Abstract
The administration of recombinant adeno-associated viral vectors (rAAV) for gene transfer induces strong humoral responses through mechanisms that remain incompletely characterized. To investigate the links between innate and adaptive immune responses to the vector, rAAVs were injected intravenously into mice deficient in cell-intrinsic components of innate responses (Toll-like receptors (TLRs), type-1 interferon (IFN) or inflammasome signaling molecules) and AAV-specific antibodies were measured. Of all molecules tested, only MyD88 was critically needed to mount immunoglobulin G (IgG) responses since MyD88−/− mice failed to develop high levels of AAV-specific IgG2 and IgG3, regardless of capsid serotype injected. None of the TLRs tested was essential here, but TLR9 ensured a Th1-biased antibody responses. Indeed, capsid-specific Th1 cells were induced upon injection of rAAV1, as directly confirmed with an epitope-tagged capsid, and the priming and development of these Th1 cells required T cell-extrinsic MyD88. Cell transfer experiments showed that autonomous MyD88 signaling in B cells, but not T cells, was sufficient to produce Th1-dependent IgGs. Therefore, rAAV triggers innate responses, at least via B cells, controlling the development of capsid-specific Th1-driven antibodies. MyD88 emerges as a critical and pivotal regulator of both T- and B-cell adaptive immunity against AAV.
Introduction
Recombinant vectors derived from adeno-associated virus (rAAV) are currently tested in several phase I/II gene therapy trials. In spite of being non-inflammatory1 and well-tolerated in animals, particularly in mice, rAAV vectors have proven to be more immunogenic than initially expected.2 In mice or primates, the adaptive immunity developing against rAAV itself is characterized by a robust humoral response and capsid serotype-specific neutralizing activity3 preventing vector readministration even with low titers.4,5 In mice, gene transfer is facilitated with serotypes inducing low levels of capsid-specific CD4 and CD8 T cells.6 In humans, there is prevalent seropositivity against several AAV capsid serotypes in particular serotypes 1 and 2 which elicit high titers of neutralizing antibodies.7,8 Administration of rAAVs gene transfer vectors can boost pre-existing natural humoral immunity in patients, triggering in some cases, the destruction of gene-modified cells by capsid epitope-specific CD4 and CD8 T cells.9,10,11 Better understanding the molecular and cellular bases for the immunogenicity of AAV, is therefore of great interest for medical applications of this viral vector.
During immune reactions, T cell help is influenced by innate responses that provide essential accessory signals for contextual information.12,13 Pathogen-associated molecular patterns are recognized by the innate system using various receptors such as the highly conserved intracellular Toll-like receptors (TLRs) located at the membrane or in endosomes (recently reviewed by ref. 13). Such pathogen-associated molecular pattern recognition mediated either through MyD88 or TRIF adaptors, leads to the production of type I interferon (IFN) or of inflammatory cytokines. Pathogenic signals may also trigger the inflammasome such as NALP3. This cytoplasmic multiprotein complex leads to the activation of the proinflammatory cytokines interleukin (IL)-1β and IL-18 and can be triggered by several microbial components and by various danger-associated host molecules.14 There is little doubt that as a virus, AAV faces some level of innate immune defenses but the specific signals and mechanisms triggered by rAAV innate recognition are only beginning to emerge. Upon intravenous injection, rAAV interacts with complement proteins which facilitate uptake of AAV by macrophages, trigger cytokine production and contribute to B cell responses to AAV2 in mice.15 Cell-intrinsic innate recognition mechanisms are also engaged in response to AAV. Both the viral genome and the capsid have been recently identified as pathogen-associated molecular patterns. Several rAAV serotypes (AAV1, AAV2, AAV9) trigger the TLR9/MyD88 and type I interferon cascade in murine and human plasmacytoid dendritic cells in vitro.16 This TLR9-induced cascade appears to be essential in mice in vivo, for the development of antibodies and of CD8 T-cell responses against both vector and transgene.16 When structured as self-complementary DNA, the viral genome of AAV2 triggers a marked inflammatory cytokine response in the liver of mice that is TLR9 dependent.17 AAV also triggers NFκB-dependent responses in mouse liver presumably through effects mediated by genomic sequences.18 Besides the viral genome, the capsid of AAV2 was also recently found to trigger innate recognition in human cell cultures. The AAV2 capsid is recognized by TLR2 in primary human liver nonhepatocyte cells in vitro, mediating NFκB-dependent cytokine production.19 Altogether, these studies provide strong evidence that innate immune recognition of AAV occurs and probably involves both the recognition of viral genome and capsid determinants. However, there is still limited information on the importance of the innate recognition for the development of T- and B-cell adaptive immune responses occurring in vivo following administration of rAAV. While an inflammatory activation is observed in the liver in response to administration of rAAV it is not clear if this inflammation has a direct link to the capacity to induce antibody or cellular responses in response to the vector. Most studies have focused on serotype 2 and the experimental systems in vivo, trigger concomitant immune responses against the vector and transgene which could be confounding factors.
To establish a link between specific innate pathways and adaptive immune responses to rAAV, we have used a battery of mice deficient in various specific molecular components of TLR, type-I IFN or IL-1/inflammasome-mediated responses and measured adaptive responses to the vector. To study vector and capsid-specific responses while avoiding reactions against transgenic antigens, we have tested rAAV vectors carrying non protein-coding sequences or coding for a naturally tolerated proteins. We have also used several AAV serotypes and rAAV1 vectors engineered with a capsid bearing CD4 epitope tags. These tools enabled us to show that the systemic intravenous (i.v.) administration of rAAV induced a strong antibody response dominated by immunoglobulin G2 (IgG2) and IgG3 subtypes, linked to the induction of a capsid-specific Th1 CD4 T cell response. Such antibody response was critically-dependent in vivo upon MyD88 signaling for both antibody and cell-mediated immunity but surprisingly not critically-dependent upon a single TLR, or upon type I IFN or IL-1/inflammasome signaling, although TLR9 innate recognition of rAAV ensured Th1-based antibody responses. Cell transfer experiments showed that B cells but not T cells, required cell-intrinsic MyD88 signaling to promote humoral rAAV1 immunity. Collectively these observations reveal that immune responses against rAAV link specific innate signals to adaptive T and B cells responses. MyD88 emerges as a precise therapeutic target for immunosuppression in the context of gene therapy.
Results
Antibody response to rAAV1 is MyD88-dependent but TLR/IL1-independent
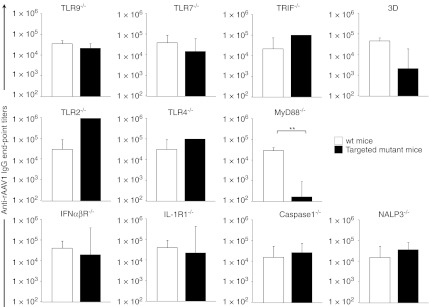
Various mutant mice with specific deficits in pattern recognition receptors (PRRs) and downstream signaling pathways were injected i.v. with rAAV1 to measure the levels of anti-rAAV1 IgG induced. We administered the rAAV1-U7ex23 vector which does not encode a protein.20 In normal mice [so-called wild-type (wt) mice], a single i.v. administration of 1011 vector genome (vg) of this rAAV1 rapidly induced rAAV1-specific IgM in about a week, followed ~2–3 weeks later by high levels of rAAV1-specific IgGs in 100% of injected wt mice (data not shown). Such antibodies were shown to be specific for rAAV1 and recognized all subunits (VP1, VP2, and VP3) of the capsid (data not shown).
The role of endosomal TLRs was evaluated as these receptors may possibly interact with rAAV during cellular infection. Mice deficient in the DNA CpG sensing TLR9 or in the RNA sensing TLR7 responded efficiently to rAAV1 by producing equivalent levels of specific IgG titers as wt control mice (Figure 1). Similarly, the anti-rAAV1 IgG response was normal in mice deficient in TRIF, which is the obligatory adaptor for the endosomal double-stranded RNA sensor TLR3, thereby excluding this PRR as being critical. To investigate the possibility that rAAV may interact with multiple but redundant endosomal TLRs, we have tested mice carrying the 3D mutation on the Unc93b1 gene controlling the trafficking and localization of endosomal TLRs, and unable to respond to TLR3, TLR8, TLR7, TLR9.21 Results show that 3D mice have a reduced IgG response to rAAV1, albeit not statistically-significant.
Figure 1.
Humoral response to adeno-associated virus serotype 1 (AAV1) vector is TLR/IL-1R and type I interferon (IFN)-R-independent but MyD88-dependent. Control C57Bl/6 or mutant TLR9−/−, TLR7−/−, TRIF−/−, 3D, TLR2−/−, TLR4−/−, MyD88 −/−, IFNαβR−/−, caspase 1−/−, NALP3−/−, IL-1R1−/− mice were infused intravenous (i.v.) with 1011 vg of rAAV1-U7mEx23 vector. Serum samples were harvested at weeks 4–5 for the measurement of anti-rAAV1 immunoglobulin G (IgG) titers. Histograms represent the geometric mean ± SEM of reciprocal end-point titers of 2 to 4 independent experiments for each targeted mutant (n = 6–13 mice per group).
The potential implication of cell surface TLRs was investigated in mice lacking either TLR2 or TLR4. Injection of the vector in these mice produced normal levels of anti-rAAV1 IgGs (Figure 1) indicating that TLR2 or TLR4 is not critically-required to respond to rAAV1. This also means that the potential presence of TLR2/4 ligands such as lipopolysaccharide in the vector preparations, which was carefully minimized by the use of endotoxin-free materials, can be ruled out as being responsible for humoral responses to our vector preparations in normal mice.
While we failed to identify a single TLR critical for responding to rAAV1, other molecules could be involved. Common paths engaged by several PRRs such as the adaptor MyD88, or downstream effectors such as antiviral or inflammatory cytokines were investigated. Administration of rAAV1 to mice deficient in MyD88 produced striking results as these mice reproducibly failed to produce specific IgG (end-point titer mean 1.7 ± 0.7 × 102 versus 3 ± 1 × 104 in wt mice, P = 0.0007) (Figure 1). In terms of cytokines, we did not observe a critical impact of type I IFN response on the development of humoral response to rAAV1. In spite of the reported need for type I IFN signaling in plasmacytoid dendritic cells to respond to rAAV2,16 we found that mice deficient in their ability to respond to type I IFNα or IFNβ and otherwise highly susceptible to viral infections,22 could produce high levels of specific IgG following i.v. administration of rAAV1, like wt mice (Figure 1). As a potential alternative, and because short-lived inflammatory reactions have been identified following rAAV1 injection in mice,1 we investigated if the inflammasome/IL-1 signaling cascade was important for anti-AAV antibody production. Mice deficient in inflammasome activation (NALP3−/− mice) or IL-1 converting enzyme (ICE−/− mice or caspase 1−/− mice) or unable to respond to IL-1 (IL1R−/− mice) exhibited normal levels of rAAV1-specific IgG responses (Figure 1). Taken together, this evaluation reveals that rAAV1 immunogenicity is critically-dependent upon MyD88 but does not involve in a critical fashion any single component of type I IFN pathway or other microbial-sensing TLRs or IL1-dependent inflammatory cytokines that was tested here.
MyD88, not TLR9, is required for adaptive humoral and cellular immunity to rAAV1
In mice, the adaptor MyD88 is crucial to develop Th1 T cell immunity23 and sufficient to induce Th1 antibodies such as IgG2 against model antigens.24 This prompted us to examine in greater detail the role of MyD88 in CD4 T cell-driven humoral responses against rAAV1. In parallel, we also investigated more closely the role of TLR9 which is described as essential for rAAV2 gene transfer-mediated immune responses.16
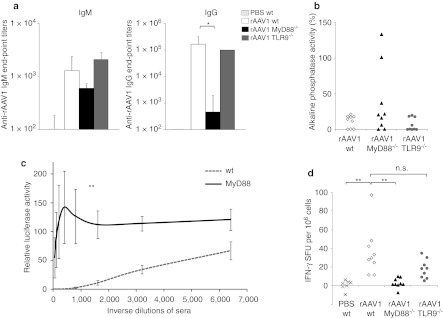
Following injection of the vector, specific IgM responses were rapidly induced in TLR9−/− or MyD88−/− mice as in normal mice (Figure 2a, left panel). However, IgG class switching occurred in TLR9−/− mice as in wt mice, but not in MyD88−/− mice (Figure 1) even after a vector boost. Thirty five days after injection of the first rAAV1-U7ex23 vector, mice were injected a second time with a rAAV1 encoding the mSeAP transgene. While wt and TLR9−/− mice exhibited high titers of anti-rAAV1 IgGs after the boost, MyD88−/− mice maintained markedly low levels of anti-rAAV1 IgGs (end-point titer mean 0.5 ± 4 × 103 in MyD88−/− mice versus 3 ± 1 × 105 in wt mice; P = 0.006) (Figure 2a, right panel). The vector injection protocol was also designed to detect neutralizing activity by measuring the level of mSeAP gene transfer obtained from the second vector.25 About a third of the MyD88−/− mice permitted gene transfer from the second vector (Figure 2b). These particular mice had also failed to respond to the first vector injection by producing negligible IgG end-point titers <102. The other MyD88−/− mice had developed low levels of antibodies which were sufficient to be neutralizing. It is known from other models, that exquisitely low antibody titers (1/20) are sufficient in vivo to block rAAV-mediated gene transfer.4,5,26 To evaluate the different groups of mice with a more discriminative measure of neutralizing activity, all sera were tested in vitro for their capacity to block the infection of 293 cells with a luciferase-encoding rAAV1 vector. This assay confirmed that on average, MyD88−/− mice immunized with rAAV1 had clearly lower serum neutralizing activity compared to wt mice since sera had to be diluted beyond 1:1,000 to permit in vitro gene transfer (Figure 2c). Taken together, these results show that the lack of MyD88 strongly impairs IgG responses to rAAV1.
Figure 2.
Involvement of MyD88 and TLR9 in neutralizing antibodies and cellular responses to recombinant adeno-associated virus serotype 1 (rAAV1). Wt, MyD88−/−, and TLR9−/− mice were injected intravenous (i.v.) with 1011 vector genome (vg) of rAAV1-U7mEx23. Serum was obtained at day 7 and day 35. At day 35, mice were re-challenged with 1011 vg of rAAV1-mSeAP then sacrificed at day 50 to measure antibodies and cellular immune responses, as well as expression of the second vector to evaluate neutralizing activity. (a) IgM levels at day 7 and immunoglobulin G (IgG) levels at day 50. Histograms represent the geometric mean ± SEM of reciprocal end-point titers of AAV1-specific IgM and IgG in 3 to 4 independent experiments (n = 9 mice per group). *Statistically-significant different titers using Student's t-test (P < 0.05). (b) Neutralizing activity in vivo at day 35. Serum alkaline phosphatase activity was measured in each mouse 15 days after rAAV1-mSeAP boost in 3 independent experiments (n = 9 mice per group). Results are expressed as a percentage relative to those found in naive mice having received the mSeAP vector (levels set as 100%). (c) Neutralization activity in vitro at day 35. These tests were performed on 293 cells incubated with rAAV1-luc mixed with serial dilutions of sera obtained 35 days post injection of rAAV1-U7mEx23 in wild type (wt) or MyD88−/− mice. Curves show the mean ± SEM of relative enzyme activity in cells 2 days after transduction in three independent experiments (n = 9 mice per group). **Statistically-different differences between the area under the curve values using Student's t-test (P < 0.05). (d) Cellular response at day 50. Spleen cells of wt, MyD88−/−, or TLR9−/− mice harvested at day 50 were stimulated with rAAV1-luc for 24 hours and assayed for interferon (IFN)-γ secretion. Each symbol represents IFN-γ spot-forming unit (SFU) (duplicate measures) of each mouse in three independent experiments (n = 9 mice per group). **Statistically-significant different group using Student's t-test (P < 0.05). n.s., not significant.
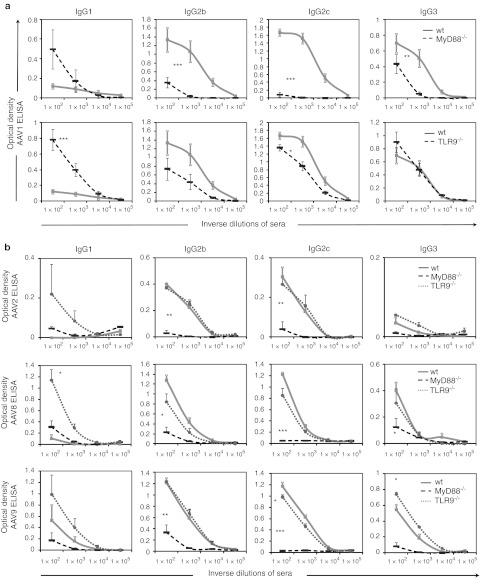
In normal mice, rAAV1 induces high levels of IgG2b and IgG2c, moderate levels of IgG3 and very low levels of IgG1 (Figure 3a,b). This particular profile suggested a response driven by a dominant IFN-γ-mediated IgG switch process. The existence of IFN-γ-mediated cellular responses were confirmed in the spleen of mice immunized with rAAV1 (Figure 2d). Following ex vivo restimulation with rAAV1, splenocytes from wt mice immunized with rAAV1 produced an average of 36 (range 11–97, n = 9 mice) IFN-γ-producing spot-forming units (SFU) per million cells which is higher than in phosphate-buffered saline (PBS)-treated controls (average 0.57 SFU, range 0–6; P = 0.003). In contrast to normal mice, MyD88−/− mice failed to develop a strong cellular IFN-γ response (average 2.6 SFU, range 0–9; P = 0.002) (Figure 2d), coherent with the marked reduction in Th1-driven IgG isotypes such as IgG2 and IgG3, observed in these mice (Figure 3a, top panel). The IgG response in TLR9−/− mice was high but qualitatively not entirely normal. Compared to normal mice, levels of IgG1 were higher and IgG2c were moderately reduced (Figure 3a, bottom panel). The cellular responses induced by rAAV1 in TLR9−/− mice tended to produce lower levels of IFN-γ than normal mice but this was not statistically-significant (average 17.6 IFN-γ SFU, range 5–34; P = 0.06) (Figure 2d).
Figure 3.
Generation of recombinant adeno-associated virus (rAAV) serotype 1, 2, 8, 9- specific Th1-type immunoglobulin G (IgG) subtypes are strongly reduced in MyD88-deficient mice and perturbed in TLR9−/− mice. Mice [wild type (wt), MyD88−/−, or TLR9−/− mice] were immunized intravenous (i.v.) with 1011 vg of rAAV1-U7mEx23, rAAV2-U7mEx23, rAAV8-mSeAP or rAAV9-mSeAP (controls received PBS) and sera were harvested at day 35 for the measurement of respectively AAV1, AAV8, AAV9-specific IgG subtypes by ELISA. Curves represent the mean ± SEM of optical density obtained at the indicated dilutions. Statistical analyses were performed by comparing area under the curve values using Student's t-test. (a) Representation of four independent experiments (n = 13 mice per group). Top and bottom panels represent the comparison of wt (plain line) mice with respectively MyD88−/− or TLR9−/− mice (dotted lines). Responses were obtained in same sets of experiments but separated for clarity. (b) Grouped representation of data from three mice per group (wt = plain gray line; MyD88−/− = black dotted line; TLR9−/− = gray finely-dotted line). From top to bottom, respectively responses to rAAV2, rAAV8 and rAAV9.
To extend these observations, we tested whether MyD88 and TLR9 were also needed for antibody responses to other AAV capsid serotypes, and results are regrouped in Figure 3b. Injection of rAAV2, rAAV8, and rAAV9 vectors encoding mSeAP into MyD88−/− and TLR9−/− mice confirmed the critical requirement for MyD88 for the production of specific IgG2b, IgG2c, and IgG3 against these two serotypes (Figure 3b). As with rAAV1, TLR9 was not required to mount AAV2, AAV8, or AAV9-specific IgG responses in mice, however IgG1 levels were elevated in the absence of TLR9 (Figure 3b).
In summary, the systemic administration of rAAV to normal mice induced the development of a strong humoral response characterized by an IgG2-skewed isotypic profile, neutralizing antibodies and accompanied by an IFN-γ-mediated cellular immune response. Administration of several serotypes showed induction of similar antibody IgG subclasses and similar requirements for TLR9 and MyD88 in controlling these responses. TLR9 was not critically-needed to produce anti-AAV IgGs but was required to maintain a high IgG2c/IgG1 ratio, typical of Th1-supported antibody responses in C57Bl/6 mice. MyD88 was necessary to develop cellular immunity and neutralizing antibody responses against rAAV1, prompting further experiments to understand the role of this adaptor in various key immune effector cells.
MyD88 controls the development of anti-capsid CD4 T cell responses
In our model, we are evaluating T cell-dependent antibodies to AAV. No specific IgG were induced by i.v. injection of 1011 vg of rAAV1-U7mex23 vector in nude BALB/c mice (data not shown) as found by others with rAAV2.27 The vector capsid being the sole source of immunogenic peptides for T cells, we hypothesize that the induction of capsid-specific CD4+ T cells triggering capsid-specific antibodies may require MyD88.
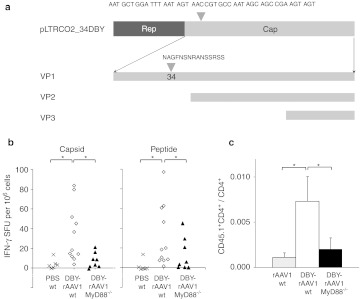
To directly test this hypothesis, we constructed a vector reporter of capsid-specific CD4 T cell responses (so-called DBY-rAAV1) by inserting the MHC class II Dby epitope of the male H-Y antigen in the N-terminal region of the VP1 capsid protein sequence, starting in AA position 34 of VP1 (Figure 4a). In the capsid of AAV2, this position is permissive to the insertion of epitope tags that are displayed at the surface of the particle.28 Particles carrying this DBY-rAAV1 chimeric capsid could be successfully assembled. Infectivity of the DBY-rAAV1 was confirmed by mSeAP production in 293 cells (data not shown). Vector production titers of the DBY-rAAV1(-U7 or -mSeAP) were reduced and averaged 3 ± 1 × 1011 vg/ml and 5 ± 2 × 1013 physical particles (pp)/ml; ratio pp/vg = 177 ± 5 (n = 2 batches of vector) in contrast to higher titers regularly obtained for batches of rAAV1-U7ex23 or rAAV1-mSeAP vectors averaging 4 ± 4 × 1012 vg/ml and 2 ± 3 × 1014 pp/ml; ratio pp/vg = 62 ± 41 (n = 10 batches of vector). In spite of this reduction in titer which may render the particles partially defective and more easily degraded, the DBY-rAAV1 tool was useful to probe T cell antigenic presentation of the capsid.
Figure 4.
MyD88 pathway involvement in anti-capsid CD4+ T cell response. (a) Schema of the construct used for the production of the DBY-rAAV1 capsid. The Dby peptide sequence is inserted in AA position 34 in VP1. (b) Capsid-specific interferon (IFN)-γ responses. Wt and MyD88−/− mice were injected intravenous (i.v.) with 5 × 1011 particles of DBY-rAAV1. Spleens were harvested at day 12 and cells were stimulated with rAAV1-luc vector (native capsid) (left panel) or with the Dby peptide (right panel) for 24 hours and assayed for IFN-γ secretion. Histograms represents mean ± SEM of IFN-γ spot-forming unit (SFU) (duplicate or triplicate measures on each point) of three independent experiments (n = 6–11 mice per group) (wild type (wt) = 6 mice; MyD88−/− + DBY-rAAV1 = 8 mice; wt + DBY-rAAV1 n = 11 mice). Statistical analysis was performed using Student's t-test (*P < 0.05). (c) In vivo T cell priming in MyD88-deficient mice. Mice (wt or MyD88−/−, both CD45.2+) were injected i.v. with 5.1011 native-rAAV1 or DBY-rAAV1 particles and the following day with 2–4 × 105 CD45.1+ [Marilyn × C57Bl/6]F1 CD90+ cells. Peripheral blood mononuclear cells were harvested at day 5 and analyzed by FACS to determine the ratio of CD45.1+CD4+/CD4+ cells in each mouse. Data are representative of two independent experiments (mice per group: PBS n = 2; MyD88−/− n = 5; wt n = 3). rAAV1, recombinant adeno-associated virus serotype 1.
In vivo, the capsid of the DBY-rAAV1 vector was efficiently processed and presented to prime naive specific CD4+ T cells. To demonstrate this point, female CD45.1+ Marilyn T cell receptor (TCR) transgenic mice in which the majority of CD4+ T cells carry the Dby-specific TCR29 were adoptively transferred into congenic CD45.2+ C57Bl/6 (wt) mice and the following day, the recipient mice were injected with rAAV1 carrying or not the Dby-tagged capsid. Subsequently the levels of transgenic T cells were monitored by FACS over time. Circulating Dby-specific T cells were clearly detected in the blood of mice injected with the DBY-rAAV1 vector at day 5 postinjection and not with the vector bearing the native capsid (data not shown), thereby validating the system.
To probe the role of MyD88 in the induction of capsid-specific T cell responses, the DBY-rAAV1 vector was injected i.v. into wt or MyD88-deficient mice and 12 days later, spleen cells were re-stimulated ex vivo with either a rAAV1 carrying a native capsid (Figure 4b, left panel) or with the Dby peptide (Figure 4b, right panel). Re-stimulation with the whole virus induced a positive IFN-γ cellular response in wt mice immunized with DBY-rAAV1 (P = 0.02 compared to PBS) thereby confirming the full immunogenicity of the DBY-rAAV1 vector used to prime the mice. Under these conditions, MyD88−/− mice failed to respond to DBY-rAAV1 (P = 0.02 compared to wt; Figure 4b, left panel). This provides an explanation for the lack of IFN-γ response observed 50 days after injection of a native vector (shown in Figure 2d) and emphasizes the crucial role of MyD88 to mount IFN-γ mediated T cell responses to rAAV1. Re-stimulation of the cells with the Dby peptide induced positive IFN-γ responses in samples from wt mice (immunization with DBY-rAAV1 compared to PBS in wt mice, P = 0.04; Figure 4b right panel). In contrast, MyD88−/− mice injected with the DBY-rAAV1 developed less IFN-γ producing cells after Dby peptide re-stimulation compared to wt mice (P = 0.02; Figure 4c, right panel). We confirmed that IL-2 was also produced specifically in response to the peptide in wt mice and IL-2-producing cells were reduced in MyD88−/− mice (IL-2-SFU per million cell = 26.2 ± 4 in wt mice (n = 11) and 9.1 ± 6.8 (n = 8) in MyD88−/− mice; P = 0.03). These data provide the first direct evidence that MyD88 signaling is required in mice to permit optimal development of capsid-specific Th1 cell responses. These results thereby provide an explanation for the strong impairment in the development of IgG2 and IgG3-mediated humoral immune responses to rAAV1 in MyD88−/− mice.
MyD88 does not play an intrinsic critical role in T cells for anti-rAAV1 immune responses
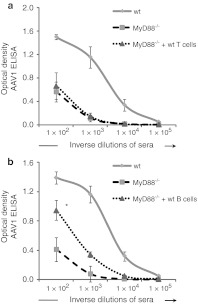
MyD88 signaling can potentially play a direct intrinsic role in T cell responses as demonstrated in another system in the case of virus-specific CD8 T cells.30 To test this hypothesis, wt T cells were adoptively-transferred into congenic MyD88−/− recipient mice and the following day, mice received the native-rAAV1 vector. The transfer of purified T cells from wt mice failed to restore antibody responses to rAAV1 even when large numbers of wt T cells (10–25 × 106 wt T cells) were injected into MyD88−/− mice. Anti-rAAV1 end-point titers remained at background levels as in nontreated MyD88−/− mice (Figure 5a).
Figure 5.
Cell-specific restoration of adeno-associated virus serotype 1 (AAV1) vector specific humoral response. Serum samples were harvested 35 days after intravenous (i.v.) injection of rAAV1 in various mice to measure anti-rAAV1 IgG titers. Curves represent the mean ± SEM of optical density obtained at the indicated dilutions of sera. Statistical analysis was performed on averaged area under the curve for sera of individual mice. (a) T cell MyD88 is not sufficient to restore anti-AAV1 antibodies. Wt and MyD88−/− mice were injected i.v. with 1011 vg of rAAV1-U7mEx23 1 day after being adoptively transferred or not with 10–25 × 106 wild type (wt) T cells purified by magnetic cell selection. Curves represent the mean ± SEM of optical density obtained at the indicated dilutions of sera obtained from two separate experiments including 6–13 mice per group. (b) B cells-MyD88 restores anti-rAAV1 humoral response. Wt- or MyD88-deficient mice were injected with rAAV1 1 day after the adoptive transfer (or not) of MACS sorted 66 × 106 wt B cells. Results from four separate experiments.
B cell-intrinsinc MyD88 is involved in anti-rAAV1 antibody responses
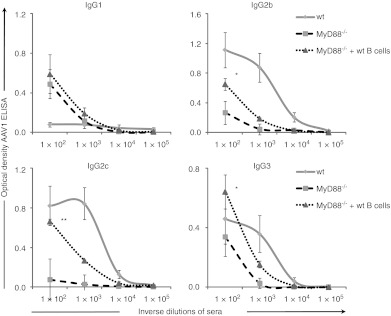
Contrary to T cell transfers, the infusion of wt B cells in MyD88−/− restored a specific anti-AAV1 IgG immune response in all four experiments performed (Figure 5b). Purified B cells were obtained either by CD19 positive selection or by negative depletion with magnetic beads. Both of these strategies provided pure cell populations containing 94–98% B220+ cells. End-point titers in wt mice were 12 328 ± 27 136 versus 285 ± 470 in MyD88−/− mice and 1,425 ± 3,379 in MyD88−/− mice + B cells, therefore a 5 fold increase in titer was due to B cell transfer. There was also a specific effect on Th1 isotypes as the transfer of wt B cells into MyD88−/− mice increased the levels of rAAV1-specific IgG2b, IgG2c, IgG3 isotypes compared to MyD88−/− controls (Figure 6).
Figure 6.
Autonomous MyD88 signaling in B cells restores IgG2c. Curves represent the mean ± SEM of optical density for measuring anti-rAAV1 immunoglobulin G (IgG) levels at indicated serum dilutions in four independent experiments (n = 8–13 mice per group). IgG subtype in wild type (wt) mice (plain line) or MyD88−/− mice (large dotted line) or MyD88−/− mice restored with wt B cells (small dotted lines). Statistical analysis was performed on averaged area under the curve for sera of individual mice. rAAV1, recombinant adeno-associated virus serotype 1.
Taken together, our data strongly suggest that the MyD88-dependent signaling intrinsic to B cells is playing an important and rather unique role in humoral rAAV1 immunity by controlling levels of Th1 antibodies.
Discussion
Adaptive immune responses to rAAV, in particular the induction of a strong humoral response against the vector capsid, represent a significant hurdle in the clinical application of this vector for gene transfer protocols in vivo. By investigating the relationship between innate immunity pathways and the development of antibody responses following rAAV administration in mice, the present study establishes the first direct link between the induction of capsid-specific T and B cell immunity and the critical role of intracellular signaling via the adaptor MyD88 particularly in B cells. The second main point of this study is the lack of critical importance that IL-1 mediated inflammation and type I IFN signals may have for the production of antibodies to AAV in mice.
Results, obtained with several capsid serotypes, confirm that cell-intrinsic innate recognition of AAV signaling through the common TLR adaptor MyD88, is essential to mount T and B cell immune responses to the AAV capsid in vivo. These data emphasize the importance of capsid-specific CD4 T cells in responses against rAAV. Indeed, a recent study shows that administering nondepleting anti-CD4 antibodies to mice can effectively prevent the induction of anti-AAV8 antibody titers.31 In our model, we show that capsid-specific CD4 T cell responses are of Th1 type based on several observations. First, a Th1-polarized antibody profile marked by high levels of IgG2c, IgG3 and low levels of IgG1, is induced in response to rAAV1, rAAV2, rAAV8, and rAAV9. We found no evidence for IgE and IgA levels in blood of mice examined (data not shown). The Dby epitope-tagged capsid allowed us to track the antigenic presentation of CD4 T cell epitopes and the development of primed CD4+ T cells, confirming the existence of capsid-specific IFN-γ- and IL-2 producing Th1 T cells. Furthermore, the Dby-tagged capsid showed the essential role of MyD88 for the productive priming and development of capsid-specific Th1 effector cells which is consistent with the known importance of MyD88 for Th1 T cell responses in other antigenic systems.23 Presumably, MyD88 is involved in licensing antigen-presenting cells (APCs) to prime naive T cells,32 through PRR-initiated signaling cascades that can upregulate MHC, costimulatory molecules and cytokines on APCs.12 Altogether, the data emphasize the important role of capsid-specific Th1 T cell responses which may therefore provide an explanation for the strong serum neutralizing activity which is observed following AAV administration in all models and in humans.
The specific cellular requirements for MyD88 signaling was investigated. A major finding is that B cell-intrinsic MyD88 controls the production of Th1-associated IgG2 antibody responses against rAAV, in particular the IgG2 (especially IgG2c) and IgG3 responses. B cells express a variety of TLRs on their surface permitting their activation and cytokine production capacity. In other immunization systems, notably with viruses, viral-like particles or bacteria, B cell-intrinsic MyD88 signaling controls the magnitude of IgG responses and in particular for the production of IgG2s.24,33,34 B cells, rather than other types of APCs, are known to efficiently recognize particulate forms of TLR ligands hereby augmenting germinal center responses.35 Elegant bone marrow chimera experiments creating a MyD88-deficient B cell compartment within a normal mouse blood system, revealed that IgG2c antibody responses against bacteria were entirely dependent on B cell-intrinsic MyD88 signaling.33,34 Therefore, in several antigenic systems, including rAAV, antibody responses are supported at least in part, by an autonomous MyD88-dependent signaling in B cells. Regardless of the exact mechanism involving MyD88 in B cells, the findings emphasize the critical role of B cells at the interface of innate and adaptive immune responses and suggest that interactions between B cells and AAV should be examined more closely.
It is currently thought that the AAV viral genome, particularly as double-stranded DNA, can interact with TLR9 and triggers via MyD88, NFκB or type I IFN signaling to induce cytokine responses which may control the development of humoral and cellular immunity against the vector and transgene.36 While we confirm the critical importance of MyD88, we also report that IgG responses to rAAV are not critically-dependent upon TLR9 or type I IFN in our model, unlike reported by Yang's laboratory.16 Several parameters could explain why different conclusions were reached in these respective studies and include: different genetic backgrounds for the TLR9-deficient strains (our mice are backcrossed on C57BL/6 background and their genotype and phenotype was validated, the mice used in the Yang study were on a BALB/c background); differences in AAV vector preparation methods (purification by double Cesium chloride gradient versus affinity columns); differences on the co-existence of an immune response to a transgene product (our system does not include a protein-coding transgene); differences in routes of administration known to impact on immunogenicity of vectors37 (we used intravenous injections, Yang et al. used intramuscular injections). These differences suggest that the degree of activation of the innate immune system by rAAV can be in part, dependent of the context. It is important to note that our results show that TLR9 contributes to antibody responses to rAAV, even though it is not critically-needed. In our model, TLR9 seems to emphasize the Th1-biased IgG responses to AAV. In the absence of TLR9, IgG2c levels are lowered and remarkably, IgG1 levels rise. In other systems, increased IgG1 responses were also observed in TLR9-deficient mice immunized with M13 phage38 or with viral-like particles loaded with Cpg.39 This may be a signature of down-regulated by IFN-γ Indeed, TLR9 is known to induce the expression of Tbet following CpG stimulation leading to IFN-γ-mediated down-regulation of IgG1 and IgE responses in mice.40 Therefore our data support the fact through TLR9 recognition, the AAV viral genome contributes to the quality of humoral responses by enforcing an optimal Th1 T cell response to the capsid of rAAV and IgG2c class switching.
The impact that inflammatory cytokine may have on the development of antibody responses to rAAV was also investigated. rAAV vectors induce much less inflammation than adenoviral vectors,1 but nevertheless can reportedly trigger rapid inflammatory responses in liver as observed by several investigators in vivo1,17 or in vitro.18,19 In our system, we find no evidence that inflammatory cytokines could be the critical factor that induces IgG responses to rAAV, since total IgGs as well as IgG subtypes (not shown) are normal in mice lacking the ability to activate IL-1 inflammation cascades as a result of deficiency in either caspase -1, IL-1R, or NALP3 inflammasome. Similarly, the role of type I IFN response was not found to ve critical as shown by normal IgG responses in IFNAR−/− mice. We also found no critical role of any TLR tested, including TLR2 which has recently been implicated in the response to the AAV2 capsid and induction of cytokines by liver sinusoidal endothelial cells and Kupfer cells.19 Our data do not exclude that the induction of cytokines in response to rAAV contributes to immune cell activation, but this is probably too transient a phenomenon, as reported,1 to be an essential factor in IgG production.
We failed to identify a single TLR that controls IgG responses to AAV, within the limits of our study. Among the endosomal PRRs which might be likely to encounter rAAV during the infection cycle, we can exclude a direct critical role of TLR9, as discussed above, but also of TLR7 and indirectly of TLR3 since we find normal IgG production in the absence of TRIF which is an obligatory signaling molecule for TLR3. We cannot exclude the possibility of redundancy. This was evaluated with 3D mice unable to sustain normal trafficking and localization of endosomal TLRs therefore unresponsive to TLR3, TLR8, TLR7, TLR9.21 Only a partial reduction in IgG response was observed in 3D mice contrasting sharply with the more severe lack of response in MyD88-deficient mice. This does not support the hypothesis that a combined activation of TLR7 and TLR9 is responsible for AAV-induced MyD88 triggering. The 3D mice also have a defect in antigenic cross presentation and in MHC class II presentation of exogenous antigens.41 It is therefore likely based on our results that the mildly (and not significantly) reduced anti-AAV1 IgG response observed in 3D mice reflects a defective antigenic presentation impacting on the priming of AAV capsid-specific CD4 T cells. The strict requirement for MyD88 and not any TLR tested or IL-1 in response to rAAV is probably due to the involvement of other receptor pathways not tested here, or due to redundant signaling, not evaluated here.
The existence of strong antibody responses induced by the rAAV vector components remains an important barrier for medical application of this vector and immunosuppressive strategies are needed. With the identification of the critical need for MyD88, for both capsid-specific CD4 Th1 T cell responses and for direct B cell activation, and with the identification of a role for TLR9 in this context, our studies confirm the importance of innate recognition of the vector components for its immunogenicity. Targeting CD4T cells with nondepleting antibodies was recently found to be a very efficient strategy to prevent the induction of neutralizing antibodies to rAAV8, although additional immunosuppression was needed to further reduce antibody titers.31 Based on our results, alternative strategies that would reduce the innate system activation would be justified. Intervening on TLR9 activation by AAV should reduce the immunogenicity of the vectors based on our data but this is not expected to completely abrogate immune responses against the vectors. Combined T and B immune-suppression should be implemented to fully control the induction of strongly neutralizing IgG2-dominated humoral responses to rAAV. Approaches targeting a common and critically-needed molecular pathway such as MyD88 could constitute an effective strategy in that regard. In addition, because of the direct role of B cell MyD88, protocols leading to B cell immunosuppression or ablation must probably be emphasized.
Materials and Methods
Recombinant AAV vector production and titration. Recombinant nonreplicative, adenovirus-free AAV2/1 vectors (rAAV1) were produced by tri- or -bitransfection of HEK293 cells. Endotoxin-free preparations of plasmids were used (Macherey-Nagel, Hoerdt, France). The adenovirus helper functions were provided by the pXX6 plasmid (a kind gift of R.J. Samulski, University of North Carolina, Chapel Hill, NC). The AAV2 rep and cap genes of AAV1 were supplied from the pLTRCO2 packaging plasmid (a kind gift from R.C. Mulligan, Harvard Medical School, Boston, MA). Capsid serotypes 2, 8, and 9 were respectively produced with the pRepCap4, pDG8, and pDF9 plasmids. To produce the chimeric capsid of the DBY-rAAV1 vector the pLTRCO2_34DBY plasmid was used in which the sequence encoding a peptide (NAGFNSNRANSSRSS) of the MHC class II male HY antigen Dby was inserted in N-terminal position 34 of the VP1 capsid protein sequence. : pGG2-CMV-muSeAP encoding the secreted form of the murine alkaline phosphatase under control of the cytomegalovirus (CMV) IE promoter-enhancer, generating the rAAV1-mSeAP vector;25 pSMD2-CMV-Luc encoding the firefly luciferase under the CMV promoter, generating the rAAV1-Luc vector and pU7_DTex23 encoding antisense sequences specific for the murine dystrophin gene and linked to a modified U7 small nuclear RNA, generating the rAAV1-U7mEx23 vector already described.20 Recombinant vectors were purified by two consecutive CsCl ultracentrifugation gradients followed by dialysis against sterile Dulbecco's PBS with Ca++ and Mg++. Vector titers were determined by quantitative PCR in comparison to a standard expression plasmid and expressed as /ml or in the case of serotype 1, titer was also measured by ELISA (PRAAV1; Progen, Heidelberg, Germany) and expressed as pp/ml. Endotoxin concentrations in the purified viral lots were measured with an end point fluorescent assay (Pyrogene rFC Assay; Lonza Group, Basel, Switzerland) and found to be inferior to 20 EU/ml.
Mice. Animal procedures were performed according to institution-approved protocols in certified EOPS animal facilities and under appropriate biological containment. MyD88−/−, TLR9−/−, TLR7−/−, TRIF−/−, TLR2−/−, TLR4−/− mice were kindly provided by S. Akira (Osaka University, Osaka, Japan), IFNαβR−/−, caspase 1−/− (ICE−/−), and IL-1R1−/− have been described respectively in refs. 22,42,43 NALP3−/− mice were kindly provided by J. Tschopp (University of Lausanne, Lausanne, Switzerland) and all of these targeted mutant mice were bred at the Orleans animal facility. Experiments with such mice were controlled on the background strain C57Bl/6 since MyD88−/−, IL-1R1−/−, TLR2−/−, TLR4−/−, TLR7−/−, TLR9−/−, 3D−/−, and NALP3−/− mice were backcrossed at least 10 times on the C57BL/6 genetic background. The Marilyn TCR transgenic, Rag2−/− mice29 were kindly provided by O. Lantz (Curie Institute, Paris, France) and were used as a source of I-Ab-restricted Dby-specific CD4 T cells either as such or after breeding to generate (Marilyn × C57Bl/6) F1 mice. C57Bl/6 wt mice, BALB/c mice and BALB/c nude mice were purchased from Charles River Laboratories (L'Arbresle, France).
Immunization and adoptive cell transfer protocols. For vector immunizations, mice were injected i.v. into the tail vein with 200 µl of vector diluted in PBS containing 1011 vg or 5 × 1011 pp as indicated. Control animals were injected with equivalent volume of PBS alone. Blood was collected by retro-orbital puncture under anesthesia and sera were stored at –80 °C. For adoptive cell transfer, mice were injected i.v. into the tail vein with 200 µl of cellular preparations containing 1–66 × 106 T or B cells, purified as described below.
Measurement of anti-AAV antibody titers. rAAV-specific serum antibody titers were evaluated by ELISA. F96 Maxisorp Nunc Immuno plates (Thermo Fisher Scientific, Roskilde, Denmark) were coated overnight at 4 °C with 109 vg of AAV1, AAV8 or AAV9 vectors and 0.1 x 109 vg of AAV2 diluted in 0.1 mol/l carbonate coating buffer (pH 9.6). Plates were washed three times and blocked with a solution of 6% milk in PBS for 2 hours at room temperature. Serial dilutions of samples in this blocking buffer (tenfold dilutions starting 10–2–10–7 were tested) were incubated on the plates for 1 hour at 37 °C. Plates were washed three times, incubated with various horseradish peroxidase-conjugated goat antibodies directed against either mouse IgG, IgG1, IgG2b, IgG2c, IgG3, or IgM (Southern Biotech, Birmingham, AL) and revealed by TMB Substrate reagent Set (BD Biosciences, San Jose, CA). Reaction was stopped with an H2SO4 solution and the optical density at 450 nm was determined using a luminometer (Discovery HT-R; BioServ, Thiais, France) with KC4 software. End point titers were defined as the inverse serum dilution giving the lowest detectable signal above threshold i.e., optical density obtained in a well without serum. The limit of detection was set at 10 end-point units and the limit of quantification was set at 100 end-point units. Results were expressed either as mean of reciprocal end-point titers, or as the average value of optical density at specific dilutions. The area under the curve values obtained from the optical densities at four dilution points (10–2, 10–3, 10–4, 10–5) were calculated for statistical analysis using GraphPad Prism software.
In vivo neutralization assay by quantification of circulating mSeAP. Determination of alkaline phosphatase concentration in immunized mice sera was performed by chemoluminescence as previously described.25 Briefly, sera were heat inactivated for 5 minutes at 65 °C to remove the endogenous alkaline phosphatase and heat resistant mSeAP was measured by addition of the reaction buffer and CSPD (1,2-dioxetane chemoluminescent substrate), according to manufacturer's instructions (Tropix; Applied Biosystems, Foster City, CA). Chemoluminescence was quantified using a luminometer (Mediators Diagnostika, Vienna, Austria). Values are given as relative enzyme activity; 100% corresponds to mSeAP activity levels in mice injected once and only with the rAAV1-mSEAP vector.
In vitro assay for anti-AAV1 neutralization activity. The neutralization activity present in the sera of immunized mice was evaluated in vitro by measuring inhibition of HEK293 cell infection by rAAV1, with slight modifications from a previously described protocol.25 HEK293 cells were plated the day before the assay in 48-wells plate in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin/streptomycin and 2 mmol/l L-glutamine (Gibco-BRL, Paisley, UK). Test sera were incubated at 56 °C for 30 minutes to inactivate complement. The AAV1-Luc vector used to infect HEK293 cells was diluted in DMEM and incubated for 1 hour at 37 °C with serial dilutions of heat-inactivated sera (twofold dilutions from 1/50 to 1/6,400). The virus-serum mixtures were added to HEK293 cells in plates at a multiplicity of infection (MOI) of 20,000 vg/cell). After 48 hours of incubation, HEK293 cells were lysed (0.2% Triton X-100 solution), mixed with assay buffer (25 mmol/l Tris-phosphate, 1 mmol/l dithiothreitol, 1 mmol/l EDTA, 15% glycerol, 8 mmol/l MgCl2, 2 mmol/l adenine triphosphate), and 167 mmol/l luciferin (Molecular Probes, Leiden, NL). Luciferase expression was quantified for 10 seconds with a luminometer (Mediators Diagnostika). Enzyme expression levels were expressed as relative light units (RLU)/s/well and standardized according to the quantity of proteins. Values are given as relative enzyme activity; 100% corresponds to Luc activity in HEK293 cells transduced in the absence of mice sera.
Magnetic cell separation. T cells and B cells were purified from freshly isolated splenocytes by magnetic cells sorting using different strategies. To prepare B cells for adoptive transfer, splenocytes were obtained by mechanic disruption of the tissue followed by removal of red blood cells with ACK lysis and magnetic immunoselection (Miltenyi Biotec, Bergisch Gladbach, Germany) using either the B cell negative selection isolation kit or CD19 microbeads for cell sorting on the autoMACS according to the manufacturer's instructions. The FACS analysis of such purified preparations of cells showed >95% B220+ cells following either negative or positive selection strategies. To prepare T cells for adoptive transfer, the residual unselected cells leftover from the CD19 positive selection experiments were labeled with the pan-T cell negative isolation kit (Miltenyi Biotec) and sorted with the autoMACS. The FACS analysis of the resulting cell populations showed >95% CD3+ cells. To prepare Dby-specific TCR transgenic T cells for adoptive transfer or for in vitro cultures, splenic T cells were obtained from [Marilyn × C57Bl/6]F1 or Marilyn mice, respectively using a direct CD90.2 magnetic bead positive selection (Miltenyi Biotec). Such purified populations contained >75% CD3+ cells as determined by FACS.
Cells staining and flow cytometric analysis. The following Abs were used for FACS analysis: pacific blue-labeled anti-CD4 (clone RM4-5; DB Bioscience, San Jose, CA), allophycocyanin-labeled anti-B220 (clone RA3-6B2; eBioscience, San Diego, CA), or anti-CD90.2 (clone 53-2.1; BD Biosciences) phycoerythrin-labeled anti-CD3 (clone 145-2C11, BD bioscience) and biotinylated anti-CD45.1 (clone A20, BD bioscience) revealed with streptavidin-allophycocyanin (BD Bioscience). Dead cells were excluded by 7-aminoactinomycin D staining. All FACS data were collected on a LSRII flow cytometer (BD Bioscience) and analyzed using the Diva software.
T-cell cytokine response assays. Multiscreen-HA Assay System 96-well Filtration plate (Millipore, Bedford, MA) were coated overnight with 5 µg/ml anti-mouse IFN-γ mAb or 3 µg/ml anti-mouse IL-2 mAb (BD Biosciences) at 4 °C, washed, and then blocked with complete medium (see above). Splenocytes were added in duplicate to the IFN-γ-coated well (1 or 2 × 106 cells/well) and cultured in the presence of the antigen, consisting either of the rAAV1-Luc vector particles (added at an MOI of 1,000 vg/cell) or DBY peptide (2 µmol/l) in case of DBY-rAAV1 immunization. Wells stimulated with ConA (5µg/ml) were included as a positive control in each test. After 24 hours, plates were washed three times with PBS/0.05% Tween 20 and three times with PBS alone before overnight incubation at 4 °C with 1 µg/ml biotinylated anti-mouse IFN-γ mAb or 1.7 µg/ml biotinylated anti-mouse IL-2 mAb (BD Biosciences) in PBS/3% fetal bovine serum. Plates were washed three times with PBS/0.05% Tween 20 and three times with PBS alone before incubation with 0.66 U/ml streptavidin-alkaline phosphatase (AP) conjugate (Roche Diagnostics, Mannheim, Germany). Plates were incubated at room temperature for 1 hour and washed with PBS/0.05% Tween 20, then with PBS alone before spot were developed using BCIP/NBT color development (Promega, Madison, WI). Spots were counted using a bioreader 2000 (BIO-SYS, Karben, Germany). SFU for each mouse are represented as the mean of duplicate measures after substraction of background values obtained with in vitro non stimulated splenocytes.
Statistical analysis. Significant differences between mean values were determined using unpaired two-tailed Student's test. For antibody production significant differences were analyzed by comparing the average values of the area under the curve for the titration of each mouse serum. P value under 0.05 was considered statistically-significant. In the graphs, *P value inferior to 0.05, **inferior to 0.005 and ***inferior to 0.001.
Acknowledgments
We are grateful to Luis Garcia for gift of the rAAV1-U7mex23 construct and to Genethon for support on plasmid and vector production and animal studies. We thank Drs Akira, Lantz, and Tschopp for kind gifts of mice and Dr Samulski for reagents. We are also grateful to Valérie Quesniaux for helpful discussions and to Philippe Moullier for careful review of this manuscript. This work was supported by the Association Française contre les Myopathies. The authors declared no conflict of interest.
REFERENCES
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS., and, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., and, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P.et al. (2000Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle J Virol 742420–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S.et al. (2006Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice Blood 1071810–1817. [DOI] [PubMed] [Google Scholar]
- Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M.et al. (2009Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins J Immunol 1826051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF.et al. (2010Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors Hum Gene Ther 21704–712. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA.et al. (2009AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Blood 1142077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., and, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., and, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A., and, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM.et al. (2008Complement is an essential component of the immune response to adeno-associated virus vectors J Virol 822727–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X., and, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B.et al. (2011The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver Blood 1176459–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayandharan GR, Aslanidi G, Martino AT, Jahn SC, Perrin GQ, Herzog RW.et al. (2011Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy Proc Natl Acad Sci USA 1083743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hösel M, Broxtermann M, Janicki H, Esser K, Arzberger S, Hartmann P.et al. (2012Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors Hepatology 55287–297. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L.et al. (2004Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping Science 3061796–1799. [DOI] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME., and, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM.et al. (1994Functional role of type I and type II interferons in antiviral defense Science 2641918–1921. [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S., and, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Pasare C., and, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Rivière C, Danos O., and, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Lorain S, Gross DA, Goyenvalle A, Danos O, Davoust J., and, Garcia L. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther. 2008;16:541–547. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Schnell MA, Tazelaar J, Hughes JV., and, Wilson JM. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol Ther. 2000;1:323–329. doi: 10.1006/mthe.2000.0045. [DOI] [PubMed] [Google Scholar]
- Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T.et al. (2000Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism J Virol 748635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O, Grandjean I, Matzinger P., and, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- Zhao Y, De Trez C, Flynn R, Ware CF, Croft M., and, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JH, Cochrane M, Cobbold S, Waldmann H, Nathwani SA, Davidoff AM.et al. (2012Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine Gene Ther 1978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Schwarz K, Manolova V, von Allmen CE, Kinzler MG, Bauer M.et al. (2010Innate signaling regulates cross-priming at the level of DC licensing and not antigen presentation Eur J Immunol 40103–112. [DOI] [PubMed] [Google Scholar]
- Barr TA, Brown S, Mastroeni P., and, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TA, Brown S, Mastroeni P., and, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185:2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L.et al. (2011Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response Immunity 34375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A., and, Herzog RW. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toromanoff A, Adjali O, Larcher T, Hill M, Guigand L, Chenuaud P.et al. (2010Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle Mol Ther 18151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi S, Yamaguchi Y, Takeuchi O, Akira S., and, Sugimura K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem Biophys Res Commun. 2010;402:19–22. doi: 10.1016/j.bbrc.2010.09.094. [DOI] [PubMed] [Google Scholar]
- Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M., and, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- Liu N, Ohnishi N, Ni L, Akira S., and, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K.et al. (2006The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9 Nat Immunol 7156–164. [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS.et al. (1995Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme Science 2672000–2003. [DOI] [PubMed] [Google Scholar]
- Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C.et al. (1997Phenotypic and functional characterization of mice that lack the type I receptor for IL-1 J Immunol 1593364–3371. [PubMed] [Google Scholar]