Abstract
Retained respiratory tract (RT) secretions, infection and exuberant inflammatory responses are core abnormalities in cystic fibrosis (CF) lung disease. Factors contributing to the destructive CF airway inflammatory processes remain incompletely characterized. The pro-oxidative inflammatory CF RT milieu is known to contain enzymatically and non-enzymatically produced regulatory lipid mediators, a panel of structurally defined oxidized metabolites of polyunsaturated fatty acids known to play a role in pathology related to inflammation. Using an extraction protocol that maximizes recoveries of sputum-spiked deuterated standards, coupled with an LC/MS/MS detection system, the present study presents a metabolomic method to assess a broad spectrum of regulatory lipid mediators in freshly obtained sputum from CF patients. A broad range of both pro-inflammatory and anti-inflammatory lipid mediators were detected including PGE2, PGD2, TXB2, LTB4, 6-trans-LTB4, 20-OH-LTB4, 20-COOH-LTB4, 20-HETE, 15-HETE, 11-HETE, 12-HETE, 8-HETE, 9-HETE, 5-HETE, EETs, diols, Resolvin E1, 15-deoxy-PGJ2 and LXA4. The vast majority of these oxylipins have not been reported previously in CF RT secretions. Whereas direct associations of individual proinflammatory lipid mediators with compromised lung function (FEV1) were observed, the relationships were not robust. However, multiple statistical analyses revealed that the regulatory lipid mediators profile taken in aggregate proved to have a stronger association with lung function in relatively stable outpatient adult CF patients. Our data reveal a relative paucity of the anti-inflammatory lipid mediator lipoxin A4 (LXA4) in CF sputum. Patients displaying detectable levels of the anti-inflammatory lipid mediator Resolvin E1 demonstrated a better lung function compared to those patients with undetectable levels. Our data suggest that comprehensive metabolomic profiling of regulatory lipid mediators in CF sputum should contribute to a better understanding of the molecular mechanisms underlying CF RT inflammatory pathobiology. Further studies are required to determine the extent to which nutritional or pharmacological interventions alter the regulatory lipid mediators profile of the CF RT and the impact of potential modulations of RT regulatory lipid mediators on the clinical progression of CF lung disease.
Keywords: Metabolomics, Regulatory Lipid Mediators, Cystic Fibrosis, Sputum, LC/MS/MS
Introduction1
Cystic fibrosis (CF) is caused by mutations in the gene encoding the CF trans-membrane conductance regulator (CFTR). Absent or defective CFTR results in abnormal salt transport and mucus hydration at epithelial surfaces where it is expressed. In the lung, respiratory tract (RT) secretions (mucus) are more viscoid, resulting in compromised mucociliary RT clearance, chronic RT infection, dysregulated and heightened inflammatory responses, and progressive lung tissue destruction [1, 2]. Therapeutic interventions include approaches designed to ameliorate the effects of mucus stasis by treatments designed to increase the hydration and removal of RT surface secretions and measures to combat the RT infection and aggressive inflammatory responses [1–8].
Inflammation represents a critical component of innate host responses to infection, and the neutrophil responses generated are critical in order to kill invading microbes via their generation of antimicrobial products. A hallmark of CF is the early development of persistent and progressively intense neutrophil and inflammatory mediator influxes into the airways [3, 6, 7, 9–12]. The heightened infected/inflammatory CF RT state with its inherent increase in the production of inflammatory mediators, including increased levels of proteolytic and oxidant-generating biomolecule species, are believed to be responsible for the eventual destruction of host RT tissues [6, 8, 13–15]. More recently, an even more expanded mis-orchestration of the immune system in CF has emerged [16]. The importance of inflammatory processes is further buttressed by the fact that most known modifiers of the CF gene phenotype that have been reported to date involve inflammatory-immune system genes [17–19] and that genomic responses to RT CFTR pertubations are associated with genes regulating inflammation [20]. This is further illustrated by the fact that activation of inflammatory signals, in the apparent absence of any microbes or exogenous stimuli, can damage and destroy RT tissues [21]. Several studies have suggested that once initiated, CF RT inflammatory responses are not well controlled [7, 11–13, 20–22]. Thus, anti-inflammatory therapies with agents such as steroids and ibuprofen, an inhibitor of arachidonic acid oxygenation pathways, and even inhibitors of NF-κB, a master regulator of inflammation, have been shown to be potentially beneficial, although many have significant side effects [3, 5, 7, 23–28]. More recently, evidence has been presented linking prostaglandin-endoperoxide synthase genes (COX-1 and COX-2) as novel modifiers of disease severity in CF patients [29].
Oxidative lipid products including, notably, the products of unsaturated lipids, are increasingly being recognized to be important contributors to chronic inflammatory diseases [24, 30–34]. Although most RT therapeutics have been focused on the main routes of arachidonic acid cyclooxygenase and lipoxygenase pathways [5, 11, 27–29, 35], their P450 and non-enzymatic oxidative products, and oxidative products of the other unsaturated lipids (e.g. 20:5 and 22:6), are also responsible for the generation of lipids capable of modifying the intensity and duration of inflammatory processes [24, 31, 33, 36, 37], including those of the plant world [38]. Recent advances have facilitated more detailed investigation of profiles of lipid products in both plasma and at inflammatory sites [31, 36, 39–43]. Further characterization of these profiles is expected to lead to new anti-inflammatory therapeutic approaches directed toward this important class of inflammatory mediators.
CF is known to be associated with abnormalities of lipid absorption and plasma lipid constituents [2, 42, 45–50]. These abnormalities are primarily secondary to dysfunctional pancreatic and hepatobiliary secretory defects [2]. CF-related lipid abnormalities are also influenced by active inflammatory processes, a measure of oxidative stress (especially in the RT) and more recently described to be influenced by aberrant CFTR modulation of lipid metabolism [51–55]. Thus far studies of amino acid and proteomic profiles of CF [55] and CF RT secretions have been detailed [56–59], but there have been few studies detailing lipidomic profiles of these secretions which bathe the RT airway surface cells. Given the non-invasive nature of collecting sputum specimens from CF patients and the advent of sophisticated analytical technology for metabolomic analyses [31, 36, 38–41, 60], we reasoned that profiling regulatory lipid mediators (also named as ‘oxylipins’ to keep the term short in rest of the paper) in CF sputum would provide a novel means of assessing the possible contributing role of the spectrum of regulatory lipid mediators to inflammatory processes in the RT of CF patients.
In the present study, we have developed and refined an efficient extraction method for sputum oxylipins, and characterized the oxylipin profile of freshly obtained sputum from patients attending an adult CF outpatient clinic. We also provide an overview of the substrates and enzymatic pathways involved in generating the identified oxylipins. Finally, we have attempted to provide preliminary correlations between oxylipin profiles and lung function using composite statistical approaches.
Experimental Procedures
Patient Demographics and Sputum Collection
Spontaneously expectorated sputum was obtained from 16 patients (10 male, 6 female; age 34 ± 16, range 20–69) attending the University of California, Davis Adult CF Clinic. Fourteen of the patients carried the del F508mutation (6 were homozygote for del F508), and all had positive sweat chloride tests. The majority of the patients had CF lung disease of moderate severity as assessed by pulmonary function tests (FEV-1 as percent of predicted; 53 ± 24% S.D., range 27–106%) [61]. Most patients were receiving standard of care CF therapy [62] including antibiotics, aggressive chest physiotherapy including aerosolized bronchodilators, pancreatic enzymes and vitamin supplements (especially the lipophilic vitamins A, D, E and K). Eight of the patients were diabetic and none were taking oral corticosteroids. Nine used inhaled preparations that included corticosteroids, several of which were confined to nasal forms alone. As with most CF patient populations, most had low BMIs (21.8 ± 2.8 S.D., range 16–29). All could produce sputum spontaneously and 13 grew predominantly P. aeruginosa from their sputa. One of the patients was studied twice over a period of approximately one month.
The current study was not powered to, nor intended to relate all of the clinical and therapeutic variables that could potentially affect sputum oxylipin profiles. FEV-1, as a percent of predicted, was used as a marker of overall CF lung disease severity. Our primary aim was to characterize and quantify the spectrum of oxylipins in the RT of a representative sample of outpatient adult with CF. Sputum samples were coded anonymously and transported on dry ice to the laboratory for analyses. The study protocol was approved by the UC Davis Institutional Review Board.
All the sputa were collected directly into a container that already had butylated hydroxytoluene (BHT) and triphenylphosphine (TPP) and a broad spectrum COX inhibitor (indomethacin). After the sputum was obtained directly into the container, the specimen was immediately frozen on dry ice. All the samples were then transported on dry ice from the clinic to the lab within 2–6 hours and then immediately stored at −80 °C until analyzed.
Sputum Processing
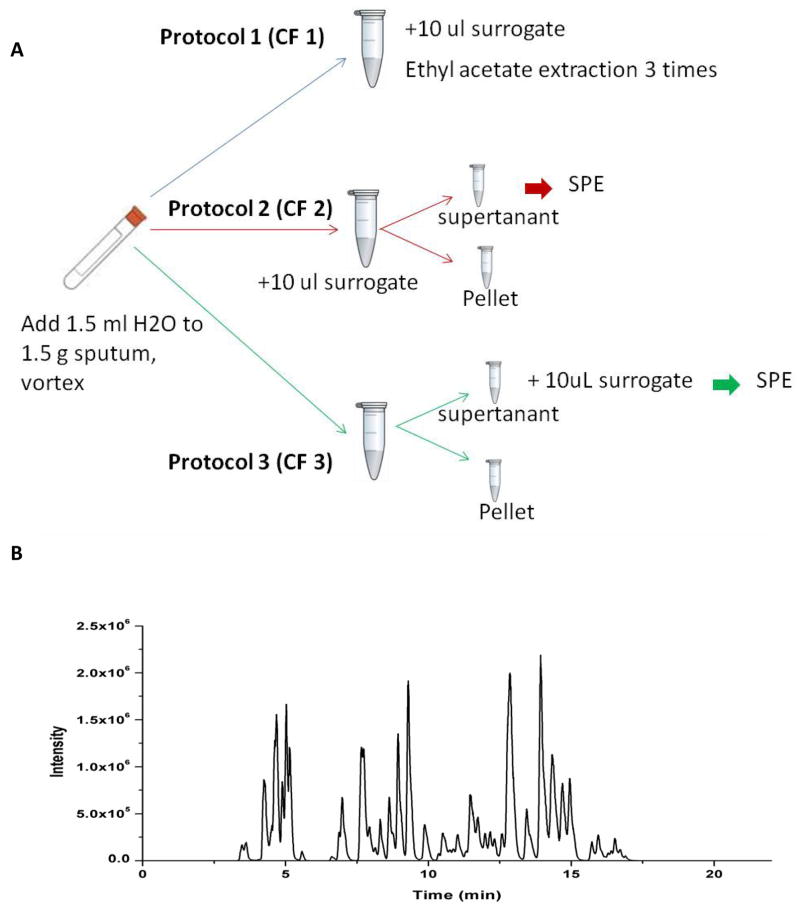
Distilled water was added to the sputum according to the sputum weight (1 ml/g sputum) and vortexed for 10 min to homogenize the sputum. The sputum was then processed by three different extraction protocols (Figure 1A) as indicated below:
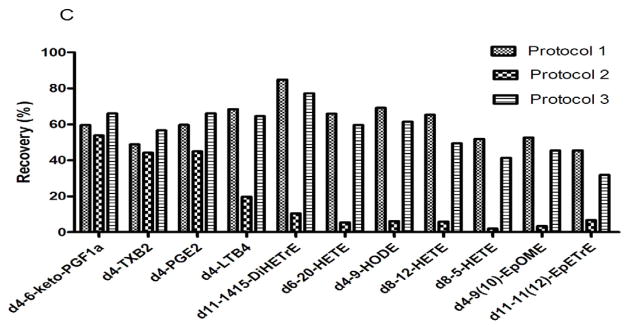
Figure 1. Schematic illustrations of extraction protocols, mass spectrometric analysis and extraction recoveries in CF sputum samples.
(A) Schematic for the extraction protocols. Protocol CF1 is liquid-liquid extraction (LLE); Protocol CF2 and CF3 is the SPE extraction protocols. To assess recoveries, the surrogate solutions (included 10 deuterated internal standards) were added to the solution before or after centrifugation respectively. (B) Representative LC/MS/MS total ion chromatogram of the profile of oxylipins in CF sputum. (C) The average recovery rate comparison among three extraction protocols. The recovery rates of the Liquid-Liquid Extraction LLE) are from 48–85%.
Protocol 1: Liquid-Liquid Extraction (LLE; CF 1)
The deuterated surrogate solutions (including 500 nmol/L d4-6-keto-PGF1α, d4-TXB2, d4-PGE2, d4-LTB4, d11-14,15-DiHETrE, d6-20-HETE, d4-9-HODE, d8-12-HETE, d8-5-HETE, d4-9(10)-EpOME, d11-11(12)-EpETrE) were added directly to sputa. After vigorous vortexing for 10 min, the mixture was extracted three times with ethyl acetate to get optimal extraction recovery. Extracts from each fraction were combined and evaporated to dryness using a SpeedVac system. The residue from each fraction was then reconstituted with 50 uL of methanol containing 200 nM 1-cyclohexyl ureido 3-dodecanoic acid (CUDA) as an internal standard. This protocol was used to determine the extraction efficiency of LLE.
Protocol 2: Solid Phase Extraction (SPE) after surrogate solution addition (CF 2)
Surrogate solution (30 uL) was added directly to sputum. The sputum sample was then centrifuged at 13,200 rpm for 10 min at 4 °C. The soluble supernatant fraction was loaded onto pretreated 60 mg Oasis-HLB cartridges (Waters Corporation, Milford, MA) according to methods previously described [60]. The SPE cartridges were then eluted with firstly 0.5 mL methanol and then 1.5 mL ethyl acetate. The eluents were evaporated to dryness using a SpeedVac system and reconstituted with 50 uL of 200 nM 1-cyclohexyl ureido-3-dodecanoic acid (CUDA) methanol solution. This protocol not only provides quantitation based upon the internal standard, but also assesses extraction efficiency. This protocol provides the extraction efficiency of the whole protocol including SPE step.
Protocol 3: SPE before surrogate solutions addition (CF 3)
This sample processing protocol was essentially the same as Protocol CF2, however, 10 uL of surrogate solutions were added after all extractions were performed. This protocol provides extraction efficiency just for the SPE step.
Oxylipin Profiling by LC/MS/MS
The liquid chromatography system used for analysis was an Agilent 1200 SL liquid chromatography series (Agilent Corporation, Palo Alto, CA). The autosampler was kept at 4 °C. Liquid chromatography was performed on an Eclipse Plus C18 2.1 × 150 mm, 1.8 μm column (Agilent Corporation, Palo Alto, CA). Mobile phase A was water with 0.1% glacial acetic acid. Mobile phase B consisted of acetonitrile/methanol (84:16) with 0.1% glacial acetic acid. Gradient elution was performed at a flow rate of 250 μL/min. Chromatography was optimized to separate all analytes in 21.5 min. Analytes are then eluted according to their polarity with the most polar analytes, prostaglandins and leukotrienes, eluting first, followed by the hydroxy and epoxy fatty acids.
The column was connected to a 4000 QTrap tandem mass spectrometer (Applied Biosystems Instrument Corporation, Foster City, CA) equipped with an electrospray source (Turbo V). The instrument was operated in negative multiple reaction monitor (MRM) mode. HPLC and LC/MS/MS protocols were essentially as previously described [60].
Quality control samples are analyzed at a minimum frequency of 10 hrs to ensure stability of the analytical calibration throughout a given analysis. Analyst software 1.5.1 was used to quantify oxylipins according to standard curves.
Statistical Approaches
A correlation analysis was employed to explore the relationship between the oxylipins and lung function (FEV1, % of predicted). Prism 4.0 (GraphPad Software Inc.,) was used to perform non-parametric (Spearman) correlation analysis. Partial Least Squares (PLS) analysis was used as the classification method for modeling the sputum oxylipin profiles. Multivariate analysis was performed using the SIMCA-P 11.5 version (Umetrics AB, Umeå, Sweden). As a powerful multivariate analysis method, PLS can be seen as a particular regression technique for modeling the association between the factors (oxylipin concentrations) of the observations (CF patients) and the responses (lung function) of the observations.
Results
Oxylipin Extraction Efficiency
To assess the recovery efficiency of the various extraction methods utilized, a surrogate solution, which includes 11 deuterated internal standards (d4-6 keto PGF1α, d4-TXB2, d4-PGE2, d4-LTB4, d11-14,15 DiHETrE, d6-20 HETE, d4-9-HODE, d8-12 HETE, d8-5 HETE, d4-9(10) EpOME, d11-11(12) EpETrE), was added to the samples at different steps during the extraction procedures. Figure 1A illustrates the three extraction protocols utilized and the stages at which the surrogate solutions were added. Extraction protocol 1 (CF 1) used liquid-liquid extraction (LLE) whereas extraction protocols 2 and 3 (CF2 and CF3) used solid phase extraction (SPE). The only difference between CF2 and CF3 is that the recovery rate of CF3 only reflects the recovery rate of the SPE, the recovery rate of CF2 actually reflects the real recovery of the whole extraction process. Figure 1B illustrates an exemplary LC/MS/MS total ion chromatogram (TIC) of oxylipins included in our profiling method.
A high recovery rate using the CF 3 protocol is expected. CF3 reflects the recovery rate of the SPE process of the relative clean matrix-supernatant. The recovery rates of the whole SPE extraction process, the one corresponding to CF 2, were much less than the ones from CF3. Extraction recovery for each of the three methods are illustrated in Figure 1B. In addition, recovery rates of the deuterated internal standards using CF 2 decreased according to their polarity. The most polar analyte (d4-6 keto PGF1α) has a comparable recovery rate with CF 3 protocol. The most non-polar analyte (d11-11(12) EpETrE) was barely recovered from sputum samples in the CF3 protocol. This suggests that the distribution of these oxylipins is related to their polarity. Given the more lipophilic properties of the insoluble pellet fraction, the oxylipins could be absorbed on some lipid, protein or DNA fragments. This indicates that the oxylipins predominantly resided in the pellet fraction.
The recovery rates using the CF1 protocol, liquid-liquid (LLE) extraction of the entire CF sputum sample, showed the most promising results. Most of the deuterated standards have 46–82% recovery rates. Compared to the ~20% recovery rates reported in the literature [63, 64], the recovery rate of our current extraction protocol is significantly improved. The reason for the better recovery rates from CF1 might be that the CF1 extraction protocol used whole sputum (both supernatant and pellet parts) for the extraction. Therefore, the LLE protocol (CF1) was utilized in the processing and analysis of all further CF sputum samples in our study.
Oxylipin Profiles in Adult CF Sputum
Table 1 shows the concentrations of oxylipins in 17 distinct patient CF sputum samples using the LLE protocol and LC/MS/MS quantitation. Of the 88 oxylipins that are included in our metabolomic profiling method, 31 oxylipins were detectable in adult CF sputum. Only 9 of them (PGE2, PGF1, PGF2α, TXB2, 6-oxo PGF1, 8-iso-PGF2α, cys-LTs) have been reported in previous studies [65–67]. To the best of our knowledge, this is the most comprehensive oxylipin profile described in CF RT secretions to date.
Table 1.
The measured oxylipin baseline in CF sputa samples a.
| Sample Name | TXB2 | PGF2a | PGE2 | 11-HETE | PGE1 | 20-COOH-LTB4 | 20-OH-LTB4 | LTB4 | 6-trans-LTB4 | 5-oxo-ETE | 5-HEPE | LTB5 | 12-HETE | 12-oxo-ETE | 12-HEPE | 9-HODE | 9-oxo-ODE | 8-HETE | 15-oxo-ETE | 15(S)-HETrE | 13-HODE | 20-HETE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||
| Pathway
|
COX
|
5-LOX
|
12-LOX
|
15-LOX
|
CYP4A/4F
|
|||||||||||||||||
| Substrates
|
AA
|
DGLA
|
AA
|
EPA
|
AA
|
EPA
|
LA
|
AA
|
DGLA
|
LA
|
AA
|
|||||||||||
| 1 | 342 | 535 | 601 | 168 | 79.1 | 3760 | 1380 | 319 | 236 | 7480 | 239 | 99.8 | 371 | 837 | 21.7 | 941 | 305 | 92.6 | 49.5 | 220 | 820 | 488 |
| 2 | 88.5 | 188 | 103 | 11.7 | 30.9 | 157 | 103 | 87.3 | 31.0 | 153 | 12.6 | 0.00 | 3070 | 3520 | 209 | 262 | 82.6 | 40.5 | 76.8 | 200 | 912 | 31.8 |
| 3 | 155 | 69.6 | 116 | 84.2 | 29.7 | 560 | 252 | 253 | 193 | 338 | 24.4 | 0.00 | 8580 | 11100 | 287 | 3260 | 3900 | 258 | 739 | 1010 | 6700 | 185 |
| 4 | 1340 | 410 | 1060 | 187 | 70.5 | 9740 | 1500 | 2630 | 238 | 3440 | 107 | 23.0 | 10800 | 8430 | 479 | 770 | 350 | 108 | 274 | 448 | 2730 | 195 |
| 5 | 11800 | 344 | 750 | 207 | 60.6 | 1240 | 293 | 201 | 95.8 | 747 | 22.0 | N.D. | 7010 | 5090 | 141 | 1030 | 293 | 278 | 507 | 667 | 2320 | 2250 |
| 6 | 864 | 524 | 745 | 829 | 180 | 2910 | 733 | 979 | 2730 | 57.3 | 267 | N.D. | 174000 | 47900 | 4700 | 3070 | 2340 | 1160 | 419 | 944 | 14400 | 657 |
| 7 | 1080 | 321 | 630 | 208 | 43.3 | 899 | 236 | 274 | 53.3 | 71.3 | N.D. | N.D. | 10200 | 4450 | 590 | 969 | 357 | 163 | 296 | 227 | 2780 | 531 |
| 8 | 691 | 372 | 1220 | 128 | 120 | 726 | 95.2 | 170 | 89.1 | 553 | 20.1 | N.D. | 10100 | 4980 | 314 | 557 | 201 | 41.7 | 111 | 224 | 1420 | 411 |
| 9 | 145 | 202 | 191 | 95.3 | 29.6 | 3500 | 396 | 1610 | 63.9 | 4240 | 119 | 41.1 | 1280 | 924 | 115 | 746 | 225 | 110 | 97.7 | 181 | 2490 | 344 |
| 10 | 219 | 32.9 | 254 | 13.0 | 8.35 | 1440 | 416 | 644 | 47.1 | 1630 | 27.1 | N.D. | 3110 | 4400 | 168 | 196 | 165 | 21.3 | 130 | 127 | 397 | 281 |
| 11 | 6840 | 152 | 228 | 22.4 | 24.9 | 1490 | 156 | 118 | 165 | 1130 | 0.00 | N.D. | 15600 | 20600 | 375 | 196 | 36.2 | 85.5 | 69.6 | 96.8 | 786 | 471 |
| 12 | 2330 | 32.9 | 85.3 | N.D. | 9.07 | 3940 | 514 | 788 | 152 | 1280 | 76.9 | 12.1 | 5510 | 4800 | 329 | 788 | 212 | 54.4 | 56.4 | 141 | 4460 | N.D. |
| 13 | 562 | 418 | 536 | 131 | 130 | 5690 | 737 | 5340 | 1370 | 23500 | 930 | 108 | 8540 | 7010 | 282 | 807 | 428 | 219 | 370 | 993 | 2380 | 335 |
| 14 | 629 | 786 | 561 | 316 | 49.8 | 4240 | 2240 | 2810 | 226 | 3990 | 259 | 70.7 | 852 | 836 | 23.1 | 766 | 403 | 269 | 259 | 420 | 1850 | 667 |
| 15 | 619 | 154 | 390 | 82.2 | 26.6 | 3080 | 2050 | 1590 | 70.9 | 2870 | 46.5 | 15.9 | 1820 | 4020 | 15.4 | 390 | 164 | 61.6 | 215 | 154 | 1520 | 51.6 |
| 16 | 101 | 133 | 67.3 | 817 | N.D. | 7.42 | 48.0 | 280 | 49.6 | 2300 | 128 | N.D. | 1310 | 777 | 115 | 2700 | 328 | 1210 | 670 | 3360 | 10700 | 845 |
| 17 | 1230 | 699 | 935 | 298 | 67.0 | 13700 | 3230 | 6750 | 574 | 13700 | 417 | 156 | 3860 | 3720 | 174 | 1790 | 572 | 353 | 406 | 799 | 5490 | 657 |
|
| ||||||||||||||||||||||
| Mean | 1710 | 316 | 498 | 212 | 56.4 | 3360 | 1240 | 1630 | 376 | 3970 | 159 | 31.0 | 15600 | 7850 | 490 | 1130 | 610 | 266 | 279 | 601 | 3650 | 494 |
| SD | 3050 | 226 | 361 | 249 | 48.4 | 3610 | 1830 | 1960 | 686 | 6090 | 232 | 48.2 | 41000 | 11400 | 1100 | 978 | 994 | 359 | 214 | 783 | 3800 | 514 |
| Patient # | CYP 2C/2J | CYP2C/2J+sEH | Multi-enzymes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA
|
LA
|
EPA
|
LA
|
LA
|
EPA
|
|||||
| 8(9)-EpETrE | 12(13)-EpOME | 9(10)-EpOME | 5,6-DiHETE | 12,13-DiHOME | 9,10-DiHOME | 9,12,13-TriHOME | 9,10,13-TriHOME | LXA4 | Resolvin E1 | |
|
|
|
|
|
|
|
|
||||
| 1 | 21.4 | 54.4 | 61.6 | 275 | N.D. | N.D. | N.D. | N.D. | N.D. | 522 |
| 2 | 69.8 | 49.1 | 47.2 | 4.09 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3 | 309 | 184 | 81.0 | 80.6 | 54.9 | 24.4 | 445 | 103 | N.D. | N.D. |
| 4 | 38.9 | 60.0 | 16.7 | 97.5 | 19.3 | 29.2 | 472 | 48.3 | N.D. | 758 |
| 5 | 259 | 68.3 | 61.0 | 27.8 | 379 | 299 | 11400 | 622 | N.D. | 48.6 |
| 6 | 4090 | 474 | 222 | 1650 | 76.4 | 37.2 | 3260 | 150 | N.D. | 49.5 |
| 7 | 66.3 | 69.5 | 107 | 12.1 | 59.0 | 27.7 | 1830 | 215 | N.D. | 280 |
| 8 | 84.5 | N.D. | 19.9 | 27.3 | 579 | 706 | 19700 | 711 | N.D. | N.D. |
| 9 | 11.3 | 58.3 | 39.7 | 80.9 | 78.7 | 34.7 | 1370 | 162 | N.D. | N.D. |
| 10 | 99.9 | 8.27 | N.D. | 39.0 | 86.8 | 7.21 | 867 | 95.2 | N.D. | N.D. |
| 11 | 163 | 8.09 | N.D. | 33.7 | 68.9 | 66.1 | 2920 | 379 | N.D. | 106 |
| 12 | 224 | 47.2 | N.D. | 196 | 29.7 | 9.55 | 784 | 37.7 | N.D. | N.D. |
| 13 | 77.9 | 36.8 | N.D. | 1560 | 67.3 | N.D. | 391 | 57.3 | N.D. | 173 |
| 14 | 30.3 | N.D. | 61.0 | 234 | 74.2 | 43.0 | 4200 | 113 | N.D. | N.D. |
| 15 | 22.4 | 99.0 | 41.4 | 278 | 12.2 | N.D. | 435 | 149 | N.D. | 67.0 |
| 16 | 6.66 | 34.3 | 42.3 | 135 | 8.68 | 2.47 | 499 | 97.0 | N.D. | N.D. |
| 17 | 251 | 0.79 | 85.1 | 412 | 77.8 | 70.8 | 2340 | 209 | N.D. | 1040 |
|
| ||||||||||
| Mean | 343 | 73.6 | 52.1 | 303 | 52.1 | 35.7 | 3540 | 332 | N.D. | 338 |
| SD | 970 | 113 | 54.7 | 504 | 415 | 308 | 7770 | 379 | N.D. | 358 |
All the concentrations are in pmol/g.
N.D. Not detected.
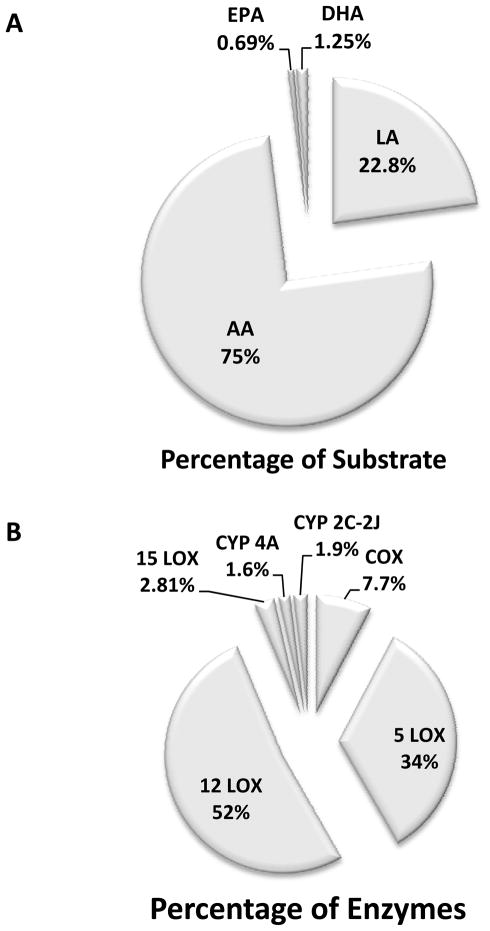
Figure 2A shows the composition of the oxylipins according to the parent fatty acid substrates. By far, the most prominent oxylipins (~75%) are derived from arachidonic acid (C20:4; AA). The arachidonic acid metabolites are typically referred to as eicosanoids, which includes many biologically important lipid mediators, such as prostaglandins and leukotrienes. The second largest fatty acid parent molecule is linoleic acid (18:2; LA). EPA and DHA represent relatively small fractions of the oxylipins detected in the current study. Figure 2B illustrates the breakdown of enzymes involved in the synthesis of the oxylipins that we have quantified in CF sputum. The largest source of oxylipins are derived from the 12-lipooxygenase (12-LOX) pathway (~52%), which produces mainly 12-HETE and 8-HETE, and 12-oxo-ETE. The second and the third most prevalent enzymes involved in the synthesis of oxylipins in CF sputum are 5-lipoxygenase (5-LOX) and cyclooxygenase (COX) pathways. The major products of 5-LOX pathways are the leukotrienes, which are potent chemokines. The products of the COX pathway include several prostaglandins and thromboxane. Many of these oxylipins are inflammatory mediators that induce inflammatory cell infiltration, although the full biological activities of many of them remain to be fully characterized, both alone and in combination.
Figure 2. Distribution of fatty acid precursors and synthetic enzymes of oxylipins profiled in sputum samples from CF patients.
(A) Distribution of oxylipins derived from parent fatty acids in CF sputum. C18:2, Linoleic acid, LA; C20:4, Arachidonic acid, AA; C20:5, Eicosapentaenoic acid, EPA; C22:6, docosahexaenoic acid, DHA. (B) Distribution of enzymes responsible for oxylipins detected in CF sputum. COX, cyclooxygenase; LOX, lipoxygenase; CYP, cytochrome P450.
Oxylipin profiles and pulmonary function
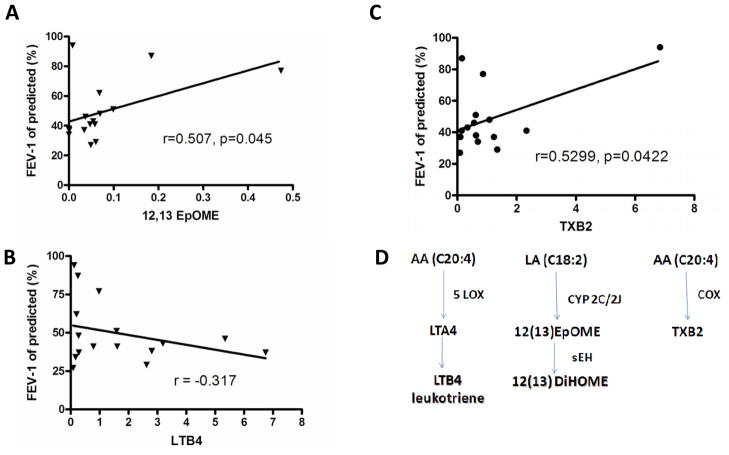
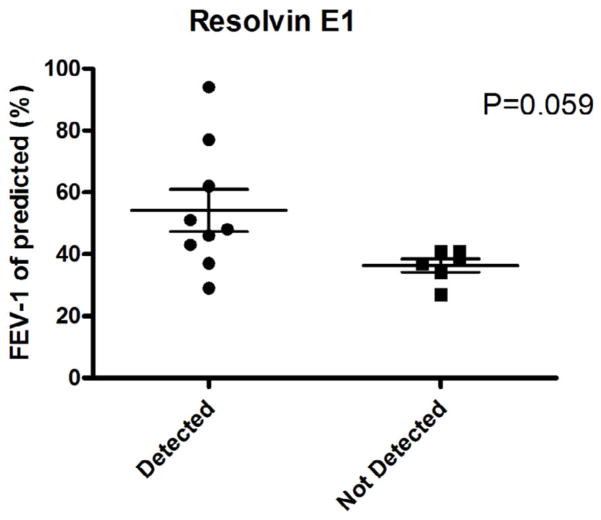
Given that our method allowed for the precise quantitation of oxylipins in adult sputum, we next examined the relationships among oxylipin levels and lung function (FEV-1; forced expiratory volume in one second, expressed as % predicted). Spearman correlation analysis was used to correlate exemplary oxylipins with lung function (FEV-1, % predicted). Figure 3 illustrates three representative examples. One of the epoxides of linoleic acid, 12(13)-EpOME, was weakly positively correlated to FEV-1 (% of predicted; r=0.507, p<0.05). 12(13)-EpOME has been regarded as a potentially toxic compound (e.g., leukotoxin), however, previous studies [68] indicate that the toxic effects are actually attributed to its hydrolysis product, the linoleic acid diol [12(13)-DiHOME]. Indeed, the epoxide of linoleic acid may actually have anti-inflammatory effects, and high levels of its diol product are clearly proinflammatory. In fact, a correlation between the EpOME/DiHOME ratio and FEV-1 % predicted was observed (data not shown). This implies that DiHOME might also have adverse effects on lung function [68]. A slight negative correlation between FEV-1 and the chemokine, LTB4, is shown in Figure 3B. Additionally, Figure 3C indicates a minor positive correlation between thromboxane B2 (TXB2) and FEV-1 (r = 0.523; p = 0.042). A summary of the synthetic pathways representative of LTB4, 12(13)-DiHOME, and TXB2 are illustrated in Figure 3D, and provide examples of 5-LOX, cytochrome P-450 (Cyp 2C/2J) and COX, respectively. As shown in Figure 4, we observed that CF patients with detectable levels of the anti-inflammatory oxylipin, Resolvin E1, displayed better lung function than those that did not have detectable levels of this oxylipin (p = 0.059).
Figure 3. Positive and negative correlations between lung function (FEV-1 % of predicted) and exemplary oxylipin concentration in CF sputum.
(A) Positive correlation between 12,13-EpOME and FEV-1. (B) Negative correlation between LTB4 and FEV-1. (C) Positive correlation between TXB2 and FEV-1. (D) Enzymatic synthetic pathways corresponding to 12,13-EpOME, LTB4 and TXB2.
Figure 4. Detection of the anti-inflammatory lipid mediator, Resolvin E1, in sputum is associated with better lung function in CF patients.
Patients were divided into two groups: Those with detectable Resolvin E1, and those who had no detectable levels. FEV-1 values were then plotted for the two separate groups. p = 0.059. The detection of limit for Resolvin E1 is 2.4 pmol/g.
Multivariate metabolomics analysis
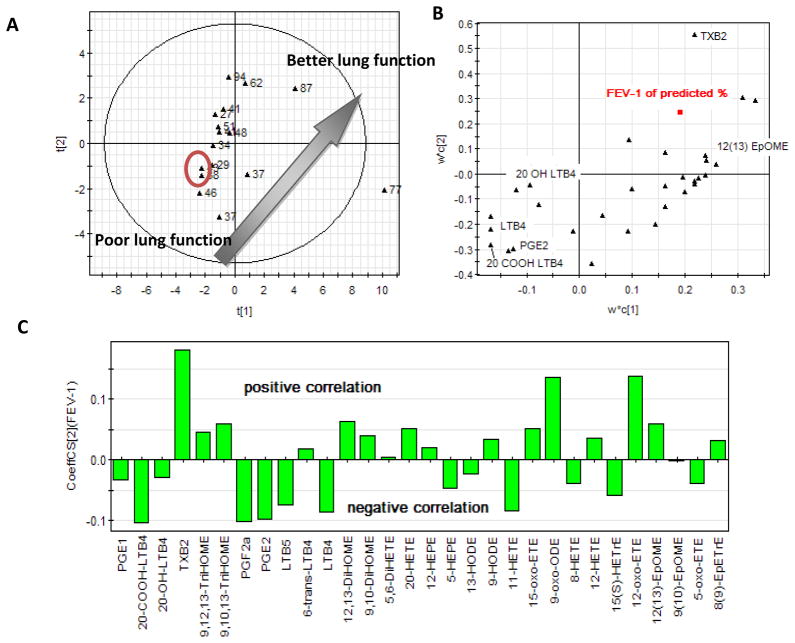
As shown in the previous sections, there are at best only weak correlations between individual oxylipins and lung function. As such, we next aimed to define whether oxylipin levels in aggregate had predictive value. To define this, we employed multivariate analysis.3 The score plot (Figure 5A) and loading plot (Figure 5B) of partial least squares (PLS) analysis based on the sputum oxylipin profiles are illustrated. The PLS technique is a good way to present the multivariate data because it can reduce the redundancy of the covariance existing in the dataset. In the score plot, each point represents a single sputum sample. The distance between points reflects the similarity of the profiles considering all of the detectable oxylipins in aggregate. From the score plot shown in Figure 5A, a clear trend can be appreciated in that the lower left points have the lowest lung function and the upper right data points have the best lung function. This suggests that the oxylipin profiles in aggregate presented by PLS score plot can reflect lung function. The two points labeled in the circle in Figure 5A are the two sputum samples acquired in the same patient at clinic visits a month apart. They are the closet points in the figure and provide some indication of the possible reproducibility of the technique temporally in the same patient. It implies the point position actually has more information about the patients themselves and which might take into account such factors as diet and magnitude of airway inflammatory processes. The loading plot shown in Figure 5B reflects correlations between the specific oxylipins and lung function. Each point in this plot corresponds to the specific oxylipins detected in the total number of patients. The closer the datum point lies to FEV-1 (% of predicted), the higher the correlation of the oxylipin to FEV-1. From the plot, it can be appreciated as examples that leukotrienes (LTB4s) are found to negatively correlate to lung function and 12(13)-EpOME is positively correlated to lung function. Figure 5C showed clearly the relationships between these oxylipins and the lung function. TXB2 correlates with lung function in a highly positive manner, while LTB4 and its metabolites negatively correlated with lung function. Moreover, PGE2 also negatively correlates with lung function.
Figure 5. Based on the cystic fibrosis patients’ sputa oxylipin profiles, the score plot (A) of the PLS (Partial Least Squares) analysis shows the clear trends which matching the lung function-FEV-1 % of predicted; the loading plot (B) of the PLS analysis indicates the important metabolites responding to the aggregation; the coefficient plot (C) of PLS indicates the positive/negative relationships between the analytes and the lung function-FEV-1 % of predicted.
As a powerful multivariate analysis method, PLS can be seen as a particular regression technique for modeling the association between the factors (oxylipins’ concentrations) of the observations (cystic fibrosis patients here) and the responses (lung function) of the observations. (A) The score plot. Each point is corresponding to each patients’ patient sample. The label is the value of the FEV-1 of predicted %. (B) The loading plot. Each point in the plot is corresponding to the variable (oxylipins or the FEV-1 of predicted %). The closer distance means the similar contribution to the score plot distribution. (C) The coefficiency plot summarizes the relationship between the analytes and the lung function. These are directly analogous to, but not identical to, coefficients obtained from multiple regression.
Discussion
The present study provides a comprehensive characterization and quantitation of RT oxylipins in adult CF patients. The LC/MS/MS method utilized in this study enabled the detection of 31 diverse oxylipins in CF sputa, which is three times more than the total detected oxylipins numbers from cumulative data from multiple papers in the literature. The representative adult study group showed a wide diversity of bioactive oxylipins, as would be expected due to the intense bacteria-infested, neutrophil-infiltrated and intensely inflamed adult CF RT. As has been shown for other biomarkers of CF RT inflammation [12, 69], the magnitude of individual pro-inflammatory bioactive lipids generally display a positive relationship with decrements in pulmonary function (Figure 3), but that is not always so clear. Interestingly, some oxylipins, such as Resolvin E1, show a positive relationship with better lung function (Figure 5). The novelty of our current metabolomic assessment of the oxylipin profile in CF sputum is that this approach allows for the use of aggregate data to predict lung function. This appears to be superior to assessing lung function/inflammatory state using individual oxylipin metabolites. The current study is discussed from a perspective technological consideration, the balance between pro-inflammatory and anti-inflammatory oxylipins, the role that bacteria (especially P. aeruginosa) may play in oxylipin formation and metabolism, clinical relevance and study limitations, and finally, conclusions.
Technical Considerations
The oxylipin profiling method reported here is robust, accurate, quantitative and rapid; all features that allow for rapid transition as a tool to assess the role that oxylipins contribute to overall RT inflammatory processes in patients with CF (and other lung diseases in which airway secretions can be collected). Additional analytes can be added to the basic method as standards become available and knowledge of the role of lipid mediators in pulmonary function expands. A meticulous assessment of extraction recoveries of the oxylipins in our protocol, as assessed using a diverse array of deuterated standards, provides confidence in quantitative metabolomic profiling of the measured oxylipins. The current profile of oxylipins detectable in our analytical method exceeds 88. Of these 88, 31 were detected in sputum from adult CF patients. Coupled with multivariate statistical analyses, the current metabolomics method provides a more accurate and comprehensive approach that better assesses lung inflammation and function, compared to using a single biomarker. The oxylipin profiles presented herein by multivariate data analysis method (PLS in current study) were able to reflect lung function very well and differentiate individual patient status (Figure 5). Whereas our current results suggest the capacity of oxylipin profiling in sputum to assess CF lung health, future studies with a larger, more diverse and detailed patient cohort will be necessary to further validate the overall translational value of this approach for the CF patient.
Table 2 lists the values of selected oxylipins reported by other investigators [65–67]. These investigators used RIA and ELISA methodologies for their determination. As shown in Table 2, it is readily apparent that the values from our current study are 10–100 times lower than the values obtained from using RIA and ELISA. Several possible reasons could contribute to the disparate results of the current study compared to the previous reports. For one, the sample groups are very different. The age ranges from the previous studies are from pediatric populations while our current study interrogated an older adult cohort with an undoubtedly more advanced disease and a more chronic and intense inflammatory RT milieu. Another possible reason comes from the nature of these three different measurement methods. Both RIA and ELISA methods are based on the recognition of the metabolites by antibodies. Therefore, the resulting concentrations may represent an overestimation due to detection of multiple highly similar metabolites. For example, the LTB4 concentration quantified by ELISA could be account for LTB4 and its bioactive metabolites such as 20-OH LTB4 and 20-COOH LTB4. The advantage of the present LC/MS/MS methodology used in the current study is that it is more highly selective.
Table 2.
Comparison of our oxylipin profiling method with that quantified by RIA and ELISA methods*
All values reported as nmol/g
Our method
Possible microbiota contribution to RT oxylipin profile
We and others have made the presumption that oxylipins detected in CF sputum arise from the host cells and tissues/lipid substrates. The presence of large numbers of bacteria (particularly P. aeruginosa) begs the question as to the possible involvement of microorganisms in the synthesis and metabolism of the analyzed RT oxylipins. Previous studies have revealed that P. aeruginosa harbor primarily shorter chain and mostly saturated fatty acids [70], thus they are not a source of the parent fatty acids (ie. AA, LA, EPA, DHA) of the oxylipins we have detected in the current study. However, recent studies have interestingly demonstrated that P. aeruginosa express a secreted cytotoxin (ExoU) with enzymatic phospholipase activity that is capable of liberating free unsaturated fatty acids (LA and AA) from host cells [71–74], including neutrophils [75] that are hightly abundant in the CF airway. Moreover, P. aeruginosa express a number of fatty acid metabolizing enzymes including dioxygenases, hydroperoxide isomerases, and arachidonate 15-lipoxygenase (LoxA; contribution to the formation of 15-HETE) [76, 77] that may directly contribute to the oxygenation of fatty acids in the CF airway [76, 78] and contribute to the virulence and airway persistence of the organism [79]. One report has revealed that P. aeruginosa may be a source of secreted inhibitors of 5-lipoxygenase [80]. Moreover, a recent study has identified an epoxide hydrolase produced by P. aeruginosa [81] that could potentially metabolize epoxide-based oxylipins to the corresponding diols which compromise the functions of these anti-inflammatory oxylipins in the CF RT. Therefore, it is reasonable to presume that P. aeruginosa may use its own molecular machinery to modulate the profiles of oxylipins in the CF airway to benefit its own survival. Not surprisingly, we find no reports in the literature on the effects of oxylipins on biology of P. aeruginosa. Future studies in this area seem warranted and may prove fruitful in further defining the mechanisms by which P. aeruginosa interact with the host in the CF airway.
Balance between pro-inflammatory and anti-inflammatory oxylipins in the CF RT
Although it appears that pro-inflammatory oxylipins predominate in the CF RT and have garnered the attention of most investigators, considerable interest has recently been placed on identifying, quantifying and establishing the functions of anti-inflammatory oxylipins [36]. Examples of classes of such anti-inflammatory lipid mediators include Resolvins, Protectins, epoxyeicosatrienoic acids (EpETrEs) and lipoxins, three of which have been quantified in the present study. Resolvin E1 is an anti-inflammatory and proresolving mediator derived from omega-3 fatty acids. Herein, we have observed that patients with detectable sputum levels of Resolvin E1 display better lung function than those patients who have no detectable levels of this anti-inflammatory mediator. While our data are reflective of a rather small cohort, they suggest that increasing the production of Resolvin E1 in the airways either by providing its parent fatty acid (EPA) in the diet, or directly instilling Resolvin E1 into the lung as an aerosol, may prove beneficial to the CF patient. Of interest, Resolvin E1 administration has already recently been shown to ameliorate toxic and septic RT inflammation and to improve survival in a mouse sepsis model [83, 84] and to counteract some of the proinflammatory circuits mediated by LTB4 [84]. However, it should also be recognized that ω-3 fatty acids (e.g. DHA and EPA) may affect antimicrobial resistance via their modulations of host immune responses [85].
Another class of anti-inflammatory lipid mediators quantified is represented by the epoxy fatty acids, particularly EETs. Enhancing EET levels in the CF airway via preventing their metabolism to diols (DHET) by inhibitors of soluble epoxide hydrolase (sEH) may provide a particularly novel and perhaps effective therapeutic approach for treating CF. Also, EETs or the corresponding ω-3 fatty acid epoxides could also conceivably be given by inhalation. It is also worth pointing out that P. aeruginosa have recently been shown to express an enzyme with sEH activity, again suggesting that P. aeruginosa may play an active role in oxylipin metabolism [81]. One study has suggested that there is a seemingly patho-physiologically important defect in Lipoxin A4 (LXA4)-mediated anti-inflammatory activity in the CF lung [86]. However, another study using a larger and more heterogeneous CF population, failed to show defects in LXA4 in CF RT secretions compared to a group of subjects with chronic bronchitis [87]. Because our current LC/MS/MS technique was less sensitive than the mentioned studies that used an ELISA method for quantitating LXA4, we were unable to detect LXA4 in any of the CF patient sputa analyzed. Thus, our observations may not allow us to comment on the possibility that modulation of LXA4 synthesis could be therapeutically useful for maintenance care of CF patients. Taken together, it seems clear that the balance of pro-inflammatory and anti-inflammatory lipid mediators may be an important consideration in inflammatory lung diseases including CF, and that strategies aimed to promote the synthesis or persistence of the latter should be further investigated [36].
Clinical Relevance and Study Limitations
The clinical implications of the present study are multifaceted. Aggressive inflammatory processes represent a dominant feature of the RT deterioration in CF [2], and thus have been viewed as a desirable target for therapeutic intervention [5, 6, 9, 12]. Bioactive oxylipins, including metabolites of arachidonic and linoleic acids, represent known contributors to pulmonary functions [36, 37, 50]. Yet, to date (including this study), only partial characterization of oxylipins in the available CF airway secretions is available. Interestingly, these profiles have perhaps been better profiled in blood and urine samples, and in bronchoalveolar fluids [42, 88] compared to the most relevant RT inflammatory milieu bathing airway epithelial cells.
The present studies demonstrate that oxylipin profiles can be potentially used to evaluate the effect of interventions with both nutritional and pharmacological strategies. In addition, these oxylipin profiles can also provide a predictive basis for designing effective interventions. For example, the impact of dietary 20:5 and 22:6 lipid administration [54, 89–91], antioxidant micronutrients [92] and statins [93, 94], are known to affect these pathways, along with a multitude of pharmaceuticals, yet their overall impact on RT oxylipin bioactivities have been incompletely characterized. More rigorous quantitative techniques for direct analysis of oxylipin profiles in RT secretions should lead to further understanding of the overall CF inflammatory airway pathobiology and lead to further developments of measures to target these pathways from both diagnostic and therapeutic perspectives.
Although we define a greater spectrum of oxylipins than previously reported in the CF airway, not all oxylipins were profiled. The present study focused on most, but not all of the arachidonic acid pathways. Further studies are needed to more fully characterize the important 5-lipoxygenase cysteinyl leukotrienes, other 20:5 and 22:6 fatty acid metabolites and oxysterols.
Because of the limited number of patients in our study, we did not attempt to further characterize the myriad of nutritional, infectious, and therapeutic factors that could potentially have affected the CF oxylipin profile beyond the associations we attempted to make with FEV1, a physiological parameter often used as a reflection of CF disease severity [95]. Further studies are necessary to better understand such related factors as: 1) the dynamic nature of RT oxylipin metabolites, 2) the relative contribution of phagocytes, epithelial cells, and even bacteria on the oxidative metabolism of unsaturated fatty acids (PUFA) in CF airways, 3) the potential modification of significant variability of diet and therapeutic drugs, and 4) oxylipin outcome contributions to CF pathobiology and disease natural history. Longitudinal studies and correlation of oxylipin profiles with other CF RT biomarkers of inflammation will be particularly insightful in this regard, as will clarification of precise cellular locations of the enzymes responsible for the generation and metabolism of oxylipins in airway CF fluids.
It is noteworthy that oxylipins are likely sensitive to the oxidative milieu including atmospheric oxygen tensions. Thus, precautions need to be taken to limit the artifacts formed by non-enzymatically-generated pathways. While we collected freshly obtained sputum into an antioxidant cocktail, we did not use the more rigorous technique of performing all sputum processing and work-up procedure in an oxygen-free environment.
On the other hand, non-enzymatic free radical-derived species usually do not have much specificity. Thus, should work-up artifact oxidations have occurred to a major degree, we should have determined comparable quantities of different isomers such as 5-HETE, 8-HETE, 12-HETE, 15-HETE, 9-HETE and 11-HETE. Our results, that showed large differences in these isomers, likely indicates that non-enzymatic free radical mechanisms were unlikely be a major concern in our study. An interesting fact is that airway secretions in CF in vivo interface directly with airway surfaces for long periods of time exposed to room air. Should “artefactual” generation of oxidized “oxylipins” be occurring during work-up they would also be likely to be formed in vivo on the surface of respiratory tract epithelial cells.
Conclusions
Studies of CF RT oxylipins, together with their metabolic profiles, as demonstrated in this paper, create a unique set of biomarkers for further characterization, biologic impact and exploitation. Sputum from CF patients is readily available and its constituency appears to exhibit some corrections with lung pathophysiology, including pulmonary function, the CF RT microbiology and other biomarkers of RT and systemic inflammatory processes. The integrative impact of nutritional, antioxidant, anti-inflammatory and therapeutic measures that modulate PUFA oxidative metabolism can now be evaluated using metabolomic oxylipin profiling. Such data should provide unique opportunities for more detailed assessment of the contribution of oxylipins on CF disease processes and rationally guide anti-inflammatory therapeutic measures in CF.
Supplementary Material
Highlights.
An extraction protocol maximizing regulatory lipid mediators (oxylipins) recoveries from CF sputum is described.
31 oxylipins in CF sputum are identified using LC/MS/MS technology.
Enzymatic pathways for generation of oxylipins in CF sputum are discussed.
The overall oxylipin profile are associated with pulmonary function in CF.
Oxylipin profiles in CF sputum are potentially diagnostically and therapeutically helpful.
Acknowledgments
The authors would like to thank the patient volunteers who participated in the study and to Prof. Karsten Gronert for his valuable advices. The work was supported by a Fellowship from the Cystic Fibrosis Research, Inc. (JY), the Cystic Fibrosis Foundation support of the UCD Adult CF Program (CEC and BMM), the NIH (HL092506, JPE and CEC), and in part by NIEHS SBRP Grant p42 ES004699, NIEHS Grant R37 ES02710, and NIH/NIEHS Grant R01 ES013933 (BDH). Partial support was provided by the American Asthma Association #09-0269. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Abbreviations: AA: arachidonic acid; CF: cystic fibrosis; CFTR: CF trans-membrane conductance regulator; CUDA: 1-cyclohexyl ureido 3-dodecanoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FEV-1: forced expiratory volume in one second; HPLC: high performance liquid chromatography; LC/MS/MS: liquid chromatography mass spectrometer/ mass spectrometer; LA: linoleic acid; LLE: liquid-liquid extraction; MRM: multiple reaction monitor; PLS: partial least squares; RT: respiratory tract; sEH: soluble epoxide hydrolase; SPE: solid phase extraction; TIC: total ion chromatography; sEH: soluble epoxide hydrolase; Regulatory lipid mediators: a panel of structurally defined oxidized metabolites of polyunsaturated fatty acids. All the regulatory lipid mediators’ common names are used in the paper according to LIPIDMAPS nomenclature. The full names are given in supplementary table1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell TS, Stecenko AA, Christman JW. Dysregulated NF-kappaB activation in cystic fibrosis: evidence for a primary inflammatory disorder. Am J Physiol Lung Cell Mol Physiol. 2001;281:L69–70. doi: 10.1152/ajplung.2001.281.1.L69. [DOI] [PubMed] [Google Scholar]
- 4.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 5.Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs. 2009;69:1903–1910. doi: 10.2165/11318500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Doring G, Gulbins E. Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol. 2009;11:208–216. doi: 10.1111/j.1462-5822.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 7.Bodas M, Vij N. The NF-kappaB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9:346–356. [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183:1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 9.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23:5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 10.Saude EJ, Lacy P, Musat-Marcu S, Mayes DC, Bagu J, Man SF, Sykes BD, Moqbel R. NMR analysis of neutrophil activation in sputum samples from patients with cystic fibrosis. Magn Reson Med. 2004;52:807–814. doi: 10.1002/mrm.20242. [DOI] [PubMed] [Google Scholar]
- 11.Koehler DR, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol. 2004;31:377–381. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 12.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijerman H. Infection and inflammation in cystic fibrosis: a short review. J Cyst Fibros. 2005;4(Suppl 2):3–5. doi: 10.1016/j.jcf.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11–12, 2003. Free Radic Biol Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratner D, Mueller C. Immune responses in Cystic Fibrosis; are they intrinsically defective? Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0399RT. [DOI] [PubMed] [Google Scholar]
- 17.Collaco JM, Cutting GR. Update on gene modifiers in cystic fibrosis. Curr Opin Pulm Med. 2008;14:559–566. doi: 10.1097/MCP.0b013e3283121cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDougal KE, Green DM, Vanscoy LL, Fallin MD, Grow M, Cheng S, Blackman SM, Collaco JM, Henderson LB, Naughton K, Cutting GR. Use of a modeling framework to evaluate the effect of a modifier gene (MBL2) on variation in cystic fibrosis. Eur J Hum Genet. 2010;18:680–684. doi: 10.1038/ejhg.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanke F, Becker T, Kumar V, Hedtfeld S, Becker C, Cuppens H, Tamm S, Yarden J, Laabs U, Siebert B, Fernandez L, Macek M, Jr, Radojkovic D, Ballmann M, Greipel J, Cassiman JJ, Wienker TF, Tummler B. Genes that determine immunology and inflammation modify the basic defect of impaired ion conductance in cystic fibrosis epithelia. J Med Genet. 2011;48:24–31. doi: 10.1136/jmg.2010.080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Liu C, Clark JC, Whitsett JA. Functional genomic responses to cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR(delta508) in the lung. J Biol Chem. 2006;281:11279–11291. doi: 10.1074/jbc.M512072200. [DOI] [PubMed] [Google Scholar]
- 21.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 22.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 24.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol. 2007;36:201–205. doi: 10.1165/rcmb.2006-0269TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpati F, Meurling L, Wretlind B, Hjelte L. Interleukin-8 in Sputum from Cystic Fibrosis Patients during Ibuprofen Treatment. In: Harrison MA, editor. Progress in Cystic Fibrosis Research. Nova Science Publisher, Inc; 2005. pp. 171–183. [Google Scholar]
- 26.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Xiang YY, Ye L, Tsui LC, Macdonald JF, Hu J, Lu WY. Nonsteroidal anti-inflammatory drugs upregulate function of wild-type and mutant CFTR. Eur Respir J. 2008;32:334–343. doi: 10.1183/09031936.00168007. [DOI] [PubMed] [Google Scholar]
- 28.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czerska K, Sobczynska-Tomaszewska A, Sands D, Nowakowska A, Bak D, Wertheim K, Poznanski J, Zielenski J, Norek A, Bal J. Prostaglandin-endoperoxide synthase genes COX1 and COX2 - novel modifiers of disease severity in cystic fibrosis patients. J Appl Genet. 2010;51:323–330. doi: 10.1007/BF03208862. [DOI] [PubMed] [Google Scholar]
- 30.Masoodi M, Eiden M, Koulman A, Spaner D, Volmer DA. Comprehensive lipidomics analysis of bioactive lipids in complex regulatory networks. Anal Chem. 2010;82:8176–8185. doi: 10.1021/ac1015563. [DOI] [PubMed] [Google Scholar]
- 31.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog in Lipid Res. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Lu Y, Hong S, Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 2007;432:275–317. doi: 10.1016/S0076-6879(07)32012-0. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-Xavier RA, Newson J, Silveira VL, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 34.Takai D, Nagase T, Shimizu T. New therapeutic key for cystic fibrosis: a role for lipoxins. Nature immunology. 2004;5:357–358. doi: 10.1038/ni0404-357. [DOI] [PubMed] [Google Scholar]
- 35.McKeever TM, Lewis SA, Smit HA, Burney P, Britton JR, Cassano PA. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med. 2005;171:966–971. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- 36.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dave A, Graham IA. Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA) Frontiers in Plant Science. 2012;3:1–6. doi: 10.3389/fpls.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gronert K. Lipid autacoids in inflammation and injury responses: a matter of privilege. Mol Interv. 2008;8:28–35. doi: 10.1124/mi.8.1.7. [DOI] [PubMed] [Google Scholar]
- 40.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spickett CM, Wiswedel I, Siems W, Zarkovic K, Zarkovic N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic Res. 2010;44:1172–1202. doi: 10.3109/10715762.2010.498476. [DOI] [PubMed] [Google Scholar]
- 42.Ollero M, Astarita G, Guerrera IC, Sermet-Gaudelus I, Trudel S, Piomelli D, Edelman A. Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J Lipid Res. 2011;52:1011–1022. doi: 10.1194/jlr.P013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massey KA, Nicolaou A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochemical Society Transactions. 2011;39:1240–1246. doi: 10.1042/BST0391240. [DOI] [PubMed] [Google Scholar]
- 44.Higdon A, et al. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 46.Iuliano L, Monticolo R, Straface G, Zullo S, Galli F, Boaz M, Quattrucci S. Association of cholesterol oxidation and abnormalities in fatty acid metabolism in cystic fibrosis. Am J Clin Nutr. 2009;90:477–484. doi: 10.3945/ajcn.2009.27757. [DOI] [PubMed] [Google Scholar]
- 47.Vaughan WJ, Lindgren FT, Whalen JB, Abraham S. Serum lipoprotein concentrations in cystic fibrosis. Science. 1978;199:783–786. doi: 10.1126/science.203033. [DOI] [PubMed] [Google Scholar]
- 48.Carlstedt-Duke J, Bronnegard M, Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci U S A. 1986;83:9202–9206. doi: 10.1073/pnas.83.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhodes B, Nash EF, Tullis E, Pencharz PB, Brotherwood M, Dupuis A, Stephenson A. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros. 2010;9:24–28. doi: 10.1016/j.jcf.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Strandvik B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2010;83:121–129. doi: 10.1016/j.plefa.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Bravo E, Napolitano M, Valentini SB, Quattrucci S. Neutrophil unsaturated fatty acid release by GM-CSF is impaired in cystic fibrosis. Lipids Health Dis. 2010;9:129. doi: 10.1186/1476-511X-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Njoroge SW, et al. Increased Delta5- and Delta6-desaturase, cyclooxygenase-2, and lipoxygenase-5 expression and activity are associated with fatty acid and eicosanoid changes in cystic fibrosis. Biochim Biophys Acta. 2011;1811:431–440. doi: 10.1016/j.bbalip.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Thomsen KF, et al. Increased elongase 6 and Delta9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis. Lipids. 2011;46:669–677. doi: 10.1007/s11745-011-3563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Njoroge SW, et al. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J Lipid Res. 2012;53:257–265. doi: 10.1194/jlr.M018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collawn JF, Fu L, Bebok Z. Targets for cystic fibrosis therapy: proteomic analysis and correction of mutant cystic fibrosis transmembrane conductance regulator. Expert Rev Proteomics. 2010;7(4):495–506. doi: 10.1586/epr.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sloane AJ, et al. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med. 2005;172:1416–1426. doi: 10.1164/rccm.200409-1215OC. [DOI] [PubMed] [Google Scholar]
- 58.McMorran BJ, Patat SA, Carlin JB, Grimwood K, Jones A, Armstrong DS, Galati JC, Cooper PJ, Byrnes CA, Francis PW, Robertson CF, Hume DA, Borchers CH, Wainwright CE, Wainwright BJ. Novel neutrophil-derived proteins in bronchoalveolar lavage fluid indicate an exaggerated inflammatory response in pediatric cystic fibrosis patients. Clin Chem. 2007;53:1782–1791. doi: 10.1373/clinchem.2007.087650. [DOI] [PubMed] [Google Scholar]
- 59.Wolak JE, Esther CR, Jr, O’Connell TM. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers. 2009;14:55–60. doi: 10.1080/13547500802688194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 62.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 63.Sampson AP, Spencer DA, Green CP, Piper PJ, Price JF. Leukotrienes in the sputum and urine of cystic fibrosis children. Br J Clin Pharmacol. 1990;30:861–869. doi: 10.1111/j.1365-2125.1990.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis. 1993;148:896–901. doi: 10.1164/ajrccm/148.4_Pt_1.896. [DOI] [PubMed] [Google Scholar]
- 65.Zakrzewski JT, Barnes NC, Piper PJ, Costello JF. Detection of sputum eicosanoids in cystic fibrosis and in normal saliva by bioassay and radioimmunoassay. Br J Clin Pharmacol. 1987;23:19–27. doi: 10.1111/j.1365-2125.1987.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid DW, Misso N, Aggarwal S, Thompson PJ, Walters EH. Oxidative stress and lipid-derived inflammatory mediators during acute exacerbations of cystic fibrosis. Respirology. 2007;12:63–69. doi: 10.1111/j.1440-1843.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 67.Zakrzewski JT, Barnes NC, Costello JF, Piper PJ. Lipid mediators in cystic fibrosis and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;136:779–782. doi: 10.1164/ajrccm/136.3.779. [DOI] [PubMed] [Google Scholar]
- 68.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasu VT, de Cruz SJ, Houghton JS, Hayakawa KA, Morrissey BM, Cross CE, Eiserich JP. Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase. Free Radic Res. 2011;45:165–176. doi: 10.3109/10715762.2010.521154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moss CW, Samuels SB, Weaver RE. Cellular fatty acid composition of selected Pseudomonas species. Appl Microbiol. 1972;24:596–598. doi: 10.1128/am.24.4.596-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato H, Feix JB, Hillard CJ, Frank DW. Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin, ExoU. J Bacteriol. 2005;187:1192–1195. doi: 10.1128/JB.187.3.1192-1195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saliba AM, Nascimento DO, Silva MC, Assis MC, Gayer CR, Raymond B, Coelho MG, Marques EA, Touqui L, Albano RM, Lopes UG, Paiva DD, Bozza PT, Plotkowski MC. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7:1811–1822. doi: 10.1111/j.1462-5822.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 73.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 74.Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorrell TC, Muller M, Sztelma K. Bacterial metabolism of human polymorphonuclear leukocyte-derived arachidonic acid. Infect Immun. 1992;60:1779–1785. doi: 10.1128/iai.60.5.1779-1785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci U S A. 2004;101:2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacEachran DP, Ye S, Bomberger JM, Hogan DA, Swiatecka-Urban A, Stanton BA, O’Toole GA. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2007;75:3902–3912. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez E, Hamberg M, Busquets M, Diaz P, Manresa A, Oliw EH. Biochemical characterization of the oxygenation of unsaturated fatty acids by the dioxygenase and hydroperoxide isomerase of Pseudomonas aeruginosa 42A2. J Biol Chem. 2010;285:9339–9345. doi: 10.1074/jbc.M109.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Twomey KB, et al. Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. ISME J. 167 doi: 10.1038/ismej.2011.167. Epub. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitamura S, Hashizume K, Iida T, Miyashita E, Shirahata K, Kase H. Studies on lipoxygenase inhibitors. II. KF8940 (2-n-heptyl-4-hydroxyquinoline-N-oxide), a potent and selective inhibitor of 5-lipoxygenase, produced by Pseudomonas methanica. J Antibiot (Tokyo) 1986;39:1160–1166. doi: 10.7164/antibiotics.39.1160. [DOI] [PubMed] [Google Scholar]
- 81.Bahl CD, Morisseau C, Bomberger JM, Stanton BA, Hammock BD, O’Toole GA, Madden DR. Crystal structure of the cystic fibrosis transmembrane conductance regulator inhibitory factor Cif reveals novel active-site features of an epoxide hydrolase virulence factor. J Bacteriol. 2010;192:1785–1795. doi: 10.1128/JB.01348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Visioli F, et al. Molecular targets of omega 3 and conjugated linoleic Fatty acids - “micromanaging” cellular response. Front Physiol. 2012;3:42. doi: 10.3389/fphys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan M, Godson C, Guiry PJ, Agerberth B, Haeggstrom JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 85.McMurray DN, Bonilla DL, Chapkin RS. n-3 Fatty acids uniquely affect anti-microbial resistance and immune cell plasma membrane organization. Chemistry and Physics of Lipids. 2011;164:626–635. doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature immunology. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 87.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur Respir J. 2006;28:581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]
- 88.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, Dahlen SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Current Pharmaceutical Biotechnology. 2011;12:1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 89.Al-Turkmani MR, Freedman SD, Laposata M. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:309–318. doi: 10.1016/j.plefa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Van Biervliet S, et al. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot Essent Fatty Acids. 2008;78:109–115. doi: 10.1016/j.plefa.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 91.Olveira G, et al. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch Bronconeumol. 2010;46:70–77. doi: 10.1016/j.arbres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunol. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 2010;3:270–279. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaddurah-Daouk R, et al. Lipidomic analysis of variation in response to simvastatin in the Cholesterol and Pharmacogenetics Study. Metabolomics. 2010;6:191–201. doi: 10.1007/s11306-010-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konstan MW, Wagener JS, Yegin A, Millar SJ, Pasta DJ, VanDevanter DR. Design and powering of cystic fibrosis clinical trials using rate of FEV(1) decline as an efficacy endpoint. J Cyst Fibros. 2010;9:332–338. doi: 10.1016/j.jcf.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.