Abstract
Therapeutics based on RNA interference (RNAi) have emerged as a potential new class of drugs for treating human disease by silencing the target messenger RNA (mRNA), thereby reducing levels of the corresponding pathogenic protein. The major challenge for RNAi therapeutics is the development of safe delivery vehicles for small interfering RNAs (siRNAs). We previously showed that cholesterol-conjugated siRNAs (chol-siRNA) associate with plasma lipoprotein particles and distribute primarily to the liver after systemic administration to mice. We further demonstrated enhancement of silencing by administration of chol-siRNA pre-associated with isolated high-density lipoprotein (HDL) or low-density lipoprotein (LDL). In this study, we investigated mimetic lipoprotein particle prepared from recombinant apolipoprotein A1 (apoA) and apolipoprotein E3 (apoE) as a delivery vehicle for chol-siRNAs. We show that apoE-containing particle (E-lip) is highly effective in functional delivery of chol-siRNA to mouse liver. E-lip delivery was found to be considerably more potent than apoA-containing particle (A-lip). Furthermore, E-lip–mediated delivery was not significantly affected by high endogenous levels of plasma LDL. These results demonstrate that E-lip has substantial potential as delivery vehicles for lipophilic conjugates of siRNAs.
Introduction
RNA interference (RNAi) is a naturally occurring cellular mechanism of regulating gene expression that is present across a wide range of species including humans. RNAi can selectively and potently silence target messenger RNA (mRNA), leading to reduced expression of the corresponding protein. Synthetic small interfering RNAs (siRNAs) that harness this endogenous pathway have emerged recently as a highly promising new class of drugs to treat human diseases. However, an siRNA, a double-stranded macromolecule with each strand comprising 19–25 nucleotides, is highly anionic and does not readily diffuse across membrane barriers. Effective cellular delivery of siRNAs remains the primary challenge for this new class of therapeutics.
Previously, we demonstrated that the conjugation of cholesterol to the 3′-end of the sense strand of an siRNA significantly improves the in vivo pharmacological properties of siRNAs.1 Subsequently, we showed that cholesterol-conjugated siRNA targeting apoB (chol-siApoB) was primarily delivered to the liver through association with endogenous lipoprotein particles when administered to mice systemically. Moreover, administration of pre-assembled complexes of high-density lipoprotein (HDL) with chol-siApoB enhanced liver apoB mRNA silencing compared to injection of chol-siApoB alone.2
HDLs are heterogeneous populations of lipoprotein particles comprised of small, nascent discoidal HDLs and larger, cholesterol ester-rich spherical HDLs. HDLs recruit excess extrahepatic cholesterol and deliver the cholesterol to the liver for biliary secretion.3,4 Scavenger receptor class B type 1 (SR-B1) mediates the transfer of HDL-cholesterol esters and lipids to cells by selective HDL lipid uptake, a non-endocytotic mechanism.5 The major lipoprotein component of HDL is apoA1, but HDL carries a population of proteins such as enzymes and other apolipoproteins including apoE.6 ApoE is present in other lipoproteins where it provides high affinity binding to low-density lipoprotein receptor (LDLR) and LDL receptor-related protein 1 (LRP1) and promotes internalization of respective lipoprotein particles.7,8,9
Reconstituted HDL-like particles (rHDL) can be readily prepared from apoA1, apoE or their variants and phosphatidylcholine.10,11,12,13 They were initially developed to study lipoprotein metabolism.12 Since then, rHDLs have been explored as potential antiatherosclerosis therapeutics14,15,16 and small molecule drug delivery vehicles,17,18 and have been used for studying membrane proteins.19,20 Biochemical and biophysical studies of rHDL molecules suggest a nascent HDL-like discoidal structure, in which apolipoproteins forming amphipathic helices surround and stabilize a lipid bilayer of 8–20 nm in diameter.21,22,23
In this study, we investigated those reconstituted particles for the delivery of chol-siApoB. We prepared them using recombinant human apoA and ApoE and named these particles as A-liposomes (A-lip) and E-liposomes (E-lip), respectively. Chol-siRNAs were complexed with A-lip and E-lip and administered into mice systemically. Uptake of chol-siRNA occurred similarly to native HDL when A-lip was used, whereas uptake of E-lip-siApoB was likely through the LDLR and its receptor family. We show that silencing of apoB mRNA was significantly improved when the E-lip-chol-siApoB complex was administered to mice compared to A-lip-chol-siApoB and that E-lip–mediated delivery may be applicable broadly to silencing gene targets in hepatocytes.
Results
Characterization of A-lip and E-lip-chol-siRNA complexes
A-lip was prepared from recombinant apoA1 and palmitoyl oleoyl phosphatidyl choline (POPC), and E-lip was prepared from recombinant apoE and dimyristoylphosphatidylcholine (DMPC), respectively. We chose POPC and DMPC as they gave particles of relatively homogeneous size. Physical characteristics, composition, and negative stain electron microscopy images of A-lip and E-lip are similar to previously reported10 and shown in Supplementary Table S1 and Figure S1. Incubation of chol-siRNA with A-lip or E-lip at molar ratios of 1:1 to 4:1 (siRNA:liposome) resulted in incorporation of 90–95% of input siRNA (Supplementary Figure S2). Association of chol-siRNA with liposomes was found to be independent of the siRNA sequence as more than 15 chol-siRNAs of different sequences were incorporated into both liposomes with similar results.
In vivo delivery of chol-siRNA by A-lip
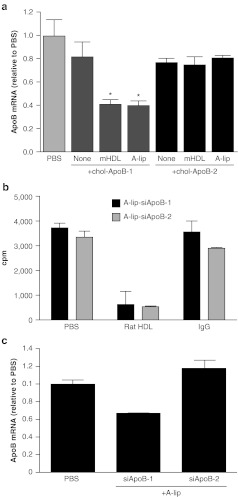
We first compared A-lip with native HDL purified from mouse plasma for delivery of chol-siApoB and silencing of hepatic apoB mRNA. For this study, we used chol-siApoB-1 with both oligonucleotide strands partially modified with a phosphorothioate backbone and containing 2′-O-methyl modifications.1,2 Complexes of A-lip or native HDL with chol-siApoB-1 with a 1:1 (mol:mol) binding stoichiometry were prepared and intravenously administered to C57BL6 mice at a dose of 50 mg/kg. Silencing of apoB mRNA was determined by quantitative PCR and branched DNA assay; both methods gave nearly identical results. Hepatic apoB mRNA was suppressed by a maximum of 40–60% with either A-lip-siApoB-1 or native HDL-chol-siApoB-1 complexes (Figure 1a), demonstrating that A-lip can deliver chol-siRNA in a fashion similar to native HDL. Consistently, binding of A-lip-chol-siRNA to rat primary hepatocytes was inhibited by purified rat HDL (Figure 1b).
Figure 1.
ApoB mRNA silencing by A-lip-chol-siApoB complexes. (a) In vivo apoB mRNA silencing activity of A-lip-chol-siApoB-1 and A-lip-chol-siApoB-2. For comparison, apoB mRNA silencing with chol-siRNA alone and chol-siApoB complexed with purified mouse HDL (mHDL) are shown. Results were shown relative to PBS control animals. N = 4 per group. Each value represents the group mean ± SD. *P < 0.05. (b) Binding of A-lip-chol-siApoB-1 and A-lip-chol-siApoB-2 to rat hepatocytes in the presence of a competitor (rat HDL, nonspecific IgG, or control PBS) using 32P- labeled chol-siRNAs at 4 °C. The assay was done in triplicate. Each value represents the group mean ± SD. (c) ApoB mRNA silencing by A-lip-chol-siApoB complexes in vitro. Mouse primary hepatocytes were incubated with 0.63 µmol/l of A-lip-chol-siApoB-1 or A-lip-chol-siApoB-2 for 6 hours at 37 °C and ApoB mRNA was quantified by qPCR. The assay was done in triplicate. Each value represents the group mean ± SD. apoB, apolipoprotein B; chol-siRNA, cholesterol-conjugated small interfering RNA; cpm, counts per minute; HDL, high-density lipoprotein; mRNA, messenger RNA; PBS, phosphate-buffered saline; qPCR, quantitative PCR.
With A-lip, we observed relatively high experiment-to-experiment variability in apoB mRNA silencing. The reason for this variation is yet to be determined. We also administered another chol-siRNA of a different sequence, chol-siApoB-2, whose parent siRNA without the cholesterol moiety was shown to be highly efficacious in non-human primate studies when formulated in lipid nanoparticles.24 With A-lip-chol-siApoB-2 or native HDL-chol-siApoB-2 complex, no apoB mRNA silencing was observed (Figure 1a). Cholesterol conjugation reduced the potency of this compound, with IC50s for apoB mRNA suppression of 500 pmol/l with chol-ApoB-2 and 50 pmol/l with chol-siApoB-1 in an in vitro cell assay with a transfection reagent. The difference in potency also reflected when mouse hepatocytes were treated with A-lip-siApoB-1 and A-lip-siApoB-2 at 0.63 µmol/l for 6 hours. Nearly 35% of apoB mRNA was silenced with A-lip-siApoB-1, but no silencing was observed with A-lip-siApoB-2 (Figure 1c). It is likely that the lack of in vivo efficacy with A-lip-chol-siRNA-2 was due to less potent siApoB-2 than siApoB-1.
E-lip–mediated chol-siRNA delivery is more effective than A-lip–mediated delivery
To improve the efficacy of liposome-mediated chol-siRNA delivery, we postulated that incorporation of apoE into liposomes would increase cellular uptake of chol-siRNA because apoE binds LDLR and other LDLR family members with high affinity,8,9 thereby potentially increasing the number of cellular entry pathways for chol-siRNA. Moreover, altering the chol-siRNA cellular entry pathway may result in an intracellular distribution that is more favorable for RNAi.
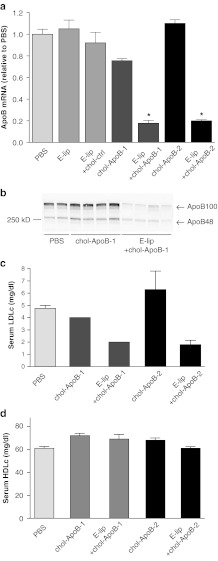
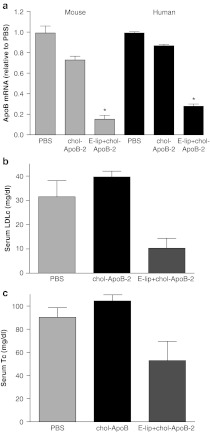
Administration of E-lip-chol-siApoB-1 complexes (1:1 binding stoichiometry) to C57BL6 mice at a dose of 30 mg/kg resulted in greater than 80% reduction of hepatic apoB mRNA (Figure 2a). Both serum apoB100 and apoB48 were lowered by 80 and 50%, respectively (Figure 2b). Furthermore, serum LDL cholesterol levels were reduced by 60% (Figure 2c). In contrast, HDL-cholesterol levels were not affected (Figure 2d). Injection of apoE alone, E-lip alone, or E-lip-chol-siRNA targeting another hepatocyte gene did not affect apoB mRNA levels (Figure 2a), indicating that the observed apoB mRNA silencing is a specific effect of the apoB siRNA.
Figure 2.
ApoB mRNA silencing, apoB protein reduction and LDL cholesterol lowering by E-lip-chol-siApoB complexes. (a) ApoB mRNA silencing by E-lip-siApoB-1 and E-lip-siApoB-2 at 48 hours after administration. Each value represents the group mean ± SD. N = 4 per group. *P < 0.05. (b) ApoB100 and apoB48 serum protein reduction by E-lip-chol-siApoB-1. Western blot analysis of serum from individual animal treated with PBS, chol-siApoB-1, or E-lip-chol-siApoB-1. (c,d) LDL-cholesterol and HDL-cholesterol levels at 48 hours after administration. apoB, apolipoprotein B; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; mRNA, messenger RNA; PBS, phosphate-buffered saline.
To further examine the ability of E-lip to facilitate chol-siRNA delivery, we also used chol-siApoB-2. In contrast to A-lip–mediated delivery, silencing of apoB mRNA was as effective with E-lip-chol-siApoB-2 as with E-lip-chol-siApoB-1 (Figure 2a) and the resultant reduction of serum LDL cholesterol was nearly identical to those with E-lip-chol-siApoB-1 (Figure 2c). We also examined hepatic apoB silencing in LDL receptor knockout (LDLR−/−) mice. Silencing apoB mRNA was less effective (55%) and reduction of apoB100 protein was similarly less effective (40%) whereas reduction of apoB48 protein was similar to that of normal C57BL6 (Supplementary Figure S3). The result suggests that the particles are indeed taken up through partly LDLR and others such as LRP1 as we had postulated.
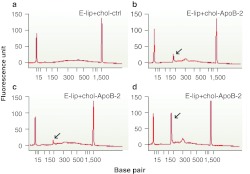
To confirm that silencing of apoB mRNA in E-lip chol-siRNA treated animals was indeed mediated by an RNAi mechanism, we performed 5′-rapid amplification of cDNA ends (RACE) analysis followed by detection of the predicted cleavage site by direct sequencing of 5′-RACE products.1,2,24 As shown in Figure 3, 5′-RACE product of the correct size and sequence was present in liver samples from animals treated with E-lip-chol-siApoB-2, but not in liver samples from animals treated with phosphate-buffered saline (PBS) or the control E-lip-chol-siRNA targeting another hepatocyte gene.
Figure 3.
Confirmation of RNAi-mediated cleavage of apoB mRNA. Capillary electrophoresis analysis of 5′-RACE PCR amplification shows the predicted specific cleavage product (arrow) in E-lip-chol-siApoB-2 (b-d) but not E-lip-chol-si-control (a) treated animals. mRNA from each individual animal was analyzed for the E-lip-siApoB-2 treated group and pooled mRNA was analyzed for the control group. apoB, apolipoprotein B; mRNA, messenger RNA; RNAi, RNA interference; 5′-RACE, 5′-rapid amplification of cDNA ends.
We extended our study to two other liver targets—protein convertase subtilisin/kexin type 9 (PCSK9) and Factor VII (FVII) to assess if the results with E-lip-chol-siApoB complex was generalizable. Administration of chol-siPCSK9 or chol-siFVII alone did not appreciably reduce their respective target mRNA. However, in complex with E-lip, ~75% of PCSK9 mRNA and 50% of FVII mRNA were silenced by chol-PCSK9 and chol-siFVII, respectively, after a single dose of 30 mg/kg (Supplementary Figure S4). Taken together, these results suggest that E-lip particles can provide an effective in vivo delivery vehicle for a broad range of chol-siRNAs targeting hepatic genes.
E-lip-chol-siApoB: tissue distribution, dose response, and duration of effect
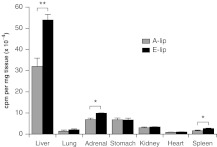
Pharmacokinetic analysis revealed plasma and liver elimination half-lives (T1/2) of 2.2 and 3.0 hours, respectively, after intravenous administration of chol-siApoB-2. Eight hours after administration of A-lip- or E-lip-chol-siApoB-2 complex, tissue distribution of the siRNA was found primarily in the liver (Figure 4) as determined using 32P-chol-siApoB.2 Uptake by the liver was significantly (70%) more with E-lip than A-lip. The results likely explain why chol-siApoB-2 is efficacious with E-lip but not with A-lip. Our previous study showed that native LDL particle-mediated uptake of chol-siRNA mainly distributed the siRNA to the liver, whereas native HDL particles mediated distribution of chol-siRNA to the adrenal and overly more than the liver per mg tissue.2 These data demonstrate that the tissue distribution of chol-siRNA delivered by both A-lip and E-lip particles resembles that of native LDL.
Figure 4.
Tissue distribution of chol-siRNA in C57BL6 mice administered by A-lip-chol-siApoB-2 or E-lip-chol-siApoB-2. Mice were injected with A-lip or E-lip complexes pre-assembled with 32P-labeled chol-siApoB-2 at a dose of 10 mg/kg. Four hours after injection, tissues were harvested and the radioactivity associated with the tissues was determined. N = 3 per group. Each value represents the group mean ± SD. *P < 0.05, **P < 0.01. chol-siRNA, cholesterol-conjugated small interfering RNA; cpm, counts per minute.
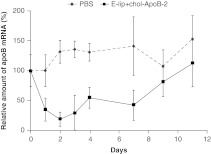
The dose dependence of silencing was steep, with small changes in dose resulting in large effects in silencing. E-lip-chol-siApoB-2 complex at a dose of 20 mg/kg resulted in apoB mRNA reduction by 20%, whereas the maximum 80–85% apoB mRNA reduction occurred at a modest increase in dose to 30 mg/kg (data not shown). These doses appeared well-tolerated as evidenced by clinical parameters and serum chemistry up to 11 days postinjection (Supplementary Figure S5). Silencing of apoB mRNA persisted until day 10 after a single dose of E-lip-chol-siApoB-2 at 30 mg/kg, by which time apoB mRNA recovered to baseline levels (Figure 5).
Figure 5.
Duration of apoB mRNA silencing in C57BL6 mice after single injection of E-lip-chol-siApoB at 30 mg/kg. N = 4 per group. Each value represents the group mean ± SD. apoB, apolipoprotein B; mRNA, messenger RNA; PBS, phosphate-buffered saline.
Silencing of ApoB mRNA in transgenic mice with increased levels of plasma LDL
Plasma lipoprotein profiles vary significantly among different species. In mice, HDL is the major liproprotein and 80% of total serum cholesterol is found in HDL. In humans, LDL is the major lipoprotein species and binds ~60% of total serum cholesterol.25,26 The relatively high concentration of LDL in humans raises the possibility that endogenous LDL may compete with E-lip for receptor binding, thereby interfering with E-lip–mediated delivery of chol-siRNA. To evaluate the effect of high levels of endogenous LDL, we tested E-lip–mediated chol-siRNA delivery in double transgenic mice that express human apoB and cholesterol ester transfer protein (CETP). These mice have high levels of LDL cholesterol in the circulation similar to that in humans.26 E-lip-chol-siApoB-2, which targets a region in apoB mRNA conserved across the human and mouse,24 was administered intravenously to these double transgenic mice at 30 mg/kg. Approximately 85% of mouse apoB mRNA and 72% of human apoB mRNA was silenced in the liver (Figure 6a), a silencing level similar to that in normal C56BL6 mice. In addition, serum LDL cholesterol and total cholesterol levels were reduced to one-third and 60% of PBS control, respectively (Figure 6b,c). Taken together, these results demonstrate that endogenous LDL does not compete significantly with E-lip.
Figure 6.
ApoB mRNA silencing and LDL cholesterol lowering in double transgenic mice expressing human apoB and CETP. (a) Reduction of mouse and human apoB mRNAs by E-lip-chol-siApoB-2 48 hours after administration. *P < 0.05. (b,c) LDL and total cholesterol levels 48 hours after administration. N = 5 per group. Each value represents the group mean ± SD. apoB, apolipoprotein B; CETP, cholesterol ester transfer protein; LDLc, low-density lipoprotein cholesterol; mRNA, messenger RNA; PBS, phosphate-buffered saline; Tc, total cholesterol.
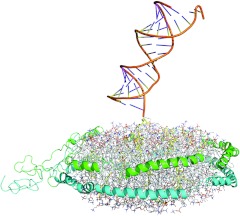
1:1 loading of siRNA per liposome is essential
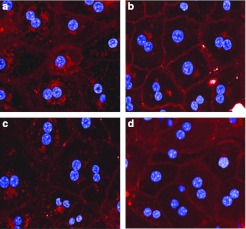
A-lip- and E-lip-siRNA complexes with a binding stoichiometry greater than 1:1 (mol:mol) demonstrated very little or no apoB mRNA silencing activity in vivo (data not shown). Figure 7 shows a molecular model of A-lip-chol-siApoB-2 complex. This model was built using the “double-belt ” model reported by Wu Z, et al.27 as the template and the structural model of chol-si-ApoB-2 we constructed. It is likely that multiple siRNAs of highly negative charge on both surfaces of the discoidal HDL are dynamic and result in a large effective size that inhibits interaction with SR-B1 or the LDLR family by steric hindrance or possibly electrostatic repulsion. To evaluate this possibility, we examined cellular uptake of Alexa-647 labeled chol-siApoB-2 by mouse primary hepatocytes. A-lip and E-lip complexes containing 1 molecule of labeled siRNA per particle (1:1 complex) and A-lip and E-lip complexes containing 1 molecule of the labeled siRNA plus 3 molecules of unlabeled siRNA per particle (4:1 complex) were prepared. When cells were incubated with A-lip or E-lip 4:1 complexes, the fluorescence was mostly localized in the plasma membranes whereas when cells were incubated with A-lip or E-lip 1:1 complexes, the fluorescence was predominantly in the cytoplasm (Figure 8). These results clearly showed that 1:1 binding stoichiometry is essential for cellular uptake of siRNA, and furthermore suggest that this stoichiometry may facilitate proper interaction of A-lip and E-lip with their receptors.
Figure 7.
Structural model of A-lip-chol-siApoB-2 complex. The model was constructed using the “double-belt ” model of discoidal HDL constructed by Wu Z, et al.27 as the template and the structural model of chol-siApoB-2. The 1:1 (siRNA:liposome) complex is shown. apoB, apolipoprotein B; HDL, high-density lipoprotein; siRNA, small interfering RNA.
Figure 8.
Uptake of Alexa647-labeled chol-siApoB by mouse primary hepatocytes. Cells were incubated with A-lip or E-lip pre-assembled with Alex-647 labeled chol-siApoB-2 with a 1:1 binding stoichiometry (1:1 complex) or the same complexes containing additional threefold excess nonlabeled chol-siApoB-2 (1:4 complex) for 60 minutes at 37 °C. Cells were stained with Hoechst and images were acquired. (a) A-lip 1:1 complex. (b) A-lip 1:4 complex. (c) E-lip 1:1 complex. (d) E-lip 1:4 complex. apoB, apolipoprotein B.
Discussion
To date, most advanced siRNA delivery technology are primarily polymer-based and lipid-based28,29,30 and they are being tested in clinical trials. In addition, conjugate-based approach is being developed.31,32,33 Despite significant progress, there exist continued needs to advance siRNA delivery methods to improve safety characteristics and therapeutic window.
Isolated serum HDL may be a viable delivery vehicle, however, HDL-like liposomal particles reconstituted from recombinant apolipoproteins may be a more practical option. These particles provide effective delivery vehicles for siRNAs and small molecule drugs for multiple reasons. First and most importantly, they have been demonstrated to be well-tolerated in humans in clinical trials.11,16 Second, they can be prepared from recombinant human apolipoproteins and naturally occurring lipids, and the reconstitution method is relatively simple, well-established, and scalable for clinical use.34 Third, the simple reconstitution method combined with protein engineering potentially allows for design of these particles with increased specificity and potency. Finally, the small particle size may allow broader tissue distribution. These considerations prompted us to investigate HDL-like liposomal particles as a delivery vehicle for chol-siRNAs.
An antisense single-stranded oligonucleotide designed to inhibit apoB100 protein synthesis has been investigated in clinic as a potential drug to lower LDL cholesterol in patients with familial hypercholesterolemia.35,36 In this study, we used double-stranded apoB siRNAs as research tool to explore the delivery of double-stranded siRNAs. We demonstrated robust and reproducible in vivo silencing of target ApoB mRNA when chol-siApoB was complexed with E-lip. Additional two different hepatocyte targets were silenced with this delivery approach, showing that it is generalizable across multiple hepatic gene targets. Furthermore, the silencing was a specific effect of the siRNA and a concomitant RNAi cleavage mechanism was demonstrated. E-lip provided more efficacious chol-siRNA delivery than A-lip. The difference in efficacy between the two delivery systems is likely due to the difference in uptake mechanisms (LDLR family members versus SR-B1) and subsequent intracellular localization of chol-siRNA.
The dose dependence of silencing with E-lip-chol-siApoB was steep with a change in dose from 20 to 30 mg/kg resulting in a change in silencing from 20 to 80%. Dose responses that have been reported from liposomal nanoparticle formulations demonstrate a dynamic range over 20-fold (5–95% silencing) with a dose range from 0.5 to 5 mg/kg with siApoB-2 without cholesterol,24 whereas chol-siApoB-1 in saline shows a change from 0 to 15% silencing in doses from 5 to 50 mg/kg.2 Further studies are needed to assess the underlying basis for the steep dose response for E-lip-chol-siRNA.
Absolute dose levels of E-lip complexes required for silencing in vivo were still high, although markedly improved as compared with uncomplexed chol-siRNAs. Biodistribution studies showed that the siRNA was mostly distributed to the liver, albeit with a relatively short elimination half-life. Increasing the circulation half-life of the E-lip chol-siRNA may help to lower the efficacious dose. In addition, these complexes with longer circulation half-time may allow delivery of chol-siRNA to other tissues such as tumors.
In summary, we demonstrated efficient in vivo delivery of chol-siRNAs to hepatocytes with E-lip complexation. Further optimization is warranted to fully develop the translational potential of this promising delivery approach which harnesses a physiologic mechanism.
Materials and Methods
Synthesis of chol-siRNAs. The chemically modified chol-siRNAs used in this study were synthesized as described previously.2 The sequences of siApoB-1, siApoB-2, and siFVII were also described previously.2,30 The sequences siPCSK9 are: siPCSK9 sense, 5′-GccuGGAGuuuAuucGGAAdT*dT-3′ chol-siPCSK9 antisense, 5′-PuuccgaauaaacuccaggcdT*dT-3′. Lower case letters represent 2′-OMe modified nucleotides; bold lower case letters represent 2′-fluoro modified nucleotides; and asterisks represent phosphorothioate linkages.
Preparation of liposome-chol-siRNA complexes. Detailed procedures for production of recombinant apoA1 and apoE can be found in Supplementary Materials and Methods. Liposomal particles were prepared using the cholate dialysis method.10 A-lip was prepared using ApoA-1 and POPC; E-lip was prepared using ApoE and DMPC (Avanti Polar Lipids, Alabaster, AL). Complex concentration was determined by measuring protein concentration. POPC and DMPC concentrations measured using Phospholipids C assay (Wako Diagnostics, Richmond, VA). Complex size determined by dynamic light scatter analysis using Malvern Nano ZS (Malvern Instruments, Worcestershire, UK). Liposome-chol-siRNA complexes were prepared by mixing either A-lip or E-lip with chol-siRNA at a molar ratio of 1.2:1 (siRNA:liposome) for 30 minutes at 37 °C and free chol-siRNA was removed on a Superdex 200 column (GE Healthcare, Piscataway, NJ). Chol-siRNA concentration determined by ion-exchange chromatography using DNAPACK PA200 (Dionex, Sunnyvale, CA).
In vivo silencing experiments. All procedures used in animal studies conducted at Alnylam were approved by the Institutional Animal Care and Use Committee and were consistent with local, state, and federal regulations as applicable. Male C57BL/6 mice were injected via tail vein with PBS, chol-siRNA or liposome-chol-siRNA complex at a volume of 0.015 ml/g. For the PCSK9 experiment, animals were maintained as described.37 Forty-eight hours after administration, blood was collected, animals were killed and liver samples harvested and frozen in liquid nitrogen unless stated otherwise. Serum ApoB protein levels were determined by western blot analysis of 0.3 µl of serum from individual animal using polyclonal anti-apoB antibody (Meridian Life Sciences, Cincinnati, OH). Bands corresponding to ApoB100 and apoB48 were quantified using the LI-COR Odyssey Imagine system (LI-COR Biosciences, Lincoln, NE). Serum cholesterol levels were determined using Olympia AU400 Serum Chemistry Analyzer (Olympus, Center Valley, PA). Frozen liver tissue was ground and tissue lysates were prepared. ApoB, PCSK9, and FVII mRNA levels were determined by quantitative reverse transcription-PCR and/or a branched DNA assay (QuantiGene 1.0 Reagent System; Affymetrix, Santa Clara, CA). The mean level of target mRNA was normalized to the mean levels of GAPDH mRNA for each sample. Group mean values were then normalized to the mean value for the control group, to obtain the relative level of target mRNA expression. One-way analysis of variance, Dunnett test was used for statistical analysis.
Cell assays. Radiolabeling of siRNAs and preparation of liposomes complexed with the labeled siRNAs were described previously.2 For competitive binding of A-lip complexed with chol-siApoB to hepatocytes, rat primary hepatocytes (100,000 cell per tube; Invitrogen, Carlsbad, CA) were incubated with 0.6 µmol/l A-lip-[32P]siApoB with PBS (control), 0.6 µmol/l A-lip-[32P]siApoB, and 6 µmol/l rat HDL, or with 0.6 µmol/l A-lip-[32P]siApoB and nonspecific IgG (1 µg/ml) in William's media E (Invitrogen) for 2 hours at 4 °C. Cells were washed with cold media three times and the radioactivity associated with cells were counted. For apoB mRNA silencing, mouse primary hepatocytes were isolated using a standard collagenase perfusion method and cultured on collagen coated 96-plates (Greiner, Kremsmunster, Austria). A-lip-siApoB-1 or A-lip-siApoB-2 (0.63 µmol/l) were added to the cells. Six hours after incubation at 37 °C, cells were harvested and apoB mRNA levels were determined by quantitative PCR as described above.
Uptake of siRNA bound E-lip and A-lip in vivo. Mice were injected by radiolabeled siRNAs bound E-lip and A-lip at 10 mg/kg (987 × 103 cpm (counts per minute) in 200 µL sample). Blood was taken 2 minutes postinjection and at indicated time points. After 8 hours, organs were harvested and weighted and radioactivity was measured in all organs. All values were normalized to the measured blood radioactivity at the 2-minute injection time point for each mouse, respectively. t-test was used for statistical analysis.
Imaging study. Mouse primary hepatocytes were isolated as above and cultured overnight on 96-well plates (Greiner) freshly coated with collagen. Cells were incubated with 1 µmol/l A-lip or E-lip pre-assembled with Alex-647 labeled chol-siApoB-2 with a 1:1 binding stoichiometry or the same complexes containing threefold excess nonlabeled chol-siApoB-2 for 15–120 minutes at 37 °C. Cells were stained with Hoechst without fixation. Images were acquired using an Opera automated spinning disc confocal system (Perkin Elmer, Waltham, MA) and data analyzed using Acapella Software (Perkin Elmer).
Modeling of the A-lip-chol-siApoB-2 complex. The solar-flare model proposed by Wu Z, et al.27 were used as the template for the liposomal particle. The molar ratio of apoA-1 versus POPC in the template is 1:100 instead of the ratio of 1:90 as in present experiments. Therefore, the excess POPC was removed from the template randomly. To relax the system, all-atom discrete molecular dynamics simulations38 were performed by iteratively constraining apoA1 proteins or lipids. After the convergence, the diameter of the resulting liposomal particle is ~10 nm in diameter. The three-dimensional structure of chol-siRNA was constructed by combining MedusaDock39 for small molecule conformation generation and all-atom discrete molecular dynamics for three-dimensional structure reconstruction. The chol-siRNA was inserted into the relaxed particle model to generate the liposome-chol-siRNA complex. Discrete molecular dynamics simulations of the complex suggest that the molecular complex is flexible due to a flexible linker between the siRNA and the cholesterol model imbedded in the HDL nano disc.
SUPPLEMENTARY MATERIAL Figure S1. Electron microscopy images of negatively stained A-lip and E-lip. Figure S2. Kinetics of E-lip and chol-siapoB-1 binding. Figure S3. ApoB mRNA silencing and protein reduction in LDLR−/− mice. Figure S4. PCSK9 and FVII mRNA silencing. Figure S5. Serum chemistry of mice treated with PBS or E-lip-siApoB-2. Table S1. Physical and biochemical characterization of A-lip and E-lip. Materials and Methods.
Acknowledgments
We thank the following people at Alnylam Pharmaceuticals. John Maraganore for helpful discussions and critical reading of the manuscript; Maryellen Livingston and Lauren Lesser for graphics assistance; Akin Akinc for contribution to early A-lip work; Maria Frank-Kamenetsky, Yosef Landesman, Amy White, Jessica Sutherland, Kathy Mills, Amy Dell, Jeff Rollins, Mara Broberg, Christopher Zurenko, Bo Pang, Sergey Shulga-Morskoy, and David Butler for assistance and helpful suggestion. M.S. was supported by an SNF Sinergia grant and is a consultant and member of the Scientific Advisory Board of Alnylam Pharmaceuticals Inc..
Supplementary Material
Electron microscopy images of negatively stained A-lip and E-lip.
Kinetics of E-lip and chol-siapoB-1 binding.
ApoB mRNA silencing and protein reduction in LDLR−/− mice.
PCSK9 and FVII mRNA silencing.
Serum chemistry of mice treated with PBS or E-lip-siApoB-2.
Physical and biochemical characterization of A-lip and E-lip.
REFERENCES
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M.et al. (2004Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs Nature 432173–178. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK.et al. (2007Mechanisms and optimization of in vivo delivery of lipophilic siRNAs Nat Biotechnol 251149–1157. [DOI] [PubMed] [Google Scholar]
- Navab M, Reddy ST, Van Lenten BJ., and, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Alexander ET, Weibel GL, Billheimer J., and, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50 suppl.:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., and, Stein Y. Atheroprotective mechanisms of HDL. Atherosclerosis. 1999;144:285–301. doi: 10.1016/s0021-9150(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC.et al. (2007Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL J Clin Invest 117746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., and, Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Mahley RW., and, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer's disease and beyond. Curr Opin Lipidol. 1999;10:207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Jonas A. Reconstitution of high density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- Atkinson D., and, Small DM. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu Rev Biophys Biophys Chem. 1986;15:403–456. doi: 10.1146/annurev.bb.15.060186.002155. [DOI] [PubMed] [Google Scholar]
- Innerarity TL, Pitas RE., and, Mahley RW. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979;254:4186–4190. [PubMed] [Google Scholar]
- Jonas A, Steinmerz A., and, Churgay L. The number of amphipathic α-helical segments of apolipoproteins A-1, E, and A-IV determines the size and functional properties of their reconstituted lipoprotein particles. J Biol Chem. 1993;268:1596–1602. [PubMed] [Google Scholar]
- Tardif JC, Grégoire J, L'Allier PL, Ibrahim R, Lespérance J, Heinonen TM.et al. (2007Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial JAMA 2971675–1682. [DOI] [PubMed] [Google Scholar]
- Newton RS., and, Krause BR. HDL therapy for the acute treatment of atherosclerosis. Atheroscler Suppl. 2002;3:31–38. doi: 10.1016/s1567-5688(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S.et al. (2006Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-1 Milano J Am Coll Cardiol 47992–997. [DOI] [PubMed] [Google Scholar]
- Rensen PC, de Vrueh RL, Kuiper J, Bijsterbosch MK, Biessen EA., and, van Berkel TJ. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv Drug Deliv Rev. 2001;47:251–276. doi: 10.1016/s0169-409x(01)00109-0. [DOI] [PubMed] [Google Scholar]
- McConathy WJ, Nair MP, Paranjape S, Mooberry L., and, Lacko AG. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs. 2008;19:183–188. doi: 10.1097/CAD.0b013e3282f1da86. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Baas BJ, Grinkova YV., and, Sligar SG. Cooperativity in cytochrome P450 3A4. J Biol Chem. 2007;282:7066–7076. doi: 10.1074/jbc.M609589200. [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Grinkova YV., and, Sligar SG. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch Biochem Biophys. 2006;450:215–222. doi: 10.1016/j.abb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, Klon AE, Sheldahl CJ, Hellinger M, De Loof H.et al. (1999A detailed molecular belt model for apolipoprotein A-1 in discoidal high density lipoprotein J Biol Chem 27431755–31758. [DOI] [PubMed] [Google Scholar]
- Martin DDO, Budamagunta MS, Ryan RO, Voss JC., and, Oda MN. Apolipoprotein A-1 assumes a “looped belt ” confirmation on reconstituted high density lipoprotein. J Biol Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- Shih AY, Freddolino PL, Sligar SG., and, Schulten K. Disassembly of nanodiscs with cholate. Nano Lett. 2007;7:1692–1696. doi: 10.1021/nl0706906. [DOI] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN.et al. (2006RNAi-mediated gene silencing in non-human primates Nature 441111–114. [DOI] [PubMed] [Google Scholar]
- Greeve J, Altkemper I, Dieterich JH, Greten H., and, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993;34:1367–1383. [PubMed] [Google Scholar]
- Grass DS, Saini U, Felkner RH, Wallace RE, Lago WJ, Young SG.et al. (1995Transgenic mice expressing both human apolipoprotein B and human CETP have a lipoprotein cholesterol distribution similar to that of normolipidemic humans J Lipid Res 361082–1091. [PubMed] [Google Scholar]
- Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM., 3rd, , Smith JD.et al. (2007The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction Nat Struct Mol Biol 14861–868. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W.et al. (2005Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs Nat Biotechnol 231002–1007. [DOI] [PubMed] [Google Scholar]
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N.et al. (2008A combinatorial library of lipid-like materials for delivery of RNAi therapeutics Nat Biotechnol 26561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratovska A., and, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–68. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR.et al. (2009Systemic administration of optimized adpamer-siRNA chimeras promotes regression of PSMA-expressing tumors Nat Biotechnol 27839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., and, Rossi JJ. Aptamer-targeted cell-specific RNA interference. Silence. 2010;1:4. doi: 10.1186/1758-907X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch PG, Förtsch V, Hodler G., and, Bolli R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996;71:155–164. doi: 10.1046/j.1423-0410.1996.7130155.x. [DOI] [PubMed] [Google Scholar]
- Raal FJ, Santos RD, Blom DJ, Marais D, Charng MJ, Cromwell WC.et al. (2010Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomized, double-blind, placebo-controlled trial Lancet 375998–1006. [DOI] [PubMed] [Google Scholar]
- Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJP.et al. (2010Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lip Res 511057–1062. [DOI] [PMC free article] [PubMed]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A.et al. (2008Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates Proc Natl Acad Sci USA 10511915–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Tsao D, Nie H., and, Dokholyan NV. Ab initio folding of proteins with all-atom discrete molecular dynamics. Structure. 2008;16:1010–1018. doi: 10.1016/j.str.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yin S., and, Dokholyan NV. Rapid flexible docking using a stochastic rotamer library of ligands. J Chem Inf Model. 2010;50:1623–1632. doi: 10.1021/ci100218t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electron microscopy images of negatively stained A-lip and E-lip.
Kinetics of E-lip and chol-siapoB-1 binding.
ApoB mRNA silencing and protein reduction in LDLR−/− mice.
PCSK9 and FVII mRNA silencing.
Serum chemistry of mice treated with PBS or E-lip-siApoB-2.
Physical and biochemical characterization of A-lip and E-lip.