Abstract
Background
We previously reported that a single prostate-specific antigen (PSA) measured at age 44–50 was highly predictive of subsequent prostate cancer diagnosis in an unscreened population. Here we report an additional seven years of follow-up. This provides a replication on an independent data set, and allows estimates of the association between early PSA and subsequent advanced cancer (clinical stage ≥T3 or metastases at diagnosis).
Methods
Blood was collected from 21,277 men in a Swedish city (74% participation rate) during 1974–1986 at age 33–50. Through 2006, prostate cancer was diagnosed in 1408 participants; we measured PSA in archived plasma for 1312 (93%) of these cases and for 3728 controls.
Results
At a median follow-up of 23 years, baseline PSA was strongly associated with subsequent prostate cancer (area-under-the-curve 0.72; 95% CI 0.70, 0.74; for advanced cancer 0.75; 95% CI 0.72, 0.78). Associations between PSA and prostate cancer were virtually identical for the initial and replication data sets with 81% (95% CI 77%, 86%) of advanced cases found in men with PSA above the median (0.63 ng/ml at age 44 – 50).
Conclusion
A single PSA at or before age 50 predicts advanced prostate cancer diagnosed up to 30 years later. Use of early PSA to stratify risk would allow a large group of men to be screened less often but increase frequency of testing on a more limited number of high-risk men. This is likely to improve the ratio of benefits to harms for screening.
Keywords: prostate cancer, prostate-specific antigen, human kallikrein 2, risk factors, case-control study
INTRODUCTION
Two randomized screening trials recently yielded interim data on the value of screening for prostate cancer based on prostate-specific antigen (PSA). In the large European trial (ERPSC), PSA-based screening resulted in a modest (≈20%) reduction of cancer mortality after 9 years.1 In the smaller US study, screening did not affect prostate cancer mortality after 7 years, although many men in the control arm underwent PSA screening both before and after randomization.2 Both studies show that PSA screening carries a high risk of overdetection, with an estimate for the European study that 1410 men would need to be screened, and 48 treated, in order to prevent one prostate cancer death. Rates of overdetection among men subject to regular screening have been estimated as high as 48%.3
One strategy to increase the benefits and decrease the harms of PSA screening is to target men at high risk. Prior studies have suggested that PSA measured in younger men can predict the risk of prostate cancer later in life.4–9 Our own work focuses on participants in the Malmö Preventive Project, a cardiovascular study from 1974–1986. As the national guidelines advise against screening, PSA testing has been uncommon in Sweden, with most prostate cancers being diagnosed clinically;7, 10–12 our cohort thus provides the natural history of the association between PSA and clinically diagnosed prostate cancer. We found that PSA measured at age 44–50 was a strong predictor of prostate cancer diagnosed up to 20 years later, with an area under the receiver operating characteristic curve (AUC) of 0.76. PSA was also associated with later diagnosis of advanced prostate cancer (AUC 0.79).10
Since our initial study, a 2006 update of the Swedish Cancer Registry has made available seven additional years’ follow-up data, and the number of participants diagnosed with prostate cancer has more than doubled. This presents an opportunity to attempt to replicate our initial findings using an independent set of cases—those with prostate cancer diagnosed after the period previously analyzed. The update also allows us to estimate more precisely the association between PSA at or before age 50 and the subsequent risk of advanced prostate cancer.
METHODS
Study cohort
The Malmö Preventive Project study cohort was previously described.7 In brief, 21,277 men aged 33–50 participated in Malmö Preventive Project during 1974–1986. This represented 74% of the men of the selected age groups living in Malmö, Sweden. Of note, the Swedish section of the European Randomized Study of Screening for Prostate Cancer (ERSPC) was based in Gothenburg, and hence there was no overlap between the two cohorts. One EDTA-anticoagulated blood sample was collected, rapidly centrifuged, and stored at −20°C until analysis. In accordance to National Guidelines, participants received no recommendations to undergo early screening for prostate cancer. Only one blood sample was collected for most men, although a subgroup was invited to give a second blood sample 6 years subsequently. The analyses of the first blood sample are presented in the current paper, and the analyses of the second sample will be reported separately. The study was approved by the ethics committee of Lund University.
Outcome ascertainment
Incident cases of prostate cancer were identified from the Swedish Cancer Registry, updated to December 31st, 2006. The registry has been shown to be highly accurate.13 A prostate cancer diagnosis was recorded for 1408 Malmö Preventive Project participants. Clinical stage at diagnosis for 1058 cases (75%) was obtained by review of case notes, procured from the one hospital in Malmö where cancer is treated. Stage at diagnosis was obtained from the National Prostate Cancer Registry in the Southern Region for an additional 221 cases (16%) and was unknown for 129 (9%). Palpable prostate cancer (clinical stage T2 or higher) was identified in 767 (54%), while advanced prostate cancer (clinical stage T3 or higher, or radiographic evidence of bone metastases at diagnosis), was identified in 385 cases (27%). Median age of cases at December 31, 1999 was 63 years, compared to 68 years at December 31, 2006. The large number of cases diagnosed after 1999 results primarily from aging, and not PSA testing, which remained infrequent in Sweden for this age group.
Matching
A nested case-control design was used. Three separate matches were conducted for each of the three study events at time of diagnosis: any prostate cancer, palpable prostate cancer, or advanced prostate cancer. For each endpoint, three controls were selected at random from the group of men alive and event-free at the follow-up time at which the case was diagnosed, using age and date of venipuncture (both ±3 months) as matching variables. Controls for palpable cases could include patients with cT1 disease, and controls for advanced cases could include patients with cT1 or cT2 disease. If three matching controls ±3 months could not be identified, we expanded the window iteratively by 1 month (i.e. to ±4 months), for a maximum window of ±12 months.
Laboratory methods
We measured free PSA, total PSA, and human kallikrein 2 (hK2) in archival blood plasma. The plasma was unthawed until analysis, which occurred 20 years or more after baseline venipuncture. We have previously demonstrated that the levels of total PSA and free PSA in archival plasma do not differ importantly from those in contemporaneously measured serum.14 To measure free and total PSA, we used the dual-label DELFIA Prostatus® total/free PSA-Assay (Perkin-Elmer, Turku, Finland),15 which is calibrated against the WHO 96/670 (PSA-WHO) and WHO 68/668 (free PSA-WHO) standards. Total hK2 was measured using a F(ab)2 fragment-antibody based modification of our in-house research assay, as it minimizes non-specific assay interference.16
Blood samples were missing or could not be analyzed for 96 of the 1408 cancer cases (7%), 49 of the 767 palpable cases (6%), and 23 of the 385 advanced cases (6%). Of the matched controls, blood samples were incomplete for 208 (5%). In total, measurements were performed for 1312 cases and 3728 controls.
Statistical methods
We conducted conditional logistic regression to investigate the association between prostate-specific kallikreins and cancer. The univariate area under the receiver operating characteristics curve (AUC) of each molecular marker was calculated directly from the molecular marker values. The multivariable AUC of a logistic regression model including all molecular markers was calculated using 10-fold cross-validation. The AUC is the probability that a randomly selected case has a higher molecular marker value (or higher predicted probability of cancer from a model) than a randomly selected control, and ranges from 0.5 (no discrimination, equivalent to a coin flip) to 1.0 (perfect discrimination).
To calculate the predicted probability of cancer for a given PSA level among men age 44–50, we used locally weighted regression (“lowess” smoothing17) with log-transformed values of PSA as the independent variable. Because of the 1:3 matching of cases and controls, 25% of the individuals in the data set had prostate cancer. Therefore, a Bayes factor was used to correct this proportion to a target incidence of prostate cancer: the risk of prostate cancer reported in Sweden in the late 1990’s, when PSA testing was still uncommon. This target incidence, 10% by age 75, provides the best estimate of the risk of clinically diagnosed prostate cancer.7 We estimated the target incidence of palpable cancer and advanced cancer to be 5.8% and 3.0%, respectively, by multiplying the risk of prostate cancer diagnosis (10%) by the proportion of palpable (or advanced) cases observed in this cohort. Percentile-based confidence intervals for both AUCs and risk estimates were obtained by bootstrap sampling with replacement, stratified for case-control status, using 2000 iterations.
For independent replication of our previous findings, we performed analyses separately for cases diagnosed through December 31st, 1999 (the group in our previous published analyses), and for those diagnosed more recently. With a longer follow-up period, the recent group of cases tended to have a longer time to diagnosis (median 24 vs 18 years) and younger age at baseline venipuncture (30% vs 11% were <44 years). To ensure that the groups were comparable, we prespecified that this analysis would be restricted to participants age 44–50 at baseline venipuncture and with a prostate cancer diagnosis 20–25 years from baseline venipuncture.
To evaluate risk stratification, we calculated Lorenz curves.18 A Lorenz curve plots centiles of the risk factor, in this case PSA, against the cumulative proportion of cases. Doing so allows one to explore alternative approaches to risk stratification by giving the proportion of cases that would be found given different proportions of the population entered into screening. To obtain a population-based distribution of PSA values for men aged 44–50, we used all the measured values for this age range, and imputed PSA values for non-measured participants: 88 participants with and 7764 without a prostate cancer diagnosis. These PSA levels were imputed by randomly sampling from the respective groups with PSA measured.
All analyses were conducted using Stata version 10.0 (Stata Corp, College Station, TX). All tests of statistical significance were two-sided.
RESULTS
Participants
Characteristics of the study cases are given in Table 1. For the outcome of any prostate cancer, most cases (1,125; 86%) had three matched controls, while 166 (13%) and 21 (2%) cases, respectively, had two or one due to insufficient blood samples. Age and date of venipuncture were ±3 months of the matched case for all but 12 controls; the maximum difference for both age and date of venipuncture was 8 months. Most cases (998; 76%) were age 44–50 years at venipuncture. Note that all PSA measurements were in accordance with WHO calibration standards, whereas those in our previous publication7 were not; accordingly, all PSA values are slightly higher than those previously reported.
Table 1.
Description of study cases.
| Any prostate cancer | Palpable prostate cancer* | Advanced prostate cancer† | |
|---|---|---|---|
| Cases through 12/31/2006 | 1408 | 767 | 385 |
|
| |||
| Cases with molecular marker data | 1312 (93%) | 718 (94%) | 362 (94%) |
|
| |||
| Age at baseline venipuncture, years | |||
| 28–39 | 192 (15%) | 71 (10%) | 26 (7%) |
| 40–43 | 114 (9%) | 55 (8%) | 25 (7%) |
| 44–50 | 998 (76%) | 588 (82%) | 309 (85%) |
| 51–52 | 8 (1%) | 4 (1%) | 2 (1%) |
|
| |||
| Median age at diagnosis, years (IQR) | 67 (63, 71) | 68 (64, 72) | 68 (64, 72) |
|
| |||
| Median time from baseline to diagnosis, years (IQR) | 23 (19, 25) | 22 (19, 25) | 22 (18, 25) |
|
| |||
| Median total PSA at diagnosis‡, ng/ml (IQR) | 10.1 (6.10, 21.9) | 13.0 (7.00, 34.2) | 29.0 (12.8, 99.1) |
|
| |||
| Clinical T stage | |||
| T1 | 492 (38%) | 0 (0%) | 10 (3%) |
| T2 | 388 (30%) | 388 (54%) | 21 (6%) |
| T3 | 297 (23%) | 297 (41%) | 297 (82%) |
| T4 | 33 (3%) | 33 (5%) | 33 (9%) |
| Unknown | 102 (8%) | 0 (0%) | 1 (0.3%) |
|
| |||
| Clinical N stage | |||
| N0 | 331 (25%) | 238 (33%) | 107 (30%) |
| N1 | 38 (3%) | 36 (5%) | 27 (7%) |
| Unknown | 943 (72%) | 444 (62%) | 228 (63%) |
|
| |||
| Clinical M stage | |||
| M0 | 655 (50%) | 445 (62%) | 202 (56%) |
| M1 | 123 (9%) | 112 (16%) | 123 (34%) |
| Unknown | 534 (41%) | 161 (22%) | 37 (10%) |
|
| |||
| WHO grade | |||
| I | 278 (21%) | 130 (18%) | 26 (7%) |
| II | 529 (40%) | 292 (41%) | 123 (34%) |
| III | 271 (21%) | 234 (33%) | 178 (49%) |
| Unknown | 234 (18%) | 62 (9%) | 35 (10%) |
|
| |||
| Matched controls with molecular marker data | |||
| 3 | 1125 (86%) | 601 (84%) | 307 (85%) |
| 2 | 166 (13%) | 103 (14%) | 48 (13%) |
| 1 | 21 (2%) | 13 (2%) | 7 (2%) |
IQR: interquartile range
Defined as clinical stage T2 or higher at diagnosis.
Defined as clinical stage T3 or higher, or presence of bone metastases at diagnosis.
PSA within 90 days before the date of prostate cancer diagnosis; available for 1145 overall cases, 655 palpable cases, and 318 advanced cases.
Median time elapsed between baseline venipuncture and prostate cancer diagnosis was 23 years (interquartile range, 19 to 25). Similar characteristics were observed for the outcomes of palpable and advanced prostate cancer. Notably, among those age 44–50 at baseline, 95% (945/998) of the overall cancer cases and 94% (289/309) of the advanced cases were diagnosed after age 60. Clinical tumor characteristics (Table 1) were available for 1210 of 1312 prostate cancer cases (92%). Of the advanced prostate cancer cases, 220 (61%) were clinical stage T3 or higher, 32 (9%) had clinical documentation of bone or nodal metastases, and 110 (30%) had both clinical stage T3 or higher and metastases. Bone scans were not routine in Sweden for much of the period of the study and many patients are therefore not classified for metastasis at diagnosis.
Association of PSA with prostate cancer
The association between PSA at or before age 50 and subsequent diagnosis of prostate cancer (Table 2) was strong and highly significant (p<0.0005; AUC 0.719; 95% CI 0.704, 0.736). For the outcomes of palpable cancers and cancers that were advanced at diagnosis, the association was strengthened (AUC 0.723 and 0.751, respectively).
Table 2.
PSA (ng/ml) in cases and controls at baseline venipuncture. Data given as median (interquartile range). All associations between PSA and outcome were significant at p<0.0005.
| Case definition | Cases | Controls | AUC | 95% CI |
|---|---|---|---|---|
| Any prostate cancer | 1.01 (0.68, 1.61) | 0.62 (0.42, 0.91) | 0.719 | 0.704, 0.736 |
| Palpable prostate cancer | 1.02 (0.68, 1.71) | 0.62 (0.41, 0.92) | 0.723 | 0.701, 0.745 |
| Advanced prostate cancer | 1.15 (0.72, 2.02) | 0.63 (0.40, 0.94) | 0.751 | 0.721, 0.781 |
As an independent replication of our initial findings, we performed analyses separately for 121 men with a diagnosis through December 31st, 1999 (who were included in our previous analysis7) versus 313 men with a more recent cancer diagnosis, all of whom were at age 44–50 at baseline venipuncture and diagnosed with cancer 20–25 years from baseline. The association between PSA and long-term risk of any, palpable, or advanced prostate cancer was virtually identical for the two separate sets of cases and controls (Table 3, Figure 1).
Table 3.
Independent replication of previous findings in participants age 44–50 at baseline and diagnosed with prostate cancer 20–25 years later. All associations between PSA and outcome were significant at p<0.0005.
| Outcome | Previously diagnosed (through December 31st, 1999) | Newly diagnosed (after December 31st, 1999) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of cases | AUC | 95% CI | Number of cases | AUC | 95% CI | |
| Any prostate cancer | 121 | 0.736 | 0.679, 0.791 | 313 | 0.736 | 0.705, 0.766 |
| Palpable prostate cancer | 77 | 0.759 | 0.685, 0.824 | 169 | 0.750 | 0.705, 0.792 |
| Advanced prostate cancer | 37 | 0.746 | 0.624, 0.839 | 88 | 0.778 | 0.719, 0.832 |
Figure 1.
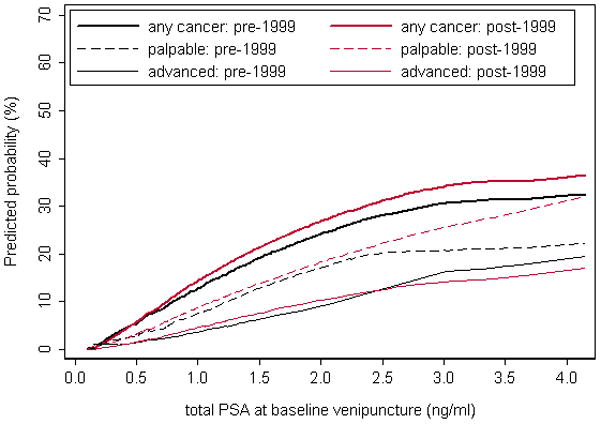
Replication of the association between PSA at age 44–50 and prostate cancer. The graph shows the predicted probability of any, palpable, or advanced prostate cancer diagnosed 20–25 years after baseline venipuncture, by PSA at age 44–50 in men with a cancer diagnosis through December 31st, 1999 (black lines) compared to recent cases with a diagnosis from January 1st, 2000 (red lines).
Predictive value of PSA according to age at blood draw and time to diagnosis
In the subgroup of 192 cases age <40 at baseline venipuncture, the long-term association between PSA and prostate cancer remained strong (p<0.0005; AUC 0.74; 95% CI 0.70, 0.78). The association between PSA and prostate cancer also persisted for cancers diagnosed ≥25 years after venipuncture (Table 4; AUC 0.67).
Table 4.
AUC for diagnosis of prostate cancer based on total PSA at baseline, calculated separately for men diagnosed at various intervals from baseline. All associations between PSA and outcome were significant at p<0.0005.
| Time to diagnosis | Any cancer | Palpable cancer | Advanced cancer | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | AUC (95% CI) | Cases | AUC (95% CI) | Cases | AUC (95% CI) | |
| ≤15 years | 107 | 0.842 (0.790, 0.891) | 69 | 0.848 (0.783, 0.907) | 47 | 0.859 (0.778, 0.929) |
| 16–19 years | 263 | 0.724 (0.688, 0.760) | 162 | 0.753 (0.706, 0.796) | 82 | 0.745 (0.681, 0.804) |
| 20–24 years | 607 | 0.725 (0.703, 0.749) | 317 | 0.727 (0.694, 0.758) | 147 | 0.756 (0.708, 0.800) |
| ≥25 years | 335 | 0.670 (0.637, 0.702) | 170 | 0.644 (0.608, 0.700) | 86 | 0.686 (0.628, 0.768) |
Risk according to PSA level at age 44–50
For risk stratification prior to prostate cancer screening, which is commonly recommended to begin at age 50,19 we regard the key age range to be 44–50 years. We therefore estimated the probability of prostate cancer diagnosis based on PSA measured at age 44–50 (Figure 2). The risk of subsequent clinically diagnosed prostate cancer was low (1–5% risk) for men with PSA <0.5 ng/ml, and was 8–15% (close to the population mean) for those with PSA 0.75–1.25 ng/mL. For PSA values above 1.50 ng/mL, corresponding to the 90th centile, the estimated risk of prostate cancer was markedly higher. The risk of palpable and advanced cancer followed a similar trend.
Figure 2.
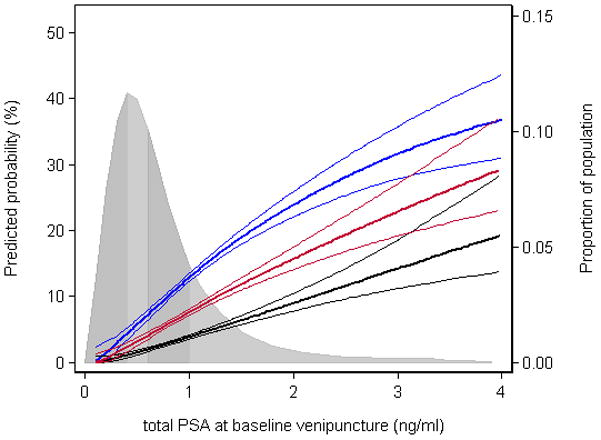
Long-term risk of prostate cancer according to PSA at age 44–50. Thick blue line, any prostate cancer; thick red line, palpable cancer; thick black line; advanced cancer. Thin lines represent 95% confidence intervals. Shaded area represents quartiles of population-based distribution of PSA values (0.42, 0.63, and 0.95 ng/mL). The curves differ from the curves for any cancer in Figure 1 because the analysis was not restricted to men diagnosed 20–25 years after venipuncture.
The relationship between PSA at age 44–50 and subsequent prostate cancer is also shown using Lorenz curves (Figure 3). These show the proportion of cancers that could be detected early by a screening program directed at the subgroup at higher risk, as defined by PSA level. In this analysis, 81% (95% C.I. 77%, 86%) of the prostate cancers that were advanced at diagnosis were in men with PSA above the median (0.63 ng/mL), and 69% (95% C.I 63%, 74%) of the advanced cancers were in men with PSA in the top tertile (≥0.83 ng/mL). Curves very similar in shape were obtained for any prostate cancer and for palpable cancer. Table 5 provides the corresponding numerical estimates of risk and cumulative proportions.
Figure 3.
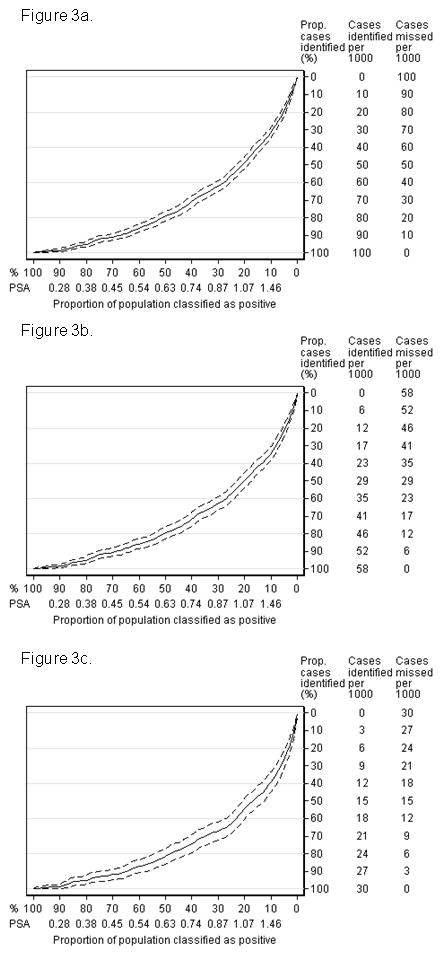
Lorenz curves for clinically-diagnosed cancer based on PSA at age 44–50. The x axes give population centiles of PSA and y axes give the cumulative number of cases. Dashed lines represent 95% confidence intervals. The curves can be used to determine the proportion of cancers that could be detected early by screening if screening were risk-stratified by PSA level at age 44–50, with only men above that level recommended for regular PSA testing. a) Any prostate cancer; b) palpable prostate cancer; c) advanced prostate cancer.
Table 5.
Absolute risk of subsequently developing any, palpable, or advanced prostate cancer by PSA level at age 44–50, and cumulative proportion of cancers above percentiles of PSA at age 44–50. The 95% confidence intervals are given in parentheses.
| Percentile | PSA (ng/ml)* | Any cancer | Palpable cancer | Advanced cancer | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Absolute risk, % | Cumulative proportion, % | Absolute risk, % | Cumulative proportion, % | Absolute risk, % | Cumulative proportion, % | ||
| 90 | 1.46 | 18 (17, 20) | 32 (29, 35) | 12 (11, 12) | 34 (30, 38) | 6.1 (5.5, 6.8) | 39 (33, 44) |
| 80 | 1.07 | 14 (13, 14) | 49 (45, 52) | 8.2 (7.7, 8.7) | 50 (45, 54) | 4.2 (3.8, 4.5) | 55 (48, 61) |
| 75 | 0.95 | 12 (12, 13) | 56 (53, 60) | 7.2 (6.7, 7.7) | 57 (53, 62) | 3.6 (3.3, 3.9) | 63 (58, 69) |
| 67 | 0.83 | 11 (10, 11) | 65 (61, 68) | 6.1 (5.7, 6.6) | 66 (62, 69) | 3.0 (2.7, 3.3) | 69 (63, 74) |
| 50 | 0.63 | 7.7 (6.9, 8.4) | 79 (76, 82) | 4.3 (3.8, 4.9) | 80 (76, 83) | 2.1 (1.8, 2.4) | 81 (77, 86) |
| 25 | 0.42 | 4.7 (3.7, 5.5) | 92 (90, 94) | 2.5 (1.8, 3.2) | 92 (89, 94) | 1.2 (0.8, 1.6) | 93 (90, 96) |
| 10 | 0.28 | 2.5 (1.2, 3.7) | 99 (98, 99) | 1.3 (0.5, 2.1) | 99 (97, 100) | 0.6 (0.2, 1.1) | 99 (97, 100) |
Imputed to the whole population from case-control data
Kallikrein markers
We took advantage of the larger study cohort to reassess additional markers as predictors of later diagnosis of prostate cancer. Free PSA, free-to-total PSA ratio and hK2 were all strongly and significantly associated with any, palpable and advanced prostate cancer (Table 6). However, combining multiple markers into a multivariable model did not improve the predictive accuracy of PSA for all men, although enhancements were observed when focusing on men with total PSA at or above the median (≥0.63 ng/ml) or the top quartile (≥0.95 ng/ml).
Table 6.
AUC for diagnosis of prostate cancer based on total PSA, free PSA, %free PSA and hK2 at age 44–50, overall and for men with total PSA above the median and above the upper quartile. 95% confidence intervals are given in parentheses.
| Model | Any cancer | Palpable cancer | Advanced cancer |
|---|---|---|---|
| All | |||
|
| |||
| Number of cases/controls | 998/2811 | 588/1627 | 309/855 |
|
| |||
| Total PSA | 0.722 (0.704, 0.740) | 0.735 (0.709, 0.758) | 0.759 (0.725, 0.790) |
| Free PSA | 0.673 (0.655, 0.693) | 0.688 (0.663, 0.712) | 0.725 (0.690, 0.757) |
| % Free PSA | 0.658 (0.641, 0.674) | 0.673 (0.650, 0.693) | 0.665 (0.634, 0.695) |
| hK2 | 0.630 (0.612, 0.652) | 0.644 (0.620, 0.672) | 0.660 (0.627, 0.697) |
| Combination | 0.728 (0.711, 0.746) | 0.741 (0.718, 0.764) | 0.759 (0.727, 0.791) |
|
| |||
| Above the median (≥ 0.63 ng/ml) | |||
|
| |||
| Number of cases/controls | 784/1366 | 465/776 | 251/413 |
|
| |||
| Total PSA | 0.657 (0.633, 0.684) | 0.675 (0.648, 0.713) | 0.711 (0.673, 0.758) |
| Free PSA | 0.590 (0.569, 0.617) | 0.609 (0.586, 0.651) | 0.661 (0.628, 0.716) |
| % Free PSA | 0.618 (0.595, 0.639) | 0.636 (0.603, 0.663) | 0.630 (0.575, 0.658) |
| hK2 | 0.580 (0.557, 0.608) | 0.600 (0.568, 0.637) | 0.610 (0.580, 0.666) |
| Combination | 0.672 (0.650, 0.697) | 0.695 (0.667, 0.729) | 0.715 (0.680, 0.766) |
|
| |||
| Above the top quartile (≥ 0.95 ng/ml) | |||
|
| |||
| Number of cases/controls | 552/663 | 333/377 | 196/204 |
|
| |||
| Total PSA | 0.618 (0.573, 0.648) | 0.653 (0.601, 0.699) | 0.671 (0.605, 0.732) |
| Free PSA | 0.547 (0.510, 0.584) | 0.583 (0.532, 0.629) | 0.620 (0.560, 0.688) |
| % Free PSA | 0.605 (0.562, 0.629) | 0.623 (0.581, 0.667) | 0.602 (0.547, 0.652) |
| hK2 | 0.600 (0.575, 0.646) | 0.619 (0.577, 0.674) | 0.630 (0.564, 0.698) |
| Combination | 0.659 (0.628, 0.695) | 0.687 (0.651, 0.745) | 0.705 (0.644, 0.769) |
DISCUSSION
We have replicated our finding that PSA measured at age 44–50 is a very strong predictor of prostate cancer clinically diagnosed many years later. Our observations now extend to cancers diagnosed more than 25 years later, and to PSA measured in men before age 40. We confirmed that our findings are not restricted to cancers of questionable clinical significance, but apply to cancers that are palpable and to those locally advanced or metastatic at diagnosis. With the greater number of cases in this update, we have the statistical power to provide more precise estimates for the association between early PSA and advanced cancers, such that even the lower bound of the confidence interval is of clear clinical relevance.
As in a prior study (7), early measurements of the additional kallikreins (free PSA, free-to-total PSA ratio, and hK2) did not importantly aid prediction beyond that of PSA alone. We noted, however, that among men with elevated PSA (≥0.95 ng/ml), the additional kallikreins moderately enhanced prediction of clinically diagnosed prostate cancers, albeit the utility of these markers may be greater if measured closer to the time of diagnosis.12 Further, several studies provided evidence that they can aid in prostate cancer screening as indication for biopsy, particularly in men with moderately elevated PSA.20, 21
Our conclusions on the long-term predictive value of PSA are consistent with several earlier studies.4–6, 8, 9. The current study is distinguished by its inclusion of a highly representative population-based sample of unscreened men, rather than those subject to PSA screening. Therefore, our study has little or no verification bias.
A limited number of studies have been conducted on the long-term association between PSA and subsequent prostate cancer diagnosis in unscreened men. In a study of 21,172 Finnish men, baseline PSA was ≥2.5 ng/ml in 95% of men diagnosed with cancer within the first 5 years and 52% diagnosed within 6–10 years. Gann et al., analyzing 366 Physicians’ Health Study participants diagnosed with prostate cancer, reported that serum PSA was elevated 5–6 years before the identification of a palpable tumor. Similar results have been reported for participants in the prospective Baltimore Longitudinal Study of Aging. However, this report includes far more cases at much longer follow-up.
A limitation of our study is that we examined cancer diagnosis, rather than morbidity or mortality from cancer. Nevertheless, the advanced cancers in our analysis represent an important endpoint, because they are highly likely to affect quality or length of life if left untreated. Another possible limitation is that, although the national guidelines continue to recommend against screening, PSA testing has become more common in Sweden in recent years,22 and some of the more recent cases may have been detected due to PSA testing. Nevertheless, differences from our previous results7, 10, 12 were slight and, perhaps most importantly, findings were highly robust for advanced cancers.
Interestingly, the participants with advanced cancer diagnosed more than 25 years after baseline were a heterogeneous subset. Most were men in their 40’s at baseline with cancer diagnosed late—after age 75. A substantial fraction, however, were men younger than 40 years at baseline, with advanced cancer diagnosed before age 60; these cancers are therefore presumably highly aggressive. That early PSA was predictive many years before diagnosis for this heterogeneous group underscores the strength of its association with cancer risk.
The predictive accuracy of PSA at age 44–50 compares favorably to that of other means of assessing prostate cancer risk: race, family history of prostate cancer, and genetic markers associated with the disease. For example, in this study early PSA had AUC 0.72 for predicting any prostate cancer, whereas 5 SNPs had an AUC of 0.57.23 A SNP near the gene encoding PSA (KLK3) has been reported to associate with prostate cancer risk, as well as with serum PSA level.24, 25 Its influence on cancer risk has, however, been disputed,26 and in any case its predictive value appears to be far less than that of an early PSA measurement.
The finding that PSA levels can indicate future prostate cancer risk for men below age 40 has intriguing biological implications. Cancer-related PSA elevations are generally held to result from the cancer disrupting the tissue architecture, thereby allowing PSA to leak into extracellular space. Under this view, a relatively high PSA level before age 40 in a man later diagnosed with cancer would be explained by the prostate already harboring prostate cancer, or possibly a premalignant condition. Addressing such issues will provide important directions for research on prostate cancer chemoprevention. We are, however, cautious about any clinical implications of PSA before age 40; for example, we see no rationale for starting PSA screening at this age.
The major clinical implication of this study is that an early PSA value could be used to individualize later screening for prostate cancer, generally recommended to begin around age 50. Based on our study results, we propose that the frequency of screening be determined by a risk appraisal based on early PSA level before age 50. Consistent with our data, a PSA at 45 would be appropriate, although there may be cases, such as men with a strong family history, in which an earlier PSA, such as at age 40, would be indicated. Men with PSA levels below the median (~0.6 ng/mL) might be expected to benefit little from subsequent annual or even biennial PSA checkups. As yet, we do not have sufficient data to suggest that these men need no further screening, but it appears reasonable to suggest that they be asked to return for a screening around age 55–60. Men with high early PSA levels - those in the top 10–25% (~ 1 ng/ml) - could be advised that they are at high risk for advanced prostate cancer and that it is critical that they return for frequent (e.g. annual or biennial) screening. Those at intermediate risk, above the median but below the top 10–25%, could be advised to return for screening at intervals of no more than 4 years, which has been found to allow detection of most prostate cancers while they are still confined to the prostate and therefore curable.1, 27 Compared to screening all men, this stratified screening strategy would also likely reduce the incidence of unnecessary prostate biopsy. Consider, for example, a man with an early PSA below median, who sometime after age 50 has a temporary elevation of PSA because of asymptomatic prostatitis. A strategy of annual screening for all men would likely lead to an unnecessary biopsy for such a man.
In conclusion, an early PSA, taken at or before age 50, is a strong predictor of advanced prostate cancer diagnosed 25 or more years later. Given that we have replicated our prior results on an independent set of cases, and that our findings are derived from a large, highly representative study that was subject to little or no verification bias, we consider our findings to be highly robust. Based on these findings, we advocate a change in the clinical paradigm for prostate cancer screening. Whereas currently the use and frequency of PSA-based screening are determined largely by national practice patterns, we propose that screening frequency be determined by individual risk as assessed from an early PSA test. Early PSA testing could also serve as the foundation for a more comprehensive risk assessment that also includes genetic markers, family history, race, and risk factors defined in the future.
Acknowledgments
We thank Ms. Gun-Britt Eriksson and Kerstin Håkansson for expert assistance with immunoassays.
Research support came from the National Cancer Institute [grant numbers R21-CA127768-01A1, P50-CA92629]; Swedish Cancer Society [3455]; Swedish Research Council (Medicine) [20095]; and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Conflicts of Interest
Dr. Hans Lilja holds patents for free PSA and hK2 Assays.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Antenor JA, Han M, Roehl KA, Nadler RB, Catalona WJ. Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J Urol. 2004;172:90–3. doi: 10.1097/01.ju.0000132133.10470.bb. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–6. doi: 10.1016/s0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 6.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–94. [PubMed] [Google Scholar]
- 7.Lilja H, Ulmert D, Bjork T, et al. Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. J Clin Oncol. 2007;25:431–6. doi: 10.1200/JCO.2006.06.9351. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Roehl KA, Antenor JA, Catalona WJ, Suarez BK, Nadler RB. Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006;67:316–20. doi: 10.1016/j.urology.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 9.Whittemore AS, Cirillo PM, Feldman D, Cohn BA. Prostate specific antigen levels in young adulthood predict prostate cancer risk: results from a cohort of Black and White Americans. J Urol. 2005;174:872–6. doi: 10.1097/01.ju.0000169262.18000.8a. discussion 76. [DOI] [PubMed] [Google Scholar]
- 10.Ulmert D, Cronin AM, Bjork T, et al. Prostate-specific antigen at or before age 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: a case-control study. BMC Med. 2008;6:6. doi: 10.1186/1741-7015-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulmert D, Serio AM, O’Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835–41. doi: 10.1200/JCO.2007.13.1490. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Ulmert D, Serio AM, et al. The predictive value of prostate cancer biomarkers depends on age and time to diagnosis: towards a biologically-based screening strategy. Int J Cancer. 2007;121:2212–7. doi: 10.1002/ijc.22956. [DOI] [PubMed] [Google Scholar]
- 13.Sandblom G, Dufmats M, Olsson M, Varenhorst E. Validity of a population-based cancer register in Sweden--an assessment of data reproducibility in the South-East Region Prostate Cancer Register. Scand J Urol Nephrol. 2003;37:112–9. doi: 10.1080/00365590310008839. [DOI] [PubMed] [Google Scholar]
- 14.Ulmert D, Becker C, Nilsson JA, et al. Reproducibility and accuracy of measurements of free and total prostate-specific antigen in serum vs plasma after long-term storage at -20 degrees C. Clin Chem. 2006;52:235–9. doi: 10.1373/clinchem.2005.050641. [DOI] [PubMed] [Google Scholar]
- 15.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label onestep immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 16.Vaisanen V, Peltola MT, Lilja H, Nurmi M, Pettersson K. Intact free prostate-specific antigen and free and total human glandular kallikrein 2. Elimination of assay interference by enzymatic digestion of antibodies to F(ab′)2 fragments. Anal Chem. 2006;78:7809–15. doi: 10.1021/ac061201+. [DOI] [PubMed] [Google Scholar]
- 17.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 18.Lee WC. Probabilistic analysis of global performances of diagnostic tests: interpreting the Lorenz curve-based summary measures. Stat Med. 1999;18:455–71. doi: 10.1002/(sici)1097-0258(19990228)18:4<455::aid-sim44>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.American Cancer Society. [accessed May 29, 2009];How is prostate cancer found? Available from URL: http://www.cancer.org/docroot/CRI/content/CRI_2_2_3X_How_is_prostate_cancer_found_36.asp.
- 20.Steuber T, Vickers A, Haese A, et al. Free PSA isoforms and intact and cleaved forms of urokinase plasminogen activator receptor in serum improve selection of patients for prostate cancer biopsy. Int J Cancer. 2007;120:1499–504. doi: 10.1002/ijc.22427. [DOI] [PubMed] [Google Scholar]
- 21.Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adolfsson J, Garmo H, Varenhorst E, et al. Clinical characteristics and primary treatment of prostate cancer in Sweden between 1996 and 2005. Scand J Urol Nephrol. 2007;41:456–77. doi: 10.1080/00365590701673625. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–9. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 24.Eeles R, Giles G, Neal D, Muir K, Easton DF. Reply to “Variation in KLK genes, prostate-specific antigen and risk of prostate cancer”. Nat Genet. 2008;40:1035–36. doi: 10.1038/ng0908-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eeles RA, Kote-Jarai Z, Giles GG, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 26.Ahn J, Berndt SI, Wacholder S, et al. Variation in KLK genes, prostate-specific antigen and risk of prostate cancer. Nat Genet. 2008;40:1032–4. doi: 10.1038/ng0908-1032. author reply 35-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roobol MJ, Grenabo A, Schroder FH, Hugosson J. Interval cancers in prostate cancer screening: comparing 2- and 4-year screening intervals in the European Randomized Study of Screening for Prostate Cancer, Gothenburg and Rotterdam. J Natl Cancer Inst. 2007;99:1296–303. doi: 10.1093/jnci/djm101. [DOI] [PubMed] [Google Scholar]
- 28.Aus G, Damber JE, Khatami A, Lilja H, Stranne J, Hugosson J. Individualized screening interval for prostate cancer based on prostate-specific antigen level: results of a prospective, randomized, population-based study. Arch Intern Med. 2005;165:1857–61. doi: 10.1001/archinte.165.16.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roobol MJ, Schroder FH, Kranse R. A comparison of first and repeat (four years later) prostate cancer screening in a randomized cohort of a symptomatic men aged 55–75 years using a biopsy indication of 3.0 ng/ml (results of ERSPC, Rotterdam) Prostate. 2006;66:604–12. doi: 10.1002/pros.20352. [DOI] [PubMed] [Google Scholar]


