were significantly higher in patients with than in those without peritoneal dissemination, even in cases of scirrhous gastric cancer, which is characterised pathologically by abundant fibrous stroma. The results suggested that the elevation of serum type IV collagen is not affected by the primary tumor, and may be a specific predictor of peritoneal dissemination. Although the precise mechanism remains unclear, we speculated that such changes in type IV collagen in reaction to peritoneal dissemination result from the destruction of the basement membrane in the peritoneum. Type IV collagen is a significant component of the basement membrane, a physical barrier that prevents cancer cells from invading the underlying stroma (32,33). Previous studies supported the importance of increased proteolytic degradation of the extracellular matrix composed of interstitial matrix and basement membrane in the process of tumor invasion and metastasis (32–36).
Matrix metalloproteinases (MMPs) are a family of highly conserved zinc-and calcium-dependent extracellular enzymes involved in the modification of the extracellular matrix (34–36). A number of studies demonstrated enhanced the tissue expression of MMP2 and 9. These MMPs are known to degrade type IV collagen and gelatine in the basement membrane in a number of malignant tumors (37–39). With regard to peritoneal dissemination, it was reported that the up-regulation of MMP2 in ovarian cancer cells is critical for their adhesion to the mesothelial lining of the peritoneum and omentum (40). In addition, Sun et al reported that type IV collagenase (MMP2/9) activity was increased in malignant ascites, including gastric cancer (41). Given these findings, it was speculated that the presence of type IV collagen in serum is related to the destruction of the basement membrane during the process of metastasis. On the other hand, type III procollagen, which is a type of fibrillar collagen, is present particularly in tissues exhibiting elastic properties, such as skin and blood vessels, and is identified in the fibrous stroma in the primary tumors of gastric cancer (42). It appears likely that type III procollagen is less affected by the destruction of the basement membrane in this situation.
The present study suggested that an analysis of the type IV collagen level improves diagnostic accuracy in cases of peritoneal dissemination in gastric cancer. Laparoscopic examination may be performed prior to chemotherapy in patients with suspected peritoneal dissemination detected based on serum type IV collagen level. Therefore, the appropriate treatment may be selected to maximise the benefit of therapy at the time of exploration. In conclusion, the serum type IV collagen level is a potentially useful novel biomarker for the peritoneal dissemination of gastric cancer. Studies in larger numbers of patients using repeated measurements of type IV collagen should be performed in order to evaluate the prognostic value of this procedure for peritoneal dissemination in gastric cancer.
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki H, Oshima A, Murakami R, Endoh S, Ubukata T. A long-term follow-up study of patients with gastric cancer detected by mass screening. Cancer. 1989;63:613–617. doi: 10.1002/1097-0142(19890215)63:4<613::aid-cncr2820630402>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Ikeguchi M, Yamamoto O, Kaibara N. Management protocol for scirrhous gastric cancer. In Vivo. 2004;18:577–580. [PubMed] [Google Scholar]
- 4.Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepatogastroenterology. 2002;49:874–877. [PubMed] [Google Scholar]
- 5.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 7.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Nagawa H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 8.Fushida S, Kinoshita J, Yagi Y, Funaki H, Kinami S, Ninomiya I, Fujimura T, Nishimura G, Kayahara M, Ohta T. Dual anti-cancer effects of weekly intraperitoneal docetaxel in treatment of advanced gastric cancer patients with peritoneal carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep. 2008;19:1305–1310. [PubMed] [Google Scholar]
- 9.Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J, Nishiyama M. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402–3407. doi: 10.1158/1078-0432.CCR-05-2425. [DOI] [PubMed] [Google Scholar]
- 10.Burke EC, Karpeh MS, Conlon KC, Brennan MF. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg. 1997;225:262–267. doi: 10.1097/00000658-199703000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gretschel S, Siegel R, Estévez-Schwarz L, Hünerbein M, Schneider U, Schlag PM. Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg. 2006;93:1530–1535. doi: 10.1002/bjs.5513. [DOI] [PubMed] [Google Scholar]
- 12.Sendler A, Dittler HJ, Feussner H, Nekarda H, Bollschweiler E, Fink U, Helmberger H, Höfler H, Siewert JR. Preoperative staging of gastric cancer as precondition for multimodal treatment. World J Surg. 1995;19:501–508. doi: 10.1007/BF00294710. [DOI] [PubMed] [Google Scholar]
- 13.Davies J, Chalmers AG, Sue-Ling HM, May J, Miller GV, Martin IG, Johnston D. Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut. 1997;41:314–319. doi: 10.1136/gut.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Düx M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr. 1999;23:913–922. doi: 10.1097/00004728-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Yang QM, Bando E, Kawamura T, Tsukiyama G, Nemoto M, Yonemura Y, Furukawa H. The diagnostic value of PET-CT for peritoneal dissemination of abdominal malignancies. Gan To Kagaku Ryoho. 2006;33:1817–1821. [PubMed] [Google Scholar]
- 16.Nakata B, Hirakawa YS, Chung K, Kato Y, Yamashita Y, Maeda K, Onoda N. Serum CA 125 level as a predictor of peritoneal dissemination in patients with gastric carcinoma. Cancer. 1998;83:2488–2492. doi: 10.1002/(sici)1097-0142(19981215)83:12<2488::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Fibrosis in the peritoneum induced by scirrhous gastric cancer cells may act as ‘soil’ for peritoneal dissemination. Cancer. 1996;77:1668–1675. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1668::AID-CNCR37>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielli GB, Corrocher R. Hepatic fibrosis and its serum markers. Dig Dis. 1991;9:303–316. doi: 10.1159/000171314. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Suou T, Kawasaki H, Yoshikawa N. Clinical significance of serum 7S collagen in various liver diseases. Clin Biochem. 1992;25:467–470. doi: 10.1016/0009-9120(92)90150-q. [DOI] [PubMed] [Google Scholar]
- 20.Jeffers LJ, Coelho-Little ME, Cheinquer H, Vargas C, Civantos F, Alvarez L. Procollagen-III peptide and chronic viral C hepatitis. Am J Gastroenterol. 1995;90:1437–1440. [PubMed] [Google Scholar]
- 21.Japanese Research Society for Gastric Cancer. The General Rules for the Gastric Cancer Study. 12th edition. Kanehara Shuppan; Tokyo: 1993. [Google Scholar]
- 22.Kasuga I, Yonemaru M, Kiyokawa H, Ichinose Y, Toyama K. Clinical evaluation of serum type IV collagen 7S in idiopathic pulmonary fibrosis. Respirology. 1996;1:277–281. doi: 10.1111/j.1440-1843.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Kataoka K, Matsumori A, Sasayama S, Yamada T, Ito H. Measuring serum aminoterminal type III procollagen peptide, 7S domain of type IV collagen, and cardiac troponin T in patients with idiopathic dilated cardiomyopathy and secondary cardiomyopathy. Heart. 1997;78:505–508. doi: 10.1136/hrt.78.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guseva NG, Anikina NV, Myllylä R, Risteli L, Risteli J, Chochlova JV. Markers of collagen and basement membrane metabolism in sera of patients with progressive systemic sclerosis. Ann Rheum Dis. 1991;50:481–486. doi: 10.1136/ard.50.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Wu Z, Zhou Q, Zhang Y, Wu D. The role of determining the levels of serum collagen type IV in diagnosing early diabetic nephropathy. Ren Fail. 2002;24:747–753. doi: 10.1081/jdi-120016063. [DOI] [PubMed] [Google Scholar]
- 26.Katayama M, Hino F, Kamihagi K, Sekiguchi K, Titani K, Kato I. Urinary fibronectin fragments (a potential tumor marker) measured by immunoenzymometric assay with domain-specific monoclonal antibodies. Clin Chem. 1991;37:466–471. [PubMed] [Google Scholar]
- 27.Basso D, Belluco C, Mazza S, Greco E, Della Rocca F, Pauletto P, Nitti D, Lise M, Plebani M. Colorectal cancer metastatic phenotype stimulates production by fibroblasts of N-terminal peptide of type III collagen: clinical implications for prognosis. Clin Chim Acta. 2001;312:135–142. doi: 10.1016/s0009-8981(01)00621-0. [DOI] [PubMed] [Google Scholar]
- 28.Mazouni C, Arun B, André F, Ayers M, Krishnamurthy S, Wang B, Hortobagyi GN, Buzdar AU, Pusztai L. Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br J Cancer. 2008;99:68–71. doi: 10.1038/sj.bjc.6604443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akazawa S, Fujiki T, Kanda Y, Kumai R, Yoshida S. Diagnostic values of serum type III procollagen N-terminal peptide in type IV gastric cancer. Gan To Kagaku Ryoho. 1985;12:861–866. [PubMed] [Google Scholar]
- 30.Korenaga D, Orita H, Maekawa S, Itasaka H, Ikeda T, Sugimachi K. Peritoneal collagen type IV concentration in adenocarcinoma of the gastrointestinal tract and its relationship to histological differentiation, metastasis, and survival. Surg Today. 1998;28:780–786. doi: 10.1007/s005950050226. [DOI] [PubMed] [Google Scholar]
- 31.Baker SG. The central role of receiver operating characteristic (ROC) curves in evaluating tests for the early detection of cancer. J Natl Cancer Inst. 2003;95:511–515. doi: 10.1093/jnci/95.7.511. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Hernandez A, Amenta PS. The basement membrane in pathology. Lab Invest. 1983;48:656–677. [PubMed] [Google Scholar]
- 33.Liotta LA. Tumor invasion and metastases – role of the extracellular matrix. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 34.Meyer T, Hart IR. Mechanisms of tumor metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 35.Conway JG, Trexler SJ, Wakefield JA, Marron BE, Emerson DL, Bickett DM. Effect of matrix metalloproteinase inhibitors on tumor growth and spontaneous metastasis. Clin Exp Metastasis. 1996;14:115–124. doi: 10.1007/BF00121208. [DOI] [PubMed] [Google Scholar]
- 36.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 37.Mori M, Mimori K, Shiraishi T, Fujie T, Baba K, Kusumoto H, Haraguchi M, Ueo H, Akiyoshi T. Analysis of MT1-MMP and MMP2 expression in human gastric cancers. Int J Cancer. 1997;74:316–321. doi: 10.1002/(sici)1097-0215(19970620)74:3<316::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.De Mingo M, Morán A, Sánchez-Pernaute A, Iniesta P, Díez-Valladares L, Pérez-Aguirre E, de Juan C, García-Aranda C, Díaz-López A, García-Botella A, Martín-Antona E, Benito M, Torres A, Balibrea JL. Expression of MMP-9 and TIMP-1 as prognostic markers in gastric carcinoma. Hepatogastroenterology. 2007;54:315–319. [PubMed] [Google Scholar]
- 39.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun XM, Dong WG, Yu BP, Luo HS, Yu JP. Detection of type IV collagenase activity in malignant ascites. World J Gastroenterol. 2003;9:2592–2595. doi: 10.3748/wjg.v9.i11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamoto T, Ooi A, Okada Y, Mai M, Nagai Y, Nakanishi I. Desmoplastic reaction of gastric carcinoma: a light-and electron-microscopic immunohistochemical analysis using collagen type-specific antibodies. Hum Pathol. 1998;19:815–821. doi: 10.1016/s0046-8177(88)80265-x. [DOI] [PubMed] [Google Scholar]