Abstract
In our recent study, replicative alphaviral vector VA7 was found to be effective against orthotopic human U87-glioma xenografts in an athymic mouse model eradicating the tumors with single intravenous (i.v.) injection. Here, we tested the efficacy of VA7 in immunocompetent orthotopic GL261 and CT-2A glioma models of C57BL/6 mouse in vivo. The cell lines were susceptible to VA7 infection in vitro, but GL261 infection was highly restricted in confluent cell cultures, and mouse interferon-β (IFNβ) pretreatment prevented the replication of VA7 in both cell lines. When mice bearing orthotopic GL261 or CT-2A tumors were administered neurotropic VA7, either i.v. or intracranially (i.c.), the vector was unable to infect the tumor and no survival benefit was achieved. Pretreatments with immunosuppressive cyclophosphamide (CPA) and rapamycin markedly lowered serum-neutralizing antibodies (NAbs) but had no effect on tumor infection or survival. Intracranial GL261 tumors were refractory also in athymic C57BL/6 mice, which have serious defects in their adaptive immunity. Implanted VA7-infected GL261 cells formed tumors with only slightly delayed kinetics and without improving survival thus excluding the participation of physical barriers and indicating robust host IFN action. Mouse and human IFNβ do not seem be species cross-reactive, which might limit the translational relevance of xenograft models in oncolytic virotherapy.
Introduction
The most common primary brain tumors such as glioblastoma multiforme are often inoperable and relatively resistant both to radiation and chemotherapy. DNA-alkylating agent temozolomide has demonstrated some efficacy in combination with standard therapy and with adenovirus-thymidine kinase /ganciclovir suicide gene therapy. Nevertheless, patients cannot be cured and they rarely live beyond 2 years after diagnosis, the median survival of patients with glioblastoma multiforme being 12–15 months.1,2 Oncolytic virotherapy is a new promising treatment strategy to tackle this devastating disease. Viruses that have entered phase 1 and phase 1/2 clinical trials in treatment of malignant glioma include herpes simplex virus-1, adenovirus, reovirus, Newcastle disease virus, and measles virus.3 Semliki Forest virus (SFV) is a novel candidate in the field of oncolytic virotherapy, and in addition to its ability to infect and kill a number of tumor cell lines in vitro, it has also proven safe and efficacious in preclinical lung adenocarcinoma, melanoma, osteosarcoma, and glioma models.4,5,6,7,8,9 SFV is an enveloped positive-strand RNA virus of the family Togaviridae with broad host range and high replication efficiency. Infection with SFV A7(74) strain and the derived vector VA7 (ref. 10) leads to transient brain viremia in mice.11 Recently, we demonstrated that intravenously (i.v.) administered VA7-EGFP virus effectively homes to the brains of athymic mice eradicating implanted human U87-Fluc glioma tumors, while sparing the healthy tissue.4 However, testing of vector safety and efficacy in immunocompetent animal models is necessary, as immunocompromised xenograft models do not feature the inflamed, yet immunosuppressive environment of human gliomas.2 Innate [complement, interferons (IFNs), tumor necrosis factor] and humoral (antibodies) immune responses can seriously impede virotherapy by preventing the infection of tumor tissue or by eradicating the therapy virus at an early stage.12 Previously, we detected IFNα/β response against VA7 in subcutaneous (s.c.) A549 human lung cancer model.6
IFNα and IFNβ belong to type I IFNs and play a crucial role in controlling the replication of SFV and other alphaviruses, and mice lacking functional IFNα/β receptor die within 48 hours post infection (p.i.).13,14,15 IFNα/β functions in preventing the widespread and lethal infection of avirulent SFV strain A7(74) in peripheral organs, but A7(74) does not display virulent pattern of neural infection even in the IFNα/β receptor–deficient mice.14,15 Nevertheless, the meningeal cells, ependymal cells, and oligodendrocytes were reported to be infected as a result of impaired type I IFN response, suggesting that separate antiviral cytokines may protect neurons and glial cells. Some tumors have acquired mutations that render them unable to produce and/or respond to IFNα/β thus enabling tumor-specific replication of IFN-sensitive viruses.2,16 On the other hand, immunosuppressive agents such as cyclophosphamide (CPA)17,18,19,20,21 and rapamycin21,22,23 have been used to inhibit virus-neutralizing immunity in order to improve oncolysis and vector efficacy.
In this study, we have studied viral oncolysis in GL261, CT-2A, and U87-Fluc glioma cell lines in vitro as well as in CT-2A and GL261 mouse glioma models in vivo. The data show that despite oncolytic efficacy in vitro, VA7-EGFP vector therapy failed to infect orthotopic tumors in vivo. Our results together with the parallel study by M. Vähä-Koskela, F. Le Boeuf, C. Lemay, N. De Silva, J.-S. Diallo, J. Cox et al. (submitted) indicate intact type I IFN response as one of the major barriers against the oncolytic efficacy of VA7-EGFP in some, but not all, syngeneic mouse tumor models. As IFNβ does not seem to feature cross-species reactivity, the immunocompromised tumor xenograft models may be of limited translational value for studies of oncolytic efficacy.
Results
Orthotopic GL261 and CT-2A gliomas are refractory to VA7-EGFP infection
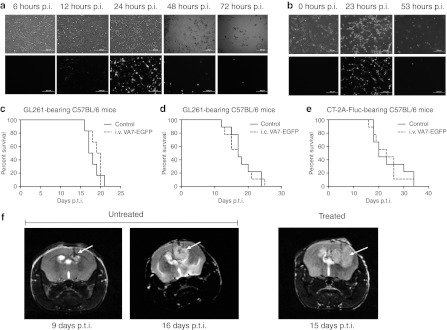
VA7-EGFP completely lysed GL261 [multiplicity of infection (MOI) = 1] and CT-2A (MOI = 0.01) cells seeded on 12-well plate at 48–72 hours p.i. as seen in fluorescence microscopy (Figure 1 a,b). In order to test the efficacy of VA7 virotherapy in vivo, we implanted GL261 and CT-2A-Fluc tumors intracranially (i.c.) into the caudate putamen of adult C57BL/6 mice. Solid tumors developed in majority of the mice, whereas some had more disseminated malignant formations. In some cases, tumor cell injection resulted in multiple tumors around the ventricles, indicating leakage of cells. Gliomas became visible in magnetic resonance imaging latest at day 9 post–tumor induction (p.t.i.) in VA7-EGFP-treated and untreated groups (Figure 1f). GL261 tumor-bearing mice were treated with VA7-EGFP either i.v. (Figure 1c) or i.c. (Figure 1d) but no survival benefit was seen. Similarly, CT-2A-Fluc tumors grew vigorously and despite the better infectivity in vitro, no survival benefit was gained by i.v. VA7-EGFP treatment (Figure 1e).
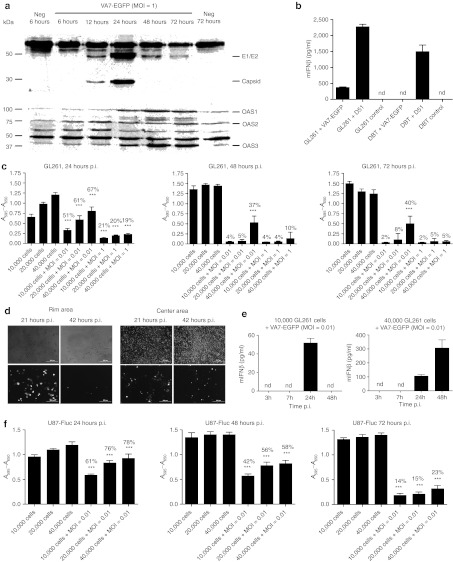
Figure 1.
VA7-EGFP infects and kills GL261 cells in vitro but not in vivo. (a) A volume of 2 × 105 GL261 cells were seeded on 12-well plate and infected the next day with VA7-EGFP, MOI = 1. Pictures were taken with fluorescence microscope at 6, 12, 24, 48, and 72 hours p.i. Upper row: phase contrast, lower row: fluorescein isothiocyanate (FITC). Bar = 200 µm. (b) A volume of 1 × 105 CT-2A seeded on 12-well plate and infected the next day with VA7-EGFP, MOI = 0.01. Pictures taken with fluorescence microscope 0, 23, and 53 hours p.i. Upper row: phase contrast, lower row: FITC. Bar = 200 µm. (c) Survival of GL261 tumor-bearing C57BL/6 mice (n = 8) treated with intravenous injections of VA7-EGFP virus on days 10, 13, and 16 p.t.i. (1 × 106 PFU/dose). Nontreated mice (n = 8) are the control group. (d) Survival of GL261 tumor-bearing C57BL/6 mice (n = 9) treated with intracranial injection of VA7-EGFP on days 6, 12, and 18 p.t.i. (1 × 106 PFU/dose). Mice (n = 9) mock treated with intracranial saline injections (at same time points) were used as a control group. (e) Survival of CT-2A-Fluc bearing C57BL/6 mice treated with intravenous injection of VA7-EGFP (1 × 108 PFU/dose, 12 days p.t.i.; n = 9). Nontreated mice (n = 9) were as a control group. (f) Representative magnetic resonance images of untreated GL261 glioma-bearing mice at 9 and 16 days p.t.i. and intravenously treated (VA7-EGFP injected at days 10 and 13 p.t.i.) mouse at day 15 p.t.i. i.c., intracranially, i.v., intravenously; MOI, multiplicity of infection; PFU, plaque-forming unit; p.i., post infection; p.t.i., post–tumor induction.
Immunosuppressive treatment with CPA and rapamycin
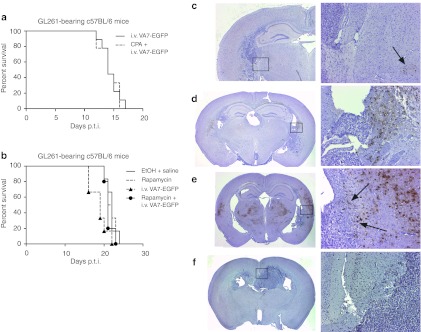
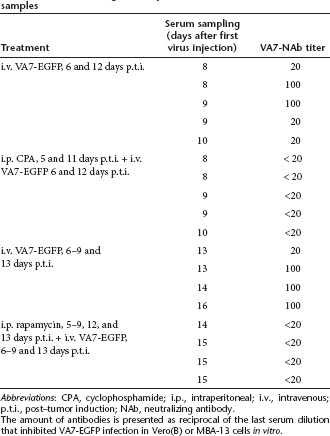
To reduce the amount of virus-neutralizing antibodies (NAbs) and the effects of innate immunity, we tested immunosuppressive drugs CPA and rapamycin in combination with the virus. Groups of GL261 tumor-bearing mice were treated with VA7-EGFP with or without preceding i.p. CPA or rapamycin treatment, but no survival benefit was gained (Figure 2a,b). A fivefold decrease of VA7-neutralizing serum antibodies upon CPA and rapamycin treatments was achieved (Table 1). Nevertheless, no virus antigen could be detected in the GL261 tumors by immunohistochemistry (Figure 2c–f), while infected glial cells could be identified in the corpus callosum following i.c. vector injection (Figure 2d) and a few virus foci were seen in healthy brain parenchyma upon i.v. injection (Figure 2c). The CPA treatment strongly enhanced VA7-EGFP replication in healthy brain tissue including neurons but not in tumor (Figure 2e). No survival advantage was gained with either combination therapy (Figure 2a,b). In contrast to CPA, rapamycin did not enhance viral central nervous system replication (Figure 2f).
Figure 2.
Immunosuppressive therapy with cyclophosphamide or rapamycin does not affect survival or infectivity in GL261 model. (a) Survival of mice (n = 9) treated with CPA and VA7-EGFP combination therapy. CPA injections were given on days 5 (3 mg) and 11 (2 mg) p.t.i. followed by intravenous injection of VA7-EGFP on the next day (days 6 and 12, 1 × 106 PFU/dose). Mice (n = 9) treated with only VA7-EGFP were used as a control group. (b) Survival of mice (n = 5) treated with rapamycin and VA7-EGFP combination therapy. Intraperitoneal (i.p.) rapamycin injection was (5 mg/kg) given on days 5–9, 12 and 13 p.t.i. Intravenous (i.v.) VA7-EGFP (1 × 106 PFU/dose) injections were administered 6–9 and 13 days p.t.i. Survival was compared with groups of mice (n = 6) given only rapamycin or VA7-EGFP. The control group (n = 6) was mock treated with 30% EtOH and saline instead of rapamycin and VA7-EGFP, respectively. (c–f) Immunohistochemistry of C57BL/6 mouse brain sections using anti-SFV antibody and hematoxylin background stain. VA7-EGFP antigen is seen as brown color. Left column: pictures taken at a magnification of ×1.25; right column: pictures taken from the same section at a magnification of ×10 (black boxes in the left column indicate the magnified areas). (c) Brain tissue section from glioma-bearing mouse given i.v. VA7-EGFP 10 and 13 days p.t.i. Mouse was killed on day 16 p.t.i. Focal infection highlighted with arrow. (d) Brain tissue section from glioma-bearing mouse administered i.c. VA7-EGFP on day 11 p.t.i. Mouse was killed on day 13 p.t.i. Infected cells of the corpus callosum are detected. (e) Brain tissue section from glioma-bearing mouse administered CPA on days 5 and 11 p.t.i. and i.v. VA7-EGFP on days 6 and 12 p.t.i. Mouse was killed on day 12 p.t.i. Enhanced infectivity of healthy brain tissue can be observed. Arrows highlight the border between infected brain tissue and uninfected GL261 glioma tissue. (f) Brain tissue section from mouse administered rapamycin at 5–9, 12, and 13 days p.t.i. and i.v. VA7-EGFP 6-9 and 13 days p.t.i. Mouse was killed on day 20 p.t.i. and infected tissue was not detected. CPA, cyclophosphamide; EtOH, ethanol; i.c., intracranial; PFU, plaque-forming unit; p.t.i., post–tumor induction.
Table 1. VA7-neutralizing antibody amounts detected from serum samples.
Orthotopic GL261 tumors in athymic C57BL/6 mice are refractory to virus
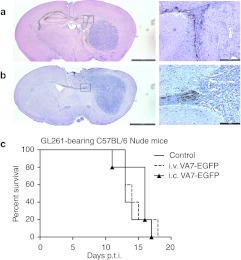
Immunohistochemical analysis of C57BL/6 nude mice killed at 2 (Figure 3a) and 4 days (Figure 3b) after VA7 i.c. injection revealed infected glial cells of corpus callosum lining the glioma tumor (similar to Figure 2d). However, no virus was detected in tumor itself (Figure 3a,b) and no survival benefit was seen (Figure 3c).
Figure 3.
VA7-EGFP is not effective in therapy of GL261 gliomas in athymic C57BL/6 mice. Immunohistochemistry of athymic C57BL/6 mouse brain sections using anti-SFV antibody and hematoxylin background stain. VA7-EGFP antigen is seen as brown color. Left column: Pictures were taken at a magnification of ×1,25 (Bar = 2,000 µm), right column: pictures were taken from the same section at a magnification of ×10 (Bar = 200 µm, black box in the left column indicates magnified area). (a) Brain section from GL261 glioma-bearing mice killed 2 days after i.c. VA7-EGFP injection. (b) Brain section from GL261 glioma-bearing mice killed 4 days after i.c. VA7-EGFP injection. (c) Survival of glioma-bearing athymic C57BL/6 mice following i.v. (n = 5) and i.c. (n = 6) VA7-EGFP therapy and untreated control mice (n = 5). i.c., intracranial; i.v., intravenous; PFU, plaque-forming unit; p.t.i., post–tumor induction; SFV, Semliki Forest virus.
Mouse IFNβ blocks infection of GL261 cells in vitro
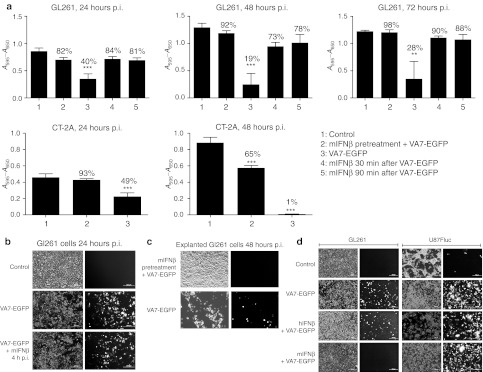
Pretreatment of GL261 cells with 100 U/ml mouse (m)IFNβ significantly (P < 0.01) inhibited VA7-EGFP infection in vitro (Figure 4a), and even when added 30 or 90 minutes p.i., rescued the cells from infection. Although majority (88–98%) of the mIFNβ-treated cells were still alive after 72 hours p.i., the infected cultures without mIFNβ treatment showed only 28% viability. Similarly, the infection was inhibited by IFNβ pretreatment also in CT-2A cells (Figure 4a). Less than 1% of the infected cells remained viable beyond 48 hours p.i., while the corresponding viability of mIFNβ-pretreated cells was 65%. No protection of GL261 cells was seen if mIFNβ was added 4 hours p.i. (Figure 4b). To test whether cells from i.c. virus–treated GL261 tumors had become irreversibly refractory, we prepared explant cultures and infected them with VA7-EGFP. The vector efficiently infected the cultures leading to complete cell lysis within 48 hours p.i. (Figure 4c). Pretreatment with 100 U/ml of mIFNβ blocked the infection similar to the inhibition of fresh GL261 cells (Figure 4c). The U87-Fluc human glioblastoma cells were readily infected with VA7-EGFP (MOI = 0.1) regardless of pretreatment with 500 U/ml of mIFNβ or hIFNβ (Figure 4d). The latter efficiently restricted VA7 infection in primary cells derived from human glioma biopsy (data not shown).
Figure 4.
Treatment with mouse IFNβ hampers VA7-EGFP infectivity and replication in GL261 and CT-2A cells in vitro. (a) Cell viability measurements performed using MTT-assay. 10,000 GL261 or CT-2A cells cultured on 96-well plate and subjected to mouse IFNβ. Pretreatment: cells were incubated in 100 U/ml mouse IFNβ 5 hours before infecting with VA7-EGFP, MOI = 1. In addition, mouse IFNβ was added to other plated cells 30 or 90 minutes p.i. with VA7-EGFP, MOI = 1. Absorbance (A595 − A650) was measured from parallel prepared plates 24, 48, and 72 hours p.i. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 as analyzed by unpaired, two-tailed t-test. (b) A volume of 1 × 105 GL261 cells cultured on 24-well plate. Top row: uninfected GL261 cells. Middle row: cells infected with VA7-EGFP, MOI = 1. Bottom row: mouse 100 U/ml IFNβ added to the cells 4 hours p.i. (c) GL261 explanted from glioma-bearing mouse given i.c. VA7-EGFP (1 × 106 PFU/dose) on days 6 and 12 p.t.i. and killed on day 17 p.t.i. Cells were seeded on 24-well plate (1 × 105 cells/well) and infected with VA7-EGFP the following day. Top row: cells pretreated 5 hours with 100 U/ml mouse IFNβ before infecting with VA7-EGFP, MOI = 1. Bottom row: cells infected with VA7-EGFP, MOI = 1. (d) Minimal cross-reactivity between human and mouse IFNβ. Left column: 1 × 105 GL261 cells cultured on 24-well plate and pretreated by incubating in culture medium containing 500 U/ml human IFNβ or 100 U/ml mouse IFNβ for 5 hours before infection with VA7-EGFP, MOI = 1. Right column: 1 × 105 U87-Fluc cells cultured on 24-well plate and pretreated by incubating in culture medium containing 500 U/ml human or mouse IFNβ for 5 hours before infecting with VA7-EGFP, MOI = 0.1. Top row: uninfected control cells. Second row: cells infected with MOI = 1. Third row: cells pretreated with human IFNβ before infection. Bottom row: cells pretreated with mouse IFNβ before infection. IFNβ, interferon-β MOI, multiplicity of infection; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; p.i. post infection.
VA7-EGFP induces activation of antiviral pathways and production of IFNβ
We then used western blotting to analyze whether VA7 infection caused induction of antiviral proteins in GL261 cells and measured oligoadenylate synthetase (OAS) expression. In concordance with fluorescence development data (Figure 1a), the levels of SFV capsid and E1/E2 envelope proteins peaked at 24 hours p.i. (Figure 5a), whereas 2′,5′-OAS isoforms 1 (OAS1) and 3 (OAS3) were increasing with time and peaked at 48 hours p.i., while the changes in isoform 2 (OAS2) were less prominent (Figure 5a). Mobilization of these cellular antiviral proteins did not, however, lead to inhibition of GL261 infection or cell lysis in vitro.
Figure 5.
VA7-EGFP induces antiviral responses in GL261 cells in vitro. (a) Viral and virus-induced antiviral proteins detected from VA7-EGFP-infected GL261 cells with western blotting. A volume of 1 × 105 GL261 cells were seeded on 12-well plate and infected with VA7-EGFP, MOI = 1. Cells were collected 6, 12, 48, and 72 hours p.i., lysed, and proteins analyzed with western blotting (15 µg protein/sample). First lane: uninfected cells collected 6 hours p.i.; second to sixth lanes: infected cells collected 6–72 hours p.i.; 7th lane: uninfected cells collected 72 hours p.i. Top picture: SFV-protein expression. E1/E2 (50 kDa) and capsid protein (30 kDa) detected with SFV-antibody. Additionally a high molecular weight band is detected in infected and uninfected samples. Bottom picture: OAS1-3 isoforms detected with OAS antibody. (b) IFNβ ELISA on GL261 and DBT cells. A total of 300,000 cells in 6-well plate were either infected with VSVΔ51-EGFP or VA7-EGFP at MOI = 0.01 or left uninfected with 1 ml medium. After 24 hours supernatants were collected and mouse IFNβ was quantified according to manufacturer's instructions (Verikine IFNb ELISA; PBLBiosource). (c) A595 − A650 measured with MTT-assay from GL261 cells. 1 × 104, 2 × 104, or 4 × 104 cells plated on 96-well plate and infected the next day with MOI = 0.01 or 1. Cell viability measured from different parallel prepared plates 24, 48, and 72 hours p.i. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 as analyzed by unpaired, two-tailed t-test. (d) Confluent centers of the wells were supplemented with subconfluent rims on a 24-well plate (50,000 GL261 cells/well in total) and infected with VA7-EGFP (MOI = 1). Pictures were taken 21 and 42 hours p.i. from the rim and center areas of the same well. (e) mIFNβ measured from 10,000 or 40,000 GL261 cells seeded on 96-well plate and infected with VA7-EGFP (MOI = 0.01) in 200 µl of culture medium. Medium was collected from wells at 3, 7, 24, and 48 hours p.i. and mIFNβ measured using ELISA kit (Verikine IFNb ELISA; PBLBiosource). Measurements were done from 2 to 3 parallel wells/time point. (f) A595 − A650 measured with MTT-assay from U87-Fluc cells infected with VA7-EGFP. A volume of 1 × 104, 2 × 104, or 4 × 104 cells were plated on 96-well plate and infected the next day with MOI = 0.01. Cell viability was measured from different parallel prepared plates at time points 24, 48, and 72 hours p.i. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 as analyzed by unpaired, two-tailed t-test. ELISA, enzyme-linked immunosorbent assay; mIFNβ, mouse interferon-β MOI, multiplicity of infection; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; nd, not detected; OAS, 2′-5′-oligoadenylate synthetase; SFV, Semliki Forest virus.
We next measured IFNβ production in culture supernatants of GL261 and DBT (Balb/c) glioma cells after infection with VA7-EGFP or Δ51 mutant of Vesicular stomatitis virus (VSV) carrying enhanced green fluorescent protein. Although relatively high levels of mIFNβ were induced by VSV Δ51 in both cell lines, markedly lower amounts were induced by VA7-EGFP in GL261 cells (Figure 5b), and no IFNβ was detected in DBT glioma cells (Figure 5b).
VA7-EGFP replication is restricted in confluent GL261 cultures in vitro
We then tested whether high cell density typical for compact GL261 tumors (Figures 2 and 3) could affect virus permissiveness by infecting confluent and subconfluent GL261 cultures in vitro. Although viability of subconfluent cultures declined to 2–5% during infection with significant (P < 0.001) decrease as compared with uninfected controls (Figure 5c), VA7 replication and spread were highly restricted in confluent GL261 cultures, and cell viability was significantly (40%, P < 0.001) higher. Similar effect was observed also in a separate study, where in GL261 cultures the subconfluent rim areas were readily infected and lysed, while the inoculated dense centers inside the same wells showed low virus permissiveness (Figure 5d). In contrast, in Vero(B) and CT-2A cultures VA7 spread was efficient regardless of cell density (Supplementary Figure S1). In order to mimic the sparse cultures in vivo, we gave i.c. VA7-EGFP injections [1 × 106 plaque-forming units (PFUs)] to C57BL/6 mice already 1 day after tumor induction. Despite the early treatment, no benefit in survival was observed (data not shown).
Next, we tested whether the refractory dense cultures produce more IFNβ as compared with sensitive sparse monolayers. The results showed that subconfluent cultures were infected and killed by VA7-EGFP despite the production of IFNβ (Figure 5e). A volume of 51.78 ± 4.96 pg/ml of IFNβ could be measured from infected subconfluent cultures 24 hours p.i. (n = 2), but declined under detection limit by 48 hours p.i. (n = 3) due to cell lysis, whereas confluent cultures showed continuously increasing IFNβ production up to 48 hours (105.42 ± 11.00 and 306.46 ± 58.02 pg/ml). IFNβ levels in samples (n = 3) collected at 3 and 7 hours from infection were below the detection limit (<31.2 pg/ml). We then studied whether VA7-EGFP replication in confluent GL261 cultures could be enhanced by adding anti-mIFNβ antibody to the culture before infection. As shown in the Supplementary Figure S2, no enhancement was gained but in contrast, slight enhancement of virus spread in confluent cultures was seen when paracellular junctions were disrupted by ethylene glycol tetraacetic acid–mediated calcium deprivation (Supplementary Figure S3).
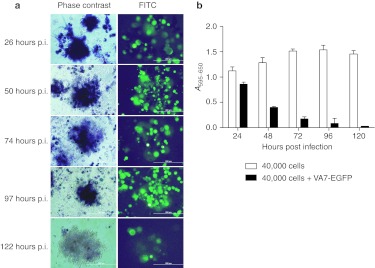
Given that high tumor cell density may compromise virus therapy especially when moderately permissive cells are used, we tested whether spherules obtained in U87 human glioblastoma cultures representing more compact cellular formation were less permissive than monolayers. As compared with GL261 cells, the U87 cells were readily infected with VA7-EGFP with significant (P < 0.001) loss of cells at 24, 48, and 72 hours p.i. (MOI = 0.01) with respective viabilities of 61–78%, 42–58%, and 14–23% (Figure 5f). We found that also the neurospheres were infected by VA7-EGFP, but in contrast to the surrounding monolayer cells that were rapidly lysed, the spheres retained their metabolic activity up to 120 hours p.i. as observed by the formation of blue formazan metabolite in 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)- assay (MOI = 0.01; Figure 6a,b).
Figure 6.
Spheres formed by U87-Fluc show prolonged survival after VA7-EGFP infection in vitro. (a) U87-Fluc spheres stay metabolically active until 122 hours p.i. upon infection. Left side: phase-contrast image indicating blue formazan metabolite in the spheres. Right side: FITC image indicating VA7-EGFP infection of the spheres. Images were taken 26–122 hours p.i. (MOI = 0.01). Bar = 200 µm. (b) MTT-assay from U87-Fluc cells infected with VA7-EGFP. A density of 4 × 104 cells plated on 96-well plate and infected the next day with MOI = 0.01. Cell viability was measured from different parallel prepared plates at times 24, 48, 72, 96, and 120 hours p.i. Data are presented as mean ± SD. FITC, fluorescein isothiocyanate; MOI, multiplicity of infection; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; p.i., post infection.
In vivo antiviral assay demonstrates robust antiviral response by the host
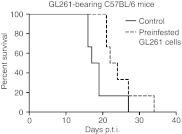
Finally, we designed “in vivo antiviral assay” and infected fresh GL261 cells for 1 hour with VA7-EGFP, washed them, and implanted into brains of immunocompetent C57BL6/J mice. Despite successful infection as determined by fluorescence microscopy from a parallel culture next day, the cells produced aggressive i.c. tumors with only slight delay on median mortality as compared with uninfected controls (23 days and 18 days, respectively, not significant, log-rank test; Figure 7).
Figure 7.
VA7-EGFP preinfected GL261 cells form intracranial gliomas when implanted to C57BL/6 mice. Subconfluent GL261 cells were infected with VA7-EGFP for 1h and implanted intracranially into c57BL/6 mice. Survival of preinfected GL261 group (n = 6) was not statistically changed (log-rank test) as compared with control group (n = 6) implanted with uninfected GL261 cells. The success of the infection was controlled using fluorescence microscopy of a parallel flask left to incubator over night. p.t.i., post–tumor induction.
Discussion
The observed discrepancy that VA7-EGFP readily lysed GL261 and CT-2A cells in vitro but neither infected tumor tissue (brain or s.c.) nor survival benefit was seen after in vivo treatments (Figures 1c–e and 2c,d) is multifaceted. The attempts to enhance the efficacy of the therapy by combining the virus with immunosuppressive drugs, CPA and rapamycin, both known to affect humoral and innate immunity, were unsuccessful. The effects of CPA are known to be pleiotropic; it can on one hand stimulate virus replication by reducing NAbs18,20 or suppressing innate immunity,17,18,19,20 but it can also stimulate antiviral IFNα/β production.24,25 Oncolytic viruses themselves are immunomodulatory and sometimes CPA/virus combinations do not produce additive or synergistic, but rather antagonistic effects.26 Rapamycin can inhibit IFNα/β production by the tumor or systemically by plasmocytoid dendritic cells thus enabling specific tumor infection when the neoplastic cells have lowered ability to respond to type I IFNs.23
Strikingly, CPA enhanced viral replication throughout the healthy brain including cortex, thalamic, hypothalamic, and periventricular regions with an infection pattern resembling the virulent SFV strains,11 whereas infected tumor cells could not be found and survival was not improved as compared with controls (Figure 2a,b,e,f). The infected brain parenchyma rather seemed to form an absolute border to the tumor lining indicating a strict cell type–specific inhibition (Figure 2e). This was probably not due to physical barriers (e.g., encapsidation) as the infection borders were evident also upon i.c. virus injections (Figure 2d). CPA counteracts Th1 responses and it has been used in treating autoimmune diseases.27,28 Antibodies and IFNγ secreted by T cells are known mediators of alphaviral clearance from central nervous system neurons,29 and thus it is not surprising that several Th1/IFNγ inhibitors in addition to CPA stimulate central nervous system replication of avirulent SFV strains.30,31 IFNγ alone inhibits only minimally VA7-EGFP replication in GL261 glioblastoma cells in vitro (data not shown) but as we have shown these cancer cells are sensitive to IFNβ, produced readily by most somatic cell types.16 As type I IFNs do not play a major role in the control of SFV neuronal replication in vivo,14,15 we speculate that the different target cells of type I and II IFNs (glial and neuronal, respectively) could explain the infection pattern induced by CPA pretreatment. This hypothesis awaits yet further corroboration. The GL261 model was recently proven resistant also to vaccinia virus even in combination with rapamycin, whereas similar combination showed partial effect in racine RG2 glioma model.22 To exclude the possibility that the therapy failure was due to residual serum NAbs despite CPA or rapamycin treatments or was caused by cellular adaptive responses, we treated orthotopic gliomas in athymic C57BL/6 mice. Virus could not be detected in tumor tissue by immunohistochemistry (Figure 3) and similar borders were seen between the infected brain parenchyma and the tumor as in the immunocompetent model. Taken together, the data strongly suggested that the therapy failure was not due to the adaptive immunity but rather mediated by components of the antiviral innate immunity, which we then proceeded to study. Intriguingly, in a parallel study by M. Vähä-Koskela, F. Le Boeuf, C. Lemay, N. De Silva, J.-S. Diallo, J. Cox et al. (manuscript submitted), VA7 demonstrated efficacy in syngeneic CT26-LacZ colon carcinoma model. This tumor model could not respond to type I IFN further proposing that syngeneic tumors per se are amenable to VA7 virotherapy as long as the cells have acquired mutations rendering them defective in their response to IFNα/β.
We saw that VA7-EGFP-infected GL261 cells are potent producers and responders to IFNβ alike (Figures 4 and 5). Mouse IFNβ was able to protect GL261 cells from VA7-EGFP infection and rescue them when administered as late as 90 minutes but not anymore 4 hours p.i. (Figure 4a,b). These data support existence of a VA7-EGFP replication threshold (quantitative or temporal) that needs to be passed before effective cell lysis is reached. Such turning point could be for example the shift from negative sense RNA synthesis to genomic plus strand RNA production occurring 4–6 hours p.i.32,33,34 The explanted GL261 cells had retained their infectivity and IFNβ sensitivity ex vivo, indicating that virus resistant tumor cell populations had not been evolved (Figure 4c). U87 human glioblastoma multiforme cells neither produce nor respond to type I IFN35 (Figure 4d) providing the most apparent explanation for their sensitivity to VA7-EGFP infection in vivo we observed earlier.4
The VA7-EGFP infection of GL261 cells was highly restricted in confluent cultures (Figure 5c and Supplementary Figure S1) resembling the poor infectivity of the dense GL261 tumors observed in situ (Figure 2d). In contrast, U87-Fluc, Vero(B), and CT-2A cell lines were highly susceptible regardless of cell density (Figure 5f and Supplementary Figure S1). Amongst the potential explanations are the known defects in IFNβ synthesis of U87 and Vero(B) cells,23,36 and we saw that also CT-2A astrocytoma cells produced markedly lower levels of IFNβ as compared with GL261 cells. We could also detect induction of intracellular antiviral proteins OAS1 and OAS3 in infected GL261 cells peaking 48 hours p.i. indicating that these cells were indeed autonomic in their type I IFN synthesis and response (Figure 5a). We observed spontaneously forming spheres in U87-Fluc cultures, which have earlier been reported to be enriched in cancer stem cells by others.37 Although susceptible to VA7-EGFP infection, their lysis was retarded until 120 hours p.i. further supporting the importance of the tumor density for infectivity. The amount of IFNβ produced by GL261 cells correlated linearly with the seeded cell number (Figure 5e) but was clearly less than the respective amount induced by VSV Δ51 used as a control. Interestingly, DBT glioma cells did not produce IFNβ upon VA7-EGFP infection (Figure 5b), but this did not translate into better in vivo efficacy (M. Vähä-Koskela, F. Le Boeuf, C. Lemay, N. De Silva, J.-S. Diallo, J. Cox et al., manuscript submitted) underlining the significant role of the tumor cell–independent local and systemic IFN response elicited by the host.
Cells in close contact might also resist infection via IFN-independent mechanisms. Recent data suggest that some dense cultures are able to transmit a rapid antiviral response through cell-to-cell gap junctions,38 and glial cells may communicate through such junctions.39 Indeed, only subconfluent GL261 rims, but not the densely growing centers inside the same wells were efficiently infected in vitro (Figure 5d). Improved infection may be obtained by disruption of paracellular junctions using ethylene glycol tetraacetic acid–mediated calcium depletion40,41,42,43 and we did observe slightly enhanced infection of confluent GL261 cell cultures upon ethylene glycol tetraacetic acid treatment (Supplementary Figure S3). Inhibition of infection upon close cellular contact may, at least in some cases, be related to the basolateral or intercellular location of the viral receptor.44 We could not observe increase in viral replication in confluent GL261 cultures upon anti-IFNβ antibody treatment (Supplementary Figure S2). This further suggested parallel existence of IFN-independent virus resistance mechanisms in the dense tumor cell cultures.
We treated orthotopic GL261 tumors with VA7 soon (24 hours p.t.i) after tumor induction mimicking sparse in vitro conditions, but saw no improvement in survival (data not shown). We then advanced to study the role of systemic and local host IFN response and designed “in vivo antiviral assay.” Antiviral assays are the most commonly used biological assays for IFN potency determinations.45 We preinfected GL261 cells for 1 hour before their i.c. implantation and could see no change in survival suggesting that physical hindrance in tumor microenvironment such as encapsulation or fibrotic barriers9 was not sufficient explanation and indeed, the in vivo therapy failure had to be accounted for host antiviral IFN activity (Figure 7). The local type I IFN production by the tumor cells themselves might have furthered the process, but was not alone enough for saving the tumor as the cells not used for grafting were successfully infected ex vivo (data not shown).
In conclusion, we report here that VA7-EGFP virotherapy does not prolong the survival of mice in CT-2A and GL261 glioma models despite obvious lytic efficacy in vitro. CPA or rapamycin immunosuppression did not improve tumor infectivity and CPA caused strong increase of viral replication in healthy brain parenchyma. This finding together with earlier reports suggests that combining Th1-type immunosuppressive therapy with neurotropic alphaviruses may pose serious safety concerns. Our data indicate that type I IFN produced both by the tumor cells themselves and, maybe even more importantly, by the host, is one of the major factors limiting the oncolytic efficacy of VA7-EGFP. This is in agreement with a recent report using Sindbis virus, a close relative of SFV, in virotherapy of different immunocompetent mouse tumor models.46 Furthermore, considering the observed importance of the tumor cell–independent host IFN response and the reported lack of cross-species reactivity of type I IFNs by us (Figure 4) and others,47,48,49 the data suggest that immunocompromised mouse xenograft models where human tumors are implanted into mouse IFN milieu might be of limited translational value in oncolytic virotherapy.
Materials and Methods
Cell cultures and viruses. Cell cultures of mouse glioma GL261 (kind gift from G. Safrany), DBT (kindly provided by Daniel Silbergeld, University of Washington, WA), CT-2A (kindly provided by Thomas Seyfried, Boston College, MA), human glioblastoma U87-Fluc (ref. 4) and mouse glial MBA-13 cells were established. GL261 and DBT cells were cultured in Dulbecco's modified Eagle's medium (D6046; Sigma-Aldrich, St Louis, MO) MBA-13 and U87-Fluc cells were cultured in minimum essential medium Eagle (M4655; Sigma-Aldrich). CT-2A cells were cultured in RPMI-1640 medium (R0883; Sigma-Aldrich). Dulbecco's modified Eagle's medium for GL261, DBT, and U87-Fluc was supplemented with 10% fetal calf serum (FCS; Autogen Bioclear, Wiltshire, UK), 1% L-glutamine, and 1% (G57513; Sigma-Aldrich) penicillin–streptomycin (P0781; Sigma-Aldrich). Minimum essential medium for MBA-13 was supplemented with 5% FCS and 1% penicillin–streptomycin. RPMI-1640 medium for CT-2A cells was supplemented with 10% FCS, 1% L-glutamine, and 1% penicillin–streptomycin. A fluorescent derivative VA7-EGFP, based on avirulent SFV A7(74) (ref. 11) was used as reporter virus. Infectivity was analyzed with fluorescence microscopy. Preparation of VA7-EGFP has been described10 and virus was produced from in vitro transcribed RNA by transfection in BHK-21 cell line cultured in Dulbecco's modified Eagle's medium with 10% FCS, 1% L-glutamine, and 1% penicillin–streptomycin. Titering of virus was done by plaque analysis.50
Infection of cell cultures and IFNβ treatment. GL261, CT-2A, or U87-Fluc cells were plated on 12- or 24-well plates (5 × 104 to 3 × 105 cells/well) and infected with VA7-EGFP (MOI = 0.01–1) the next day. IFNβ pretreatment was done by incubating the cells in culture medium containing 100 or 500 U/ml of mouse or human recombinant IFNβ (Sigma-Aldrich) for 5 hours before infection with VA7-EGFP. Addition of IFNβ was done by replacing the medium used in infection step with medium containing IFNβ. The infection was monitored with a fluorescence microscope.
Cell viability measurements. Cell viability was measured using MTT Cell Proliferation Kit I (Roche) according to manufacturer's instructions. A volume of 1 × 104 to 4 × 104 GL261, CT-2A, or U87-Fluc cells were plated on 96-well plates and infected with VA7-EGFP (MOI = 0.01 or 1.0) in 100 µl of culture medium. IFNβ treatment was done by incubating GL261 cells 3.5 hours in 100 µl of medium containing 100 U/ml mouse IFNβ. In addition, 100 U/ml mouse IFNβ was added to VA7-EGFP infected cells 30 or 90 minutes after infection (MOI = 1). MTT reagent was added 24, 48, or 72 hours p.i. followed by solubilization buffer 4 hours later. Absorbance at wavelength 590 nm and 650 nm was measured with VICTOR-plate reader (PerkinElmer, Waltham, MA) the following day. Results are presented by mean absorbance (A595 subtracted by A650) ± SD. Statistical analysis was done using unpaired, two-tailed t-test (GraphPad Prism; GraphPad Software, La Jolla, CA). Cell viability was determined by calculating the mean absorbance (A595 − A650) of treated sample divided by mean absorbance of respective untreated control and indicated as percentage (marked above the columns).
In vivo animal experiments. Gliomas were implanted to groups of normal C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) by injecting 1 × 105 GL261 or 5 × 104 firefly luciferase expressing CT-2A-Fluc cells suspended in 5 µl of OPTI-MEM (Sigma-Aldrich) i.c. 2 mm right from bregma in 3 mm depth. The cells were stored on ice before injection. For tumor induction, the animals were sedated with ketamine and medetomidine (75 mg/kg and 1 mg/kg, respectively) and kept under 2% isoflurane gas anesthesia during the operation. Antisedative drug atipamezole (1 mg/kg, i.p.) was administered after operation and carprofen (5 mg/kg, s.c.) and buprenorphine (0.05–0.1 mg/kg, s.c.) were used for analgesia. Tumor development was monitored with T2-weighted magnetic resonance imaging using 9.7 T vertical magnet (Oxford Instruments, Oxford, UK). Mice were weighed daily and their condition and possible distress were monitored carefully. If signs of severe distress (e.g., paralysis) or weight loss of >20% were observed, the animal was euthanized. The animal experiments were performed at biosafety 2 containment level obeying the guidelines of the local committee of animal welfare.
First study group (n = 8) was given three i.v. injections of VA7-EGFP (1 × 106 PFU/dose) on days 10, 13, and 16 p.t.i.. In this study, an untreated tumor-bearing group (n = 8) was used as a control. To enhance the probability of in vivo tumor infection, we performed a second study where the mice (n = 9) were given three i.c. injections of VA7-EGFP (1 × 106 PFU/dose). Injections were given on days 6, 12, and 18 p.t.i. In this study, the control group (n = 9) was given i.c. saline injections instead of virus. CT-2A-Fluc bearing groups of mice were given one i.v. injection of VA7-EGFP (1 × 108 PFU) 12 days p.t.i. (n = 9). Control group (n = 9) received no treatment.
Immunosuppressive combination therapy employing CPA with VA7-EGFP virus was carried out by giving the mice (n = 9) i.p. injections of CPA on day 5 (3 mg) and 11 (2 mg) p.t.i. and i.v. injection of VA7-EGFP (1 × 106 PFU/dose) on day 6 and 12 p.t.i. The control group (n = 9) for this study was administered only VA7-EGFP. For rapamycin (diluted in 30% ethanol) combination therapy studies, the gliomas were induced as previously by injecting 1 × 104 GL261 cells i.c. to C57BL/6 mice. The combination therapy group (n = 5) was given i.p. 5 mg/kg rapamycin on days 5–9, 12, and 13 p.t.i. and VA7-EGFP (1 × 106 PFU/dose i.v.) was given on days 6–9 and 13 p.t.i. Control groups (n = 6) were given only rapamycin or VA7-EGFP injections. Third control group (n = 6), was given injections of 30% ethanol i.p. and saline i.v. instead of rapamycin and VA7-EGFP.
In experiments utilizing athymic C57BL/6 nude mice (B6.Cg/NTac-Foxn1nu NE10), 1 × 105 GL261 cells were implanted i.c. as described previously. Groups of mice were treated with i.v. (n = 5) or i.c. (n = 6) VA7-EGFP at days 6 and 12 p.t.i. or left untreated as control (n = 5). Mice were monitored as previously.
In the “in vivo antiviral assay” subconfluent GL261 cells grown in 175-cm2 culture flask were infected with VA7-EGFP (~1.4 × 108 PFUs) for 1 hour, washed and implanted i.c. into immunocompetent C57BL/6 mice. Survival of preinfected GL261 group (n = 6) was compared with control group (n = 6) implanted with uninfected GL261 cells. The success of the infection was controlled by fluorescence microscopy of a parallel flask left to incubator over night.
Immunohistochemical analysis of brain tissue samples. After CO2 killing, the mice were perfused with 4% formalin and the brains were collected. Brain tissue samples were first stored at +4°C in 4 % paraformaldehyde (dissolved in phosphate-buffered saline) and transferred to 70% ethanol on the following day. Samples were embedded in paraffin and sliced into 7-µm sections with microtome. Immunohistochemistry was performed to detect SFV antigens in paraffin-embedded brain tissue sections using polyclonal rabbit antibody and Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Explanted GL261 glioma cells. Explanted GL261 cell cultures were established from glioma tissue samples taken from orthotopic tumors of C57BL/6 mice after i.c. VA7-EGFP treatment. Glioma samples were pressed through fine metal mesh onto tissue culture plates and cultured under normal conditions in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% FCS, 1% L-glutamine, and 1% penicillin–streptomycin. Explant GL261 cultures were analyzed for VA7-EGFP infectivity and IFNβ-sensitivity as described previously.
Detection of VA7-EGFP NAbs in serum samples. Blood samples from the mice were collected immediately after killing and serum was separated using Microvette 500 Z-gel tubes (SARSTEDT). Serum samples were stored at −20 °C. Titering of NAbs was carried out on either MBA-13 or Vero (B) cells seeded onto 12-well plates (2 × 106 cells /well). Fivefold serial dilutions (1:20, 1:100, 1:500, and 1:2,500) were made from the serum samples and 40 µl of the dilutions were mixed with 100 µl of VA7-EGFP sample (containing 30–50 PFU) and incubated for 30 minutes at room temperature. Serum from an untreated mouse was used as a control. After incubation the mixtures were transferred onto 12-well plates containing the cells in 0.5 ml medium. The cells were observed with fluorescence microscopy 24 and 48 hours after infection. Neutralization titer was defined as reciprocal of the last dilution that completely inhibited VA7-EFGP infection visualized as cells expressing EGFP. The choice of cells [MBA or Vero (B)] did not affect the titering result. No neutralization was observed in the untreated samples.
Western blot analysis. Cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (15 µg protein/sample, measured with Bradford method) and transferred to 0.1 µm Protran nitrocellulose membrane (Schleicher & Schuell, Keene, NH). Membranes were incubated in anti-SFV or anti-OAS (kind gift from Ilkka Julkunen, KTL, Finland) polyclonal antibody followed by peroxidase-conjugated secondary antibody. Precision Plus Protein Dual Color was used as marker (BioRad, Hercules, CA). Blots were developed using chemiluminescence detection to X-ray film (Kodak, Rochester, NY).
Measuring IFNβ with enzyme-linked immunosorbent assay. A volume of 3 × 105 GL261 or DBT cells were seeded in 6-well plate and either infected with VA7-EGFP or VSV carrying EGFP (VSV Δ51-EGFP) at MOI = 0.01 or left uninfected with 1 ml medium. After 24 hours supernatants were collected and kept at −20 °C until analyzed. Measurement was done from two parallel wells using Verikine IFNβ ELISA kit (PBL InterferonSource, Piscataway, NJ) according to manufacturer's instructions. To measure IFNβ production from subconfluent and confluent GL261 cultures, cells were seeded on 96-well plate (1 × 104 or 4 × 104 cells/well, respectively) and infected with VA7-EGFP (MOI = 0.01) in 200 µl of culture medium. Supernatants were collected 3, 7, 24, and 48 hours p.i. and kept at −20 °C until analyzed as above using half diluted samples (two or three parallels) with Verikine IFNβ ELISA kit (PBL InterferonSource) according to manufacturer's instructions.
SUPPLEMENTARY MATERIAL Materials and Methods. Figure S1. Inhibition of VA7-EGFP infectivity in confluent GL261 but not in confluent Vero(B) or CT-2A cultures in vitro. Figure S2. Anti-mIFNβ antibody does not enhance VA7-EGFP infectivity. Figure S3. EGTA pretreatment slightly enhances VA7-EGFP infection in GL261 cells in vitro.
Acknowledgments
We thank the group of Olli Gröhn for assistance with mouse magnetic resonance imaging and Ivana Kholova for expertise help in histopathological analysis. The study was financially supported by the Finnish Cancer Foundations, Academy of Finland, Cancer Center of Eastern Finland (CCEF no. 22122010), Kuopio University Hospital (EVO), and Oskar Öflund Foundation.
Supplementary Material
Inhibition of VA7-EGFP infectivity in confluent GL261 but not in confluent Vero(B) or CT-2A cultures in vitro.
Anti-mIFNβ antibody does not enhance VA7-EGFP infectivity.
EGTA pretreatment slightly enhances VA7-EGFP infection in GL261 cells in vitro.
REFERENCES
- Määttä AM, Samaranayake H, Pikkarainen J, Wirth T., and, Ylä-Herttuala S. Adenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant glioma. Curr Gene Ther. 2009;9:356–367. doi: 10.2174/156652309789753365. [DOI] [PubMed] [Google Scholar]
- Zemp FJ, Corredor JC, Lun X, Muruve DA., and, Forsyth PA. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21:103–117. doi: 10.1016/j.cytogfr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Parker JN, Bauer DF, Cody JJ., and, Markert JM. Oncolytic viral therapy of malignant glioma. Neurotherapeutics. 2009;6:558–569. doi: 10.1016/j.nurt.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä JE, Vähä-Koskela MJ, Ruotsalainen JJ, Martikainen MW, Stanford MM, McCart JA.et al. (2010Intravenously administered alphavirus vector VA7 eradicates orthotopic human glioma xenografts in nude mice PLoS ONE 5e8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola A, Hinkkanen A, Yongabi F, Furu P, Määttä AM, Liimatainen T.et al. (2008Oncolytic Semliki forest virus vector as a novel candidate against unresectable osteosarcoma Cancer Res 688342–8350. [DOI] [PubMed] [Google Scholar]
- Määttä AM, Liimatainen T, Wahlfors T, Wirth T, Vähä-Koskela M, Jansson L.et al. (2007Evaluation of cancer virotherapy with attenuated replicative Semliki forest virus in different rodent tumor models Int J Cancer 121863–870. [DOI] [PubMed] [Google Scholar]
- Määttä AM, Mäkinen K, Ketola A, Liimatainen T, Yongabi FN, Vähä-Koskela M.et al. (2008Replication competent Semliki Forest virus prolongs survival in experimental lung cancer Int J Cancer 1231704–1711. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE., and, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Kallio JP, Jansson LC, Heikkilä JE, Zakhartchenko VA, Kallajoki MA.et al. (2006Oncolytic capacity of attenuated replicative semliki forest virus in human melanoma xenografts in severe combined immunodeficient mice Cancer Res 667185–7194. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Tuittila MT, Nygårdas PT, Nyman JK, Ehrengruber MU, Renggli M.et al. (2003A novel neurotropic expression vector based on the avirulent A7(74) strain of Semliki Forest virus J Neurovirol 91–15. [DOI] [PubMed] [Google Scholar]
- Tuittila MT, Santagati MG, Röyttä M, Määttä JA., and, Hinkkanen AE. Replicase complex genes of Semliki Forest virus confer lethal neurovirulence. J Virol. 2000;74:4579–4589. doi: 10.1128/jvi.74.10.4579-4589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–538. doi: 10.1517/14712598.1.3.525. [DOI] [PubMed] [Google Scholar]
- Grieder FB., and, Vogel SN. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257:106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- Fragkoudis R, Breakwell L, McKimmie C, Boyd A, Barry G, Kohl A.et al. (2007The type I interferon system protects mice from Semliki Forest virus by preventing widespread virus dissemination in extraneural tissues, but does not mediate the restricted replication of avirulent virus in central nervous system neurons J Gen Virol 88Pt 123373–3384. [DOI] [PubMed] [Google Scholar]
- Ryman KD, Klimstra WB, Nguyen KB, Biron CA., and, Johnston RE. α/β interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden G, Mohamed MR, Rahman MM., and, Bartee E. Cytokine determinants of viral tropism. Nat Rev Immunol. 2009;9:645–655. doi: 10.1038/nri2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J.et al. (2006Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses Proc Natl Acad Sci USA 10312873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D.et al. (1999Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses Nat Med 5881–887. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN.et al. (2000Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant J Virol 744765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM.et al. (2008Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus Clin Cancer Res 14259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC.et al. (2009Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide Clin Cancer Res 152777–2788. [DOI] [PubMed] [Google Scholar]
- Lun X, Chan J, Zhou H, Sun B, Kelly JJ, Stechishin OO.et al. (2010Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma Mol Ther 181927–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E.et al. (2010Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production Proc Natl Acad Sci USA 1071576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F.et al. (2000Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer Blood 952024–2030. [PubMed] [Google Scholar]
- van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S.et al. (2009Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth PLoS ONE 4e6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon C, Diaz RM, Wongthida P, Galivo F, Kottke T, Thompson J.et al. (2011Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intratumoral oncolytic virus and cyclophosphamide Mol Ther 19140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhani N, Gorman MP, Branson HM, Stazzone L, Banwell BL., and, Chitnis T. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72:2076–2082. doi: 10.1212/WNL.0b013e3181a8164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radis CD, Kahl LE, Baker GL, Wasko MC, Cash JM, Gallatin A.et al. (1995Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study Arthritis Rheum 381120–1127. [DOI] [PubMed] [Google Scholar]
- Binder GK., and, Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Scallan MF., and, Fazakerley JK. Aurothiolates enhance the replication of Semliki Forest virus in the CNS and the exocrine pancreas. J Neurovirol. 1999;5:392–400. doi: 10.3109/13550289909029480. [DOI] [PubMed] [Google Scholar]
- Erälinna JP, Röyttä M, Hukkanen V, Zinhu D, Salmi AA., and, Salonen R. Selective downregulation of Th1 response by Linomide reduces autoimmunity but increases susceptibility to viral infection in BALB/c and SJL mice. J Neuroimmunol. 1998;88:165–176. doi: 10.1016/s0165-5728(98)00115-5. [DOI] [PubMed] [Google Scholar]
- Sawicki DL., and, Sawicki SG. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980;34:108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki DL, Sawicki SG, Keränen S., and, Kääriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981;39:348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki SG, Sawicki DL, Kääriäinen L., and, Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981;115:161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Wollmann G, Robek MD., and, van den Pol AN. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J Virol. 2007;81:1479–1491. doi: 10.1128/JVI.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca JD., and, Pitha PM. Transcriptional and posttranscriptional regulation of exogenous human β interferon gene in simian cells defective in interferon synthesis. Mol Cell Biol. 1986;6:2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH.et al. (2008Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87 Cancer Lett 265124–134. [DOI] [PubMed] [Google Scholar]
- Patel SJ, King KR, Casali M., and, Yarmush ML. DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci USA. 2009;106:12867–12872. doi: 10.1073/pnas.0809292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Yi C, Luo X, Xu S, Yu Z, Tang Y.et al. (2011Glial gap junctional communication involvement in hippocampal damage after middle cerebral artery occlusion Ann Neurol 70121–132. [DOI] [PubMed] [Google Scholar]
- Bilello JP, Cable EE, Myers RL., and, Isom HC. Role of paracellular junction complexes in baculovirus-mediated gene transfer to nondividing rat hepatocytes. Gene Ther. 2003;10:733–749. doi: 10.1038/sj.gt.3301937. [DOI] [PubMed] [Google Scholar]
- Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M.et al. (2000Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo Am J Respir Cell Mol Biol 22129–138. [DOI] [PubMed] [Google Scholar]
- Esclatine A, Lemullois M, Servin AL, Quero AM., and, Geniteau-Legendre M. Human cytomegalovirus infects Caco-2 intestinal epithelial cells basolaterally regardless of the differentiation state. J Virol. 2000;74:513–517. doi: 10.1128/jvi.74.1.513-517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Davidson BL, Melchert P, Slepushkin VA, van Es HH, Bodner M.et al. (1998Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia J Virol 729818–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT., and, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A. Biological assays for interferons. J Immunol Methods. 2002;261:21–36. doi: 10.1016/s0022-1759(01)00570-1. [DOI] [PubMed] [Google Scholar]
- Huang PY, Guo JH., and, Hwang LH. Oncolytic sindbis virus targets tumors defective in the interferon response and induces significant bystander antitumor immunity in vivo. Mol Ther. 2012;20:298–305. doi: 10.1038/mt.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck CJ, Dickson PV, Ng CY, Zhou J, Hall MM, Gray JT.et al. (2006Antitumor efficacy of AAV-mediated systemic delivery of interferon-β Cancer Gene Ther 1399–106. [DOI] [PubMed] [Google Scholar]
- Qin XQ, Beckham C, Brown JL, Lukashev M., and, Barsoum J. Human and mouse IFN-β gene therapy exhibits different anti-tumor mechanisms in mouse models. Mol Ther. 2001;4:356–364. doi: 10.1006/mthe.2001.0464. [DOI] [PubMed] [Google Scholar]
- Alexenko AP, Ealy AD., and, Roberts RM. The cross-species antiviral activities of different IFN-tau subtypes on bovine, murine, and human cells: contradictory evidence for therapeutic potential. J Interferon Cytokine Res. 1999;19:1335–1341. doi: 10.1089/107999099312795. [DOI] [PubMed] [Google Scholar]
- Santagati MG, Itäranta PV, Koskimies PR, Määttä JA, Salmi AA., and, Hinkkanen AE. Multiple repeating motifs are found in the 3′-terminal non-translated region of Semliki Forest virus A7 variant genome. J Gen Virol. 1994;75 (Pt 6):1499–1504. doi: 10.1099/0022-1317-75-6-1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of VA7-EGFP infectivity in confluent GL261 but not in confluent Vero(B) or CT-2A cultures in vitro.
Anti-mIFNβ antibody does not enhance VA7-EGFP infectivity.
EGTA pretreatment slightly enhances VA7-EGFP infection in GL261 cells in vitro.