Abstract
Research on polycyclic aromatic hydrocarbons and their derivatives has received significant attention from the scientific community. The present study involved the synthesis of several novel 6,12-disubstituted chrysene derivatives. Nitration of chrysene with nitric acid produced 6,12-dinitrochrysene which when reduced yielded 6,12-diaminochrysene. A coupling reaction of 6,12-diaminochrysene with an acid in the presence of isobutylchloroformate produced amide. The reduction of amide produced an amine. The amino was converted to a hydrochloride salt. The new compounds were characterized through different types of analytical data. One of these compounds demonstrated marked activity in vivo against a colon cancer cell line. Inhibition of the growth of this tumor was best noted at day 20 when each treatment regimen inhibited the average tumor volume by 50%. In a number of in vivo tests in various regimens, the hydrochloride salt demonstrated consistent inhibition of the growth of the cancer HT-29 cell line. Despite the research progress in polycyclic aromatic compounds, the use of these types of molecules as anticancer agents has not been reported systematically.
Keywords: chrysene derivative, colon cancer cell line, in vivo test
Introduction
Research on polycyclic aromatic hydrocarbons and their derivatives has received significant attention from chemists, biologists and medical investigators (1). However the use of these molecules as anticancer agents has yet to be investigated systematically. Bair et al reported the effectiveness of polyaromatic compounds against tumor cell lines (2). The compounds were believed to interact with DNA by intercalation and to inhibit topoisomerase II. Intercalation was also confirmed for napthalimide, amonafide and mitonafide derivatives, and their antitumor activity was suggested to result from this interaction (3–6).
To extend a previously published investigation involving biologically active aromatic compounds, this study describes the synthesis of several novel 6,12-disubstituted chrysene derivatives. Notably, one of these compounds demonstrated marked activity in vivo against a colon cancer cell line.
Materials and methods
Materials
Chrysene, nitric acid, hydrazine hydrate, isobutylcholoformate, lithium aluminum hydride, tetrahydrofuran, hydrochloric acid, and the colon cancer HT-29 cell line were used in the study.
Synthesis
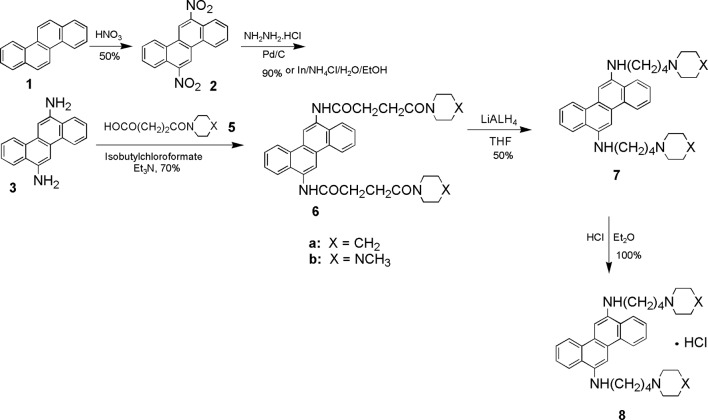
Chrysene 1 on nitration with nitric acid produced 6,12-dinitrochrysene 2 and this compound was then reduced by hydrazine hydrate/Pd-C to yield 6,12-diaminochrysene 3. 6,12-Diaminochrysene 3 was then condensed with acid 5 in the presence of isobutylchloroformate to yield amide 6. Amide 6 on reduction yielded amine 7. Amine 7b was converted to its salt 8 (7–12).
Experiment
N, N-(6,12-chrysenyl)-bis-(4-(4N-methylpipera-zinyl)-butane-1,4-diamine (8): mp 166–168°C; IR (film): 3383, 2932, 2849, 2798, 1594, 1524, 1452, 1357, 1277, 1225, 1162, 1113, 1048, 1012; cm−1H NMR (CDCl3): δ1.6 (m, 8H), 2.3 (s, 6H), 2.5 (m, 20H), 3.5 (t, 4H), 7.6 (m, 6H), 8.0 (d, 2H, J = 8 Hz), 8.6 (d, 2H, J = 8 Hz); UV: 370, 286, 234 with a mass of 567, 531, 313, 304, 284, 263, 230, 216, 206, 154 and 130 was used. The analysis was calculated for C36H50N6: C, 76.3, H, 8.9, N, 14.8. The result obtained was: C, 76.61, H, 8.88, N, 14.67.
Preparation of salt 8
The hydrochloride salt 8 of the amine 7 was prepared by mixing it with an excess hydrochloric acid solution in ether for 1 h and filtering the residue (100% yield). The salt was characterized after regenerating the parent amine by the basification-extraction procedure.
In vivo results
Compound 8 activity against the human colon cancer HT-29 cell line
Table I shows a typical in vivo experiment.
Table I.
Compound 8 activity against the colon cancer HT-29 cell line in vivo.
| Tumor volume/mouse Day |
||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 11 | 13 | 17 | 20 | 25 | 27 | |
| Controls (17) | 85.9 mm3 | 240.8 mm3 | 285.8 mm3 | 404.1 mm3 | 450.0 mm3 | 8/17 |
| 13 (10) 35 mg/kg a.m. 25 mg/kg p.m i.p. x 5 |
50.1 mm3 | 91.4 mm3 | 129.3 mm3 | 204.0 mm3 | 276.0 mm3 | 1/10 |
| 13 (5) 35 mg/kg a.m. i.p. x 5 10 mg/kg 6 h later i.p. x 5 15 mg/kg 12 h later i.p. x 5 |
43.2 mm3 | 92.0 mm3 | 97.7 mm3 | 201.3 mm3 | 216.6 mm3 | 1/5 |
Tumor-bearing mice were sacrificed when their tumors reached an estimated 1 cm3. Measurements were in 2 dimensions, and tumor volume was calculated by the formula: TV= (LxW) 2/2. Numbers in parentheses indicate the effective no. of mice. The no. of mice sacrificed when the tumors reached 1 cm3 over the effective number of mice at 27 days is shown. i.p., intraperitoneally.
Following the injection of 1.25×106 cells, tumor growth was evident in the majority of mice at day 11, with a rapid increase in the average tumor volume at day 25. Growth of this tumor in the control mice was consistently between 85 and 100%. When tumors achieved a volume of 1 cm3 the animals were sacrificed. As shown in Table I, 47% of the control mice were sacrificed by day 27, and this result was quite typical of many runs. When treated with 8 either BID (twice daily) or TID (three times a day), inhibition of the growth of tumors in terms of tumor growth was consistently evident. Subsequently, on day 27 only 10% of the 8 BID and 20% of the 8 TID mice were sacrificed. Another indication of the inhibition of growth of this tumor was best evidenced at day 20 when each regimen inhibited the average tumor volume by 50%.
In a number of in vivo tests compound 8 in various regimens demonstrated consistent inhibition of the growth of HT-29 cells. Although we did not achieve 100% inhibition of the tumor growth it should be noted that there are no agents in clinical use against this tumor that are curative.
Discussion
The in vivo results demonstrated that compound 8 was active against the colon cancer HT-29 cell line. Notably, this compound is a hydrochloride salt and is soluble in water. We are also encouraged by the finding that in repeated in vitro testing by the NCI Program, additional human colon cancers demonstrated even greater sensitivity to this agent.
Figure 1.
Scheme of the synthesis of the 12-disubstituted chrysene derivatives.
Acknowledgements
We gratefully acknowledge the financial support for this project from NIH/NCI (B.K.B.) and the Golden Family Fund (F.F.B.); as well as the shared resources of the pharmacology core facility of the University of Texas M.D. Anderson Cancer Center.
References
- 1.Harvey RG. Polycyclic Aromatic Hydrocarbons. Wiley-VCH; NY: 1997. [Google Scholar]
- 2.Bair KW, Andrews CW, Tuttle RL, Knick VC, Cory M, McKee DD. 2-(Arylmethyl)amino-2methyl-1,3-propanediol DNA intercalators: An examination of the effects of aromatic ring variation on antitumor activity and DNA binding. J Med Chem. 1991;34:1983–1990. doi: 10.1021/jm00111a010. [DOI] [PubMed] [Google Scholar]
- 3.Malviya VK, Liu PY, Alberts DS, Surwit EA, Craig JB, Hanningan EV. Evaluation of amonafide in cervical cancer, phase II. A SWOG study. Am J Clin Oncol. 1992;15:41–44. doi: 10.1097/00000421-199202000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Sami SM, Dorr RT, Solyion AM, Alberts DS, Remers WA. Amino-substituted2-[2/-(dimethylamino)ethyl]-1,2-dihydro-3H-dibenz[de, h]isoquinoline-1,3-diones. Synthesis, anti-tumor activity and quantitative structure-activity relationship. J Med Chem. 1995;38:983–993. doi: 10.1021/jm00006a018. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima T, Kawai Y, Nakayama T, Yamaguchi T, Yoshida A, Urasaki Y, Imamura S, Kamiya K, Tsutani H, Ueda T, Nakamura T. Superior cytotoxic potency of mitoxantrone in interaction with DNA: comparison with that of daunorubicin. Oncol Res. 1996;8:95–100. [PubMed] [Google Scholar]
- 6.Denny WA, Rewcastle GW, Ganguley BC. Potential antitumor agents. 59 Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of minimal DNA-intercalating agents which may not act via topoisomerase II. J Med Chem. 1990;33:814–819. doi: 10.1021/jm00164a054. [DOI] [PubMed] [Google Scholar]
- 7.Banik BK, Becker FF. Polycylic aromatic compounds as anticancer agents: structure-activity relationships of new chrysene and pyrene derivatives. Bioorg Med Chem. 2001;9:593. doi: 10.1016/s0968-0896(00)00297-2. [DOI] [PubMed] [Google Scholar]
- 8.Becker FF, Mukhopadhyay C, Hackfeld L, Banik I, Banik BK. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg Med Chem. 2000;8:2693–2699. doi: 10.1016/s0968-0896(00)00213-3. [DOI] [PubMed] [Google Scholar]
- 9.Banik I, Becker FF, Banik BK. Stereoselective synthesis of β-lactams with polyaromatic imines: entry to new and novel anticancer agents. J Med Chem. 2003;46:12–15. doi: 10.1021/jm0255825. [DOI] [PubMed] [Google Scholar]
- 10.Samajdar S, Becker FF, Banik BK. Surface-mediated highly efficient regioselective nitration of aromatic compounds by bismuth nitrate. Tetrahedron Lett. 2000;41:8017–8020. [Google Scholar]
- 11.Banik BK, Banik I, Becker FF. Indium/ammonium chloride-induced selective reduction of aromatic nitro compounds. Org Syn. 2004;81:188. [Google Scholar]
- 12.Ghatak A, Becker FF, Banik BK. Samarium induced alkyl halide mediated reductive coupling of detones. Tetrahedron Lett. 2000;41:3793–3796. [Google Scholar]