Abstract
Neisseria gonorrhoeae encodes five lytic transglycosylases (LTs) in the core genome, and most gonococcal strains also carry the gonococcal genetic island that encodes one or two additional LTs. These peptidoglycan (PG)-degrading enzymes are required for a number of processes that are either involved in the normal growth of the bacteria or affect the pathogenesis and gene transfer aspects of this species that make N. gonorrhoeae highly inflammatory and highly genetically variable. Systematic mutagenesis determined that two LTs are involved in producing the 1,6-anhydro PG monomers that cause the death of ciliated cells in Fallopian tubes. Here, we review the information available on these enzymes and discuss their roles in bacterial growth, cell separation, autolysis, type IV secretion, and pathogenesis.
Introduction
Neisseria gonorrhoeae, a Gram-negative obligate human pathogen, causes the sexually transmitted disease gonorrhea, which is manifested as cervicitis in women and urethritis in men. Each year over 88 million new gonococcal infections are reported worldwide.63 Because gonorrhea is asymptomatic in 50%–80% of infected women,43 many fail to seek treatment, often resulting in complications such as pelvic inflammatory disease, chronic pelvic pain, and ectopic pregnancy (Aral et al., 1988). Other possible outcomes of untreated infections in men and women include sterility, disseminated gonococcal infection, and arthritis. No vaccine is available to protect against gonococcal infections, and treatment of this disease is becoming more difficult due to the development and spread of antibiotic-resistant strains. Since the advent of antibiotic therapies, N. gonorrhoeae has developed resistance to every drug used to treat gonococcal infections (e.g., sulfanilamides, penicillin, tetracycline, and ciprofloxacin).42 Currently, the cephalosporins are the only remaining class of antibiotics to which N. gonorrhoeae has not yet developed widespread resistance, though resistance is on the rise in Asia.3,42 The development of new therapies is critical to prevent a once-treatable disease from becoming untreatable.
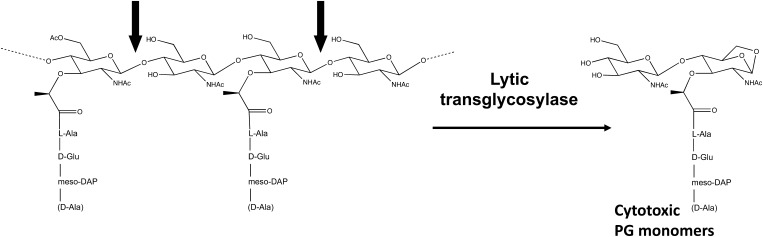
One target of numerous antibiotics, including the cephalosporins, has been the peptidoglycan (PG) layer of the cell wall. Providing the cell with structural support and protection against osmotic stress, PG is a dynamic molecule that is constantly remodeled to allow for cell expansion, division, and the insertion of cellular structures (e.g., flagella, pili, and secretion systems) within the cell wall.33,36 Three classes of enzymes are involved in this remodeling: amidases, endopeptidases, and lytic transglycosylases (LTs). The focus of this review is the LTs, which cleave the glycan backbone at the N-acetylmuramic acid-β-1,4-N-acetylglucosamine (MurNAc-GlcNAc) linkages, resulting in the formation of nonreducing 1,6-anhydro bonds on the MurNAc residues (Fig. 1). A PG fragment containing one GlcNAc-1,6-anhydro-MurNAc disaccharide unit linked to a peptide is referred to as an anhydro PG monomer, while fragments containing multiple disaccharide units are known as multimers. During growth, gonococci release mostly anhydro PG monomers, in addition to some anhydro PG multimers, and these fragments have been shown to contribute to the pathogenicity of the organism. Thus, an interest of our lab is the role of gonococcal LTs in the formation of these toxic PG fragments. Here, we review the LTs found in N. gonorrhoeae and discuss their roles in the biology and virulence of this pathogen.
FIG. 1.
Lytic transglycosylases cleave the N-acetylmuramic acid-β-1,4-N-acetylglucosamine bond in peptidoglycan (PG). The 1,6-anhydro PG monomers created by this reaction cause inflammation and cell death in several biological systems. The peptide chains of PG may have a terminal meso-DAP or D-Ala.
PG Fragments Released by N. gonorrhoeae Contribute to Disease
During logarithmic growth of Gram-negative bacteria, about half of the PG is degraded in each generation.22 In Escherichia coli, most of the PG fragments are efficiently recycled, with roughly 5%–8% released to the surrounding milieu.23,25 In contrast, N. gonorrhoeae releases significantly more PG fragments into its environment,47 despite having a functional PG recycling system.18 These fragments, which include 1,6-anhydro tetrapeptide monomer and 1,6-anhydro tripeptide monomer, have been demonstrated to kill ciliated cells when applied to Fallopian tube tissue in organ culture, replicating the damage observed in patients with pelvic inflammatory disease.40 In addition, when injected into rats, both soluble, large PG fragments and PG monomers were found to induce systemic arthritis,16 similar to the arthritis observed in patients with disseminated gonococcal infection. The large PG fragments gave the most potent response, and PG isolated from a strain with O-acetylation at the C-6 position on the MurNAc of PG was more arthropathic than PG from a strain that does not acetylate.16 Thus, both large fragments released by autolysis and small fragments released during normal growth may generate the inflammation seen in various types of gonococcal infections.
The mechanism by which gonococcal PG induces these inflammatory responses is not known. The inflammatory response to the bacteria and bacterial products appears to be responsible for most or all of the pathogenesis of gonococcal diseases. The damage to Fallopian tube cells is particularly of interest since it has been directly linked to PG fragments and because the cell sloughing is responsible for tubal-factor infertility resulting from gonococcal pelvic inflammatory disease.40 The mechanism of ciliated cell death in the Fallopian tube may be the same as the ciliated cell death that occurs in tracheal tissue during Bordetella pertussis infection. Studies with B. pertussis demonstrated that 1,6-anhydro disaccharide-tetrapeptide PG monomers were responsible for the ciliated cell death, and this molecule was referred to as tracheal cytotoxin, TCT.21,50 Mechanistic studies using hamster tracheal rings demonstrated that the cell death occurred through inflammatory cytokine induction.31 TCT triggered an interleukin-1 (IL-1) response in epithelial cells, IL-1 induced nitric oxide production, and the nitric oxide killed the more sensitive ciliated cells.30 The receptor for TCT for initiating this response is not known. Another possibility for the mechanism of ciliated cell death in Fallopian tubes may be through the PG receptor Nod1.20 In humans, Nod1 responds to disaccharide-tripeptide PG monomers and results in inflammatory cytokine production.37 Since studies examining the effects of PG monomers on Fallopian tube cells used a mixture of tripeptide and tetrapeptide PG monomers,40 either one of these may be responsible for ciliated cell death.
Studies of cytokine induction by N. gonorrhoeae in Fallopian tube tissue have shown increased IL-1β, IL-8, and tumor necrosis factor (TNF)-α.38 TNF-α was also found to correlate with the tissue damage seen during infection.39 Thus, these studies suggest that tissue damage is mediated by the large inflammatory response. An attempt to correlate Fallopian tube damage with nitric oxide levels was not successful,19 suggesting differences between the Fallopian tube ciliated cell damage and that seen in the hamster tracheal ring model of pertussis.30 In addition to inflammatory cytokine induction in the Fallopian tubes and trachea, PG fragments are known to induce similar responses in other tissues. Studies of Shigella flexneri infections have demonstrated that the Nod1 response to PG fragments in intestinal cells results in IL-8 production.41 Similarly, human monocytes in blood were shown to produce IL-6 and IL-1beta in response to PG monomers.14 Since N. gonorrhoeae produces both the disaccharide-tetrapeptide PG monomer (TCT) and the disaccharide-tripeptide PG monomer that is the human Nod1 agonist, and since a variety of cell types respond to one of these two molecules, it is likely that PG fragments are involved in the damaging inflammatory response that occurs in many forms of gonococcal infection.
We plan to examine the mechanism of PG damage to Fallopian tube cells using both purified PG fragments and N. gonorrhoeae mutants affected in PG fragment release. As we have performed multiple studies of PG fragment release using gonococcal strain MS11,8–12,17,18,34,35 we have begun our studies of damage to Fallopian tube tissue by determining whether strain MS11 would cause cell damage and sloughing as has been seen with other gonococcal strains (Fig. 2). Human Fallopian tubes in organ culture were infected with strain MS11 for 18 hours. Examination of uninfected tissue by light microscopy showed actively beating cilia. Upon examination by scanning electron microscopy, the tissue appeared undamaged, and ciliated cells were present in the epithelium (Fig. 2A). By contrast, examination of infected Fallopian tube tissue showed no beating cilia. Further, scanning electron microscopy showed that infection had caused extensive sloughing of ciliated cells (Fig. 2B). A few gonococci could be observed attached to the tissue.
FIG. 2.
Human Fallopian tube tissue in organ culture either uninfected (A) or infected with N. gonorrhoeae strain MS11 (B). Infected tissue shows many sloughed ciliated cells. A few gonococci can also be seen (arrows).
Gonococci Have Five to Seven LTs
Since the toxic PG monomers released by gonococci have the 1,6-anhydro bond,53 they must be produced by LTs (Fig. 1). N. gonorrhoeae encodes between five and seven LTs, depending on whether a given strain carries the gonococcal genetic island (GGI), a 57-kb variable region of the chromosome containing genes involved in type IV secretion.13,26 All sequenced strains of N. gonorrhoeae have the LT-encoding genes ltgA, ltgB, ltgC, ltgD, and ltgE, which all have E. coli homologues.10 Eighty percent of gonococcal strains carry the GGI,13 and all forms of the GGI in N. gonorrhoeae encode LtgX, a homologue of the F-plasmid geneX product Orf169.26,35 Most versions of the GGI (79%) also carry the LT gene atlA (66% of N. gonorrhoeae strains overall).13 AtlA is a homologue of lamba endolysin (Table 1).
Table 1.
Function of Lytic Transglycosylases in Peptidoglycan Monomer Release
| Gonococcal lytic transglycosylase | Escherichia coli homologue | Monomer release by gonococcal mutant (% of wt) |
|---|---|---|
| LtgA | Slt70 | 62 |
| LtgB | MltC | 96 |
| LtgC | MltA | 83 |
| LtgD | MltB/Slt35 | 38 |
| LtgE | MltD | 105 |
| GGI-encoded lytic transglycosylases | ||
|---|---|---|
| LtgX | F-plasmid Orf169 | |
| AtlA | Lambda endolysin | 111 (ΔGGI) |
GGI, gonoccocal genetic island.
To identify the genes involved in PG monomer production, each of the seven genes was systematically mutated, and the mutants were analyzed for monomer release in pulse-chase experiments. Briefly, the mutants were grown in medium containing [3H]-glucosamine to metabolically label the PG, and the released PG fragments were analyzed by size-exclusion chromatography followed by scintillation counting.8–10,34,35 Single mutations in ltgB, ltgC, and ltgE resulted in levels of released PG monomers comparable to wild type,9,10,34 whereas single mutations in ltgA and ltgD reduced monomer release to 62% or 38%, relative to the wild type.8,10 A double mutant in ltgA and ltgD was completely abolished in PG monomer production, highlighting the role of LtgA and LtgD in generating these toxic PG fragments.10
LTs Involved in PG Monomer Release: LtgA and LtgD
In addition to releasing significantly less PG monomer, the ltgA mutant released less free disaccharide but more PG multimers.8 This mutant grew without any apparent growth defects, having a doubling time comparable to that of the wild type. Its colony and cellular morphologies were also similar to those of the wild type, demonstrating that ltgA is not required for growth or cell separation. Interestingly, the ltgA mutant was also more resistant to cell death in stationary phase and more resistant to autolysis under conditions that are favorable for N-acetylmuramyl-L-alanine amidase activity.8,28 Not only are LTs involved in cell separation and cell growth, but they are also believed to be involved in autolysis; these findings suggest that LtgA may be acting directly in cell lysis or indirectly by facilitating the activity of other PG-degrading enzymes. Further support for the idea that the LTs act in autolysis came from an examination of PG fragments released during lysis under nongrowth conditions. Although most fragments released during autolysis are large fragments of PG, the second most abundant fragments are the PG monomers, suggesting that LTs are active in autolysis.17 In addition, the ltgA mutant was reduced in PG turnover during logarithmic-phase and early stationary-phase growth, supporting the notion that LtgA functions as an LT.
LtgA (NGO2135) is predicted to be 616-amino-acid long with a molecular weight of 67.5 kDa. LtgA is 33% similar to its E. coli homologue, Soluble lytic transglycosylase 70 (Slt70), a well-characterized exo-muramidase with a similar fold to lysozyme.8,56 Slt70 cleaves macromolecular PG at the β-1,4-linkages between MurNac and GlcNAc residues to form 1,6-anhydromuropeptides.4 The exo-muramidase activity of Slt70 has been demonstrated in vitro, and crystal structures of this enzyme alone and in complex with the inhibitor bulgecin or with a 1,6-anhydromuropeptide have been solved.56,57,61 Three distinct domains, comprised mostly of alpha helices, form a doughnut-like superstructure with the active site situated in a deep groove within the C-terminal domain. The active site contains a single catalytic glutamate and a substrate binding site that can accommodate six N-acetylaminosugar units. A substrate requirement of Slt70 is that the PG contains peptides linked to the glycan strands; unsubstituted glycan strands lacking peptides are not cleaved by Slt70. Most of the similarity between LtgA and Slt70 is found in the C-terminal regions of the proteins, with the C-terminus of LtgA containing the active site glutamate and several other conserved residues involved in substrate binding in Slt70.8 While Slt70 is a soluble, periplasmic enzyme, LtgA is predicted to be a lipoprotein that is inserted in the inner leaflet of the outer membrane, as the N-terminus contains a predicted signal sequence cleavage site as well as a near-consensus lipoprotein processing site.8 We speculate that differences such as this may explain why gonococci release significantly more PG fragments than E. coli do; that is, LtgA may be localized in a way that favors PG monomer release over recycling.
To determine whether LtgA can function like its E. coli homologue, soluble constructs of LtgA were made and analyzed for their ability to degrade macromolecular PG in vitro. We found that LtgA had robust enzymatic activity, solubilizing the majority of the macromolecular PG and forming 1,6-anhydro PG monomers as the major product (our unpublished data). PG that was O-acetylated at the C-6 hydroxyl group of MurNAc was degraded to a much lesser extent than unacetylated PG; like lysozyme and other LTs, LtgA may be blocked by O-acetylation.6,11,49 Current studies are aimed at determining whether LtgA can cleave other substrates such as soluble PG fragments. The increase in released multimeric PG fragments observed in the ltgA mutant suggests that these might serve as additional substrates for LtgA.
Like the ltgA single mutant, the ltgD mutant released significantly less PG monomer and free disaccharide but released more PG multimers than the wild type.10 The ltgA ltgD double mutant was completely abolished for PG monomer and free disaccharide release, but it released significantly more PG multimers than the wild type. The presence of these multimers shows that the ltgA ltgD mutant could degrade macromolecular PG to soluble multimers but could not break them down further into monomers. In addition, LtgA and LtgD might be involved in producing free disaccharides, given the absence of released free disaccharides by the ltgA ltgD mutant. In order for free disaccharides to be released, both an LT and the amidase AmiC are necessary17; it is possible that LtgA and/or LtgD fulfill the role of the LT for this reaction. Another possible explanation for the lack of released free disaccharide by the ltgA ltgD mutant is that this mutant is unable to recycle larger PG fragments and has an increased metabolism of free disaccharide. Pulse-chase experiments were performed with the ltgA ltgD mutant and the wild type, and the cytosol of each strain was examined for radiolabeled UDP-MurNAc pentapeptide, an indicator that PG fragments have been liberated by the cell and transported back to the cytoplasm for recycling.10 This precursor was only present in the cytoplasm of the wild type, indicating that the ltgA ltgD mutant does not recycle liberated PG fragments. Thus, LtgA and LtgD appear to be involved in providing the cell with PG fragments, such as monomers and free disaccharide, for recycling, a process that allows a cell to recover nutrients and possibly sense its growth state.44 Although LtgA and LtgD are required for normal PG recycling, they are not required for growth or cell separation, as ltgA, ltgD, and ltgA ltgD mutants grew without any apparent growth or morphological defects.
LtgD (NGO0626) is predicted to be 363-amino-acid long with a molecular weight of 38.5 kDa. LtgD is 52% similar to its E. coli homologue, Membrane-bound lytic transglycosylase B (MltB), a lipoprotein with exo-muramidase activity.10,15 Slt35 is a naturally occurring, enzymatically active, soluble form of MltB in which the first 35 amino acids have been proteolytically cleaved. The exo-muramidase activity of Slt35 has been demonstrated in vitro,15 and crystal structures of Slt35 alone and in complex with bulgecin or PG fragments have been solved.58,60 Slt35 is an ellipsoidal protein comprised of three domains, with the catalytic core domain resembling a lysozyme fold with an EF-hand calcium-binding domain. This EF-hand was shown to be important for the stability of Slt35, and it may also have a role in facilitating protein-folding or protein–protein interactions.59 Crystal structures of the catalytic core domain revealed a sugar-binding site that can accommodate four N-acetylaminosugar units and two peptide-binding sites, and these are located in close proximity to the active site glutamate located within a deep cleft formed by the alpha and core domains.60 The catalytic residues, PG binding residues, EF-hand motif, and amino acids lining the hydrophobic pocket of Slt35 are conserved in the LtgD amino acid sequence.10,59
A predicted lipoprotein, LtgD, has a predicted signal sequence cleavage site and a conserved lipoprotein processing site. A soluble form of LtgD lacking the leader sequence and lipoprotein processing site was constructed and tested for its ability to degrade macromolecular PG in vitro. Not surprisingly, LtgD degraded insoluble PG to form 1,6-anhydro PG monomers, though not to the extent that LtgA did [unpublished data]. Like LtgA, LtgD solubilized acetylated PG to a lesser extent than unacetylated PG, consistent with the notion that O-acetylation may block the activity of these LTs.
The question remains why N. gonorrhoeae has two LTs with apparently redundant functions. One speculation is that LtgA is more efficient than LtgD at cleaving macromolecular PG, while LtgD may preferentially act on soluble PG fragments. Recently, a soluble form of MltB from E. coli was shown to degrade both macromolecular PG and a synthetic PG dimer,55 while none of the other 5 E. coli LTs examined were capable of degrading the latter. Testing the ability of LtgA or LtgD to degrade this PG dimer will likely yield important insights into the specificities of these LTs. One hypothesis is that these LTs work together, possibly in a complex with each other or other PG-associated proteins, with LtgA generating soluble fragments that serve as the substrates for LtgD. Another scenario is that these LTs are localized to different parts of the cell and function independently (e.g., one is located at the septum while another is located mid-wall or at the poles). Current work in our lab is aimed at dissecting the process by which LtgA and LtgD form and release PG monomers, important virulence factors in gonococcal infections.
An LT Involved in Cell Separation: LtgC
Of the seven LTs, the only one shown to have a significant effect on growth is LtgC. LtgC (NGO2048) is a predicted 46-kDa protein and exhibits 47% similarity to E. coli MltA. It has a predicted signal sequence and lipoprotein processing site.9 The sequence characteristics suggest that LtgC should be an outer membrane lipoprotein. In Neisseria meningitidis the LtgC homologue was originally named GNA33 (Genome-derived Neisseria antigen 33) as it was identified as a possible vaccine candidate for N. meningitidis.45 It met the criteria as a protein exported from the cytoplasm that elicited an antibody response that was protective in serum bacteriocidal assays. Further investigation revealed that antibodies directed at GNA33 also bound to porin PorA, and the bacteriocidal effects of the antisera were likely due to this cross reaction, not to binding of GNA33.24 Interestingly, mutation of gna33 in N. meningitidis led to an infection defect in infant rats. The mutant was unable to establish bacteremia and was cleared from the animals.1 The mutant showed a cell separation defect similar to what is observed with the gonococcal ltgC mutant.1,9 Transcription and translation of gna33 appears to be complex. Upstream of gna33 is a stem-loop structure, and this structure reduced transcription of gna33.52 Factors affecting transcription have not been identified, but it may be advantageous for Neisseria to produce LtgC only after the septum has been formed.
Study of N. gonorrhoeae LtgC may shed light on these phenotypes observed in meningococci. ltgC mutation leads to lack of cell separation9 (Fig. 3). These cells have a single cell wall that is shared by the bacteria in the clump of unseparated cells.9 Also, these bacteria have a single outer membrane covering the clump. This unusual growth characteristic may make the outer membrane unstable and increase the sensitivity of the bacteria to environmental conditions and chemicals in the milieu as is seen in the other gonococcal mutant defective in cell separation, the amiC mutant.17 ltgC mutants are more autolytic than wild type and release outer membrane proteins.1,9 The ltgC mutant was not actually reduced in growth rate, since protein accumulation in cells was the same as that of the wild type. Only the way that the bacteria grew—in clumps—and the increased degree of autolysis differed from wild type.9
FIG. 3.
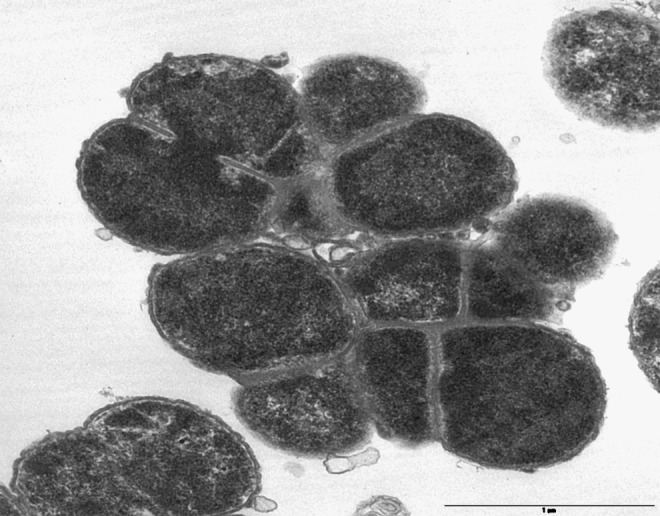
This thin section electron micrograph demonstrates that mutation of ltgC causes a defect in cell separation. Wild-type gonococci grow as single cocci or diplococci (not shown), but ltgC mutants show decreased cell separation and grow in clumps that share a single, connected cell wall.
The crystal structure of gonococcal LtgC (also known as MltA) has been solved and compared to that of E. coli MltA.46 Asp405 was identified as a likely catalytic residue analogous to the glutamate residues that catalyze cleavage by some other LTs, such as LtgA. The most striking difference between the structure of gonococcal LtgC and E. coli MltA is the extra domain found inserted in LtgC. The function of this domain is unknown, but Powell et al. speculate that this domain may participate in binding other PG synthesis or degradation proteins.46
LTs of Unknown Function: LtgB and LtgE
In contrast to LtgA, LtgD, and LtgC, the functions of LtgB and LtgE are not clear. Of the two enzymes, LtgB has been more thoroughly investigated. LtgB (NGO1033) is a predicted 22.5-kDa protein with similarity to E. coli MltC.34 A complete deletion of ltgB was made in N. gonorrhoeae, and the mutant was not affected in PG fragment release.34 However, the enzyme does appear to act in PG degradation. Expression of ltgB in E. coli resulted in cell lysis when the gene was co-expressed with the lambda lysis genes S and Rz, showing that the LtgB can functionally replace lambda's LT gpR. Using this E. coli lysis assay, mutations were characterized for their effects on LtgB function. Surprisingly, substitution of the predicted active site residue, E117A, had no effect on LtgB function while substitution of a nearby glutamate, E115N, led to loss of LtgB function.34 These results suggest that there is more to learn about the mechanism of LtgB activity and about its function in gonococci.
LtgE (NGO0608) is a predicted 72-kDa protein with 56% similarity to E. coli MltD. An insertion mutation in ltgE had no effect on PG monomer release, though PG multimer release appeared very slightly elevated.10 In Helicobacter pylori the MltD homologue is thought to act as an endo-type LT. Also, an mltD mutant showed decreased autolysis in stationary-phase culture.7 It is unknown whether the gonococcal ltgE mutant is affected in these processes.
LTs Involved in Type IV Secretion: AtlA and LtgX
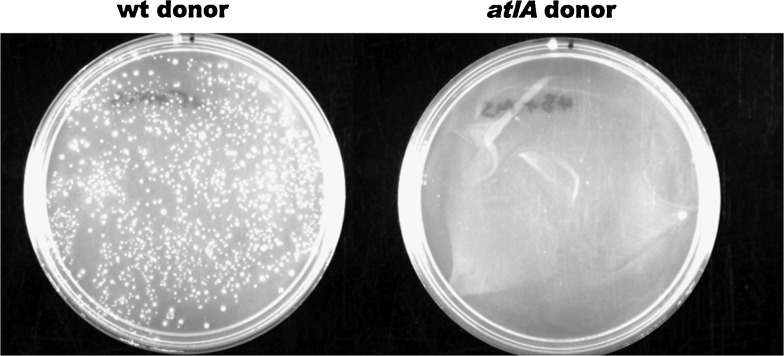
AtlA was the first LT identified in N. gonorrhoeae and was initially characterized as a possible autolysin, thus explaining its name, autolysin A (AtlA).12 An atlA mutant showed reduced death in late stationary-phase culture, after 32 hours in liquid culture. It should be noted that the major autolytic events still occurred in this mutant with over 90% of bacteria dying by 24 hours, but the remaining 10% of cells died more slowly than in the wild type.12 It seemed possible that this slight reduction in autolysis might also result in a reduction in DNA donation for natural transformation, since it was assumed that DNA donation occurred by autolysis and death of the donor. However, in coculture transformation assays, it was discovered that differences in DNA donation were not delayed until late stationary-phase autolysis, but rather DNA donation was seen to occur in log-phase culture.13 The atlA mutant was found to differ in DNA donation, showing a 500-fold reduction in DNA donation for natural transformation in coculture (Fig. 4). atlA was found to be in an operon with type IV secretion genes, and mutations in these genes also gave defects in DNA donation.27 Subsequent analysis demonstrated that AtlA is necessary for the secretion of chromosomal DNA by the gonococcal type IV secretion system.13,26,35
FIG. 4.
A wild-type donor strain (JD1545, cnp::mTnCm3 recA6) efficiently donates the chloramphenicol resistance marker to a wild-type recipient strain (MS11 Spc) when the two strains are grown together, yielding over 10,000 transformants per milliliter of log-phase culture (left plate). An atlA mutant (JD1543, cnp::mTnCm3 recA6 atlA::ermC) donates the chloramphenicol resistance marker very poorly in coculture with recipient strain MS11 Spc, yielding sometimes 10 or 100 transformants. No transformants were obtained in this experiment (right plate).
AtlA (NG5025) is a predicted 20.5-kDa protein with 56% similarity to lambda endolysin, gpR.12 Lambda endolysin has been studied extensively and has been shown to be an LT.5 Similarly, AtlA was purified as a fusion protein with maltose binding protein and was shown to function as an LT in vitro, digesting gonococcal sacculi and generating 1,6 anhydro PG monomers.35 A point mutation causing a substitution at the putative active site residue, E48A, resulted in a nonfunctional protein in vitro and in vivo. An interesting difference between AtlA and the phage lysins is that AtlA carries an N-terminal extension of 28 amino acids. This sequence does not have the characteristics of a signal sequence. A deletion within this region (delta 2-26) eliminated DNA secretion during gonococcal growth.35 We speculate that the N-terminal extension is either necessary for AtlA interaction with another type IV secretion protein or for localization.
The type IV secretion gene cluster in the GGI also contains a second LT gene, ltgX. LtgX (NG5004) is similar to LTs found in other type IV secretion systems, including Orf169, the geneX product encoded in the E. coli F-plasmid.26 LtgX is a predicted 17-kDa protein with 58% similarity to Orf169. Little is known about the function of LtgX, except that it is required for type IV secretion by gonococci. Deletion of ltgX in N. gonorrhoeae resulted in a strain that did not secrete detectable levels of DNA into the medium during log-phase growth.35
Several questions remain regarding the function of LTs in type IV secretion. LTs are speculated to cause a localized break in the PG in order to allow assembly of the apparatus or passage of substrates, but no one has been able to demonstrate this function directly.36 No type IV secretion system other than the N. gonorrhoeae system has been found to require two LTs. It is unclear why gonococci would need two LTs. If the purpose is to punch a hole in the cell wall, then is one hole not enough? Could it be that one LT facilitates assembly of the apparatus and the second opens the passageway for substrates? Other possibilities were considered in the study by Kohler et al.,35 including the possibilities that AtlA was directly lysing cells that produced it or that AtlA acted in allolysis,54 lysing other cells in the population in order to harvest their DNA for natural transformation. Using a sensitive measure of autolysis, release of [3H]-labeled RNA, Kohler et al. found that AtlA did not act to lyse cells that produced it or to lyse other cells in the population to harvest their contents. Autolysis was found to occur to a measurable degree during growth in a portion of the population whether AtlA was present or not.35 These and earlier studies failed to find any association of lysis with AtlA during the growth period when DNA is secreted.13,26 Further, studies demonstrating that DNA secreted by the gonococcal type IV secretion system is single-stranded indicate that autolysis is not the method of DNA release.51 Thus, it appears that AtlA and LtgX must be acting in the assembly and/or function of the type IV secretion system, and determination of their specific roles will require further experimentation.
Model for Function of LTs and Areas for Further Study
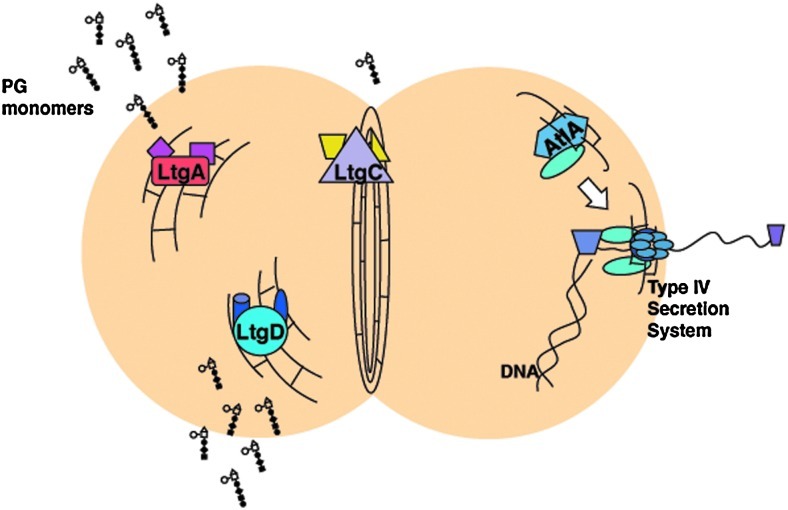
The study of LTs in N. gonorrhoeae has allowed us to assign functions to these proteins and to begin to understand their roles in various cellular processes. This situation is somewhat different from the study of E. coli LTs. Because of the larger number of LTs and their ability to substitute for each other in function, multiple mutations are necessary to observe a phenotype in E. coli.29 Except for mutations in ltgB and ltgE, we have a phenotype for each of the LT mutants in PG fragment release, cell separation, or type IV secretion. Even in the case of LtgA and LtgD, where both proteins function in PG fragment production, each mutation gave a reduction in fragment release.8,10 Thus, it appears that gonococcal LTs each have their own role and they do not effectively substitute for each other. The model (Fig. 5) illustrates the roles determined for several of the gonococcal LTs. LtgA and LtgD act in breakdown of the cell wall. They are likely bound to other PG-synthesizing and PG-degrading enzymes as part of a complex of proteins that act in PG metabolism, as proposed by Höltje.32 They may even be bound in complex with one another. Their location relative to the septum, mid-wall, or pole is unknown. LtgC is required for normal cell separation and may interact with other PG degradation proteins, such as amidase AmiC, which is also required for this process.17 LTs are also required for assembly and function of secretion systems, and in gonococci, LtgX and AtlA are required for type IV secretion. AtlA is hypothesized to introduce a localized break in the cell wall to allow assembly of the type IV secretion system. This system pumps single-stranded chromosomal DNA out of the cell where it can be taken up by other gonococci through natural transformation.51
FIG. 5.
Model for the function of lytic transglycosylases in Neisseria gonorrhoeae. Lytic transglycosylases LtgA and LtgD are responsible for production and release of the toxic PG monomers. LtgC may make a small contribution to PG monomer production. LtgC acts along with AmiC to split the septum after division thus facilitating cell separation. AtlA is required for type IV secretion and may act to create a localized break in the cell wall to allow assembly of the type IV secretion system.
Although the study of LTs has been fruitful for understanding the functions of these enzymes, several questions remain or have arisen in these studies. With regard to LtgA and LtgD and the production of toxic PG fragments, it is not clear why gonococci release larger amounts of PG monomers than E. coli or other commensal species. Do LtgA and LtgD localize to the outer membrane as predicted and does the location of these enzymes favor PG fragment release instead of recycling? Are LtgA and LtgD part of a protein complex that facilitates export of PG fragments across the outer membrane? For cell growth, are LtgA and LtgD localized to a growth zone at the septum of these cocci, or are they found elsewhere or throughout the periplasm? Similarly, is LtgC part of a protein complex involved in septum splitting and cell separation? Since meningococcal ltgC (gna33) mutants are defective in infection,1 could inhibitors of cell separation be used as novel antibiotics? Questions about the type IV secretion LTs are listed in the previous section, but the big question about these is what is their actual role in facilitating secretion system assembly and function? For all the LTs, it is not known how their functions are controlled or regulated. Gonococci acetylate their PG at the C-6 hydroxyl group of MurNAc for approximately half of the MurNAc residues,48 and we have found that LtgA and LtgD are inhibited by acetylation as expected. However, mutations in the PG acetylation genes pacA, pacB, or the O-acetyl PG esterase ape1 (NGO0532)62 do not lead to growth defects, and a pacA mutant analyzed for PG fragment release showed no differences in amounts or types of PG monomers released.11 PG from this pacA mutant could be fully digested by lysozyme, showing that it lacked acetylation. An explanation for the fragment release data is that Ape1 is part of the complex of PG degradation proteins and deacetylates PG before the LTs digest it. However, it does not appear that the acetylation is protecting the cell wall from uncontrolled breakdown by the LTs or that deacetylation is necessary for cell growth. Control of LTs may occur partly by transcriptional and translational regulation as was observed for LtgC homologue GNA33 by Serruto.52 Also, localization and binding to the degradation/synthesis complex may limit LT activity to its appropriate place and conditions.
Acknowledgment
This work was supported by NIH grant R01AI047958 awarded to J.P.D.
Disclosure Statement
No competing financial interests exist.
References
- 1.Adu-Bobie J. Lupetti P. Brunelli B. Granoff D. Norais N. Ferrari G. Grandi G. Rappuoli R. Pizza M. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture and virulence. Infect. Immun. 2004;72:1914–1919. doi: 10.1128/IAI.72.4.1914-1919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aral S.O. Mosher W.D. Cates W., Jr Self-reported Pelvic Inflammatory Disease in the United States, 1988. JAMA. 1991;266:2570–2573. [PubMed] [Google Scholar]
- 3.Barry P.M. Klausner J.D. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert. Opin. Pharmacother. 2009;10:555–577. doi: 10.1517/14656560902731993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beachey E.H. Keck W. Pedro M.A.d. Schwarz U. Exoenzymatic activity of transglycosylase isolated from Escherichia coli. Eur. J. Biochem. 1981;116:355–358. doi: 10.1111/j.1432-1033.1981.tb05342.x. [DOI] [PubMed] [Google Scholar]
- 5.Bienkowska-Szewczyk K. Lipinska B. Taylor A. The R gene product of bacteriophage lambda is the murein transglycosylase. Mol. Gen. Genet. 1981;184:111–114. doi: 10.1007/BF00271205. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn N.T. Clarke A.J. Characterization of soluble and membrane-bound family 3 lytic transglycosylases from Pseudomonas aeruginosa. Biochemistry. 2002;41:1001–1013. doi: 10.1021/bi011833k. [DOI] [PubMed] [Google Scholar]
- 7.Chaput C. Labigne A. Boneca I.G. Characterization of Helicobacter pylori lytic transglycosylases Slt and MltD. J. Bacteriol. 2007;189:422–429. doi: 10.1128/JB.01270-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloud K.A. Dillard J.P. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 2002;70:2752–2757. doi: 10.1128/IAI.70.6.2752-2757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloud K.A. Dillard J.P. Mutation of a single lytic transglycosylase causes aberrant septation and inhibits cell separation of Neisseria gonorrhoeae. J. Bacteriol. 2004;186:7811–7814. doi: 10.1128/JB.186.22.7811-7814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloud-Hansen K.A. Hackett K.T. Garcia D.L. Dillard J.P. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J. Bacteriol. 2008;190:5989–5994. doi: 10.1128/JB.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillard J.P. Hackett K.T. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 2005;73:5697–5705. doi: 10.1128/IAI.73.9.5697-5705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillard J.P. Seifert H.S. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol. Microbiol. 1997;25:893–901. doi: 10.1111/j.1365-2958.1997.mmi522.x. [DOI] [PubMed] [Google Scholar]
- 13.Dillard J.P. Seifert H.S. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 2001;41:263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 14.Dokter W.H.A. Dijkstra A.J. Koopmans S.B. Stulp B.K. Keck W. Halie M.R. Vellenga E. G(Anh)MTetra, a natural bacterial cell wall breakdown product induces interleukin-1beta and interleukin-6 expression in human monocytes. J. Biol. Chem. 1994;269:4201–4206. [PubMed] [Google Scholar]
- 15.Engel H. Smink A.J. van Wijngaarden L. Keck W. Murein-metabolizing enzymes from Escherichia coli: existence of a second lytic transglycosylase. J. Bacteriol. 1992;174:6394–6403. doi: 10.1128/jb.174.20.6394-6403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming T.J. Wallsmith D.E. Rosenthal R.S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 1986;52:600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia D.L. Dillard J.P. AmiC functions as an N-acetylmuramyl-L-alanine amidase necessary for cell separation and can promote autolysis in Neisseria gonorrhoeae. J. Bacteriol. 2006;188:7211–7221. doi: 10.1128/JB.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia D.L. Dillard J.P. Mutations in ampG or ampD affect peptidoglycan fragment release from Neisseria gonorrhoeae. J. Bacteriol. 2008;190:3799–3807. doi: 10.1128/JB.01194-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García K.P. Rubilar P.S. Vargas M.F. Cárdenas H. Rios M.A. Orihuela P.A. Vargas R.H. Fuhrer J. Heckels J.E. Christodoulides M. Velásquez L.A. Nitric oxide is not involved in Neisseria gonorrhoeae-induced cellular damage of human Fallopian tubes in vitro. Biol. Res. 2010;43:39–50. [PubMed] [Google Scholar]
- 20.Girardin S.E. Boneca I.G. Carneiro L.A. Antignac A. Jéhanno M. Viala J. Tedin K. Taha M.K. Labigne A. Zähringer U. Coyle A.J. DiStefano P.S. Bertin J. Sansonetti P.J. Philpott D.J. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 21.Goldman W.E. Klapper D.G. Baseman J.B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect. Immun. 1982;36:782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodell E.W. Recycling of murein by Escherichia coli. J. Bacteriol. 1985;163:305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodell E.W. Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J. Bacteriol. 1985;162:391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granoff D.M. Moe G.R. Giuliani M.M. Adu-Bobie J. Santini L. Brunelli B. Piccinetti F. Zuno-Mitchell P. Lee S.S. Neri P. Bracci L. Lozzi L. Rappuoli R. A novel mimetic antigen eliciting protective antibody to Neisseria meningitidis. J. Immunol. 2001;167:6487–6496. doi: 10.4049/jimmunol.167.11.6487. [DOI] [PubMed] [Google Scholar]
- 25.Greenway D.L. Perkins H.R. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J. Gen. Microbiol. 1985;131:253–263. doi: 10.1099/00221287-131-2-253. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton H.L. Domínguez N.M. Schwartz K.J. Hackett K.T. Dillard J.P. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton H.L. Schwartz K.J. Dillard J.P. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 2001;183:4718–4726. doi: 10.1128/JB.183.16.4718-4726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hebeler B.H. Young F.E. Autolysis of Neisseria gonorrhoeae. J. Bacteriol. 1975;122:385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidrich C. Ursinus A. Berger J. Schwarz H. Höltje J.-V. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 2002;184:6093–6099. doi: 10.1128/JB.184.22.6093-6099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiss L.N. Lancaster J.R. Corbett J.A. Goldman W.E. Epithelial autotoxicity of nitric oxide: role in the respiratory cytopathoology of pertussis. Proc. Natl. Acad. Sci. U. S. A. 1994;91:267–270. doi: 10.1073/pnas.91.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiss L.N. Moser S.A. Unanue E.R. Goldman W.E. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect. Immun. 1993;61:3123–3128. doi: 10.1128/iai.61.8.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Höltje J.V. A hypothetical holoenzyme involved in the replication of the murein sacculus of Escherichia coli. Microbiology. 1996;142(Pt 8):1911–1918. doi: 10.1099/13500872-142-8-1911. [DOI] [PubMed] [Google Scholar]
- 33.Höltje J.V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohler P.L. Cloud K.A. Hackett K.T. Beck E.T. Dillard J.P. Characterization of the role of LtgB, a putative lytic transglycosylase in Neisseria gonorrhoeae. Microbiology. 2005;151:3081–3088. doi: 10.1099/mic.0.28125-0. [DOI] [PubMed] [Google Scholar]
- 35.Kohler P.L. Hamilton H.L. Cloud-Hansen K. Dillard J.P. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 2007;189:5421–5428. doi: 10.1128/JB.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koraimann G. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell Mol. Life Sci. 2003;60:2371–2388. doi: 10.1007/s00018-003-3056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magalhaes J.G. Philpott D.J. Nahori M.A. Jehanno M. Fritz J. Le Bourhis L. Viala J. Hugot J.P. Giovannini M. Bertin J. Lepoivre M. Mengin-Lecreulx D. Sansonetti P.J. Girardin S.E. Murine Nod1 but not its human orthologue mediates innate immune detection of tracheal cytotoxin. EMBO Rep. 2005;6:1201–1207. doi: 10.1038/sj.embor.7400552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maisey K. Nardocci G. Imarai M. Cardenas H. Rios M. Croxatto H.B. Heckels J.E. Christodoulides M. Velasquez L.A. Expression of proinflammatory cytokines and receptors by human fallopian tubes in organ culture following challenge with Neisseria gonorrhoeae. Infect. Immun. 2003;71:527–532. doi: 10.1128/IAI.71.1.527-532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGee Z.A. Clemens C.M. Jensen R.L. Klein J.J. Barley L.R. Gorby G.L. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb. Pathog. 1992;12:333–341. doi: 10.1016/0882-4010(92)90096-7. [DOI] [PubMed] [Google Scholar]
- 40.Melly M.A. McGee Z.A. Rosenthal R.S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 1984;149:378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- 41.Nigro G. Fazio L.L. Martino M.C. Rossi G. Tattoli I. Liparoti V. De Castro C. Molinaro A. Philpott D.J. Bernardini M.L. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell Microbiol. 2008;10:682–695. doi: 10.1111/j.1462-5822.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohnishi M. Golparian D. Shimuta K. Saika T. Hoshina S. Iwasaku K. Nakayama S. Kitawaki J. Unemo M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?.: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–l3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pariser H. Asymptomatic gonorrhea. Med. Clin. North Am. 1972;56:1127–1132. doi: 10.1016/s0025-7125(16)32338-0. [DOI] [PubMed] [Google Scholar]
- 44.Park J.T. Why does Escherichia coli recycle its cell wall peptides? Mol. Microbiol. 1995;17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- 45.Pizza M. Scarlato V. Masignani V. Giulianl M.M. Arlco B. Comanducci M. Jennings G.T. Baldl L. Bartolini E. Capecchi B. Galeotti C.L. Luzzi E. Manetti R. Marchetti E. Mora M. Nutti S. Ratti G. Santini L. Savino S. Scarselli M. Stornl E. Zuo P. Broeker M. Hundt E. Knapp B. Blair E. Manson T. Tettelin H. Hood D.W. Jeffries A.C. Saunders N.J. Granoff D.M. Venter J.C. Moxon E.R. Grandl G. Rappuoli R. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 46.Powell A.J. Liu Z.J. Nicholas R.A. Davies C. Crystal structures of the lytic transglycosylase MltA from N. gonorrhoeae and E. coli: insights into interdomain movements and substrate binding. J. Mol. Biol. 2006;359:122–136. doi: 10.1016/j.jmb.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal R.S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect. Immun. 1979;24:869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthal R.S. Blundell J.K. Perkins H.R. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect. Immun. 1982;37:826–829. doi: 10.1128/iai.37.2.826-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal R.S. Folkening W.J. Miller D.R. Swim S.C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect. Immun. 1983;40:903–911. doi: 10.1128/iai.40.3.903-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenthal R.S. Nogami W. Cookson B.T. Goldman W.E. Folkening W.J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 1987;55:2117–2120. doi: 10.1128/iai.55.9.2117-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salgado-Pabón W. Jain S. Turner N. van der Does C. Dillard J.P. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol. Microbiol. 2007;66:930–947. doi: 10.1111/j.1365-2958.2007.05966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serruto D. Galeotti C.L. The signal peptide sequence of a lytic transglycosylase of Neisseria meningitidis is involved in regulation of gene expression. Microbiology. 2004;150:1427–1437. doi: 10.1099/mic.0.26780-0. [DOI] [PubMed] [Google Scholar]
- 53.Sinha R.K. Rosenthal R.S. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 1980;29:914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinmoen H. Knutsen E. Havarstein L.S. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7681–7686. doi: 10.1073/pnas.112464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suvorov M. Lee M. Hesek D. Boggess B. Mobashery S. Lytic transglycosylase MltB of Escherichia coli and its role in recycling of peptidoglycan strands of bacterial cell wall. J. Am. Chem. Soc. 2008;130:11878–11879. doi: 10.1021/ja805482b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thunnissen A.-M.W.H. Dijkstra A.J. Kalk K.H. Rozeboom H.J. Engel H. Keck W. Dijkstra B.W. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature. 1994;367:750–753. doi: 10.1038/367750a0. [DOI] [PubMed] [Google Scholar]
- 57.Thunnissen A.-M.W.H. Rozeboom H.J. Kalk K.H. Dijkstra B.W. Structure of the 70 kDa soluble lytic transglycosylase complexed with bulgecin A. Implications for the enzymatic mechanism. Biochemistry. 1995;34:12729–12737. doi: 10.1021/bi00039a032. [DOI] [PubMed] [Google Scholar]
- 58.van Asselt E.J. Dijkstra A.J. Kalk K.H. Takacs B. Keck W. Dijkstra B.W. Crystal structure of Escherichia coli lytic transglycosylase Slt35 reveals a lysozyme-like catalytic domain with an EF-hand. Structure. 1999;7:1167–1180. doi: 10.1016/s0969-2126(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 59.van Asselt E.J. Dijkstra B.W. Binding of calcium in the EF-hand of Escherichia coli lytic transglycosylase Slt35 is important for stability. FEBS Lett. 1999;458:429–435. doi: 10.1016/s0014-5793(99)01198-9. [DOI] [PubMed] [Google Scholar]
- 60.van Asselt E.J. Kalk K.H. Dijkstra B.W. Crystallographic studies of the interactions of Escherichia coli lytic transglycosylase Slt35 with peptidoglycan. Biochemistry. 2000;39:1924–1934. doi: 10.1021/bi992161p. [DOI] [PubMed] [Google Scholar]
- 61.van Asselt E.J. Thunnissen A.-M.W.H. Dijkstra B.W. High resolution crystal structures of the Escherichia coli lytic translycosylase Slt70 and its complex with a peptidoglycan fragment. J. Mol. Biol. 1999;291:877–898. doi: 10.1006/jmbi.1999.3013. [DOI] [PubMed] [Google Scholar]
- 62.Weadge J.T. Clarke A.J. Identification and characterization of O-acetylpeptidoglycan esterase: a novel enzyme discovered in Neisseria gonorrhoeae. Biochemistry. 2006;45:839–851. doi: 10.1021/bi051679s. [DOI] [PubMed] [Google Scholar]
- 63.WHO. 2011. Emergence of multi-drug resistant Neisseria gonorrhoeae—threat of global rise in untreatable sexually transmitted disease. whqlibdoc.who.int/hq/2011/WHO_RHR_11.14_eng.pdf. [Oct 20;]. p. 2011.whqlibdoc.who.int/hq/2011/WHO_RHR_11.14_eng.pdf (Online.)