Abstract
Background
Bone bruises located on the lateral femoral condyle and posterolateral tibia are commonly associated with anterior cruciate ligament (ACL) injuries and may contribute to the high risk for knee osteoarthritis after ACL injury. The resultant footprint (location) of a bone bruise after ACL injury provides evidence of the inciting injury mechanism.
Purpose/Hypothesis
(1) To analyze tibial and femoral articular cartilage pressure distributions during normal landing and injury simulations, and (2) to evaluate ACL strains for conditions that lead to articular cartilage pressure distributions similar to bone bruise patterns associated with ACL injury. The hypothesis was that combined knee abduction and anterior tibial translation injury simulations would demonstrate peak articular cartilage pressure distributions in the lateral femoral condyle and posterolateral tibia. The corollary hypothesis was that combined knee abduction and anterior tibial translation injury conditions would result in the highest ACL strains.
Study Design
Descriptive laboratory study.
Methods
Prospective biomechanical data from athletes who subsequently suffered ACL injuries after testing (n = 9) and uninjured teammates (n = 390) were used as baseline input data for finite element model comparisons.
Results
Peak articular pressures that occurred on the posterolateral tibia and lateral femoral condyle were demonstrated for injury conditions that had a baseline knee abduction angle of 5°. Combined planar injury conditions of abduction/anterior tibial translation, anterior tibial translation/internal tibial rotation, or anterior tibial translation/external tibial rotation or isolated anterior tibial translation, external tibial rotation, or internal tibial rotation resulted in peak pressures in the posterolateral tibia and lateral femur. The highest ACL strains occurred during the combined abduction/anterior tibial translation condition in the group that had a baseline knee abduction angle of 5°.
Conclusion
The results of this study support a valgus collapse as the major ACL injury mechanism that results from tibial abduction rotations combined with anterior tibial translation or external or internal tibial rotations.
Clinical Relevance
Reduction of large multiplanar knee motions that include abduction, anterior translation, and internal/external tibial motions may reduce the risk for ACL injuries and associated bone bruises. In particular, prevention of an abduction knee posture during initial contact of the foot with the ground may help prevent ACL injury.
Keywords: bone bruise, ACL, articular cartilage, knee injury
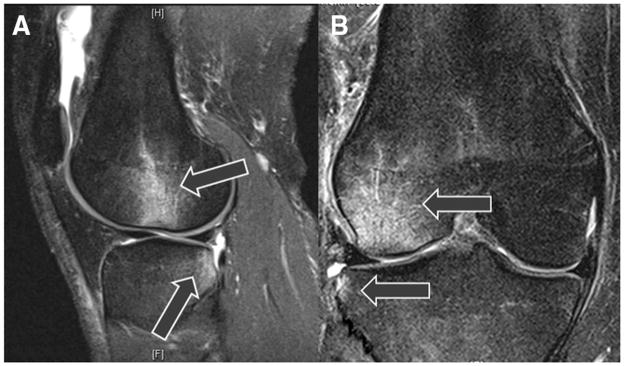
Anterior cruciate ligament (ACL) injury is a common, often devastating, injury. It is well documented that women demonstrate a 4- to 6-fold higher injury rate compared with men participating in similar sports.1,2,15,25 During the ACL injury event, the large external forces that incite ligament disruption likely also lead to violent impact of the tibial and femoral articular cartilage, which transfers into the subchondral bone and often causes a bone bruise “footprint” that is a result of the mechanism of injury (Figure 1).43 Clinical imaging studies of acute ACL injury demonstrate that hyperintense signals in the subchondral tibia and femur (bone bruises) occur in more than 80% of patients who sustain complete ACL disruption.49 Bone bruises are commonly found on the posterolateral tibia and lateral femoral condyle on imaging studies after acute ACL injury (Figure 1) and likely reflect the tibial and femoral cartilage impact that occurs at the time of injury.20,33,49 The locations of bone bruises may provide insight into the directions (anatomic planes) that lead to ACL injury.
Figure 1.
Magnetic resonance images of bone bruise patterns (lateral femoral condyle and posterolateral tibial plateau) associated with acute anterior cruciate ligament (ACL) injury that the finite element model data are compared to determine relevance of injury condition. A, sagittal plane view showing the more posterior location of the tibial bruise relative to the femoral bruise. The arrows point to the hyper-intense signals associated with bone bruises. B, frontal plane view showing lateral compression of the femur and tibia.
The objectives of this study were (1) to analyze tibial and femoral articular cartilage pressure distributions during normal landing and injury simulations and (2) to evaluate ACL strains for conditions that lead to articular cartilage pressure distributions similar to bone bruise patterns associated with ACL injury. We hypothesized that combined anterior tibial shear and knee abduction injury simulations would demonstrate articular cartilage pressure distributions in locations similar to bone bruise patterns found on imaging studies after acute ACL injuries. We also hypothesized that this same kinematics would lead to high ACL strains.
MATERIALS AND METHODS
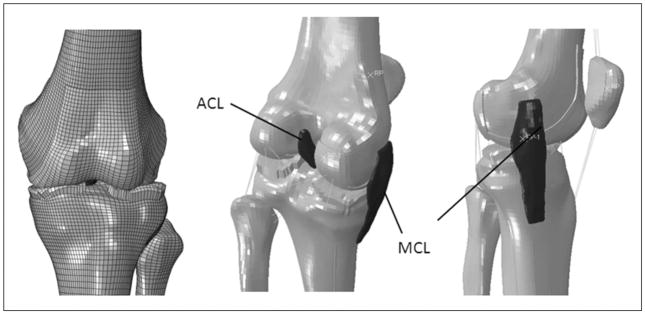
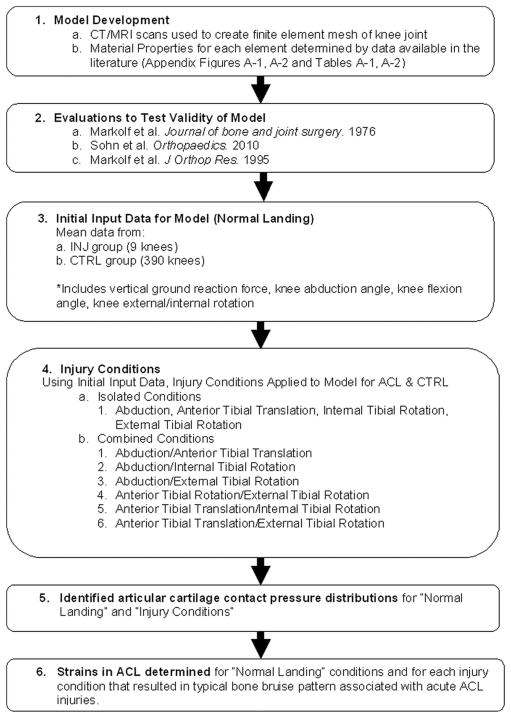
Institutional review board approval and written, informed consent forms were obtained before testing for all patients. A dynamic finite element knee model (Figure 2) (Appendix Figure A-1, available in the online version of this article at http://ajs.sagepub.com/supplemental/) was created from computed tomography and magnetic resonance images of a skeletally mature, young adult, female athlete to determine articular cartilage pressure distributions during normal landing and injury simulations (Abaqus 6.10 software, Simulia, Providence, Rhode Island). Details of model development methods are described in the Appendix (Appendix Tables A1–3 and Figures A1–3, available online).39 Figure 3 demonstrates a flow chart of the methods sequence. The model was subjected to validity evaluations and model simulations to analyze articular pressure distributions during normal landing and injury.
Figure 2.
Knee finite element model that demonstrates the femur, tibia, fibula, patella anterior cruciate ligament (ACL), and medial collateral ligament meshes.
Figure 3.
Flow chart describing the methods of the study.
The finite element model was subjected to evaluations based on cadaveric experimental data available in the literature and from previous cadaveric tests conducted by some of the current investigators (Appendix Figures A-2 and A-3, available online, provides detailed results). The first criterion for model confirmation was that the Pearson correlation was good (r = .50–.75) to excellent (r > .75) for the model results compared with cadaveric data available in the literature.38,46 The second criterion for model confirmation was that the differences between finite element model simulations and cadaveric data were less than 2 standard deviations (SDs) from the mean of the cadaveric data.31
We observed an excellent correlation (r = .97, P < .001) between the cadaveric results and predicted laxity in the finite element model. The finite element model predicted knee laxity values similar to those of Markolf et al,30 with anterior-posterior, external-internal, and varus-valgus laxities at 0°, 20°, and 45° of knee flexion for the finite element model within 2 SDs of the predicted mean for the cadaveric investigations. We used data from 10 human cadaveric lower extremities tested by Sohn et al48 to confirm the articular cartilage pressure distributions for the finite element model during weightbearing conditions. We observed a correlation (r = .888, P < .01) between the finite element predictions and the cadaveric data for articular cartilage pressures, with the model results within 2 SDs of the mean of the cadaveric data (Appendix Table A-3, available online). Markolf et al28 also examined combined loading states that generate high ACL forces (varus-valgus, internal-external tibial rotation, and anterior tibial force). These loading conditions were repeated in the model (knee flexion angles of 0°–40°). Again, an excellent correlation (r = .92, P < .001) was observed between the finite element predicted forces and the cadaveric results (Appendix Figures A-2 and A-3, available online). The finite element data were within 2 SDs of the reported cadaveric means for each testing condition.
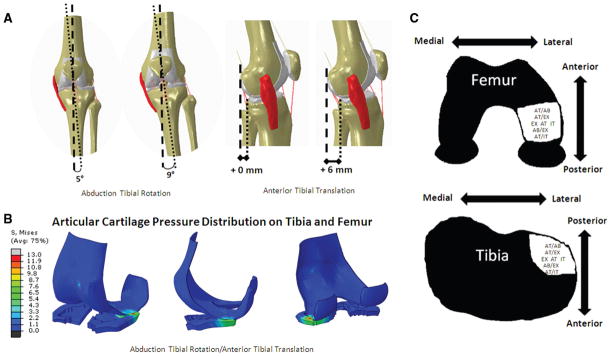
The femur and tibia were divided into 6 separate sections each in order to describe articular cartilage pressure distributions (Figure 4). These sections were defined as anterior-lateral (AL), anterior-medial (AM), middle-lateral (ML), middle-medial (MM), posterior-lateral (PL), and posterior-medial (PM) by drawing a sagittal plane divider and 2 frontal plane dividers (Figure 4). This technique was previously described in detail and was adapted to allow for comparisons to current clinical techniques for localizing articular cartilage abnormalities (International Cartilage Repair Society format).4,39 Because the PL tibial and ML femoral sections are the most common bone bruise locations after ACL injury, articular cartilage contact pressure locations in the PL and ML sections were considered likely ACL injury mechanisms (Figures 1 and 5).33 Peak ACL strains were identified for each condition that resulted in peak articular cartilage pressures in the PL tibia and ML femur. Peak ACL strain was determined by the midsub-stance ACL element that had the maximum critical value.
Figure 4.

Illustration of the sections used to describe pressure locations that demonstrate articular cartilage pressures during a loading scenario. The various colors indicate the stresses in the menisci and articular cartilage (further description in Figure 5). A, femur and tibia articular cartilage and menisci. B, tibia articulation surface divided into sections. Femur articular surface viewed posteriorly (C) and superiorly (D) divided into sections. The sections for the tibia and femur are divided into anterior-lateral (AL), middle-lateral (ML), posterior-lateral (PL), anterior-medial (AM), middle-medial (MM), and posterior-medial (PM).
Figure 5.
Injury conditions that resulted in peak articular cartilage contact pressure locations in the posterolateral tibia and lateral femur. A, combined abduction tibial rotation and anterior tibial translation injury conditions. B, the articular cartilage pressure distribution during abduction tibial rotation and anterior tibial translation in the injury (INJ) group. C, loading conditions that resulted in articular cartilage stress patterns similar to the bone bruise patterns associated with anterior cruciate ligament (ACL) injury on the femur and tibia.
Kinematic and kinetic data during a drop vertical jump maneuver from female athletes before their athletic seasons were used as baseline input for the finite element model. Mean kinematic and kinetic data at initial contact during landing from a drop vertical jump for athletes who went on to subsequent injury (INJ) after testing (n = 9 knees) and control (CTRL) (n = 390 knees) athletes (who did not have subsequent ACL injury) were used as baseline input for the model.16 Methods for biomechanical data collection are described in Ford et al9 and Hewett et al.16 A static trial was used as the athlete’s neutral (zero) alignment with subsequent measures relative to this position.10
Three-dimensional coordinates for the tibial and femoral markers were identified from the in vivo data (see Ford et al for marker placement) and used to define baseline input rotational and translational boundary conditions for the finite element model at corresponding node locations.9,16 The coordinate system described by Grood and Suntay14 was used to define the knee joint coordinate system of the finite element model with respect to the 3-dimensional knee joint motions, and a geometric center axis was used to apply flexion rotations.34 A vertical ground-reaction force was applied to the finite element model as a compressive force across the tibiofemoral joint. A 600-N quadriceps tension and 400-N hamstrings tension were applied to the model to simulate in vivo muscle resistance and compressive joint forces.46,51 Baseline “normal” landing conditions included a knee abduction angle of 5° (INJ) or −3.4° (CTRL) with a 20° knee flexion angle and a vertical ground-reaction force (600 N) for both groups.16
We used the finite element model to simulate ACL injury mechanisms for each group (INJ, CTRL) by single planar loading conditions for abduction rotation (abduction of the tibia relative to the femur), internal tibial rotation, external tibial rotation, and anterior tibial translation (Figure 3). Combined injury conditions were examined for combined abduction/anterior tibial translation, abduction/internal tibial rotation, abduction/external tibial rotation, anterior tibial translation/internal tibial rotation, and anterior tibial translation/external tibial rotation. Because video analyses indicate that female athletes have 4° higher abduction angles compared with their initial contact angle during the ACL injury event, abduction injury simulations consisted of a 4° increase in abduction angle relative to baseline initial contact landing conditions.22 Anterior tibial translation injury simulations consisted of 6-mm increases in anterior tibial translation. Normal anterior tibial translation laxity is approximately 3 mm (depending on flexion angle and applied force), and ACL deficiency leads to an increase of 3 mm (or more) of anterior tibial translation laxity compared with the healthy contralateral limb.6,29,30 Internal and external tibial rotation injury simulations included 9° increases in the respective rotations because weightbearing axial rotations are approximately 5° to 7° in each direction, and Olsen et al36 reported approximately 9° of internal or external rotation of the tibia during ACL injury.47 The articular cartilage contact pressure locations were determined for each injury scenario and compared with the common bone bruise locations after acute ACL injury.
RESULTS
Table 1 summarizes the location of peak articular cartilage pressure distributions during normal (noninjury producing) landing, isolated single-plane injury conditions, and combined injury conditions. Peak articular pressures that matched the typical bone bruise pattern associated with ACL injury were found for the following conditions in the INJ group: combined abduction/anterior tibial translation, anterior tibial translation/internal tibial rotation, or anterior tibial translation/external tibial rotation and isolated anterior tibial translation, external tibial rotation, or internal tibial rotation (Figure 5). Only the combined abduction/anterior tibial translation condition resulted in articular cartilage pressure locations isolated to the quadrants similar to bone bruise patterns (Table 1). Although other conditions resulted in peak pressures that occurred in the PL and ML quadrants, the patterns overlapped into other quadrants as well (Table 1). No injury conditions in the CTRL group led to peak pressures in the locations that matched the typical bone bruise pattern (Table 1 and Figure 5).
TABLE 1.
Section Locations of Articular Cartilage Pressure Distributions for Each Injury Condition for Injury (INJ) and Control (CTRL) Groupsa
| INJ
|
CTRL
|
|||
|---|---|---|---|---|
| Tibia | Femur | Tibia | Femur | |
| Normal landing | ML, PM | ML, MM | MM, PM | MM |
| Abduction tibial rotation | ML, PL | ML | MM, ML | ML, MM |
| Anterior tibial translation | PL, ML | ML, MM | PM | MM |
| Internal tibial rotation | PL, ML, PM | ML, MM | PL, MM | ML, MM |
| External tibial rotation | PL, MM | ML, MM | ML, PM | ML, MM |
| Abduction/anterior tibial translation | PL | ML | PM, PL | ML, MM |
| Abduction/internal tibial rotation | ML, PL | ML | PM, PL | ML, MM |
| Abduction/external tibial rotation | PL, ML | ML | MM, ML | ML, MM |
| Anterior tibial translation/internal tibial rotation | PL, PM | ML, MM | PM | MM |
| Anterior tibial translation/external tibial rotation | PL, PM | ML, MM | PM | MM |
ML, middle-lateral; PM, posterior-medial; MM, middle-medial; PL, posterior-lateral.
The increases in ACL strains from normal landing conditions for the scenarios that resulted in peak articular cartilage distributions similar to bone bruises associated with the ACL are listed in Table 2. The highest ACL strain occurred in the combined abduction/anterior tibial translation condition with a 4.6-fold (358%) increase from normal landing conditions in the INJ group. Combined anterior tibial translation/internal tibial rotation for the INJ group also resulted in high ACL strains (3.9-fold, 289% increase in strain relative to normal landing conditions). Combined anterior tibial translation/external tibial rotation resulted in a 3.7-fold (270%) increase to ACL strain for the INJ group, and combined abduction/external tibial rotation for the INJ group resulted in a 2.0-fold (99%) increase in ACL strain. Isolated anterior tibial translation, internal tibial rotation, and external tibial rotation resulted in a 3.6-fold (264%) increase, 1.2-fold (16%) increase, and 0.8-fold (16%) decrease from the normal landing condition ACL strain, respectively.
TABLE 2.
Change in Peak Anterior Cruciate Ligament (ACL) Strain for Injury Conditions (Relative to Normal Landing) That Resulted in Articular Cartilage Contact Pressure Locations in Posterolateral Tibia and Lateral Femoral Condylea
| ACL Strain | |
|---|---|
| Normal landing | — |
| Internal tibial rotation | 1.2-fold (16%) increase |
| External tibial rotation | 0.8-fold (16%) decrease |
| Abduction/anterior tibial translation | 4.6-fold (358%) increase |
| Abduction/external tibial rotation | 1.6-fold (62%) increase |
| Anterior tibial translation/internal tibial rotation | 3.9-fold (289%) increase |
| Anterior tibial translation/external tibial rotation | 3.7-fold (270%) increase |
Peak ACL strain was determined by the midsubstance ACL element that had the maximum critical value.
DISCUSSION
Bone bruises are commonly associated with ACL injuries, and the resultant location of these bone bruises (commonly found on the lateral femoral condyle and posterolateral tibia) provides evidence to the injury mechanism. We developed and evaluated a finite element model of a knee of a young female athlete to analyze tibial and femoral articular cartilage pressure distributions during normal landing and injury simulations. The objectives of this study were (1) to analyze tibial and femoral articular cartilage pressure distributions during normal landing and injury simulations and (2) to evaluate the ACL strains for conditions that lead to articular cartilage pressure distributions similar to bone bruise patterns associated with ACL injury.
The tibiofemoral osteokinematic planar (sagittal, frontal, transverse motions) contributions to the mechanisms of ACL injury are a current debate in recent journal articles and symposia at sports medicine conferences.24,40,41,50,52 Video studies of ACL injuries provide evidence of 2 predominant loading patterns: (1) valgus collapse of the knee (a combination of knee valgus, hip internal rotation, and tibial rotation) or (2) anterior tibial shear.3,21,40 Systematic reviews of the literature related to ACL injury mechanisms by Shimokochi et al45 and Quatman et al41 concluded that ACL injuries are more likely to occur because of multiplanar rather than single-planar mechanisms, in which anterior tibial shear, valgus knee collapse, and internal or external tibial rotations represent the most likely contributors to the injury mechanism. Despite the large amount of literature related to ACL injury, the relationship and additive nature of these multiplanar loading conditions to ACL strain and injury remain unclear.
In vivo biomechanical data and video analyses indicate that increased lower extremity abduction loads and movements are associated with increased ACL strain and risk of injury.3,17,21,22 Data from the current investigation support abduction as a component of the ACL injury mechanism. Although isolated abduction injury simulations did not result in the typical bone bruise pattern, combined abduction/anterior tibial translation and combined abduction/external tibial rotation lead to articular cartilage pressure distributions on the lateral femur and posterolateral tibia. In addition, the current study demonstrated that combined abduction/anterior tibial translation, anterior tibial translation/internal tibial rotation, anterior tibial translation/external tibial rotation and isolated anterior tibial translation, external tibial rotation, or internal tibial rotation only resulted in peak pressure distributions occurring in the areas similar to the bone bruise pattern associated with ACL injury in athletes who had an abduction angle of 5° at initial contact during landing (INJ group). Thus, these lateral tibial and femoral bone bruises likely result from lateral compression of the femur and tibia. The more posterior tibial location of the bone bruise relative to the femur is most likely to result from abduction combined with either anterior tibial translation or internal or external tibial rotation during the injury event.
Most ACL injuries occur under weightbearing conditions, and modeling studies indicate that the ACL can impinge on the notch during combined abduction/external tibial rotation.12 Results from this study also indicate that external tibial rotation combined with anterior tibial translation or abduction rotation in an athlete who lands with an abducted knee posture may result in a bone bruise pattern that has been associated with ACL injuries. An abducted knee posture at initial contact is likely important for this type of mechanism because combined anterior tibial translation/external tibial rotation in the CTRL group did not result in the classic bone bruise pattern. However, the ACL strain for combined abduction/external tibial rotation was lower than the abduction/anterior tibial translation condition ACL strain.
Anterior shear of the proximal tibia is the most obvious ACL loading mechanism, and decreasing knee flexion angles increase the anterior shear force at the tibia.28,44 As video studies indicate that noncontact ACL injuries usually occur at low flexion angles, and women have been reported to have less knee flexion during sports movements, it is theorized that a powerful quadriceps force at low knee flexion angles could produce enough anterior shear force at the tibia to cause ACL rupture.¶ Although anterior tibial translation conditions led to high ACL strains, isolated anterior tibial translation conditions only resulted in the typical bone bruise pattern in the INJ group that had an abducted knee posture during initial contact of landing from the jump. Moreover, there were high articular cartilage pressures noted on the medial aspect of the joint as well.
Cadaveric data indicate that combined knee abduction with internal tibial rotation, external tibial rotation, or anterior tibial forces leads to considerably larger ACL forces than internal or anterior tibial forces alone.28 For the current study, the ACL strains were higher for tibial abduction conditions coupled with anterior tibial translations or internal tibial rotations than isolated abduction, anterior translation, or internal tibial rotation conditions. Thus, multiplanar load conditions appear to be additive for ACL strains and likely increase risk of ACL injury.
The underlying microstructural damage to the articular cartilage and subchondral bone that occurs as a result of the large impact forces sustained by the tibia and femur may lead to cartilage thinning and osteochondral defects, in addition to the acute consequences of an initial ACL injury and concomitant bone bruise. This damage may serve as a precursor to osteoarthritis that accelerates the progression of degenerative changes in the knee.7,11,18,27 Recent reports also indicate that ACL injuries/reconstructions not only affect anterior-posterior stability but also knee joint motion in all 6 degrees of freedom. This multi-planar loss of knee stability may alter the tibiofemoral cartilage contact, which in combination with a commonly reported torn meniscus, may represent a secondary causative factor for knee osteoarthritis subsequent to ACL deficiency.19 The characterization of the kinematic and kinetic variables that contribute to ACL injury is essential in the design of the most effective prevention strategies. If bone bruise footprints can be utilized to define ACL injury mechanisms, specific neuromuscular training interventions designed to prevent the lower extremity mechanics that contribute to these bruise patterns may help athletes avoid the disability associated with posttraumatic knee osteoarthritis.
Limitations
All models have inherent limitations, and there are likely differences between experimental conditions and the real-world biomechanics of sports. High loading rate simulations are challenging and may require model simplifications, and it may not be possible to fully confirm injury models under these conditions. Inherent damage associated with injury biomechanics limits a researcher’s ability to conduct robust high rate mechanical characterization on any single specimen. However, we used both information from a large in vivo cohort study and data provided by the finite element model to extend the existing knowledge base and to appraise working hypotheses about reducing injury risk (both ACL and associated bone bruises) without reporting exact stress and strain results. Instead, the relationships between structures and changes in the locations of concentrated forces were evaluated between test conditions to help us understand, but perhaps not accurately predict, exact articular cartilage stresses. The methodology utilized in this study is both innovative and unique in that it embraces a new “in sim” methodological approach that shifts the current experimental paradigm to incorporate a multifaceted integration of in vivo, in vitro (cadaveric), and in silico (computer modeling) methods.42 In sim methodology can be used to provide a platform for a more comprehensive understanding of the complex joint biomechanics that occur during ACL injury.42 This model serves as an initial step in the development of a model to evaluate ACL injury mechanisms. Further development will focus on the incorporation of high rate and injury biomechanics data, not available at this stage. As this is a preliminary study and given the limited validation data to represent high loading conditions, only the location of pressure distributions associated with injury mechanisms is reported at this point. This model is needed to guide further study.
CONCLUSION
The results of this study support a valgus collapse injury mechanism that results from tibial abduction rotations combined with anterior tibial translation or external or internal tibial rotations. Reduction of knee abduction motions is possible with neuromuscular training that may help decrease the risk for ACL injury and associated bone bruises in female athletes.13,15,26,35 The large impacts sustained by the tibia and femur during ACL injuries may be a significant precursor event that accelerates the progression of degenerative changes in the knee. As demonstrated by the results of this study, the lateral tibial and femoral bone bruises associated with acute ACL injury may occur as a result of lateral joint compression. The more posterior location of bone bruises on the tibial plateau relative to the femur may indicate that the tibia shifts anteriorly or rotates internally relative to the femur during the injury event. Prevention programs that target reduction of large knee abduction, anterior translation, and internal and external tibial motions during sports activities may help reduce the risk for sustaining ACL injuries with associated bone bruises.
Acknowledgments
The authors thank Keith Kenter, Catherine Quatman-Yates, Jason Levine, Ata Kiapour, Krishna Mallik, Mark Paterno, Laura Schmitt, and Sam Wordeman for their stimulating discussions about these topics.
Footnotes
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
One or more authors has declared the following potential conflict of interest or source of funding: National Institutes of Health grants R01-AR049735, R01-AR056259, and R03-AR057551. Funding support from the University of Toledo College of Medicine Pre-Doctoral Fellowship, the American College of Sports Medicine Foundation Plus One Active Research Grant on Wellness Using Internet Technology.
References
- 1.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23(6):694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 3.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85 (Suppl 3):109–115. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane JL, Lloyd DG, Buttfield A, Seward H, McGivern J. Characteristics of anterior cruciate ligament injuries in Australian football. J Sci Med Sport. 2007;10(2):96–104. doi: 10.1016/j.jsams.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 7.Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, Fowler PJ. Occult osteochondral lesions after anterior cruciate ligament rupture: six-year magnetic resonance imaging follow-up study. Am J Sports Med. 1999;27(4):489–494. doi: 10.1177/03635465990270041301. [DOI] [PubMed] [Google Scholar]
- 8.Ferretti A, Papandrea P, Conteduca F, Mariani PP. Knee ligament injuries in volleyball players. Am J Sports Med. 1992;20(2):203–207. doi: 10.1177/036354659202000219. [DOI] [PubMed] [Google Scholar]
- 9.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 10.Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech. 2006;21(1):33–40. doi: 10.1016/j.clinbiomech.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Fowler PJ, Regan WD. The patient with symptomatic chronic anterior cruciate ligament insufficiency: results of minimal arthroscopic surgery and rehabilitation. Am J Sports Med. 1987;15(4):321–325. doi: 10.1177/036354658701500405. [DOI] [PubMed] [Google Scholar]
- 12.Fung DT, Hendrix RW, Koh JL, Zhang LQ. ACL impingement prediction based on MRI scans of individual knees. Clin Orthop Relat Res. 2007;460:210–218. doi: 10.1097/BLO.0b013e31804d2339. [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36(8):1476–1483. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- 14.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three- dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 15.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 16.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 17.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DL, Bealle DP, Brand JC, Jr, Nyland J, Caborn DN. The effect of a geographic lateral bone bruise on knee inflammation after acute anterior cruciate ligament rupture. Am J Sports Med. 2000;28(2):152–155. doi: 10.1177/03635465000280020301. [DOI] [PubMed] [Google Scholar]
- 19.Jung HJ, Fisher MB, Woo SL. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:9. doi: 10.1186/1758-2555-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan PA, Walker CW, Kilcoyne RF, Brown DE, Tusek D, Dussault RG. Occult fracture patterns of the knee associated with anterior cruciate ligament tears: assessment with MR imaging. Radiology. 1992;183(3):835–838. doi: 10.1148/radiology.183.3.1584943. [DOI] [PubMed] [Google Scholar]
- 21.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 22.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32(4):395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 24.Lin CF, Gross M, Ji C, et al. A stochastic biomechanical model for risk and risk factors of non-contact anterior cruciate ligament injuries. J Biomech. 2009;42(4):418–423. doi: 10.1016/j.jbiomech.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Malone TR, Hardaker WT, Garrett WE, Feagin JA, Bassett FH. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J South Orthop Assoc. 1993;2:36–39. [Google Scholar]
- 26.Mandelbaum BR, Silvers HJ, Watanabe D, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing the incidence of ACL injuries in female athletes: two-year follow up. Am J Sports Med. 2005;33(6):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 27.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 28.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 29.Markolf KL, Graff-Radford A, Amstutz HC. In vivo knee stability: a quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60(5):664–674. [PubMed] [Google Scholar]
- 30.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee: the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58(5):583–594. [PubMed] [Google Scholar]
- 31.McLean SG, Su A, van den Bogert AJ. Development and validation of a 3-D model to predict knee joint loading during dynamic movement. J Biomech Eng. 2003;125:864–874. doi: 10.1115/1.1634282. [DOI] [PubMed] [Google Scholar]
- 32.McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103(901):537–539. [PubMed] [Google Scholar]
- 33.Mink JH, Deutsch AL. Occult cartilage and bone injuries of the knee: detection, classification, and assessment with MR imaging. Radiology. 1989;170(3 Pt 1):823–829. doi: 10.1148/radiology.170.3.2916038. [DOI] [PubMed] [Google Scholar]
- 34.Most E, Axe J, Rubash H, Li G. Sensitivity of the knee joint kinematics calculation to selection of flexion axes. J Biomech. 2004;37(11):1743–1748. doi: 10.1016/j.jbiomech.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk? athletes. BMC Musculoskelet Disord. 2007;8:39. doi: 10.1186/1471-2474-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 37.Pandy MG, Shelburne KB. Dependence of cruciate-ligament loading on muscle forces and external load. J Biomech. 1997;30(10):1015–1024. doi: 10.1016/s0021-9290(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 38.Portney LG, Watkins MP. Foundations of Clinical Research. Norwalk, Connecticut: Appleton & Lange; 2000. [Google Scholar]
- 39.Quatman CE. Anterior Cruciate Ligament Injury Mechanisms in Female Athletes: A Finite Element Investigation [thesis] Toledo, Ohio: Engineering Center for Orthopaedic Research Excellence, University of Toledo; 2009. [Google Scholar]
- 40.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quatman CE, Quatman-Yates CC, Hewett TE. A ‘plane’ explanation of anterior cruciate ligament injury mechanisms: a systematic review. Sports Med. 2010;40(9):729–746. doi: 10.2165/11534950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Quatman CE, Quatman CC, Hewett TE. Prediction and prevention of musculoskeletal injury: a paradigm shift in methodology. Br J Sports Med. 2009;43(14):1100–1107. doi: 10.1136/bjsm.2009.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders TG, Medynski MA, Feller JF, Lawhorn KW. Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. Radiographics. 2000;20(Spec No):S135–S151. doi: 10.1148/radiographics.20.suppl_1.g00oc19s135. [DOI] [PubMed] [Google Scholar]
- 44.Sell TC, Ferris CM, Abt JP, et al. Predictors of proximal tibia anterior shear force during a vertical stop-jump. J Orthop Res. 2007;25(12):1589–1597. doi: 10.1002/jor.20459. [DOI] [PubMed] [Google Scholar]
- 45.Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. J Athl Train. 2008;43(4):396–408. doi: 10.4085/1062-6050-43.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin CS, Chaudhari AM, Andriacchi TP. The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech. 2007;40(5):1145–1152. doi: 10.1016/j.jbiomech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Shultz SJ, Shimokochi Y, Nguyen AD, Schmitz RJ, Beynnon BD, Per-rin DH. Measurement of varus-valgus and internal-external rotational knee laxities in vivo part I: assessment of measurement reliability and bilateral asymmetry. J Orthop Res. 2007;25:981–988. doi: 10.1002/jor.20397. [DOI] [PubMed] [Google Scholar]
- 48.Sohn DH, Balasubramanian S, Demetropoulos C, Yang K, Guettler J, Jurist KA. Biomechanical verification that PCL reconstruction is unnecessary in the muscle-stabilized knee. Orthopedics. 2010;33(5) doi: 10.3928/01477447-20100329-12. [DOI] [PubMed] [Google Scholar]
- 49.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20(4):382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 50.van den Bogert AJ, McLean SG. Comment on “a stochastic biomechanical model for risk and risk factors of non-contact anterior cruciate ligament injuries”. J Biomech. 2009;42(11):1778–1779. doi: 10.1016/j.jbiomech.2009.03.055. author reply 1780–1782. [DOI] [PubMed] [Google Scholar]
- 51.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41 (Suppl 1):i47–i51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu B, Lin CF, Garrett WE. Lower extremity biomechanics during the landing of a stop-jump task. Clin Biomech (Bristol, Avon) 2006;21(3):297–305. doi: 10.1016/j.clinbiomech.2005.11.003. [DOI] [PubMed] [Google Scholar]