Abstract
Huntington’s disease (HD) is an autosomal dominant inherited and progressive neurodegenerative disorder with motor dysfunction and cognitive deficits. Although, there are no treatments to delay the appearance and the progression of HD, there are potential drugs currently in preclinical and clinical trials that are focused on HD therapy. The signaling pathways involved in HD are not yet clearly elucidated; however, expression of mutant huntingtin protein is considered a key factor in the induction and/or progression of HD. The demonstration that the onset and progression of HD in models of transgenic mice, in particular, are delayed or improved by the application of neurotrophic factors has emphasized their importance in neuroprotection in HD. In addition, other compounds targeting the HD gene or mutant huntingtin protein are currently in preclinical and clinical testing and may show promising neuroprotective effects. There are current patented drugs that are currently being considered as potential therapeutics for HD. These patented drugs may provide promising therapy for HD.
Keywords: Huntington’s disease, neurodegenerative diseases, neuroprotection, oxidative stress
1. INTRODUCTION
Huntington’s disease (HD) is an autosomal dominant inherited and progressive neurodegenerative disorder with symptoms that include motor dysfunction, cognitive deficits and psychiatric symptoms [1, 2]. Motor dysfunction is a major problem in HD. The CAG trinucleotide repeat expansion within exon 1 of the huntingtin gene on chromosome 4 is the key factor in HD (Huntington’s Disease Collaborative Research Group, [3]). The huntingtin gene encoding polyglutamine extends in the N-terminal domain of the huntingtin protein. The degree of the pathology is impacted by the length of the polyglutamine. Individuals who carry CAG repeats from 6 to 35 do not develop HD; however, when the number of repeats exceeds 35, the gene encodes a version of huntingtin protein that can lead to HD [4, 5]. It is noteworthy that incomplete penetrance is around 36–39 CAG repeats [6]. The causality of HD is mainly the accumulation of the polyglutamine extended huntingtin protein fragments in the cytoplasm and nucleus. Although, the mechanism of action of neurodegeneration in HD is still not identified, a key factor in the induction of HD is the mutant huntingtin protein. The formation of neuronal intranuclear inclusions containing mutant huntingtin protein may be the cause of neurodegeneration as demonstrated in animal models of HD [7]. The increases of intranuclear inclusions were found to be associated with HD symptoms [8].
HD is characterized by neuronal dysfunction and degeneration in the striatum and cortex [9, 10]. The neuropathology may involve atrophy and degeneration of GABAergic medium spiny neurons in the striatum that are connected to the cortical areas [11]. HD therapy has been a major problem, since there is currently no treatment for preventing or attenuating the progression of the HD. Patients suffering from HD are generally treated with neuroleptics or anticonvulsants to monitor only some of the symptoms. On the other hand, transplantation has been considered the only approach to replace degenerated neurons [12]. Transplantation of fetal neuroblasts into the striatum has been suggested to be safe and potentially therapeutic in HD patients [13, 14]. Furthermore, patients grafted with human fetal neuroblasts into the striatum show improvements in cognition and motor function [15]. Interestingly, a patent indicates the use of a method for treating HD using human teratocarcinoma cell line (hNT) [16]. The patent provides methods and compositions for the transplantation of differentiated hNT neurons for the treatment of HD. These methods are aimed to improve the motor skills of patients suffering from HD who might have been transplanted with hNT neurons. It has been demonstrated that transplantation of hNT neurons in lesion rat and monkey models improved motor dysfunction [17].
Moreover, neurotrophic factors have been considered as potential therapeutic proteins that play a key role in neuroprotection and neurogenesis. Findings demonstrate that there is an association between the mutant huntingtin protein and neurotrophic factors (for review see Ref. [18]). There are at least three types of neurotrophic factors that have been considered to be potentially used for treatment of the progression of HD. Among them are: brain-derived neurotrophic factor (BDNF), fibroblast growth factor-2 (FGF-2) and glial cell line-derived neurotrophic factor (GDNF). The deficits of endogenous neurotrophic factors are considered critical cause of the progression of HD and other neurodegenerative diseases [19–21].
Other non-trophic factor compounds and methods have been suggested as drug candidates for the treatment of HD. The identification of these drugs is based on the clear understanding of the signaling pathways involving mutant huntingtin protein, which is a major inducer for neuronal death in HD. Here, the discussion describes some of the drugs in pre-clinical and clinical trials for the treatment of the progression of HD and other patented drugs for potential treatments of the progression and/or prevention of HD.
2. THERAPEUTIC EFFECTS OF NEUROTROPHIC FACTORS IN HD
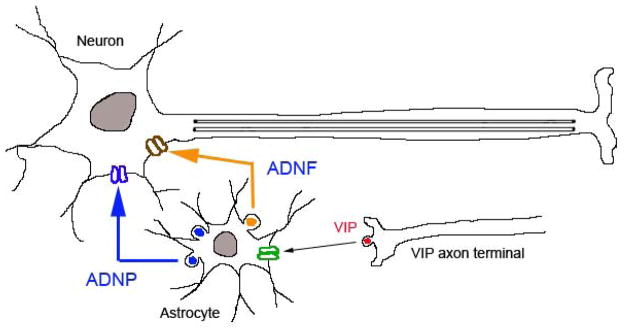
Neurotrophic factors are identified based on their activity in preventing neuronal death. These proteins have been shown to promote survival or prevent oxidative stress that induces cell death. Neurotrophic factors may increase the neuronal metabolism, cell growth and processes that can lead to the growth of new axons and the reestablishment of synaptic connections. These may result in neuroprotection, cell restoration and improvement of cellular function. The first neurotrophic factor identified is nerve growth factor (NGF). NGF is a member of a family of trophic factors termed neurotrophins, which include BDNF. GDNF and FGF-2 are also identified as growth factors that have a key role in neuroprotection. On the other hand, the use of derived peptides from activity-dependent neurotrophic factor (ADNF) and activity-dependent neuroprotective protein (ADNP) have been considered as trophic peptides for the attenuation of neurodegeneration in Alzheimer’s disease (AD) and Amyotrophic Lateral Sclerosis (ALS). Both ADNF and ADNP are synthesized and released from astroglia [22, 23]. The release of these trophic factors is regulated by vasoactive intestinal peptide (VIP) [24] as shown in Fig. (1). We have recently demonstrated that the neuroprotective effects of these derived peptides ADNF-9 (SALLRSIPA) and NAP (NAPVSIPQ) from ADNF and ADNP, respectively, in oxidative stress models [25, 26]. Thus, these derived peptides, ADNF-9 and NAP, have been shown to protect neuronal degeneration against the insults of oxidative stress and other related neurodegenerative models in vitro and in vivo (for review see Ref. [26, 27]).
Fig. 1.
Model shows the mechanism of release of activity dependent neurotrophic factor (ADNF) and activity dependent neuroprotective protein (ADNP). The release of vasoactive intestinal peptide (VIP) stimulates glial target receptor and induce vesicular excytosis contained ADNF and ADNP. ADNF and ADNP maintain neuronal survival in neurodegenerative diseases through unknown mechanism.
The pharmacological actions of neurotrophic factors are considered promising new therapeutic agents for the treatment of HD. There are at least three neurotrophic factors that have been tested in pre-clinical and clinical settings for the treatment of the progression of HD. The outcomes of these neurotrophic factors are discussed in this review. There are also other neurotrophic factors tested in other neurodegenerative diseases that might be considered potential drugs for the treatment of HD.
2.1. BDNF
BDNF is found to be an important trophic factor for the treatment of HD. It is noteworthy that the level of BDNF is found to be downregulated in HD patients [28–30]. In accordance, downregulation of BDNF was found to be associated with CAG repeats [31]. Deficit in BDNF levels is associated with alteration of BDNF transport by mutant huntingtin protein [32, 33]. In general, normal huntingtin protein is found to enhance vesicular transport of BDNF along microtubules, but mutant huntingtin can alter this mechanism. Regulating the levels of BDNF in the corticostriatal pathway might promote cell survival and consequently delay the progression of HD. BDNF was found to be produced in cortex and transported in the corticostriatal pathway in the medium spiny neurons [34, 35], which are the neurons most affected by HD. This suggests that therapeutic approaches targeting the increase of BDNF levels might be a potential strategy to slow the progression of HD (for review see Reference [18]).
BDNF has been shown to be linked mechanistically with the underlying genetic defect in HD (for review see Ref. [36]). BDNF is considered as a potent factor to prevent cell death, as shown in vitro, and to delay the progression of HD, as demonstrated in animal models [31, 37–41]. Studies have assessed the effects of upregulation of BDNF using chemically induced disease. Thus, delivery of BDNF by protein infusion, intrastriatal injection of adenovirus expressing BDNF, or implantation of cells expressing BDNF induced neuroprotection in striatum that was exposed to toxins [41–43]. Moreover, studies using HD mouse models showed that BDNF is neuroprotective [7]. Thus, BDNF administration reversed the increased of GABAergic function found in HD mouse models [44]. The delivery of BDNF using osmotic minipump into the striatum in mice overexpressing exon 1 of human mutant huntingtin protein was associated with elevated expression of encephalin, which is affected mostly in HD [31]. This study also demonstrated delayed motor impairment and extended survival time in these animal models. Another study using a combination of BDNF-adenovirus vector delivery and noggin molecule showed promoting neurogenesis, striatal neuronal regeneration, and delayed motor impairment and extended the survival time in HD mouse models [45].
Similar to HD, BDNF is also a potential neurotrophic factor for treatment of AD. Deficits of cholinergic neurons are possibly the cause of cognitive deterioration, which is one of the major symptoms of AD [21]. The use of BDNF in AD is more effective for ameliorating the cholinergic functions [46]. In addition, BDNF mediates synaptic plasticity and cognitive function [47]. In humans suffering from AD, BDNF mRNA and protein were found to be decreased in cholinergic neurons in the cortex and hippocampus. A deficit in pro-BDNF protein also was found in the parietal cortex in AD [48]. It is clear that the reduction of BDNF levels in AD, particularly in cholinergic neurons, indicates that this neurotrophic factor is considered a key factor in AD. The acetylcholinesterase inhibitors, antioxidants, and glutamate antagonists have been used mostly in clinical settings for the treatment of AD [49–51]. A patent relates to novel analogs of choline and methods of use for treatment of AD, HD and other neurodegenerative diseases [52]. Together, these findings provide ample information about the uses of BDNF in several neurodegenerative diseases including HD.
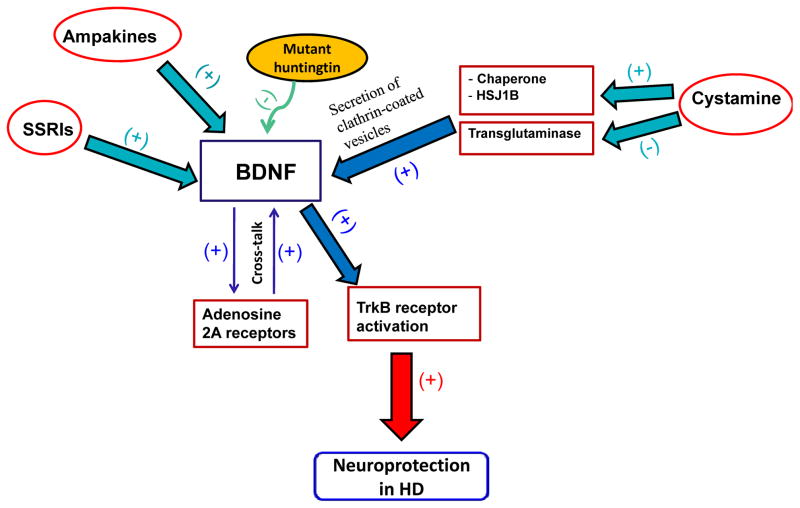
Although, the mechanism of action of BDNF in the prevention of the effects of mutant huntingtin protein is unclear, studies have shown that there is cross-talk between adenosine 2A receptors and BDNF (for review see Ref. [53]). The adenosine 2A receptors play a role in the mechanism of action involving the transactivation of BDNF receptor TrkB and also regulate the effect of BDNF on the synaptic neurotransmission as shown in Fig. (2). On the other hand, the molecular mechanisms underlying downer-gulation of BDNF level by mutant huntingtin protein might be associated with dysregulation of BDNF exon IV and VI transcription [32].
Fig. 2.
Molecular and pharmacological mechanism of actions of BDNF in the prevention against the effects of mutant huntingtin protein. First, adenosine 2A receptors play a role in the mechanism of action involving the transactivation of BDNF receptor TrkB. Second, cystamine has a neuroprotective effect that is mediated through the upregulation of chaperone, HSJ1B, and the inhibition of transglutaminase. These proteins are key players in the secretion of clathrin-coated vesicles containing BDNF. Third, ampakines and SSRIs are also suggested to increase the expression or activity of BDNF. Together, these compounds may induce BDNF expression, which leads to neuroprotection. (+) Stimulatory effect; (−) Inhibitory effect.
There are compounds that regulate the levels of BDNF in the brain. Cystamine, an inhibitor of transglutaminase, is a neuroprotective drug that inhibits caspase-3 activation [54] and increases the levels of antioxidants, glutathione and L-cysteine [54, 55]. The neuroprotective effect of cystamine is mediated through upregulation of chaperone, HSJ1B, and the inhibition of transglutaminase. These proteins are key players in the secretion of clathrin-coated vesicles containing BDNF [56]. Moreover, this latter study showed also that an FDA-approved drug, cysteamine, was effective in increasing BDNF levels and consequently inducing neuroprotective effects in HD mouse models. In addition, cysteamine up-regulates serum BDNF levels in mouse and primate models of HD [56]. Together, these findings suggest that cysteamine is considered a potential compound for the treatment of HD and BDNF levels in serum might be used as biomarker to determine the efficacy of the treatment of HD.
Moreover, a patent provides novel methods to improve or prevent cognitive dysfunction in pre-symptomatic or asymptomatic patients carrying mutations in the huntingtin gene [57]. These novel methods involve the increase of the expression or activity of the BDNF protein in the brains of HD patients using several compounds (e.g. ampakines, desipramine, afobazole, and others). In certain embodiments, this patent indicates that increasing the BDNF level or activity in humans comprises administering glutamate AMPA receptor modulators (e.g. ampakines) to upregulate the expression or activity of BDNF. This patent provides the use of compounds that increase the level or activity of BDNF in a mammal for the treatment or prevention of cognitive dysfunction in a pre- or asymptomatic mammal having one or more mutations in the huntingtin gene [57].
BDNF can also be regulated by activation of the serotonergic system as discussed in our previous review (for review, see Ref. [58]). Studies have shown that administration of selective serotonin re-uptake inhibitors (SSRIs) increased the levels of BDNF in hippocampus [59–62]. This suggests that SSRIs might be used to modulate the level of BDNF in HD.
2.2. FGF-2
Fibroblast growth factor-2 (FGF-2) has been shown to be neuroprotective against exposure to toxins or excitatory amino acids [63]. FGF-2 upregulates the level of normal huntingtin protein in a dose-dependent manner, which indicates its involvement in the expression of normal huntingtin levels [63]. FGF-2 has been found to be protective in striatal neurons [64–66]. FGF-2 increases neurogenesis and induces neuroprotection, which has a positive effect on the survival of R6/2 transgenic mice [67]. This suggests that an increase of neurogenesis in striatum with administration of FGF-2 may have an effect on the migration of nascent neurons, which become medium spiny neurons; these neurons are replaced in HD. A patent involves the identification of FGF-2 that stimulates neurogenesis, and induction of migration of newborn cells in the striatum and cortex; this patent indicates FGF-2 as a neuroprotective factor that may extend the lifespan of patients suffering from HD and other neurodegenerative diseases [68]. In certain embodiments, this patent suggests methods of promoting neurogenesis and neuroprotection by upregulating the expression or availability of endogenous FGF-2 or by administering FGF-2 in a sufficient amount to induce neuroprotection. The methods consist of administration of FGF-2 by systemic route or directly into the brain. Increased FGF-2 expression can be monitored by radiation treatment, anti-depressant or 2-adrenergic receptor agonist administrations [68].
2.3. GDNF
A third trophic factor is GDNF, which has been considered a potential neurotrophic factor for the treatment of neurodegenerative diseases, including HD [69–73]. GDNF is a glycosylated disulfide-bonded homodimer which has closest structural homology to the transforming growth factor-β (TGF-β) family of neurotrophic proteins [74, 75]. The delivery of GDNF using viral vector technology has been shown to be effective in N171-82Q transgenic HD mice [71]. The administration of GDNF induced neuroprotection and overcome the behavioral deficits found in this HD mouse model. However, GDNF viral delivery fails to improve the behavioral deficits in the R6/2 mouse model [76]. This might be due to the fact that R6/2 mice have a larger number of repeats as compared to N171-82Q transgenic mice. Together, these findings suggest that GDNF has limited therapeutic effects that may depend on the CAG repeats. On the other hand, a patent provides a method for preventing or reducing cell death mediated by NMDA receptor agonist by administering GDNF [77]. This new developed method relates, in particular, to the treatment of HD and other neurodegenerative diseases. Similar to its neuroprotective role in HD, GDNF has been found to attenuate the loss of nigrostriatal dopaminergic neurons in animal models of PD and in clinics [74, 78–86], and prevents neurodegeneration of motoneurons in mutant SOD1, ALS mouse models [87–89]. The pool of target thera-pies contains several trophic factors that may protect and maintain the functionality of the motoneurons [90, 91]. Together, these findings suggest that GDNF plays an important role in neurodegenerative diseases, including HD.
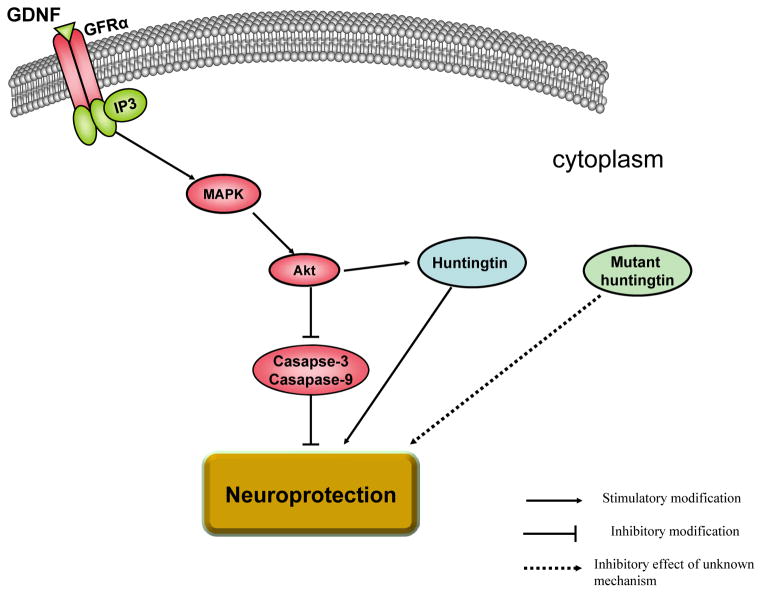
In regards to the mechanism of actions of GDNF in neuroprotection, it has been demonstrated that GDNF binds to GFRα1/Ret receptor complex and in turn activates the inositol triphosphate and the mitogen-activated protein kinase intracellular cascades [92]. This leads to activation of Akt which in turn inactivates caspases 9 and 3, consequently inhibiting cell death as shown in Fig. (3). Importantly, huntingtin and mutant huntingtin proteins are phosphorylated proteins. For example, huntingtin can be phosphorylated by Akt [93–97], which is considered a survival kinase that can lead to neuroprotection Fig. (3). Note that the expression of mutant huntingtin protein can lead to the reduction of phosphorylation of huntingtin protein at Ser421 [95, 97]. This is one of the mechanisms involved in neurodegene-ration in HD.
Fig. 3.
Molecular mechanism of actions of GDNF in neuroprotection. GDNF binds to GFRα1/Ret receptor complex and in turn activates the inositol triphosphate (IP3) and the mitogen-activated protein kinase (MAPK) intracellular cascades. MAPK activates Akt, which consequently inactivates caspases 3 and 9 and thus inhibits cell death. Moreover, Akt activates normal huntingtin protein, which in turn maintains cell survival. However, when mutant huntingtin protein is expressed, it may induce downregulation of phosphorylated normal huntingtin protein leading to cell death.
2.4. Other Potential Neurotrophic Factors for the Treatment of the Progression of HD
There are other neurotrophic factors that are potentially useful for the treatment of the progression or delay of HD. For example, Insulin-like growth factor (IGF) is another factor that has been suggested to play a role in neuroprotection in HD. The neuroprotection is mediated through the prosurvival kinase Akt that has an effect in the phosphorylation of huntingtin protein at Ser421 [94]. The signaling pathways involving IGF-1 and Akt are affected in HD animal models [93]. Akt has an inhibitory effect on mutant huntingtin protein, thus preventing cell death [98]. Akt may induce phosphorylation of ADP-ribosylation factor-interacting protein 2 (arfaptin 2), which is a key factor in cell survival. Arfaptin 2 phosphorylation inhibits the blockade of proteasome caused by the mutant huntingtin protein [98]. IGF-1 has also been considered as a potent neurotrophic factor for the treatment of ALS [99, 100]. Together, these findings suggest that IGF-1 might be a potential neurotrophic factor for the treatment of HD.
VEGF is also another trophic factor that might be suggested for the treatment of HD. VEGF has been considered as a promising factor for the treatment of ALS. In animal models, the role of VEGF in ALS has been investigated through the establishment of a new line of transgenic mice with the deletion of the hypoxia-inducible response element in the VEGF promoter. In this case, there was reduction in the expression of VEGF, progressive muscle weakness, and degeneration of motoneurons that are characteristic of ALS [101]. In addition, the levels of endogenous VEGF were found low in the cerebrospinal fluid, which is identified early in ALS patients [102]. Based on these findings, it is warranted to investigate the role of VEGF in HD.
A synthetic hybrid peptide, Colivelin, composed of ANDF-9 and AGA-(C8R)HNG17, is a potent derivative of humanin (bioactive peptide with anti-AD activity) and has been shown to improve motor performance and prolong the survival of mutant SOD1 mice models of ALS [103]. The addition of humanin to the synthetic peptide ADNF-9 prevents the degradation of this peptide and consequently increases its efficiency for neuroprotection. Colivelin is considered a new trophic factor to improve motor performance and prolong survival of ALS mouse models. The advantage of using the complex ADNF-9-Humanin is based on its stability and its ability to cross the blood-brain barrier [104]. Colivelin has also been found to have a neuroprotective effect in fetal alcohol exposure model, as demonstrated recently by our laboratory [105]. A patent filed from our laboratory indicates the neuroprotective role of Colivelin against oxidative stress [106]. Since Colivelin has been found to have neuroprotective effects in ALS and AD models, investigating its role in HD is warranted. Importantly, a patent indicates the use of Colivelin for the treatment and prevention of HD and other neurodegenerative diseases [107]. It has been demonstrated that in mice treated intracerebroventricularly with β-amyloid, Colivelin has a potent neuroprotective effect on β-amyloid-induced memory dysfunction [108]. In regard to humanin alone, studies have shown that the rescue activity of humanin is mediated by formyl peptide receptor-like 1, a G-protein coupled receptor (GPCR humanin receptor) [109]. A patent has been disclosed for methods of examining whether a potential drug is considered a modulator of the GPCR humanin receptor [110]. Moreover, there are other humanin receptors besides the formyl peptide receptor-like 1 that can play a role in neuroprotection [111].
Moreover, another derived peptide from ADNP, NAP, was found to inhibit β-amyloid aggregation by binding to its 25–35-fragment at high affinity and consequently preventing its toxic effect [112]. NAP also was found to interact with tubulin (principle unit for axonal transport) or stimulate neuronal target receptor for neuronal protection processes [112]. Regarding the second derived peptide, ADNF-9 has been tested in a model of apolipoprotein E (ApoE)-deficient mouse; apolipoprotein deficiency is considered one of the risk factors of AD. Daily injections of ADNF-9 to newborn ApoE-deficient mice were found to improve the acquisition of developmental reflexes and prevent short-term memory deficits [22]. ADNF-9 has been suggested also to aggregate with β-amyloid and consequently block its toxic effect [108]. There is a patent that relates to the use of ADNF-9 peptide for treatment of neurotoxicity induced by chemicals or by disease process [113]. In addition, another patent relates to the treatment of oxidative stress in a patient for reducing a condition associated with fetal alcohol syndrome in a subject and methods of enhancing learning and memory [114]. Moreover, another patent relates to ADNF-9 peptide for its neuroprotective action and its uses thereof for the treatment of neurological deficiencies and for the prevention of cell death [115].
3. POTENTIAL THERAPEUTIC COMPOUNDS AND METHODS FOR THE TREATMENTS OF HD
Research projects are testing other compounds and methods for the treatment of HD. The discovery of these new drugs and methods has been possible due to the identification of the signaling pathways involving the gene and/or mutant huntingtin protein and the implications of glutamatergic system in HD.
3.1. Potential Compounds Targeting Glutamatergic System for the Treatment of HD
HD is one of the diseases that show dysregulation of glutamate [2, 116–118]. Importantly, a decline in glutamate uptake has been observed in HD transgenic mouse models [119–121] as well as HD patients post-mortem [122]. Dysregulation in glutamate uptake might be associated with a deficit in glutamate transporter 1 (GLT1 or EAAT2), which plays a critical role in HD and other neurodegenerative diseases [119, 123]. GLT1 was found dysfunctional in HD mouse models [119, 120, 124]. We recently demonstrated that a deficit in glutamate uptake in the R6/2 mouse model can be reversed with the ceftriaxone [121], which is a β-lactam antibiotic that upregulates more specifically the level of GLT1 [125]. It is noteworthy that a phase III clinical trial of ceftriaxone for treatment of ALS is already underway (for review see Ref. [126]).
Glutamate receptors have been important targets for the treatment of HD. For example, guanidine derivatives, which show high binding affinity to phencyclidine (PCP) receptors and low affinity to sigma receptors, inhibit NMDA receptors. These compounds are suggested to be neuroprotective and might be useful in the treatment of HD and other neurodegenerative diseases [127]. Moreover, another patent provides a method for producing a therapeutic vaccine, which consists of NMDA-NR1 subunit expressed in insect cells, to produce recombinant proteins which are encapsulated in poly-lactide-co-glycolic acid microparticles and used for oral immunization for the treatment of HD and other neurodegenerative diseases [128].
A patent provides a pharmacological composition and a method for treating HD using Naaladase (N-acetylated-a-linked acidic dipeptidase) inhibitors [129]. N-acetylas-partylglutamate (NAAG) has been suggested to play a role as a potential storage form of glutamate. NAALADase can induce hydrolysis of NAAG into N-acetylaspartate (NAA) and glutamate. The inhibition of NAALADase might be neuroprotective in diseases involving excess glutamatergic transmission, and this may include HD and other neurodegenerative diseases [130]. Moreover, NAAG can also be considered as an agonist to metabotropic glutamate receptors and as a mixed agonist/antagonist to NMDA receptors. These suggest that inhibition of NAALADase may increase NAAG levels that can lead to neuroprotection associated with NAAG actions toward these glutamatergic receptors.
Another patent provides a method for the treatment of HD by slowing the onset and/or the progression of HD or preventing the development of HD using hydrogenated pyrido[4,3-b] indoles, including Dimebon [131]. Dimebon was characterized as a low-affinity NMDA receptor antagonist (for review see Ref. [132]). Although Dimebon is considered to have weak action, it is suggested that the drug has a mechanism of action involving mitochondria [132]. On the other hand, studies have shown that Dimebon has clinical relevance that might result from the inhibition of alpha-adrenergic receptors (alpha1A, alpha1B, alpha1D, and alpha2A), histamine H1 and H2 receptors and serotonin 5-HT2c, 5-HT5A, 5-HT6 receptors [133].
In regard to mitochondrial dysfunction in HD, there is a patent that indicates a method of ameliorating HD by administration of a formulation that includes mitochondrial coenzyme Q10 [134]. This formulation contains Hydro-Q Sorb®, which is composed of coenzyme Q10 and cyclo-dextrin. In addition, another patent indicates the uses of compositions and methods for the treatment of mitochondrial dysfunction in neurodegenerative diseases, including HD [135]. The methods consists of administering pyrimidine nucleotide precursors, which regulate mitochondrial biosynthesis.
Moreover, there are compounds that are suggested to be effective in alleviating and/or reversing neurodegeneration in HD and other neurodegenerative diseases [52, 136–138]. The patented compounds are substituted pyrrolines that are used as kinase inhibitors for the treatment of disorders involving kinase. In addition, another patent provides the use of Efaroxan or therapeutically-acceptable salts, in their racemic forms or in the forms of optically-active isomers, for the treatment of HD [128]. Efaroxan is a 2-[2-(2-ethyl-2,3-dihydrobenzofuranyl)]-2-imidazoline that has antagonistic properties on the α 2-adrenergic receptors. The patent indicates the advantages of Efaroxan in reducing the excitotoxic effects of quinolinic acid in the striatum, as well as its advantage in the prevention of disorders involving glutamate receptors in neurotoxicity.
3.2. Blockade of Mutant HD Gene for Symptomatic Treatment
A patent provides methods for suppressing the mutant huntingtin gene by using a double-stranded RNA (dsRNA) [139]. This patent provides a method for targeting a specific sequence of mRNA immediately upstream of CAG repeats in the HD gene; the mutant huntingtin gene expression is suppressed by using a dsRNA homologous to the sequence. The patent indicates also that a short siRNA (short double-stranded RNA) having bp as short as around 21–23 bp may be as effective as dsRNA homologous to a specific RNA sequence in a region immediately upstream of CAG repeats. The dsRNA is suggested to be used as an inhibitor of mutant huntingtin gene to delay the progression or prevent neurodegeneration in HD [139]. Moreover, a patent indicates the identification of a new nucleotide sequence as an anti-sense strand that complements at least part of the HD gene [140]. The patent also relates to pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier that has an effect on inhibiting the expression of the mutant huntingtin gene. There is also another patent that relates to methods and assays for identifying compounds that modulate the aberrant conformation, aggregation or expression of mutant huntingtin protein [141]. This patent provides polypeptide, nucleic acid targets and siRNA sequences to modulate the expression of mutant huntingtin protein [141]. In addition, a patent provides the use of small interfering RNA and methods of treating HD and other neurodegenerative diseases [142]. Another patent provides the use of these siRNA and methods to treat HD [143]. The methods consist of surgically implanted catheters that discharge siRNA vectors targeting huntingtin gene. Furthermore, another patent indicates the use of isolated nucleic acid duplex sequences for reducing huntingtin gene [144]. These sequences, molecules and methods provide treatment for HD by reducing the mRNA without causing death, locomotor impairment or cellular dysfunction, as demonstrated in primates [144].
In addition, a patent provides apoptosis modulators that interact with the HD gene [145]. This patent indicates the identification of proteins designated as huntingtin-interacting proteins (HIP-1) that interact differentially with the gene product of a normal (16 CAG repeat) and an expanded (>44 CAG repeat) HD gene. The HIP-1 protein isolated from a yeast two-hybrid screen is encoded by a 1.2 kb cDNA devoid of stop codons expressing ~400 amino acid poly-peptide. The present patent provides a new class of apoptotic modulators which are referred to as HIP-apoptosis modulating proteins.
3.3. Targeting Mutant Huntingtin Protein Using Selective Antibodies
In the focus of mutant huntingtin protein, a patent relates to the generation and characterization of anti-huntingtin antibodies binding on the mutant huntingtin protein [146]. This patent indicates the generation of antibodies, particularly monoclonal antibodies including antibody fragments such as single-chain variant fragments that bind to the mutant huntingtin protein. The antibodies may have a neuroprotective effect that is mediated through the prevention of mutant huntingtin protein aggregation and the regulation of its toxic effects. In one embodiment of this patent, the antibodies are suggested to bind to an epitope within a polyproline region of the mutant huntingtin protein having a more than 5 consecutive proline residues, which may inhibit its aggregation [146]. The idea of the uses of antibodies against proteins or peptides involved in neurodegenerative diseases is in accordance with a patent providing the uses of anti-amyloid antibodies for the treatment of AD [147].
3.4. Uses of Selective Compound Aggregating Mutant Huntingtin Protein
Geldanamycin is a compound that has been shown to inhibit mutant huntingtin protein aggregation [148]. Geldanamycin is a benzoquinone ansamycin antibiotic that binds to Hsp90 (Heat Shock Protein 90) and alters its function. Hsp 90 is associated with another protein called HSF-1 (Heat Shock Factor 1). Geldanamycin binds to Hsp 90 and consequently makes this protein unable to associate with HSF-1 [148]. Free HSF-1 induces expression of Hsp70 and Hsp40, which consequently unfold and promote the degradation of misfolded mutant huntingtin protein through the proteasome [148, 149]. On the other hand, a patent indicates the use of a method to modulate the aggregation of polyglutamine protein in HD and proteins in other neurodegenerative diseases [150]. For example, the Y-27632, an inhibitor of the Rho-associated kinase p160ROCk, reduces polyglutamaine protein aggregation at micromolar concentrations and reduces neurodegeneration in a Drosophila model of polyglutamaine disease [150].
3.5. Utilization of Electrical Stimulation for the Treatment of HD Symptoms
A patent relates to introducing one or more stimulating drugs to the brain and/or applying electrical stimulation to the brain for the treatment of HD [151]. An implantable system control unit may induce electrical pulses delivered via electrodes implanted in the brain and/or drug infusion pulses delivered via a catheter implanted in the brain of HD patients. The goal of this method is to adjust the activity of a specific brain region with stimulation.
CURRENT & FUTURE DEVELOPMENTS
HD is characterized by neuronal dysfunction and degeneration that are suggested to be caused by the expression of mutant huntingtin protein. Although, there are no treatments to slow or prevent the progression of HD, neurotrophic factors, selective compounds, and methods are considered therapeutic tools for the treatment of HD to overcome neuronal dysfunction and behavioral abnormalities. In HD therapy, BDNF, GDNF, and FGF-2 have been well studied and showed promising effects on neuroprotection in HD. On the other hand, since the key factor in HD is the mutant huntingtin protein, new compounds and methods have been identified and are considered to be potentially effective in targeting the HD gene or mutant huntingtin protein. Moreover, targeting glutamatergic system has been a key player in the treatment of HD. There are current patents that are in preclinical and clinical testing and may show promising therapeutic effects. It is important to note that there are patented compounds or methods to monitor the regulatory effects of neurotrophic factors and glutamatergic system in HD. Importantly, these treatments might be combined with new patented drugs targeting the mutant huntingtin protein involved in the progression of HD. A combination of several methods might be a key role in the prevention or delay of the progression of HD.
Acknowledgments
I would like to thank the National Institute of Health-NIAA for their continuous support (R21AA017735; R21AA-016115). I would also like to thank Charisse Montgomery for editing this review article.
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Martin JB, Gusella JF. Huntington’s disease. Pathogenesis and management. N Engl J Med. 1986;315 (20):1267–76. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA. Polyglutamine pathogenesis: Emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron. 2002;35 (5):819–22. doi: 10.1016/s0896-6273(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 3.(HDCRG), HsDCRG. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 4.McMurray CT. Huntington’s disease: New hope for therapeutics. Trends Neurosci. 2001;24 (11 Suppl):S32–8. doi: 10.1016/s0166-2236(00)01997-4. [DOI] [PubMed] [Google Scholar]
- 5.Myers RH, Marans KS, MacDonald ME. Huntington’s disease. In: Wells RD, Warren ST, editors. genetic instabilities and hereditary neurological diseases. Academic Press; San Diego: 1998. pp. 301–23. [Google Scholar]
- 6.Brocklebank D, Gayan J, Andresen JM, Roberts SA, Young AB, Snodgrass SR, et al. Repeat instability in the 27–39 CAG range of the HD gene in the Venezuelan kindreds: Counseling implications. Am J Med Genet B Neuropsychiatr Genet. 2009;150B (3):425–9. doi: 10.1002/ajmg.b.30826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90(3):537–48. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 8.Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington’s disease with 140 CAG repeats. J Comp Neurol. 2003;465(1):11–26. doi: 10.1002/cne.10776. [DOI] [PubMed] [Google Scholar]
- 9.de la Monte SM, Vonsattel JP, Richardson EPJ. Morp’hometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. J Neuropathol Exp Neurol. 1988;47(5):516–25. doi: 10.1097/00005072-198809000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EPJ. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57 (5):369–84. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Peschanski M, Bachoud-Levi AC, Hantraye P. Integrating fetal neural transplants into a therapeutic strategy: The example of Huntington’s disease. Brain. 2004;127:1219–28. doi: 10.1093/brain/awh145. [DOI] [PubMed] [Google Scholar]
- 13.Bachoud-Levi A, Bourdet C, Brugieres P, Nguyen JP, Grandmougin T, Haddad B, et al. Safety and tolerability assessment of intrastriatal neural allografts in five patients with Huntington’s disease. Exp Neurol. 2000;161(1):194–202. doi: 10.1006/exnr.1999.7239. [DOI] [PubMed] [Google Scholar]
- 14.Rosser AE, Barker RA, Harrower T, Watts C, Farrington M, Ho AK, et al. Unilateral transplantation of human primary fetal tissue in four patients with Huntington’s disease: NEST-UK safety report ISRCTN no 36485475. J Neurol Neurosurg Psychiatry. 2002;73 (6):678–85. doi: 10.1136/jnnp.73.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachoud-Levi AC, Remy P, Nguyen JP, Brugieres P, Lefaucheur JP, Bourdet C, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356(9246):1975–9. doi: 10.1016/s0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- 16.Freed CR, Kaddis FG. US6254865. Method of treating Huntington’s disease using HNT neurons. 2001
- 17.Hurlbert MS, Gianani RI, Hutt C, Freed CR, Kaddis FG. Neural transplantation of hNT neurons for Huntington’s disease. Cell Transplant. 1999;8(1):143–51. doi: 10.1177/096368979900800106. [DOI] [PubMed] [Google Scholar]
- 18.Alberch J, Perez-Navarro E, Canals JM. Neurotrophic factors in Huntington’s disease. Prog Brain Res. 2004;146:195–229. doi: 10.1016/s0079-6123(03)46014-7. [DOI] [PubMed] [Google Scholar]
- 19.Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev. 1998;27(1):1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 20.Kruttgen A, Saxena S, Evangelopoulos ME, Weis J. Neurotrophins and neurodegenerative diseases: Receptors stuck in traffic? J Neuropathol Exp Neurol. 2003;62(4):340–50. doi: 10.1093/jnen/62.4.340. [DOI] [PubMed] [Google Scholar]
- 21.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63(1):71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 22.Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72(3):1283–93. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- 23.Zusev M, Gozes I. Differential regulation of activity-dependent uroprotective protein in rat astrocytes by VIP and PACAP. Regul Pept. 2004;123(1–3):33–41. doi: 10.1016/j.regpep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I. Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol. 2004;1(3):193–9. doi: 10.1017/S1740925X05000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sari Y. Activity-dependent neuroprotective protein-derived peptide, NAP, preventing alcohol-induced apoptosis in fetal brain of C57BL/6 mouse. Neurosci. 2009;158(4):1426–35. doi: 10.1016/j.neuroscience.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected, in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Brain Res Rev. 2006;52(1):107–18. doi: 10.1016/j.brainresrev.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Gozes I. Activity-dependent neuroprotective protein: From gene to drug candidate. Pharmacol Ther. 2007;114(2):146–54. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866 (1–2):257–61. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 29.Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24(9):2270–6. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Sci. 2001;293(5529):493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 31.Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci. 2004;24(35):7727–39. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gambazzi L, Gokce O, Seredenina T, Katsyuba E, Runne H, Markram H, et al. Diminished activity-dependent brain-derived neurotrophic factor expression underlies cortical neuron microcircuit hypoconnectivity resulting from exposure to mutant huntingtin fragments. J Pharmacol Exp Ther. 2010;335 (1):13–22. doi: 10.1124/jpet.110.167551. [DOI] [PubMed] [Google Scholar]
- 33.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118(1):127–38. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 35.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24(17):4250–8. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–22. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 37.Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27 (43):11758–68. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, et al. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci. 2007;27(16):4424–34. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, et al. Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington’s disease. J Neurochem. 2005;93(5):1057–68. doi: 10.1111/j.1471-4159.2005.03047.x. [DOI] [PubMed] [Google Scholar]
- 40.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95(1):55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 41.Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther. 2004;9(5):682–8. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, et al. Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol Res. 2005;52(2):133–9. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, et al. Increased GABAergic function in mouse models of Huntington’s disease: reversal by BDNF. J Neurosci Res. 2004;78 (6):855–67. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- 45.Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117(10):2889–902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler J, Thal LJ, Gage FH, Fisher LJ. Cholinergic strategies for Alzheimer’s disease. J Mol Med. 1998;76(8):555–67. doi: 10.1007/s001090050250. [DOI] [PubMed] [Google Scholar]
- 47.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahnestock M, Garzon D, Holsinger RM, Michalski B. Neurotrophic factors and Alzheimer’s disease: Are we focusing on the wrong molecule? J Neural Transm Suppl. 2002;(62):241–52. doi: 10.1007/978-3-7091-6139-5_22. [DOI] [PubMed] [Google Scholar]
- 49.Lane RM, Kivipelto M, Greig NH. Acetylcholinesterase and its inhibition in Alzheimer disease. Clin Neuropharmacol. 2004;27(3):141–9. doi: 10.1097/00002826-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 51.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336 (17):1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 52.Buccafusco JJ, Terry AV, Beach J, Jonnala RR. US6881738. Analogs of choline for neuroprotection and cognitive enhancement in neurodegenerative disorders. 2005
- 53.Tebano MT, Martire A, Chiodi V, Ferrante A, Popoli P. Role of adenosine A(2A) receptors in modulating synaptic functions and brain levels of BDNF: A possible key mechanism in the pathophysiology of Huntington’s disease. Scientific World J. 2010;10:1768–82. doi: 10.1100/tsw.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278(6):3825–30. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 55.Fox JH, Barber DS, Singh B, Zucker B, Swindell MK, Norflus F, et al. Cystamine increases L-cysteine levels in Huntington’s disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91(2):413–22. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 56.Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116 (5):1410–24. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons D, Lynch G, Kramar E. US20080176793. Upregulating activity or expression of BDNF to mitigate cognitive impairment in asymptomatic Huntington’s subjects. 2008
- 58.Powrozek TA, Sari Y, Singh RP, Zhou FC. Neurotransmitters and substances of abuse: Effects on adult neurogenesis. Curr Neurovasc Res. 2004;1(3):251–60. doi: 10.2174/1567202043362225. [DOI] [PubMed] [Google Scholar]
- 59.Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry. 1998;44 (5):324–35. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 60.Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav. 2002;73 (2):317–26. doi: 10.1016/s0091-3057(02)00844-4. [DOI] [PubMed] [Google Scholar]
- 61.Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17(8):2785–95. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262(1):1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 63.Haque NS, Isacson O. Neurotrophic factors NGF and FGF-2 alter levels of huntingtin (IT15) in striatal neuronal cell cultures. Cell Transplant. 2000;9(5):623–7. doi: 10.1177/096368970000900507. [DOI] [PubMed] [Google Scholar]
- 64.Bjugstad KB, Zawada WM, Goodman S, Free CR. IGF-1 and bFGF reduce glutaric acid and 3-hydroxyglutaric acid toxicity in striatal cultures. J Inherit Metab Dis. 2001;24(6):631–47. doi: 10.1023/a:1012706908779. [DOI] [PubMed] [Google Scholar]
- 65.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6(5):474–86. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 66.Zhou D, DiFiglia M. Basic fibroblast growth factor enhances the growth of postnatal neostriatal GABAergic neurons in vitro. Exp Neurol. 1993;122(2):171–88. doi: 10.1006/exnr.1993.1118. [DOI] [PubMed] [Google Scholar]
- 67.Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, et al. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102(50):18189–94. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellerby L, Greenberg D, Anderson J, Jin K. US20090111748. Fibroblast growth factor-2 promotes neurogenesis and neuroprotection and prolongs survival in Huntington’s disease. 2009 doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed]
- 69.Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, et al. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182(1):107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 70.Kim BT, Rao VL, Sailor KA, Bowen KK, Dempsey RJ. Protective effects of glial cell line-derived neurotrophic factor on hippocampal neurons after traumatic brain injury in rats. J Neurosurg. 2001;95(4):674–9. doi: 10.3171/jns.2001.95.4.0674. [DOI] [PubMed] [Google Scholar]
- 71.McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, et al. Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2006;103 (24):9345–50. doi: 10.1073/pnas.0508875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perez-Navarro E, Arenas E, Reiriz J, Calvo N, Alberch J. Glial cell line-derived neurotrophic factor protects striatal calbindin-immunoreactive neurons from excitotoxic damage. Neuroscience. 1996;75 (2):345–52. doi: 10.1016/0306-4522(96)00336-3. [DOI] [PubMed] [Google Scholar]
- 73.Watabe K, Ohashi T, Sakamoto T, Kawazoe Y, Takeshima T, Oyanagi K, et al. Rescue of lesioned adult rat spinal motoneurons by adenoviral gene transfer of glial cell line-derived neurotrophic factor. J Neurosci Res. 2000;60(4):511–9. doi: 10.1002/(SICI)1097-4547(20000515)60:4<511::AID-JNR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 74.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Sci. 1993;260(5111):1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 75.Poulsen KT, Armanini MP, Klein RD, Hynes MA, Phillips HS, Rosenthal A. TGF beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neurons. Neuron. 1994;13(5):1245–52. doi: 10.1016/0896-6273(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 76.Popovic N, Maingay M, Kirik D, Brundin P. Lentiviral gene delivery of GDNF into the striatum of R6/2 Huntington mice fails to attenuate behavioral and neuropathological changes. Exp Neurol. 2005;193(1):65–74. doi: 10.1016/j.expneurol.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Martin D, Miller G. US5741778. Method for treating Huntington’s diease using glial cell line-derived neurotrophic factor GDNF protein product. 1998
- 78.Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886(1–2):82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 79.Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol. 2005;58(2):224–33. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- 80.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20(12):4686–700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Sci. 2000;290(5492):767–73. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 82.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–95. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 83.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59(3):459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 84.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurol. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 85.Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: A two-year outcome study. Ann Neurol. 2005;57(2):298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 86.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102 (2):216–22. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 87.Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13(9):1047–59. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 88.Lu YY, Wang LJ, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, et al. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci Res. 2003;45(1):33–40. doi: 10.1016/s0168-0102(02)00195-5. [DOI] [PubMed] [Google Scholar]
- 89.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22(16):6920–8. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson CE, Yamamoto Y, Livet J, Arce V, Garces A, deLapeyriere O. Role of neurotrophic factors in motoneuron development. J Physiol Paris. 1998;92(3–4):279–81. doi: 10.1016/s0928-4257(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 91.Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res. 2000;301(1):71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- 92.Eketjall S, Fainzilber M, Murray-Rust J, Ibanez CF. Distinct structural elements in GDNF mediate binding to GFRalpha1 and activation of the GFRalpha1-c-Ret receptor complex. EMBO J. 1999;18(21):5901–10. doi: 10.1093/emboj/18.21.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colin E, Regulier E, Perrin V, Durr A, Brice A, Aebischer P, et al. Akt is altered in an animal model of Huntington’s disease and in patients. Eur J Neurosci. 2005;21(6):1478–88. doi: 10.1111/j.1460-9568.2005.03985.x. [DOI] [PubMed] [Google Scholar]
- 94.Humbert S, Bryson EA, Cordelieres FP, Connors NC, Datta SR, Finkbeiner S, et al. The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves Huntingtin phosphorylation by Akt. Dev Cell. 2002;2(6):831–7. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 95.Pardo R, Colin E, Regulier E, Aebischer P, Deglon N, Humbert S, et al. Inhibition of calcineurin by FK506 protects against polyglutamine-huntingtin toxicity through an increase of huntingtin phosphorylation at S421. J Neurosci. 2006;26(5):1635–45. doi: 10.1523/JNEUROSCI.3706-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rangone H, Poizat G, Troncoso J, Ross CA, MacDonald ME, Saudou F, et al. The serum- and glucocorticoid-induced kinase SGK inhibits mutant huntingtin-induced toxicity by phosphorylating serine 421 of huntingtin. Eur J Neurosci. 2004;19(2):273–9. doi: 10.1111/j.0953-816x.2003.03131.x. [DOI] [PubMed] [Google Scholar]
- 97.Warby SC, Chan EY, Metzler M, Gan L, Singaraja RR, Crocker SF, et al. Huntingtin phosphorylation on serine 421 is significantly reduced in the striatum and by polyglutamine expansion in vivo. Hum Mol Genet. 2005;14(11):1569–77. doi: 10.1093/hmg/ddi165. [DOI] [PubMed] [Google Scholar]
- 98.Rangone H, Pardo R, Colin E, Girault JA, Saudou F, Humbert S. Phosphorylation of arfaptin 2 at Ser260 by Akt Inhibits PolyQ-huntingtin-induced toxicity by rescuing proteasome impairment. J Biol Chem. 2005;280(23):22021–8. doi: 10.1074/jbc.M407528200. [DOI] [PubMed] [Google Scholar]
- 99.Wilczak N, de Keyser J. Insulin-like growth factor system in amyotrophic lateral sclerosis. Endocr Dev. 2005;9:160–9. doi: 10.1159/000085764. [DOI] [PubMed] [Google Scholar]
- 100.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9 (11):1371–81. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 101.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–8. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 102.Devos D, Moreau C, Lassalle P, Perez T, De Seze J, Brunaud-Danel V, et al. Low levels of the vascular endothelial growth factor in CSF from early ALS patients. Neurol. 2004;62 (11):2127–9. doi: 10.1212/01.wnl.0000129913.44351.a3. [DOI] [PubMed] [Google Scholar]
- 103.Chiba T, Yamada M, Sasabe J, Terashita K, Aiso S, Matsuoka M, et al. Colivelin prolongs survival of an ALS model mouse. Biochem Biophys Res Commun. 2006;343(3):793–8. doi: 10.1016/j.bbrc.2006.02.184. [DOI] [PubMed] [Google Scholar]
- 104.Matsuoka M, Hashimoto Y, Aiso S, Nishimoto I. Humanin and colivelin: Neuronal-death-suppressing peptides for Alzheimer’s disease and amyotrophic lateral sclerosis. CNS Drug Rev. 2006;12(2):113–22. doi: 10.1111/j.1527-3458.2006.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sari Y, Chiba T, Yamada M, Rebec GV, Aiso S. A novel peptide, colivelin, prevents alcohol-induced apoptosis in fetal brain of C57BL/6 mice: signaling pathway investigations. Neurosci. 2009;164(4):1653–64. doi: 10.1016/j.neuroscience.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sari Y, Chiba T, Aiso S. US057413. Colivelin as a neuroprotective factor. 2009
- 107.Chiba T, Kita Y, Matsuoka M, Terashita K, Aiso S, Nishimoto I, Nishimoto T. US20080227699. Therapeutic agent for neurodegenerative diseases. 2008
- 108.Chiba T, Yamada M, Hashimoto Y, Sato M, Sasabe J, Kita Y, et al. Development of a femtomolar-acting humanin derivative named colivelin by attaching activity-dependent neurotrophic factor to its N terminus: characterization of colivelin-mediated neuroprotection against Alzheimer’s disease-relevant insults in vitro and in vivo. J Neurosci. 2005;25(44):10252–61. doi: 10.1523/JNEUROSCI.3348-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu ZX, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172(11):7078–85. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 110.Villegas S, Kelner GS, Unett DJ, Gatlin J. US20080125491. Human G protein-coupled receptor and modulators thereof for the treatment of cell death-related disorders. 2008
- 111.Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77(24):3092–104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 112.Ashur-Fabian O, Segal-Ruder Y, Skutelsky E, Brenneman DE, Steingart RA, Giladi E, et al. The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides. 2003;24(9):1413–23. doi: 10.1016/j.peptides.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 113.Gozes I, Miller J. US7452867. Use of ANDF polypeptides for treating peripheral neurotoxicity. 2008
- 114.Brenneman DE, Castellon R, Spong CY, Hauser JM, Gozes I. US7427590. Neurotrophic components of ADNF I complex. 2008
- 115.Brenneman DE. US6174862. Neurotrophic peptides of activity dependent neurotrophic factor. 2001
- 116.DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington’s disease. Trends Neurosci. 1990;13(7):286–9. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- 117.Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neurosci. 1981;6(5):863–73. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 118.Harper PS. Huntington’s Disease. 2 W.b. Saunders; London: 1996. [Google Scholar]
- 119.Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125(Pt 8):1908–22. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- 120.Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, et al. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8(5):807–21. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- 121.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neurosci. 2008;153(1):329–37. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hassel B, Tessler S, Faull RL, Emson PC. Glutamate uptake is reduced in prefrontal cortex in Huntington’s disease. Neurochem Res. 2008;33(2):232–7. doi: 10.1007/s11064-007-9463-1. [DOI] [PubMed] [Google Scholar]
- 123.Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001;58(3):365–70. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- 124.Estrada-Sanchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol Dis. 2009;34(1):78–86. doi: 10.1016/j.nbd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 125.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433 (7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 126.Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: A systematic assessment. Neurol. 2006;67(1):20–7. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 127.Weber E, Keana JFW. US6251948. Tri-and tetra-substituted guanidines and their use as excitatory amino acid antagonists. 2001
- 128.Reddy JR. US20090175891. Preventive and therapeutic vaccine for Huntington’s disease. 2009
- 129.Slusher BS, Wozniak K. US20050080139. Naaladase inhibitors for treating huntington’s disease. 2005
- 130.Jackson PF, Slusher BS. Design of NAALADase inhibitors: A novel neuroprotective strategy. Curr Med Chem. 2001;8(8):949–57. doi: 10.2174/0929867013372797. [DOI] [PubMed] [Google Scholar]
- 131.Hung D. US20070117834. Methods and compositions for treating Huntington’s disease. 2007
- 132.Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23(8):518–23. doi: 10.1358/dnp.2010.23.8.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferrante RJ. US7901675. Method of using coenzyme Q10 to treat Huntington’s disease. 2011
- 135.Von Borstel RW. US7915233. Compositions and methods for treatment of mitochondrial diseases. 2011
- 136.Alanine A, Buettelmann B, Neidhart M-PH, Jaeschke G, Pinard E, Wyler R. US6432985. Neuroprotective substituted piperidine compounds with activity as NMDA NR2B subtype selective antagonists. 2002
- 137.Griffin JH, Zlokovic BY. US7074402. Neuroprotective, antithrombotic and anti-inflammatory uses of activated protein C (APC) 2006
- 138.Zhang H-C, Kuo G-H, Maryanoff BE, Ye H, O’Neill D, Shen L, Demarest K, Conway BR, McComsey DF. US7125878. Substituted pyrroline kinase inhibitors. 2006
- 139.Ichiro K, Wanzhao L, Wang Y-L, Wada K, Goto J, Murata M. US7589189. Inhibition of the expression of Huntington gene. 2009
- 140.Sah DW-Y, Hadwiger P, Roehl I, Bramlage B, Tan P, Vornlocher HP, Bumcrot D. US7749978. Compositions and mehtods for inhibiting expression of Huntingtin gene. 2010
- 141.Fischer DF, Janssen RA, De Pril R, Van Steenhoven DM, Kwak S, Howland DS, Signer E. WO2009098196. Molecular targets and compounds, methods to identify the same, useful in the treatment of neurodegenerative diseases. 2009
- 142.Kaemmerer WF. US7605249. Treatment of neurodegenerative disease through intracranial delivery of siRNA. 2009
- 143.Kaemmerer WF, Kaytor MD. US7732591. Compositions, devices and methods for treatment of Huntington’s disease through intracranial delivery of sirna. 2010
- 144.Kaemmerer WF, Kaytor MD. US7902352. Isolated nucleic acid duplex for reducing Huntington gene expression. 2011
- 145.Kalchman M, Hayden MR, Hackam A, Chopra V, Goldberg P. US6235879. Apoptosis modulators that interact with the Huntington’s disease gene. 2001
- 146.Khoshnan A, Ko J, Patterson PH. US20080107657. Antibodies that bind to an epitope on the Huntington’s disease protein. 2008
- 147.Mercken M, Benson JM. US20050129695. Anti-amyloid antibodies, compositions, methods and uses. 2005
- 148.Sittler A, Lurz R, Lueder G, Priller J, Lehrach H, Hayer-Hartl MK, et al. Geldanamycin activates a heat shock response and inhibits Huntingtin aggregation in a cell culture model of Huntington’s disease. Hum Mol Genet. 2001;10(12):1307–15. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 149.Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, Mestril R, et al. Progressive decrease in chaperone protein levels in a mouse model of Huntington’s disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet. 2004;13(13):1389–405. doi: 10.1093/hmg/ddh144. [DOI] [PubMed] [Google Scholar]
- 150.Diamond MI, Pollitt SK. US7803559. Protein aggregation regulators. 2010
- 151.Whitehurst TK, Pianca AM. US20040225335. Treatment of Huntington’s disease by brain stimulation. 2004