Abstract
Previous studies showed that dietary calcium D-glucarate (CG) inhibited benzo[a]pyrene (B[a]P)-induced A/J mouse lung tumorigenesis, suppressing cell proliferation and chronic inflammation and inducing apoptosis during late post-initiation stages. The present study aimed to investigate changes in the homeostasis of cytokines in blood serum, as well as alterations in biomarkers of inflammation and apoptosis in lung tissue caused by dietary CG during early post-initiation stages of B[a]P-induced lung tumorigenesis. Two doses of 3 mg of B[a]P were given intragastrically to A/J mice 2 weeks apart. CG administration in the AIN-93G diet (2 and 4%, w/w) commenced at 2 weeks following the second dose of B[a]P. The levels of interleukin (IL)-6, IL-10 and tumor necrosis factor α (TNFα) in blood serum were investigated by FCAP array analysis. Two weeks after the second dose of B[a]P, approximately 8- and 28-fold increases of TNFα and IL-6, respectively, occurred in the blood serum and an approximately 16% decrease of IL-10 levels compared to the untreated control group was noted. At 4 weeks after the second dose of B[a]P and after 2 weeks of CG administration in the diet, the 2 and 4% CG diets significantly reduced the levels of IL-6 and TNFα (by 70 and 33%, respectively). In a dose-related manner, the diets also increased the level of anti-inflammatory cytokine IL-10 compared to the B[a]P group. At 6 weeks after the second dose of B[a]P, the cytokine levels in the serum continued to show a decrease in the CG-treated groups. These events are accompanied by an increased level of cleaved caspase-9 product with a molecular weight of 37 kDa. In conclusion, dietary D-glucarate decreases the level of proinflammatory cytokines, increases the level of the anti-inflammatory cytokine IL-10 during early post-initiation stages of B[a]P-induced lung tumorigenesis in A/J mice and affects apoptotic induction.
Keywords: lung cancer, tumorigenesis, inflammation, apoptosis, cytokines
Introduction
Lung cancer is the most frequently diagnosed major neoplastic disease and the most common cause of cancer mortality in males and females in the United States, and worldwide (1–3). Cigarette smoking is the primary cause of lung cancer (1,4). The exact molecular alterations promoted by smoking in lung tissue that result in lung cancer development and impact survival have yet to be elucidated. Lung cancers may appear as non-small cell lung carcinomas (adenocarcinomas, squamous and large cell carcinomas), small cell carcinomas or other less frequent mixed types (5). In 2006, the age-standardized mortality rates for lung cancer in Europe were 64.8 for males and 15.1 for females per 100,000 individuals (European standard) (1). Concomitantly, the US standard age-adjusted mortality rates were 67.5 for males and 40.2 for females per 100,000 persons (2). Patients with early-stage non-small cell lung cancer (NSCLC), who undergo curative resection, have a substantial risk of developing metastases (6). The identification of sensitive and specific biomarkers predictive of unfavorable prognosis may therefore have a clinically significant impact on NSCLC treatment strategies. Such biomarkers may also aid in the selection of patients for further therapy (7–16).
The lung, as a crucial and specialized organ that uptakes oxygen and releases carbon dioxide, is simultaneously vulnerable to numerous insults from inhaled toxic agents. Such unrelenting physical, chemical and biological insults, including pollutants, toxins, carcinogens and gases, render the lung susceptible to varying degrees of oxidative injury. Inhaled toxic agents stimulate the generation of reactive oxygen/nitrogen species (ROS/RNS), which in turn provoke inflammatory responses and cause the release of proinflammatory cytokines and chemokines. These biomolecules subsequently stimulate the influx of polymorphonuclear leukocytes (PMNs) and monocytes into the lung, in order to combat the invading pathogens or toxic agents. However, persistent inhalation of toxic agents and other pollutants may induce chronic inflammation and lung injury. During constant inflammation, ROS/RNS generation is enhanced, resulting in recurring DNA damage, activation of proto-oncogenes by initiating signal transduction pathways and inhibition of apoptosis. Therefore, it is likely that during chronic inflammation constant release of proinflammatory cytokines and chemokines in the lung predisposes individuals to lung cancer (17,18).
Labilization of lysosomal enzymes is often associated with the general process of inflammation (19). The present study investigated the effect of a tobacco smoking-related carcinogen, benzo[a]pyrene (B[a]P), on the activity of the lysosomal enzyme-β-glucuronidase (βG) and its correlation with other biomarkers of inflammation in the lung. Moderate oxidative stress (20) was found to rapidly induce partial lysosomal rupture, followed by apoptosis and further loss of intact lysosomes. The release of hydrolytic enzymes from the lysosomal compartment to the cytosol is a crucial initiating event in the apoptotic process (20). Regarding inflammation, βG is known to be released from granulocytes, including neutrophils (21). It has been reported that the levels of proinflammatory cytokines, such as interleukin 1 (IL-1) and C-reactive protein, circulating markers of inflammation, correlated well with βG activity in the serum of patients with inflammatory disorders (20). Published data (22) add in vivo human evidence to previous animal data (23–27) that βG is a potential biomarker useful for monitoring pulmonary inflammation caused by human exposure to coal dust, asbestos fibers, crystalline silica dust, diesel engine exhaust, and tobacco smoke. In a murine model, concomitant with the morphologic changes noted with the increasing duration of tobacco smoke exposure, the alveolar and pulmonary macrophage population size increased approximately 8-fold compared to the control values. Following smoke exposure, lung βG activities were increased to more than double those of the control animals. It is suggested that macrophage changes and elevated activity of lung βG reflect initial alterations that lead to permanent pulmonary pathology (26).
B[a]P was shown to suppress systemic immunity in experimental animals, which may contribute to the growth of the chemically-induced tumors. However, its effects on lung immunity after inhalation, a common route for human exposure in urban areas, has yet to be determined. Kong et al (28) examined intratracheal B[a]P instillation on lung natural killer (NK) cell activity, alveolar macrophage (AM) functions and susceptibility to tumor cell challenge in Fischer 344 rats. Although exposure to B[a]P did not alter cell recovery after lavage, histologic changes were observed as evidenced by granulomatous inflammation and squamous metaplasia. A marked suppression of tumor necrosis factor α (TNFα) and IL-1 secretion in LPS-and/or cytokine-activated alveolar macrophages occurred. IL-1 was found to be reduced through day 100 following exposure. B[a]P exposure yielded the increased growth of MADB106 metastatic tumor cells in the lung.
Our long-term aim was to investigate the potential role of the immune system impairment, including the role of βG and D-glucaric acid (GA), in the early detection and prevention of lung cancer. GA is a natural, apparently non-toxic compound produced in small amounts by mammals, including humans, as well as by certain plants (29). GA is an end product of the D-glucuronic acid pathway in mammals (29). The oxidation of D-glucuronic acid or its lactone results in products that hydrolyze spontaneously in aqueous solution to yield the potent βG inhibitor, D-glucaro-1,4-lactone (1,4-GL), non-inhibitory D-glucaro-1,4-lactone -glucaro-6,3-lactone (6,3-GL) and GA, all of which are excreted in urine (30). GA was identified as a normal constituent of urine (30) and serum (31). GA is considered to be a major serum organic acid (32).
However, significant differences in the urinary excretion of GA were reported (33,34) in apparently healthy individuals. Urinary excretion of GA increases following exposure to xenobiotics. Thus, it is an indirect indicator of hepatic microsomal enzyme induction by xenobiotic agents (35). GA excretion in cancer patients and tumor-bearing rats was found to be significantly lower than in healthy controls (36). In mice with experimental tumors and in cancer patients, an uninvolved liver exhibited a reduced GA level (29). The cancer tissue itself lacked the GA synthesizing system (29).
The physiological function of GA has yet to be elucidated. The formation of 1,4-GL, an inhibitor of βG, from one of the products of its hydrolytic action may be regarded as a negative feedback mechanism (37,38). Accumulation in the body of free aglycons, normally excreted as glucuronides, may not only be aggravated by the elevated βG activity, but also by the depressed synthesis of 1,4-GL. This lactone does not directly affect the UDP-glucuronosyltransferase activity (39). However, by inhibiting βG, net glucuronidation is enhanced. Therefore, this lactone shows potential in the chemoprevention of cancer (40–42). Mounting evidence (41,42) from short and long-term models shows potential control of the various stages of the carcinogenic process by the βG inhibitor D-glucaro-1,4-lactone and its precursors, such as D-glucaric acid salts (D-glucarates) and 2,5-di-O-acetyl-D-glucaro-1,4:6,3-dilactone (DAGDL) (43). D-glucaric acid and 1,4-GL are found in certain vegetables and fruits (44,45). Thus, the consumption of fruits and vegetables naturally rich in D-glucaric acid, or self-medication with D-glucaric acid derivatives, offers a novel chemopreventive approach.
The present study aimed to investigate the changes in the homeostasis of cytokines in the blood serum, as well as the level of biomarkers of inflammation and apoptosis in the lung tissue caused by dietary calcium D-glucarate (CG) during early post-initiation stages of B[a]P-induced lung tumorigenesis. The inflammatory microenvironment is thought to play a key part in tumorigenesis, aided by the presence of proinflammatory cytokines. Cytokines are released in response to a diverse range of cellular stresses, such as infection and inflammation, including response to carcinogens (46–49). Interleukins are crucial biomolecules that regulate inflammatory and immune responses. Interleukin 10 (IL-10) suppresses proinflammatory mediators such as IL-1 and IL-6. The effects of IL-10 are generally thought to be immunosuppressive, inhibit the synthesis of a variety of proinflammatory cytokines and down-regulate the immune response. Numerous proinflammatory cytokines are found in the microenvironment of various tumors and cytokines, such as TNFα which promote the transformation of pre-cancerous cells to malignant ones (47). Proinflammatory cytokines also affect later stages of tumor progression, including angiogenesis and metastasis. The longer the inflammation persists, the higher the risk of associated carcinogenesis.
Our overall hypothesis is that D-glucarate deficiency and/or high βG levels are markers of an increased risk for lung cancer, and that D-glucarate supplementation reduces the risk of lung cancer by suppressing target cell proliferation and inflammation and inducing apoptosis (41,42,50,51).
Materials and methods
Reagents and diets
B[a]P was obtained from Aldrich Chemical Co. (Milwaukee, WI, USA). CG and 1,4-GL were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Experimental diets, based on the AIN-93G diet, containing 70 or 140 mmol/kg diet of CG (2 and 4%, w/w) were purchased from Dyets Inc. (Bethleham, PA, USA) and the AIN-93G diet was used as the control diet.
Animal care and treatment
Female A/J mice, 5–6 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The animals were housed under conditions of constant temperature and humidity, and were maintained on a 12-h light/dark cycle with ad libitum access to food and water. The animal procedures were performed in accordance with the NIH Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center, San Antonio, USA. Briefly, 40 mice were randomly allocated into four groups (10 mice per group): group 1, vehicle-treated; group 2, B[a]P-treated; group 3, B[a]P-treated and maintained on a 2% calcium glucarate diet; and group 4, B[a]P-treated and maintained on a 4% calcium glucarate diet. The mice were treated by gavage with 3 mg B[a]P in cotton seed oil. Two doses of 3 mg of B[a]P were given intragastrically to A/J mice 2 weeks apart. CG administration in the AIN-93G diet (2 and 4%, w/w) commenced at 2 weeks after the second dose of B[a]P. No change occurred in the calcium content of CG diets in comparison to the control AIN-93G diet. The mice were sacrificed at 2, 4, 6 and 10 weeks after the second dose of B[a]P. Immediately upon sacrifice by CO2 asphyxiation, blood was collected by cardiac puncture and the lungs were perfused with cold phosphate-buffered saline and harvested. Normal lungs from vehicle-treated mice and the lungs excised from carcinogen-treated mice were frozen in liquid nitrogen and stored at −80°C.
Cytokine analysis
Whole blood samples, collected in sterile tubes, were allowed to coagulate for ~2 h at 4°C prior to centrifugation. The sera were preserved at −70°C until cytokines measurement. Cytometric bead array mouse inflammation kit (BD Biosciences, San Diego, CA, USA) was used according to the manufacturer's instructions to simultaneously detect mouse IL-6, IL-10, interleukin 12p70 (IL-12p70), interferon-γ (IFN-γ) and TNFα in the serum. Briefly, dilution of the standards and mixed mouse inflammation capture beads were prepared according to the manufacturer's specifications. The reagents and test samples were transferred to the appropriate assay tubes. A mouse inflammation PE detection reagent was then added to the assay tubes which were incubated for 2 h at room temperature. Following incubation, 1 ml of wash buffer was added and the reaction mixture was centrifuged at 200 × g for 5 min. The supernatant was discarded and 300 μl of wash buffer was added to resuspend the bead pellets. The samples were analyzed at the University of Texas Cytometric Core Facility, San Antonio, USA. The results were analyzed using the FCAP array software (BD Biosciences).
Immunohistochemistry
The tissues were prepared for histological evaluation by using conventional paraffin sections and H&E staining at the University of Texas Pathology Core Facility, San Antonio, USA. The murine lung sections were deparaffinized and rehydrated. Endogenous peroxidase activity was inhibited with 3% H2O2, followed by antigen retrieval. The slides were then blocked with 2.5% normal goat serum (Vector Laboratories, Burlingame, CA, USA). For the immunocytochemical localization of cleaved caspase-9 and BrdU in the paraffin sections, the avidin-biotin complex technique (Vector Laboratories) with 3,3′-diamino-benzidine as a peroxidase substrate (Sigma) were employed, according to the manufacturer's instructions. Cleaved caspase-9 antibody detects the endogenous level of the 37 kDa subunit of mouse caspase-9 only after cleavage at aspartic acid 353. Anti-cleaved caspase-9 and anti-BrdU antibodies were purchased from Cell Signaling (Danvers, MA, USA) and Lab Vision (Fremont, CA, USA), respectively. At least 10 sections on each slide were viewed, counted and photographed using an Olympus BX41 microscope.
Statistical analysis
To verify the statistical significance of the results, a two-tailed unpaired Student's t-test was conducted. The B[a]P group was compared to the control group and the CG groups were compared to the B[a]P group. P<0.05 was considered to be statistically significant. The results are expressed as the mean ± SD and were repeated an average of three times, unless otherwise stated.
Results
The present study aimed to investigate the changes in the homeostasis of cytokines in the blood serum caused by dietary CG and the diversities in other biomarkers of inflammation during early post-initiation stages of B[a]P-induced lung tumorigenesis. The groups treated with B[a]P and fed CG diets were compared to the positive controls, treated only with B[a]P. The B[a]P group was compared to the untreated control group.
Interleukin 6
IL-6 is a cytokine originally known to be a regulator of immune and inflammatory responses. An elevated expression of IL-6 was found to be present in multiple epithelial tumors (48). IL-6 not only regulates cell proliferation, cell survival and metabolism, but may also act on signaling, suggesting it plays a role in tumorigenesis. IL-6 levels increased 28-fold 2 weeks after the second dose of B[a]P in comparison to the negative group (Fig. 1A). Four weeks after the second dose of B[a]P, included in the diet of 2 and 4% CG, a decrease of the IL-6 level by 74.3 and 70.6%, respectively, was observed (Fig. 1B). Six weeks after the second dose of B[a]P was administered the IL-6 level in the serum was still lower compared to the B[a]P control. The 2 and 4% CG diet reduced the IL-6 level by 41.4 and 49.5%, respectively (Fig. 1C). As shown in Fig. 1D, 10 weeks after the second dose of B[a]P the 2 and 4% CG diets reduced the IL-6 level by 37.4 and 76.3%, respectively, compared to the B[a]P group (Fig. 1D).
Figure 1.
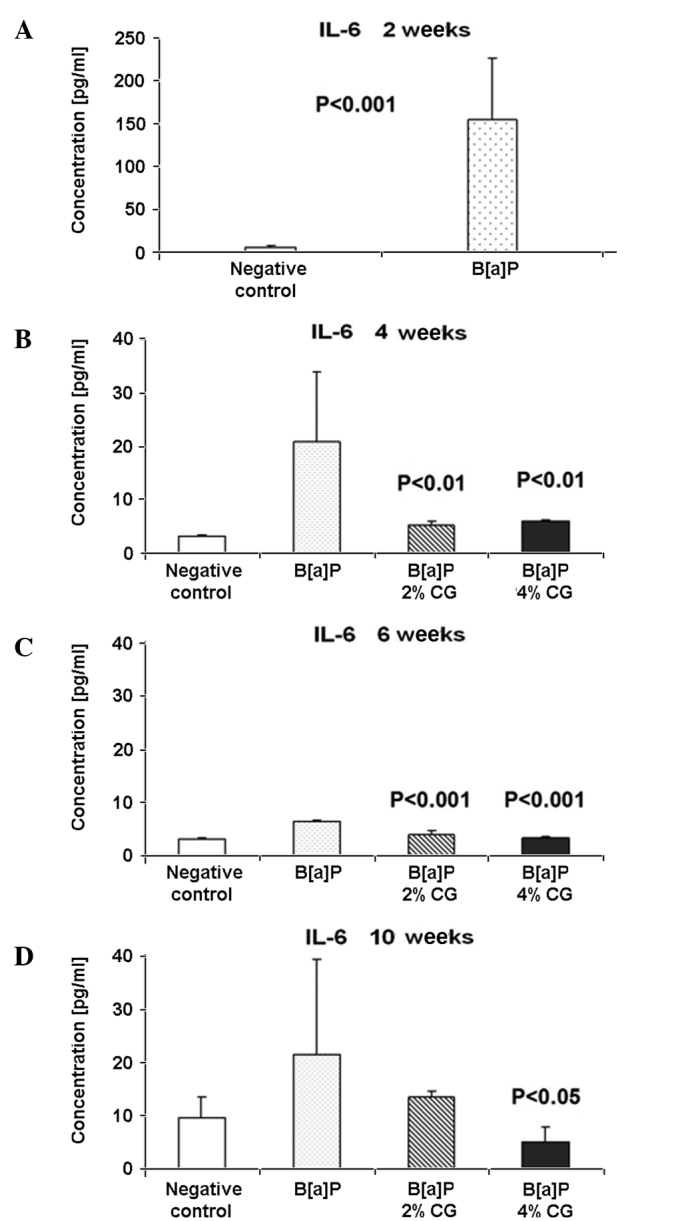
Dietary D-glucarate effect on the serum level of interleukin (IL)-6 during the early post-initiation stages of benzo[a]pyrene (B[a]P)-induced lung tumorigenesis in A/J mice. The expression of proinflamatory IL-6 was significantly elevated in the B[a]P group at 2 weeks after the second dose of B[a]P. The decrease of IL-6 is also statistically significant in the remaining calcium D-glucarate (CG) CG groups. Statistical analysis was performed using the Student's t-test. Values are the means ± SD. The B[a]P group was compared to the control group and the CG groups were compared to the B[a]P group.
Tumor necrosis factor α
The pathways linking inflammation and promotion of tumor growth are not well characterized, but it is well known that TNFα is a significant mediator of inflammation. Despite the apoptotic induction of pathological cells at the site of inflammation, TNFα stimulates the growth of fibroblasts. However, 2 weeks after the second dose of B[a]P, the TNFα level increased by ~8-fold, compared to the untreated control group (Fig. 2A). Four weeks after the last dose of B[a]P and following CG administration in the diet for 2 weeks, both 2 and 4% CG reduced the TNFα level by 42.4 and 33.8%, respectively (Fig. 2B). Six weeks following the second dose of B[a]P, the TNFα level in the serum was reduced in the CG-treated groups. The 2% CG diet decreased the TNFα level by 16.2% and the 4% CG diet by 28.8%, in comparison to the B[a]P group (Fig. 2C). Ten weeks after the second dose of B[a]P, the 4% CG diet reduced the TNFα level by 11.3% compared to the B[a]P group. Almost no changes occurred in the TNFα level in the case of the 2% CG diet (Fig. 2D).
Figure 2.
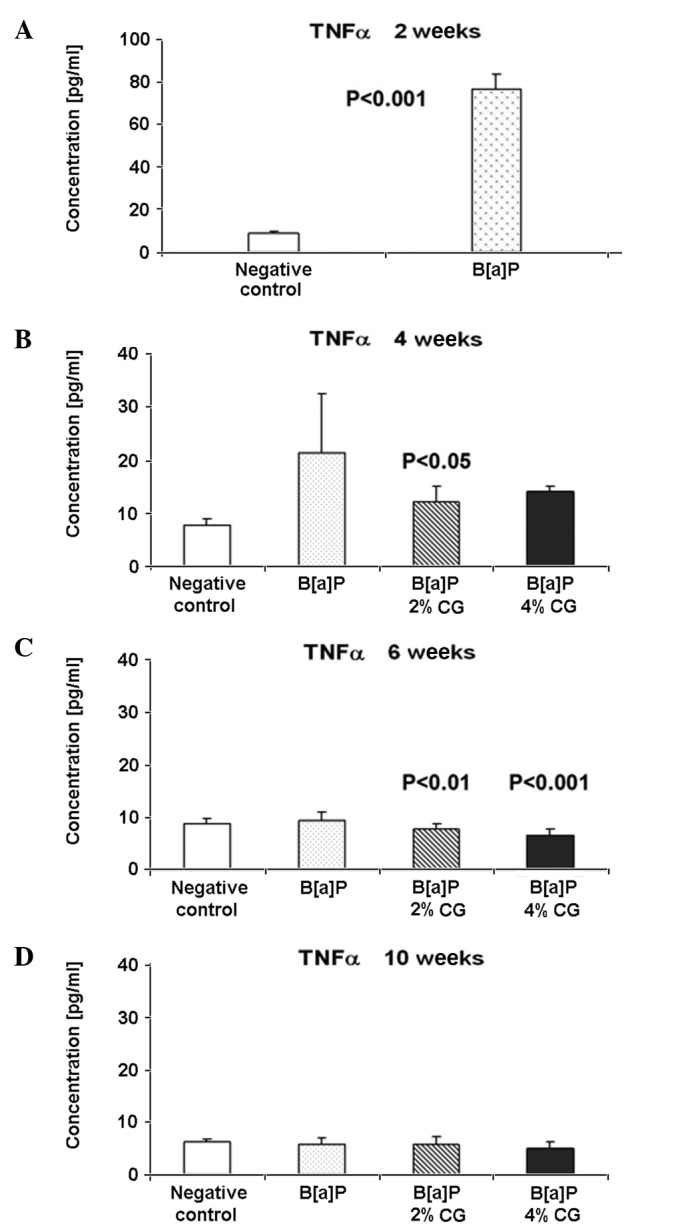
Dietary D-glucarate effect on the serum level of tumor necrosis factor α (TNFα) during early post-initiation stages of benzo[a]pyrene (B[a]P)-induced lung tumorigenesis in A/J mice. Student's t-test was used to compare the difference between the groups. Values are the means ± SD. The B[a]P group was compared to the control group and the calcium D-glucarate groups were compared to the B[a]P group. P<0.05 was considered to be statistically significant.
Interleukin 12p70
The level of IL-12p70 was evaluated. As shown in Fig. 3A, the IL-12p70 level was 34.5% higher in the B[a]P group compared to the negative control, 2 weeks after the second dose of B[a]P. Four weeks after the second dose of B[a]P, an increase of the IL-12p70 level of 111.49% in the 2% CG diet and one of 97.9% in the 4% CG diet was noted in mice fed with 2 and 4% CG (Fig. 3B). Six weeks following the last dose of B[a]P, a decrease of the IL-12p70 level of 10.7 and 21.4% for the 2 and 4% CG diets, respectively, was observed in the serum (Fig. 3C). The IL-12p70 level was reduced 10 weeks after the last treatment with B[a]P by 23.2 and 21.1%, respectively, for the 2 and 4% CG diets (Fig. 3D).
Figure 3.
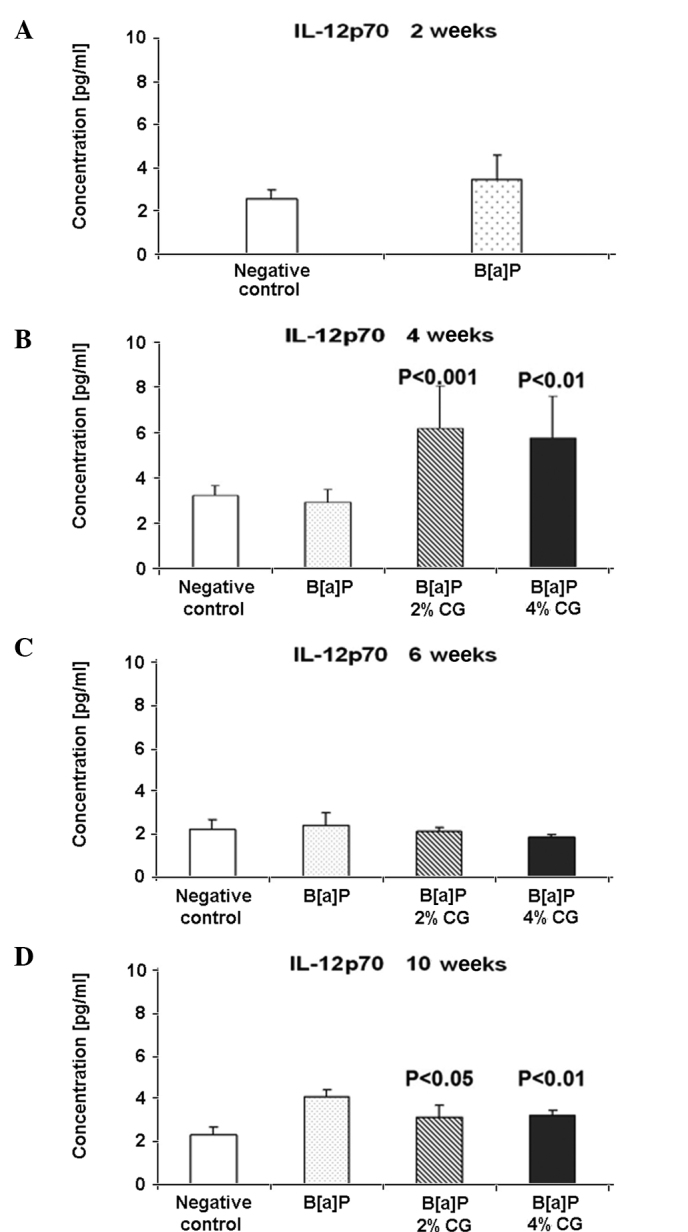
Dietary D-glucarate effect on the serum level of interleukin (IL)-12p70 during the early post-initiation stages of benzo[a]pyrene (B[a]P)- induced lung tumorigenesis in A/J mice. Two and 10 weeks after the second dose of B[a]P, statistically significant differences were observed between the calcium D-glucarate (CG) groups and the positive B[a]P group. Statistical analysis was performed using the Student's t-test. Values are the means ± SD. The B[a]P group was compared to the control group and the CG groups were compared to the B[a]P group.
Interleukin-10
In contrast various proinflammatory cytokines, IL-10 indicates potent antitumor activities. IL-10 is synthetized in monocytes and lymphocytes and is regarded as a key anti-inflammatory immune-regulating cytokine. A 16.6% decrease of the IL-10 level in the serum was observed 2 weeks after the second dose of B[a]P compared to the untreated control group (Fig. 4A). Four weeks after the second dose of B[a]P and CG administration in the diet, the 2 and 4% CG diets increased the level of anti-inflammatory cytokine IL-10, in a dose-related manner, by ~3-fold compared to the B[a]P group. The IL-10 level was elevated by 249.4 and 288.5% for the 2 and 4% CG in the diet, respectively (Fig. 4B). However, six weeks after the second dose of B[a]P, the IL-10 level decreased by 51.5 and 42.1% in the cases of the 2 and 4% CG diets, respectively (Fig. 4C). Notably, the IL-10 level was below detection at 10 weeks after the second dose of B[a]P.
Figure 4.
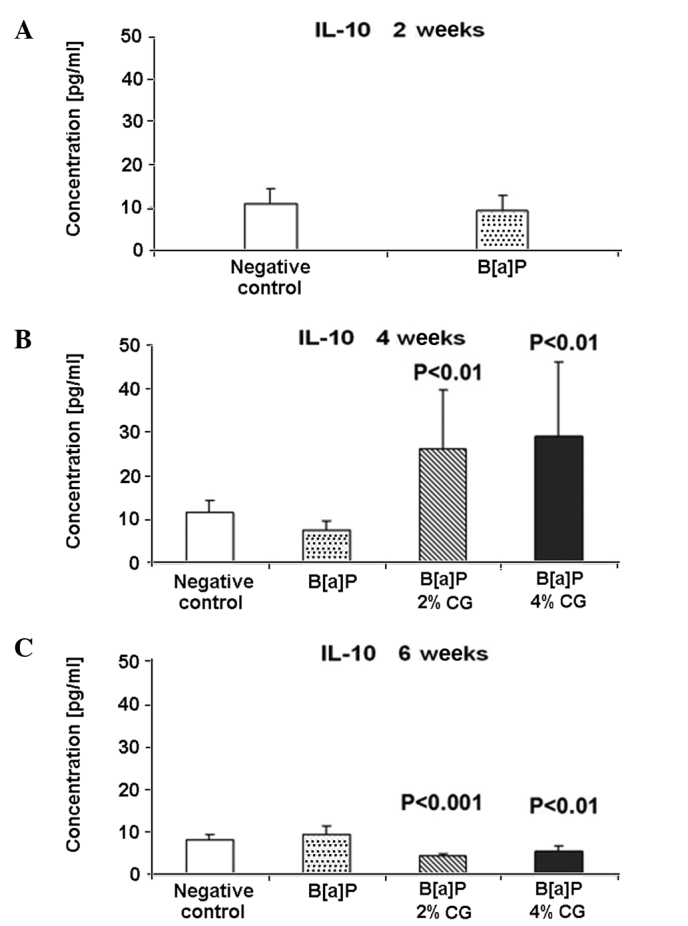
Dietary D-glucarate effect on the serum level of interleukin (IL)-10 during the early post-initiation stages of benzo[a]pyrene (B[a]P)- induced lung tumorigenesis in A/J mice. The level of IL-10 was significantly elevated in the calcium D-glucarate (CG) groups at 4 weeks after the second dose of B[a]P, and significantly reduced in the CG groups at 6 weeks after the second dose of B[a]P. Student's t-test was used to compare the difference between the groups. Values are the means ± SD. The B[a]P group was compared to the control group and the CG groups were compared to the B[a]P group. The level of IL-10 was below detection at 10 weeks after the second dose of B[a]P.
Interferon-γ
IL-12 was investigated to elucidate its correlation with IFN-γ, a cytokine that exhibits immunologic functions. Fig. 5A shows that the IFN-γ level increased by 58.4% 2 weeks after the last dose of B[a]P. CG administration in the diet 4 weeks after B[a]P treatment caused an increase of the IFN-γ level by 12.5 and 54% in the cases of the 2 and 4% CG diets, respectively (Fig. 5B). However, the same CG concentration 6 weeks after B[a]P treatment decreased the IFN-γ level of 40.4 and 36.7%, respectively (Fig. 5C). The IFN-γ level in the serum 10 weeks after the last dose of B[a]P showed an increase of 15.6 and 20.6% in the case of the 2 and 4% CG diets, respectively, compared to the B[a]P group (Fig. 5D).
Figure 5.
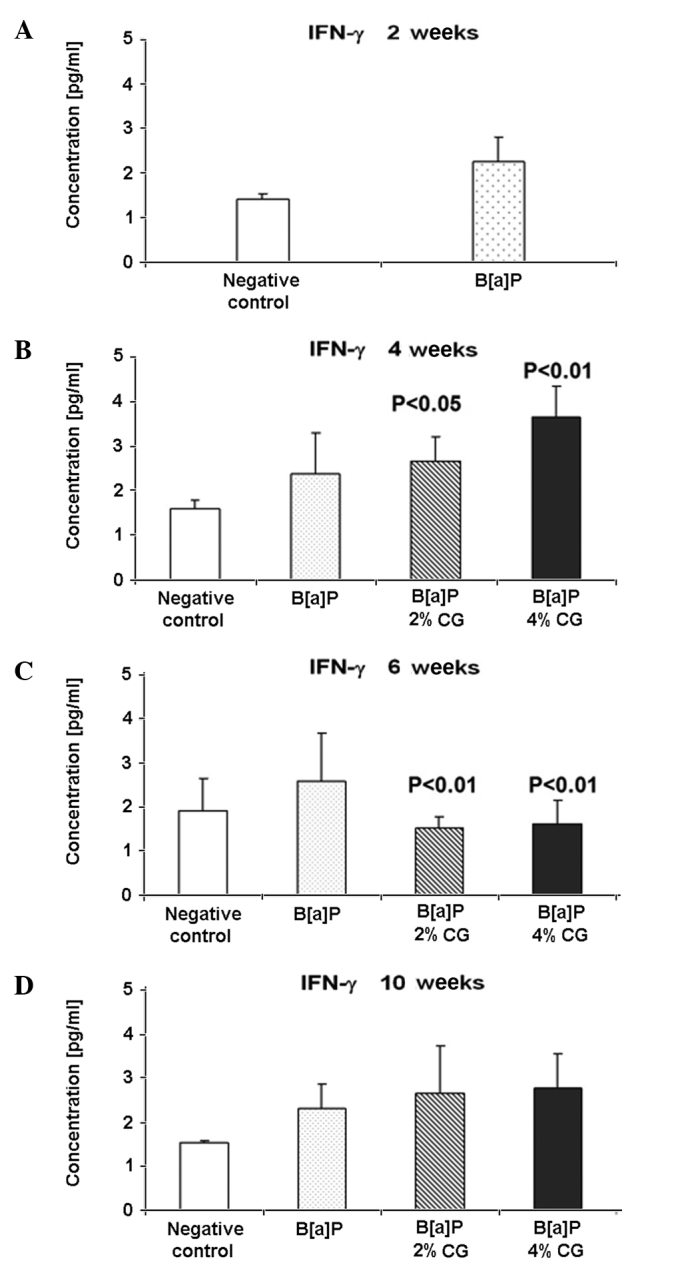
Dietary D-glucarate effect on the serum level of IFN-γ during the early post-initiation stages of benzo[a]pyrene (B[a]P)-induced lung tumorigenesis in A/J mice. The level of IFN-γ was significantly elevated in the calcium D-glucarate (CG) groups 4 weeks after the second dose of B[a]P and significantly reduced in the CG groups 6 weeks after the second dose of B[a]P. Statistical analysis was performed using the Student's t-test. Values are the means ± SD. The B[a]P group was compared to the control group and the CG groups were compared to the B[a]P group.
Caspase-9 activation
Apoptotic cells were detected by cleaved caspase-9 antibodies identifying the caspase 9 subunit with a molecular weight of 37 kDa. The percentage of lung cells stained with these antibodies 2 weeks after the second dose of B[a]P increased by 73.3% as compared to the untreated control group (Fig. 6A). Feeding the mice for 4 weeks with a diet containing 2 and 4% CG after the last dose of B[a]P elevated the percentage of cells stained with the used antibody by 12 and 7.2%, respectively (Fig. 6B). Six weeks following the second dose of B[a]P, the percentage of cells stained to yield the caspase-9 product increased by 0.1 and 8.4%, respectively, in the 2 and 4% CG diets (Fig. 6C). Notably, the highest percentage of cells immunostained with anticleaved caspase-9 antibody, i.e., 32.3 and 67.5%, was observed 10 weeks after the second dose of B[a]P after mice were fed with a diet containing 2 and 4% CG, respectively (Fig. 6D).
Figure 6.
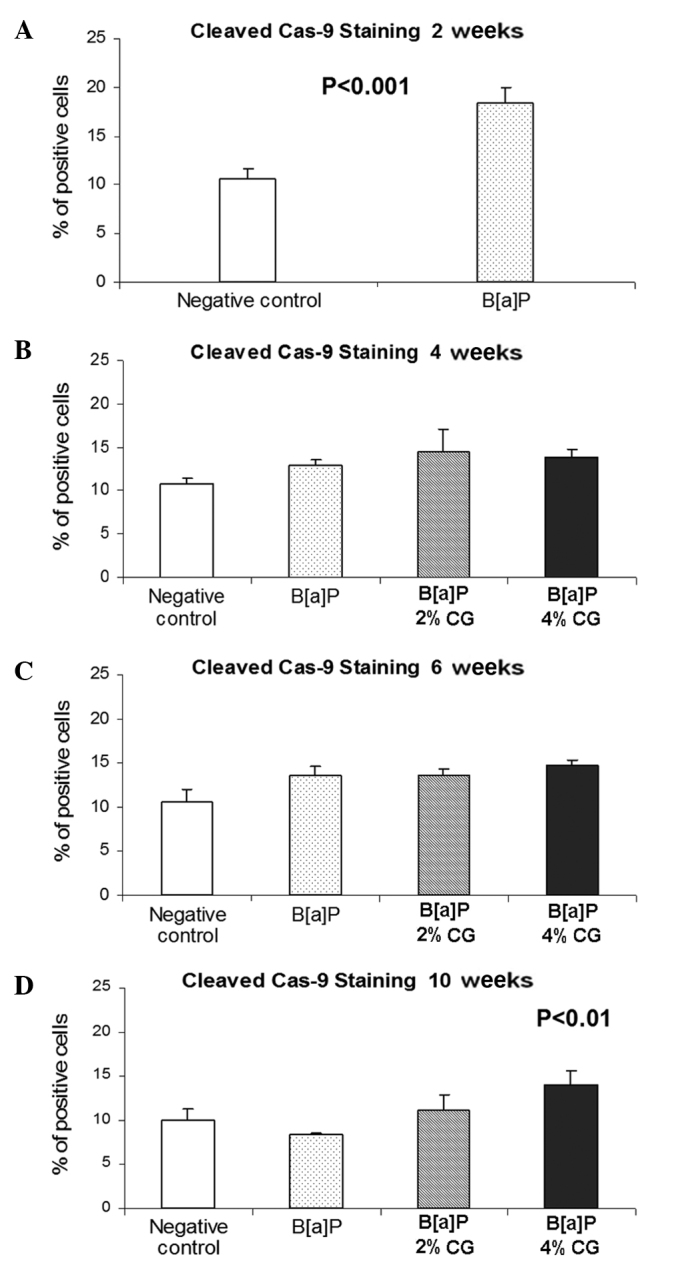
Dietary D-glucarate effect on the percentage of the cleaved caspase-9 positive cells during the early post-initiation stages of benzo[a]pyrene (B[a]P)-induced lung tumorigenesis in A/J mice. At least 10 sections on each slide were viewed and counted. Values are the means ± SD. Statistical analysis was performed using the Student's t-test. The calcium D-glucarate groups were compared to the B[a]P group and the negative control was compared to the B[a]P group. Dietary D-glucarate had a proapoptotic effect as evidenced by the significantly increased level of cleaved caspase-9-stained cells in the lung tissue.
Proliferation rate evaluation
The percentage of lung cells stained with anti-BrdU antibody 2 weeks after the last dose of B[a]P was significantly elevated, i.e., by 233% as compared to the untreated control group (Fig. 7A). Results showed that 4 weeks after the last dose of B[a]P uptake and administration of 2 and 4% CG in the diet, the population of stained cells reduced by 13.3 and 35.6%, respectively (Fig. 7B). As shown in Fig. 7C, the duration of mice CG feeding gradually decreased BrdU staining of the studied cells in the concentration of the glucaric acid derivative, by 21.2 and 49.2%, respectively, in the case of the 2 and 4% CG diets. Notably, the diet containing CG, 10 weeks after the second dose of B[a]P, significantly reduced the level of the cells stained with anti-BrdU antibody in the lung tissue by 45.2 and 67.2% in the cases of the 2 and 4% CG diets, respectively (Fig. 7D).
Figure 7.
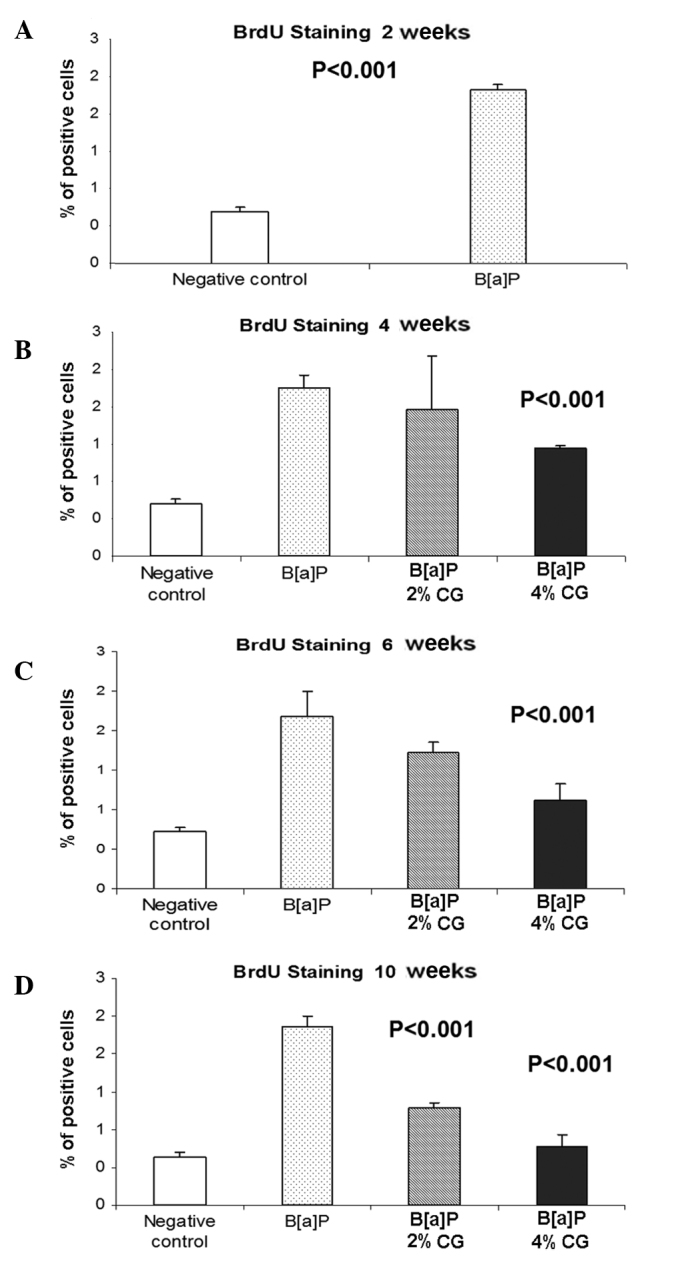
Dietary D-glucarate effect on the percentage of the BrdU-positive cells during the early post-initiation stages of benzo[a]pyrene (B[a]P)- induced lung tumorigenesis in A/J mice. Values are the means ± SD. Statistical analysis was performed using the Student's t-test. The groups fed with calcium D-glucarate were compared to the B[a]P group and the untreated group was compared to the B[a]P group. Dietary D-glucarate exhibited anti-proliferative effects as evidenced by the significantly decreased population of BrdU-stained cells in the lung tissue.
Discussion
The association of various inflammatory cell types with cancer is not a recent development. Virchow reported dense areas of inflammatory cells at the periphery of tumor as early as 1863 (49,52). Inflammatory response appears to contribute to cancer growth and spread, as well as to immune suppression and antitumor response, concomitantly with emerging paradigms for the roles of matrix metalloproteinases (MMPs) in cancer development. A number of independent studies using human clinical samples show that inflammation has an impact on epithelial cell turnover (53,54). More significantly, proliferation in the setting of chronic inflammation predisposes humans to carcinoma in the breast, large intestine, gastric mucosa and other tissues (49). Evidence regarding the importance of inflammation during neoplastic progression originates from a study concerning cancer risk among long-term users of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs). A large body of data indicates that the use of these drugs reduces colon cancer risk by 40–50% and may be preventive for lung, esophagus and stomach cancer (52,55). The mechanism(s) underlying the chemopreventive effects of NSAIDs concerns their ability to inhibit cyclooxygenases (COX-1 and COX-2). COX-2 converts arachidonic acid to prostaglandins, which in turn induces inflammatory reactions in damaged tissues (56). Thus, inflammatory cells elicit host defense and stimulate progression. Inflammation is a complex phenomenon, involving the summation of initiation and maintenance signals originating from inflammatory cells and the tissue environment (48,49).
The inflammatory response is divided into three phases: acute, sub-acute and chronic. The acute phase is characterized by rapid onset, blood vessel dilatation, edema and leukocyte infiltration. This phase may last only a few days. The sub-acute phase is characterized by further leukocyte and phagocyte infiltration, which may continue for 3–4 weeks. Chronic inflammation persists for longer periods of time and may result from the persistence of the injuring agent in the tissue. During the course of chronic inflammation, cytokines attract additional activated lymphocytes as well as other inflammatory cells to the site of insult, thereby amplifying and prolonging inflammation. The inflammatory component of developing neoplasm includes a diverse leukocyte population, i.e., macrophages, neutrophils, eosinophils and mast cells. This diverse leukocyte population is intermittently loaded with an assorted array of cytokines, cytotoxic mediators, such as ROS, serine-, cysteine- and metallo-proteinase, membrane-perforating agents and soluble mediators of cell apoptosis, such as TNFα, ILs and IFNs (54,57,58). PMNs are the most abundant circulating blood leukocytes. They provide the first line of defense against infection and release soluble chemotactic factors and proteases that alter the microenvironment and guide the recruitment of non-specific and specific immune effector cells (57).
Mast cells release inflammatory mediators and factors known to enhance angiogenic phenotypes, including heparin, heparanase, histamine, metallo- and serine proteinases, and various polypeptide growth factors, such as tumor growth factor (TGF) and vascular endothelial growth factor (VEGF) (59). Eosinophils, which possess numerous bioactive molecules in their granules, are recruited to tissue as a host defense against parasites or during allergic responses (60), but are resident in the mammary gland and contribute to morphogenesis (61). Macrophages produce a number of potent angiogenic cytokines, growth factors, neutrophil chemoattractants and proteases. Macrophage infiltration is closely associated with the depth of invasion of primary melanoma due, in part, to macrophage-regulated tumor-associated angiogenesis. Macrophages express numerous bioactive molecules, including proteases, arachidonate metabolites, TGF-α, TNFα and IL-1 (60–64). In response to macrophage expression, melanocytes express IL-8 and VEGF, thereby inducing angiogenesis through paracrine control (63,65). Notably, macrophages and eosinophils also contribute to mammary development (61). Macrophages are not unique among inflammatory cells in the potentiation of neoplastic processes. PMNs, mast cells and activated T-lymphocytes also contribute to malignancies by releasing proteases, angiogenic factors and chemokines (49). Previous data indicate that mast cells and neutrophils potentiate the actions of HPV16 oncogenes (59,65) and amplify neoplastic cell proliferation and angiogenesis largely by the release of MMP-9.
Our previous studies in the mouse lung tumorigenesis model showed that dietary CG inhibited B[a]P-induced mouse lung tumorigenesis, in part, by inhibiting the enzyme βG (66), suppressing cell proliferation and chronic inflammation, and by inducing apoptosis during the late post-initiation stages of lung tumorigenesis in A/J mice (50). The present study aimed to investigate the changes in the homeostasis of cytokines in the serum, as well as the diversities of biomarkers of inflammation and apoptosis in the lung tissue caused by dietary CG during the early post-initiation stages of B[a]P-induced lung tumorigenesis. Although 2 and 4% CG diets (w/w) exerted physiological changes in lung tissues via the decreased level of B[a]P-induced TNFα, only 4% CG in the diet significantly decreased the number of adenomas. Changes likely to occur in the level of IL-6, IL-10 and TNFα in the serum were investigated using FCAP array analysis. IL-6 was found to be a key mediator of the acute phase response. TNFα belongs to the same group of stimulators of the acute phase response, but also causes apoptosis. IL-12p70 is crucial in the immune response to microorganisms and tumors, activating NK cells and T-lymphocytes, which in turn initiates IFN-γ production and antigen-specific Th1 responses (24,25).
Two weeks after the second dose of B[a]P, an increase of the TNFα and IL-6 levels by approximately 8- and 28-fold, respectively, was observed in the serum along with an approximately 17% decrease of the IL-10 level compared to the untreated control group. An increase of the IFN-γ level of 58% and of the IL-12p70 level of 34% was simultaneously observed in the B[a]P-treated group, in comparison to the untreated group.
Four weeks after the second dose of B[a]P and CG administration in the diet for 2 weeks, the 4% CG diet reduced the IL-6 and TNFα levels, but also increased the level of anti-inflammatory cytokines IL-10, IFN-γ and IL-12p70, compared to the B[a]P group. The levels of IL-6 and TNFα decreased by 70 and 34%, while that of IL-10 increased by approximately 3-fold. The IFN-γ and IL-12p70 levels increased by 54 and 98%, respectively, in the 4% CG group. At 6 weeks after the second dose of B[a]P, the cytokine levels in the serum continued to be decreased in the CG-treated groups. The 4% CG diet reduced the level of IL-6 by 49%, TNFα by 29%, IFN-γ by 37%, IL-12p70 by 21% and the level of IL-10 was reduced by 42% compared to the B[a]P group. Ten weeks after the last treatment with B[a]P, the 4% CG diet reduced the IL-6, IL-12p70 and TNFα levels by 76, 21 and 11%, respectively, compared to the B[a]P group. The IFN-γ level in the serum 10 weeks after the last dose of B[a]P showed an increase of 21% in the case of 4% CG, compared to the B[a]P group.
The percentage of cells stained for cleaved caspase-9 at 2 weeks after the second dose of B[a]P increased by 73%, as compared to the untreated control group. At 4 weeks after the second dose of B[a]P, the 4% CG diet increased the percentage of cells stained with anti-cleaved caspase-9 antibody in the lung tissue by 7%, and at 6 weeks by 8%. Ten weeks after the second dose of B[a]P, the 4% CG diet increased the percentage of cells stained with anti-cleaved caspase-9 antibody in the lung tissue by 67%.
Regulating cell proliferation is crucial in cancer prevention, since this process plays a key role in carcinogenesis, including the initiation and promotion steps. Chemopreventive agents suppress carcinogen-induced hyper-proliferation of cells in the target organs during the initiation, as well as the post-initiation events. Therefore, effective agents usually suppress cell proliferation and inhibit the occurrence of malignant lesions (67,68). The percentage of lung cells stained with anti-BrdU antibody 2 weeks after the last dose of B[a]P increased by 233%, as compared to the untreated control group. The results showed that 4 weeks after the last dose of B[a]P uptake and administration of 4% CG in the diet the population of stained cells was reduced by 35.6%. Moreover, 6 weeks after the last dose of B[a]P, this concentration of CG diet gradually decreased the BrdU-stained cells by 49.2%. The diet containing CG, 10 weeks after the second dose of B[a]P, significantly decreased the level of cells stained with anti-BrdU antibody in the lung tissue by 67.2%.
In conclusion, dietary D-glucarate reduces the level of proinflammatory cytokines and increases the level of anti-inflammatory cytokine IL-10 during the early post-initiation stages of B[a]P-induced lung tumorigenesis in A/J mice. Dietary D-glucarate exhibits proapoptotic effects, as evidenced by the increased levels of cleaved caspase-9 in the lung tissue.
Moreover, CG may prevent lung cancer in tobacco smokers and ex-smokers by enhancing apoptosis and suppressing the acute inflammation and proliferation induced by tobacco-related carcinogens. CG supplementation has the potential to reduce the risk of lung cancer development in high-risk individuals.
Acknowledgements
This study was supported by the NIH grants RO1CA102747, RO1CA079065, P30 CA 54174-16S1 and the American Cancer Research Center and Foundation (ACRCF).
References
- 1.Ferlay J, Autire P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Top Ten Cancer. 2006. United States Cancer Statistics. [Google Scholar]
- 3.Landi MT, Consonni D, Rotunno M, et al. Environment and Genetics in Lung cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health. 2008;8:203–213. doi: 10.1186/1471-2458-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K, Bowman ED, Simon R, et al. Laser capture microdissection and microarray expression analysis of lung adenocarcinoma reveals tobacco smoking- and prognosis-related molecular profiles. Cancer Res. 2002;62:3244–3250. [PubMed] [Google Scholar]
- 5.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adeno-carcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 6.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 8.Garber ME, Troyanskaya OG, Schluens K, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adeno-carcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 11.Endoh H, Tomida S, Yatabe Y, et al. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J Clin Oncol. 2004;22:811–819. doi: 10.1200/JCO.2004.04.109. [DOI] [PubMed] [Google Scholar]
- 12.Xi L, Lyons-Weiler J, Coello MC, et al. Prediction of lymph node metastasis by analysis of gene expression profiles in primary lung adenocarcinomas. Clin Cancer Res. 2005;11:4128–4135. doi: 10.1158/1078-0432.CCR-04-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerson M, Carbone D. Genomic and proteomic profiling of lung cancers: lung cancer classification in the age of targeted therapy. J Clin Oncol. 2005;23:3219–3226. doi: 10.1200/JCO.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 14.He P, Varticovski L, Bowman ED, et al. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuro-endocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1169–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. New England J Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 17.Reddy SP. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr Mol Med. 2008;8:376–383. doi: 10.2174/156652408785160925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, Sharma S, Dubinett SM. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5:811–815. doi: 10.1513/pats.200809-100TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giri SN, Hollinger MA. Effect of cadmium on lung lysosomal enzymes in vitro. Arch Toxicol. 1995;69:341–345. doi: 10.1007/s002040050181. [DOI] [PubMed] [Google Scholar]
- 20.Shimoi K, Saka N, Nozawa R, et al. Deglucuronidation of a flavonoid, luteolin monglucuronide, during inflammation. Drug Metab Disp. 2001;29:1521–1524. [PubMed] [Google Scholar]
- 21.Marshall T, Shult P, Busse WW. Release of lysosomal enzyme β-glucuronidase from isolated human eosinophils. J Allergy Clin Immunol. 1988;82:550–555. doi: 10.1016/0091-6749(88)90964-5. [DOI] [PubMed] [Google Scholar]
- 22.Cobben NA, Drent M, De Vries J, Wouters EF, van Dieijen-Visser MP, Henderson RF. Serum β-glucuronidase activity in a population of ex-coalminers. Clin Biochem. 1999;32:659–664. doi: 10.1016/s0009-9120(99)00070-3. [DOI] [PubMed] [Google Scholar]
- 23.DiMatteo M, Antonini JM, van Dyke K, Reasor MJ. Characteristics of the acute phase pulmonary response to silica in rats. J Toxicol Environ Health. 1996;47:93–108. doi: 10.1080/009841096161951. [DOI] [PubMed] [Google Scholar]
- 24.DiMatteo M, Reasor MJ. Modulation of silica-induced pulmonary toxicity by dexamethasone-containing liposomes. Toxicol Appl Pharmacol. 1997;142:411–421. doi: 10.1006/taap.1996.8057. [DOI] [PubMed] [Google Scholar]
- 25.Hirano S, Shimada T, Osugi J, Kodama N, Suzuki KT. Pulmonary clearance and inflammatory potence intratracheally instilled or acutely inhaled nickel sulfate in rats. Arch Toxicol. 1994;68:548–554. doi: 10.1007/s002040050112. [DOI] [PubMed] [Google Scholar]
- 26.Matulionis D, Traurig HH. In situ response of lung macrophages and hydrolase activities to cigarette smoke. Lab Invest. 1977;37:314–326. [PubMed] [Google Scholar]
- 27.Pappas P, Sotiropoulou M, Karamanakos P, et al. Acute-phase response to benzo[a]pyrene and induction of rat ALDH3A1. Chem Bil Interac. 2003;144:55–62. doi: 10.1016/s0009-2797(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 28.Kong LY, Luster MI, Dixon D, O'Grady J, Rosenthal GJ. Inhibition of lung immunity after tracheal instillation of benzo[a]pyrene. Am J Resp Cit Care Med. 1994;150:1123–1129. doi: 10.1164/ajrccm.150.4.7921446. [DOI] [PubMed] [Google Scholar]
- 29.Levy G, Conchi J. Glucuronic Acid: Free and Combined. Academic Press; New York: 1966. β-Glucuronidase and the hydrolysis of glucuronides; pp. 301–364. [Google Scholar]
- 30.Marsh CA. Metabolism of D-glucarolactone in mammalian systems. Identification of D-glucaric acid as a normal constituent of urine. Biochem J. 1963;86:77–86. doi: 10.1042/bj0860077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui M, Fukuo A, Watanabe Y, Wanibe T, Okada M. Studies on the glucaric acid pathway in the metabolism of D-glucuronic acid in mammals. IV. Fluorometric method for the determination of D-glucaric acid in serum. Chem Phar Bull. 1972;20:845–848. doi: 10.1248/cpb.20.845. [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal HJ, Lucuta VL, Blumenthal DC. Specific enzymic assay for D-glucarate in human serum. Anal Biochem. 1990;185:286–293. doi: 10.1016/0003-2697(90)90294-j. [DOI] [PubMed] [Google Scholar]
- 33.Colombi A, Maroni M, Antonini C, Fait A, Zocchetti C, Foa V. Influence of sex, age and smoking habits on the urinary excretion of D-glucaric acid. Clin Chim Acta. 1983;128:349–358. doi: 10.1016/0009-8981(83)90334-0. [DOI] [PubMed] [Google Scholar]
- 34.Mocarelli P, Brambilla P, Colombo L, et al. A new method for D-glucaric acid excretion measurement that is suitable for automated instruments. Clin Chem. 1988;34:2238–2290. [PubMed] [Google Scholar]
- 35.Batt HM, Siest G. Laboratory tests as indirect indications of the activity of drug metabolizing enzymes use of glucaric acid and gamma-glutamyl-transpeptidase. Dev Clin Biochem. 1980;2:178–192. [Google Scholar]
- 36.Yokoyama M, Matsuoka S, Wakui A. Recent Adv Chemother, Proc Int Congr Chemother 14th. Univ Tokyo Press; Tokyo: 1985. Activation of tegafur and urinary excretion of D-glucaric acid in tumor-bearing hosts; pp. 113–115. [Google Scholar]
- 37.Dohrmann RE. β-Glucuronidase. Springer-Verlag; Berlin: 1969. [Google Scholar]
- 38.Masaki H. Metabolic pathway of dermal D-glucaric acid synthesis from D-glucuronolactone. D-Glucaric acid and D-glucuronolactone dehydrogenase in human skin. β-Gucuronidase feedback mechanism. Nippon Hifuka Gakkai Zashi. 82:151–158. [PubMed] [Google Scholar]; Chem Abstr. 1972;78:2709b–27062u. [Google Scholar]
- 39.Dutton GJ. Glucuronidation of Drugs and Other Compounds. CRC Press; Boca Raton, FL: 1980. [Google Scholar]
- 40.Clark AG, Fischer FJ, Milburn P, Smith RL, Williams RL. The role of gut flora in the enterohepatic circulation of stilboestrol in the rat. Biochem J. 1988;112:17–18. doi: 10.1042/bj1120017pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walaszek Z. Potential use of D-glucaric acid derivatives in cancer prevention. Cancer Lett. 1990;54:1–8. doi: 10.1016/0304-3835(90)90083-a. [DOI] [PubMed] [Google Scholar]
- 42.Walaszek Z. Chemopreventive properties of D-glucaric acid derivatives. Cancer Bull. 1993;45:453–457. [Google Scholar]
- 43.Yoshimi N, Walaszek Z, Mori H, Hanausek M, Szemraj J, Slaga TJ. Inhibition of azoxymethane-induced rat colon carcinogenesis by potassium hydrogen D-glucarate. Int J Oncol. 2000;16:43–48. doi: 10.3892/ijo.16.1.43. [DOI] [PubMed] [Google Scholar]
- 44.Walaszek Z, Szemraj J, Hanausek M, Adams AK, Sherman U. D-Glucaric acid content of various fruits and vegetables and cholesterol lowering effects of dietary D-glucarate in the rat. Nutr Res. 1996;16:673–682. [Google Scholar]
- 45.Zoltaszek R, Hanausek M, Kiliańska ZM, Walaszek Z. The biological role of D-glucaric acid and its derivatives potential use in medicine. Adv Hyg Exptl Med. 2008;62:451–462. [PubMed] [Google Scholar]
- 46.Kishimoto T. Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 47.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 48.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2001;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoltaszek R, Hanausek M, Slaga TJ, Walaszek Z. Proapoptotic effects of D-glucarate on chemically induced lung tumorigenesis in A/J mice. Proc Am Assoc Cancer Res. 2007;48:795. [Google Scholar]
- 51.Zoltaszek R, Grzelak M, Hanausek M, Kilianska Z, Slaga TJ, Walaszek Z. Effects of dietary D-glucarate on biomarkers of inflammation during early post-initiation stages of benzo[a]pyrene (B[a]P)-induced lung tumorigenesis in A/J mice. Proc Am Assoc Cancer Res. 2008;49:532. [Google Scholar]
- 52.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe M. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. PNAS USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–173. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 55.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 56.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 57.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in anti-tumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 58.Wahl LM, Kleinman HK. Tumor-associated macrophages as targets for cancer therapy. J Natl Cancer Inst. 1998;90:1583–1584. doi: 10.1093/jnci/90.21.1583. [DOI] [PubMed] [Google Scholar]
- 59.Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothenberg ME. Eosinophilia. N Eng J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 61.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 62.Lin EY, Nguyen AV, Russel RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human angiogenesis. Cancer Chemother Pharmacol. 1999;43:S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- 64.Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- 65.Coussens LM, Tinkel CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walaszek Z, Hanausek-Walaszek M, Webb TE. Dietary glucarate-mediated reduction of sensitivity of murine strains to chemical carcinogenesis. Cancer Lett. 1986;33:25–32. doi: 10.1016/0304-3835(86)90098-4. [DOI] [PubMed] [Google Scholar]
- 67.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 68.Boivin D, Blanchette M, Barrette S, Moghrabi A, Béliveau R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007;27:937–948. [PubMed] [Google Scholar]


