Abstract
It is unknown how to use hESC to effectively treat hearts with postinfarction LV remodeling. Using a porcine model of postinfarction LV remodeling, this study examined the functional improvement of enhanced delivery of combined transplantation of hESC-derived endothelial cells (ECs) and hESC-derived smooth muscle cells (SMCs) with a fibrin 3D porous scaffold biomatrix. To facilitate tracking the transplanted cells, the hESCs were genetically modified to stably express green fluorescent protein and luciferase (GFP/Luc). Myocardial infarction (MI) was created by ligating the 1st diagonal coronary artery for 60 min followed by reperfusion. Two million each of GFP/Luc hESC-derived ECs and SMCs were seeded in the 3D porous biomatrix patch and applied to the region of ischemia/reperfusion for cell group (MI+P+C, n=6), whereas biomatrix without cell (MI+P, n=5), or saline only (MI, n=5) were applied to control group hearts with same coronary artery ligation. Functional outcome (1 and 4 weeks follow-up) of stem cell transplantation was assessed by cardiac magnetic resonance imaging (MRI). The transplantation of hESC-derived vascular cells resulted in significant LV functional improvement. Significant engraftment of hESC-derived cells was confirmed by both in vivo and ex vivo bioluminescent imaging (BLI). The mechanism underlying the functional beneficial effects of cardiac progenitor transplantation is attributed to the increased neovascularization. These findings demonstrate a promising therapeutic potential of using these hESC-derived vascular cell types and the mode of patch delivery.
Keywords: Stem cells, myocardial infarction, heart failure
INTRODUCTION
Myocardial infarction (MI) often induces a period of left ventricular (LV) remodeling. When LV remodeling occurs, an initial period of hemodynamic stability is followed by the development of LV dysfunction that may eventuate in congestive heart failure (CHF). The mechanisms that contribute to the transition from the compensated state to CHF remain unclear, but may be related to progressive contractile dysfunction of the border zone (BZ) region of viable myocardium that surrounds the infarct 1, 2.
Both experimental and clinical evidence demonstrate that cellular transplantation can improve the LV contractile performance of failing hearts 3–6,7. The underlying mechanisms remain unclear. Transplanted cells may regenerate myocytes and new vessels, and they may also release cytokines that exert trophic effects on host cardiac cells. We hypothesize that the beneficial effects of BZ stem cell engraftment result in increased neovascularization in the ischemic zone (IZ) and possibly BZ, and paracrine effects on stressed native cardiomyocytes in the BZ. This results in stabilization of BZ bioenergetic and contractile function, which in turn is associated with attenuated myocyte apoptosis and expansion of the BZ size.
Although it is a consistent observation in the literature that cellular transplantation improves LV contractile function, the cell engraftment rate a few weeks after the transplantation is usually very low 8–12. Therefore, it is clear that majority of cells transplanted to the heart do not demonstrate durable engraftment. Further, the majority of transplanted cells that do engraft do not differentiate into host cardiac myocytes cell phenotypes 6, 11–13.
We have recently developed a novel 3D porous fibrin biomaterial that can bind to growth factors to create an optimal microenvironment for stem cells to reside 7, 14–16. The biomaterial can also function to control prolonged release of growth factors (SDF-1α) to mobilize the endogenous cardiac progenitors to the injury site enhancing the repair. Using swine and mouse models of hearts with acute myocardial infarction, we have recently shown that bone marrow derived mesenchymal stem cells (MSC) embedded in a novel 3D porous biomaterial “patch” that attached to the surface of the myocardial infarction, resulted in a remarkable increase of engraftment rate and significant functional improvements a few weeks after transplantation 7, 14–16.
Additionally, we have recently developed a novel method in differentiating hESC into endothelial (hESC-ECs) and smooth muscle (hESC-SMCs) cells, which can provide an ample source for clinical cellular therapy application in patients with heart failure 17.
In the present study, we hypothesized that the trophic effects of cellular therapy are associated with increased neovascularization and regional myocardial contractile function, reduction of LV wall stresses and myocytes apoptosis, and possibly mobilization of the endogenous cardiac progenitors to the injury site for repair. Using a well established clinically relevant pig model of postinfarction LV remodeling, and an enhanced cell delivery by the 3D porous fibrin biomatrix, we examined whether the transplantations of mixture of hESC-ECs and hESC-SMCs can ameliorate LV dysfunction and hypertrophy in hearts with post-infarction LV remodeling. Immuno-suppression was achieved by using the established xeno-transplantation protocol. LV infarct size and chamber function was examined by magnetic resonance imaging (MRI). Additionally, we used an immunodeficient mouse model of postinfarction LV remodeling to assess the time course of the in vivo cell engraftment rate using molecular bioluminescent imaging (BLI). The results demonstrated that the fibrin patch enhanced delivery of hESC-ECs and hESC-SMCs resulted in a significant cell engraftment, which is accompanied by improvement of LV chamber function, reduction of infarct size and increase of neovascularization at both infarct zone and peri-infarct border zone, suggesting a promising novel cellular therapy using hESC-derived vascular cells with the novel biomatrix delivery mode.
METHODS
All experiments were performed in accordance with the animal use guidelines of the University of Minnesota, and the experimental protocols were approved by the University of Minnesota Research Animal Resources Committee. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23).
Embryonic Stem Cell Culture and Vascular Differentiation
Human embryonic stem cell line H9 (Wicell, Madison, WI) was maintained on mouse embryonic fibroblasts and genetically modified to express green fluorescent protein and firefly luciferase (GFP/Luc), as previously described 18. Endothelial cells (hESC-ECs) and smooth muscle cells (hESC-SMCs) were derived from the H9 GFP/Luc cell line as previously described and cultured under EC and SMC conditions, respectively 17. Briefly, undifferentiated hESCs were co-cultured with M210 mouse stromal cells to induce mesoderm differentiation. After 13–15 days, CD34+CD31+ hESC-derived vascular progenitor cells were sorted by magnetic nano-particle selection system. Cells were cultured on fibronectin-coated plates with cytokine containing media to support EC development. Subsequently, to generate SMCs, these cells were cultured in media containing PDGFBB and TGF-beta. Immediately prior to transplantation, cells were harvested using 0.05% Trypsin-1mM EDTA (Invitrogen, Carlsbad, CA), counted, pelleted and resuspened in 30 μL PBS solution (Hyclone/Thermo Scientific, Waltham, MA) for mouse studies or in 1 mL fibrinogen solution (Sigma-Aldrich) for pig studies.
Mouse Studies
Immunodeficient NOD/SCID mice 12 weeks of age weighing 17–20 g were used for the infarct model. Acute myocardial infarction (AMI) was induced by left coronary artery ligation according to a previously described method 19. Fifteen minutes post ligation of left anterior descending (LAD) coronary artery, mice were randomized into three groups that received saline only (MI, n=13), hESC-derived ECs (MI+EC, n=8) or a mixture of hESC-derived ECs and SMCs (MI+EC+SMC, n=13). Cell transplantation was achieved with 3 injections (total volume of 30 μL) into the peri-infarct regions with a 31-gauge needle. 1 ×106 cells (100% hESC-EC or a 50/50 mixture of hESC-EC and hESC-SMC) were delivered to MI+EC and MI+EC+SMC groups, respectively. The functional outcome of cell transplantation was assessed by echocardiography 10 with 4-week follow-up while in vivo cell engraftment rate was measured with BLI 20. Following the 4 week echocardiogram, mice were sacrificed and the hearts excised. Mouse heart infarct size was measured by using NIH Image J software and saved tissue was subject to cryosection and immunofluorescent staining 10. Methods detailing mouse AMI surgery, cardiac echocardiography, in vivo BLI, measurement of infarct size and immunohistochemistry are described in the Online Supplemental Materials.
Swine studies
Swine Model of Myocardial Ischemia/Reperfusion Model
Details of the animal model of postinfarction LV remodeling secondary to myocardial ischemia/reperfusion have been described previously 7, 11. Briefly, young Yorkshire female swine (~20kg; Manthei Hog Farm, Elk River, MN) were anesthetized with pentobarbital (30 mg/kg, iv), intubated, and ventilated with a respirator with supplemental oxygen. A left thoracotomy was performed. The root of 1st diagonal coronary artery from left anterior descending coronary artery (LAD) was ligated for 60 min followed by reperfusion to create MI, which resulted in 10% LV mass damage. Other drugs administrated during open-chest surgery include Lidocaine (2 mg/kg iv bolus before ligation followed by 1 mg/(kg·min) iv for 70 minutes) and nitroglycerine (0.5 g/(kg·min) iv for 70 minutes starting 10 minutes before ligation). If ventricular fibrillation occurred, electrical defibrillation was performed immediately. Fifteen minutes after reperfusion, surviving pigs were randomized to ligation only (MI, n=5), open fibrin patch transplantation (MI+P, n=5) and cell seeded fibrin patch transplantation (MI+P+C, n=6) groups. The chest was then closed. Animals received standard post-operative care including analgesia until they ate normally and became active.
Fibrin Patch-based Stem Cell Transplantation
A fibrin patch was employed as the vehicle to deliver stem cell as described previously in detail 7 (Online Supplemental Materials).
Immunosuppression
All pigs received clinical protocol of immunosuppression for xeno-transplantation with Cyclosporine 30 mg/kg per day with food as previously described in details 12.
MRI Methods
MRI was performed on a 1.5 Tesla clinical scanner (Siemens Sontata, Siemens Medical Systems, Islen NJ) using a phased-array 4-channel surface coil and ECG gating as previously described in details 21 (Online Supplemental Materials).
Tissue Preparation and Immunohistochemistry
After the MRI experiments were completed, the animals were anesthetized, the thoracotomy incision was reopened. Hemodynamics was measured. The heart was then explanted. The LV was sectioned in a bread-loaf manner into 6 transverse sections (1 cm in thickness) from apex to base. Detailed immunohistochemistry methods have been described previously 9, 10 (Online Supplemental Materials).
Analysis of Myocardial Vascular Density
Vascular density was assessed using the methods as previously described in details 11 (Online Supplemental Materials).
Statistics and Data Analysis
The repeated measures ANOVA was applied to compare the measurements of systolic thickening fraction, ejection fraction and scar size across treatment groups and different time points (before MI, 1 week, and 4 weeks after MI). The significance level of type I error (p < 0.05) was used. The Bonferroni correction for the significance level was used to take into account multiple comparisons 22. All values are expressed as mean ± standard deviation (SD). All statistical analyses were performed in Sigmastat version 3.5 (San Jose, CA).
RESULT
Endothelial and Smooth Muscle Cells Derived from Human Embryonic Stem Cells
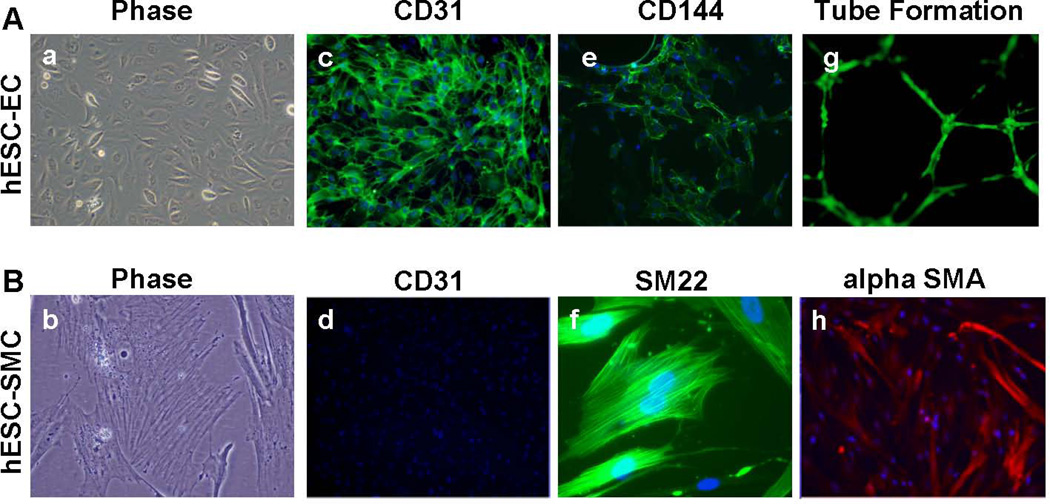
As previously demonstrated, hESCs co-cultured with stromal cells gave rise to a population of CD34+CD31+ cells 23. These hESC-derived CD34+CD31+ cells function as vascular progenitor cells that can be placed into secondary culture systems to produce homogeneous populations of endothelial and smooth muscle cell types (hESC-ECs and hESC-SMCs), respectively 17. Here, we derived hESC-ECs and hESC-SMCs from the H9 hESC line that has stable expression of GFP and luciferase (GFP/Luc). These hESC-ECs exhibit classic endothelial cell morphology, express endothelial surface markers, and form capillary structures on Matrigel (Figure 1A). hESC-SMCs also assumed appropriate morphology, expressed intracellular smooth muscle cell markers, and lacked expression of endothelial surface marker CD31 (Figure 1B).
Figure 1.
Endothelial and Smooth Muscle Cells Derived from Human Embryonic Stem Cells.
Using GFP/Luc expressing H9 human embryonic stem cells, distinct populations of endothelial and smooth muscle cells were derived from a common CD34+CD31+ vascular progenitor cell population. Panel A (left to right) hESC-ECs showed classic cobblestone morphology, expressed CD31 (PECAM), CD144 (VE-Cadherin), and formed capillary structures on Matrigel. Original magnification: 40×. Panel B (left to right) hESC-SMCs assumed appropriate filamentous morphology, lacked expression of endothelial marker CD31, and expressed smooth muscle makers SM22 and alpha-smooth muscle actin (alpha SMA). Original magnification: 40× (alpha-smooth muscle actin, CD31) and 80× (phase, SM22). Panel a–f and h were acquired from GFP− cells to avoid overlapping of immunofluorescence (Alexa Fluor 488) and GFP expression.
Significant Improvement of Cardiac Function and Cell Engraftment in Infarcted Mouse Hearts Treated with hESC-ECs and hESC-SMCs
We have previously demonstrated interaction between hESC-ECs and hESC-SMCs in vitro 17. Data from present study demonstrated these cell populations significantly improved LV chamber function and regional myocardial contractile performance in vivo while transplantation of hESC-ECs alone showed moderate improvement when compared to hearts that did not receive a cellular treatment and exposed to an identical LAD ligation protocol (Figure 2a–b). Since the hESC-derived cells maintained stable Luc expression, we were able to use bioluminescent imaging (BLI) to demonstrate persistence of the hESC-derived cells up to 8 weeks post-treatment (Figure 2c–e). BLI illustrated persistence of luminescent signal, indicating engraftment of human cells, in hearts that received hESC-EC injections and co-injections of hESC-ECs and hESC-SMCs (Figure 2c). Measurable signal from engrafted cells in the left ventricle was detected throughout the course of the 4 week study and in excised hearts (Figure 2e). Some animals receiving both hESC-ECs and hESC-SMCs were assessed for luciferase signal beyond the 4 week study. Remarkably, the luminescent signal actually increases at later time points (8 weeks), demonstrating stable engraftment and expansion of these cells (Figure 2d). Additional immunohistochemical studies demonstrated development of GFP+ vascular cells at 4 weeks post transplant, again demonstrating survival and function of these hESC-derived cells in hearts with postinfarction LV remodeling (Figure 2f).
Figure 2.
Significant Improvement of Cardiac Function and Cell Engraftment in Infarcted Mouse Hearts Treated with hESC-ECs and SMCs.
Cell therapy was delivered to the ischemic region of the LV after LAD coronary artery ligation. Ejection fraction (panel a) and shortening fraction (panel b) were measured 4 weeks post-infarction in mice receiving no injection (MI), 106 hESC-ECs (MI+EC), and 5×105 each hESC-EC and SMC (MI+EC+SMC). Significant improvement in LV function was achieved in mice that received combined cellular therapy (p<0.05). Transplanted GFP/Luc cells were tracked in vivo via bioluminescent imaging (BLI, panel d–e) and cell engraftment was confirmed post mortem by immunofluorescent staining against GFP (panel f). Panel d, quantification of luciferase signal from mouse hearts receiving cellular injection throughout the course of the experiment. The BLI intensity curves corresponding to panel d and e are highlighted in bold.
Because of the significant difference in heart rate between mouse and human (600 beats compared to 70 beats per minute), and the arrhythmia concerns that associated with the human cells engraftment in the mouse heart, we combined these novel cell types derived from hESC in a 3D porous biodegradable fibrin patch to a clinical relevant pig model of ischemia-reperfusion and post-infarction LV remodeling. To assess the therapeutic potential of the cellularized patch, we examined LV chamber and regional myocardial contractile function, LV wall thickness and systolic wall stresses, scar size and chamber function.
Swine studies
Anatomic Data
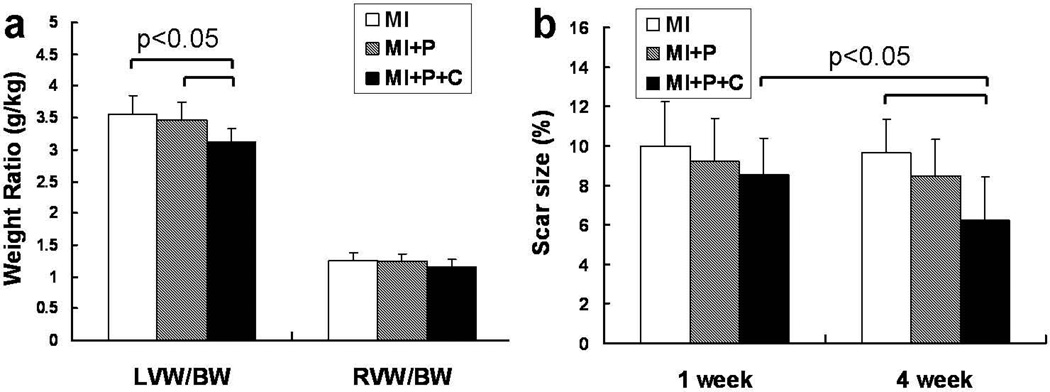
Cell transplantation resulted in a significant decrease in left ventricular hypertrophy as reflected by a decrease in LVW/BW (Figure 3a, p<0.05) in the cell treated hearts as compared to the hearts in both of the control groups. The myocardial infarct size data measured by MRI are illustrated in Figure 3b. At week 1 post LAD ligation the infarct size was about 9 percent of total LV, and was not significantly different among the 3 groups (p=NS). However, at week 4 the infarct size was significantly smaller in cell treated group than the MI group (p<0.05).
Figure 3.
a, heart weight to body weight ratio (g/kg) at 4 weeks after MI. b, scar size as measured by delayed contrast-enhanced cardiac MRI at 1 week and 4 weeks after MI.
LV Contractile Functional Data
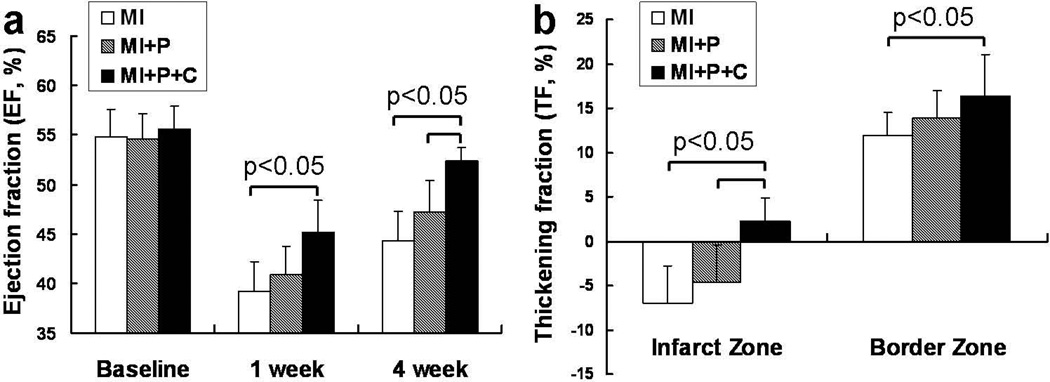
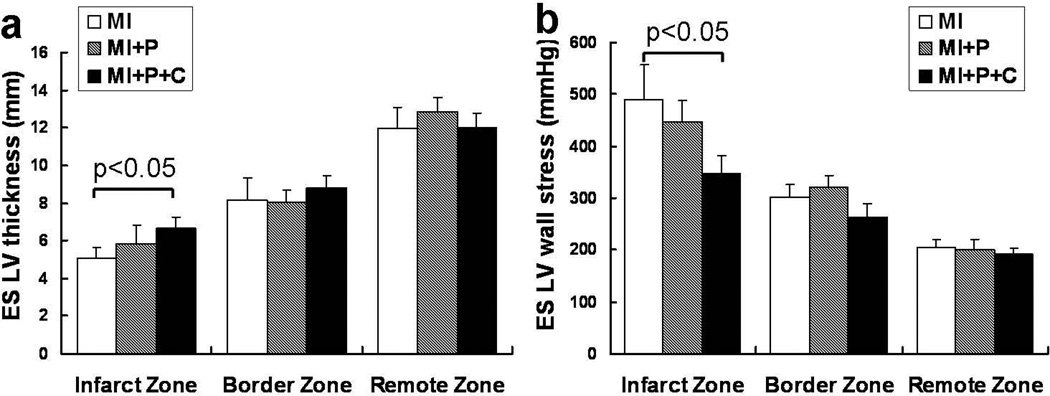
The temporal changes in ejection fraction (EF) as measured by MRI are illustrated in Figure 4a. There was no significant difference among the 3 groups at baseline. However, the fibrin patch-based cell transplantation showed a significant improvement in ejection fraction, which was seen as early as 7 days (MI+P+C (45.2±3.1) vs. MI (39.2±3.0), p<0.05). The beneficial effect in LV chamber function persisted up to 4 weeks (Figure 4a, p<0.05). Similarly, the regional LV systolic thickening fraction in the infarct zone was also significantly improved in cell treated group compared to the other two control groups (Figure 4b, p<0.05). In addition, the cell treatment resulted in reduction of the left ventricular thinning in the infarct zone seen at 4 weeks after MI, as depicted by significantly lower across the LV wall thickness in the saline treated- or open patch treated- groups (Figure 5a, p<0.05). This increased LV wall thickness in the infarct zone was accompanied by a significant lower systolic LV wall stresses as compared to MI control group (Figure 5b, p<0.05).
Figure 4.
Temporal change of LV contractile function in terms of ejection fraction (a) and thickening fraction (b) as measured by cine cardiac MRI. The cell transplanted group showed significant LV functional improvement as compared to the control groups.
Figure 5.
Alleviation of LV wall stress by increased LV thickness from stem cell transplantation. a, end-systolic LV wall thickness at 4 weeks after MI. b, end-systolic LV wall stress at 4 weeks after MI.
Vascular Density
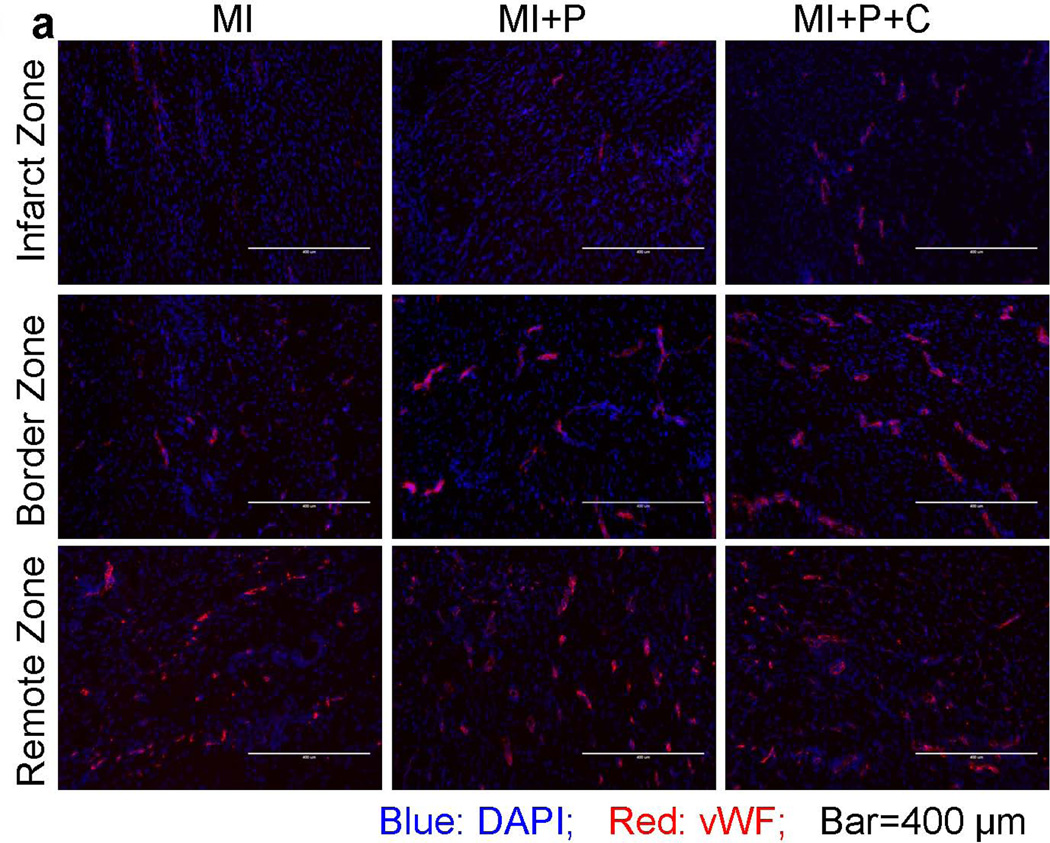
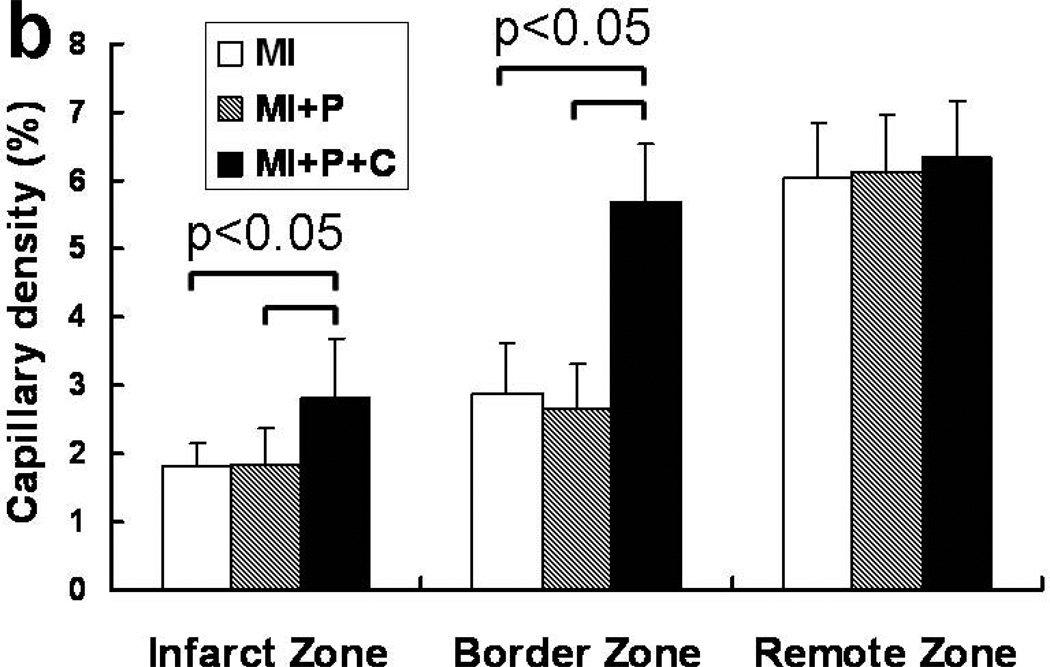
To determine the mechanisms underlying the beneficial effects of cell transplantation, we investigated the effects of cell transplantation on neovascularization and angiogenesis in the post-MI hearts. At 4 weeks after cell transplantation, immunofluorescence staining for vWF antibody indicated significant angiogenesis in stem cell treated hearts, with more vWF expressing capillaries being present in both infarct and peri-infarct regions of cell treated compared with the other two groups (Figure 6a). Quantitative evaluation of vWF-positive capillary fractional area per high-power field (20×) indicated that vascular density was significantly greater in the cell treated group than the control groups of saline-treated and open patch-treated hearts (Figure 6b). In the peri-infarct region of cell transplanted hearts, double vWF+/GFP+ cells were observed via co-staining of vWF and GFP, however, few cells were stained positive for both the myocyte marker TnT or MHC, and GFP (data not shown). These data suggested that transplanted hESC-derived cells may rescue ischemia threatened myocytes from apoptosis, promote angiogenesis through a paracrine effect.
Figure 6.
Increased capillary density from stem cell transplantation. a, immuno-fluorescent staining of vWF at 4 weeks after MI. b, quantification of vWF-positive capillary density.
Cell Engraftment
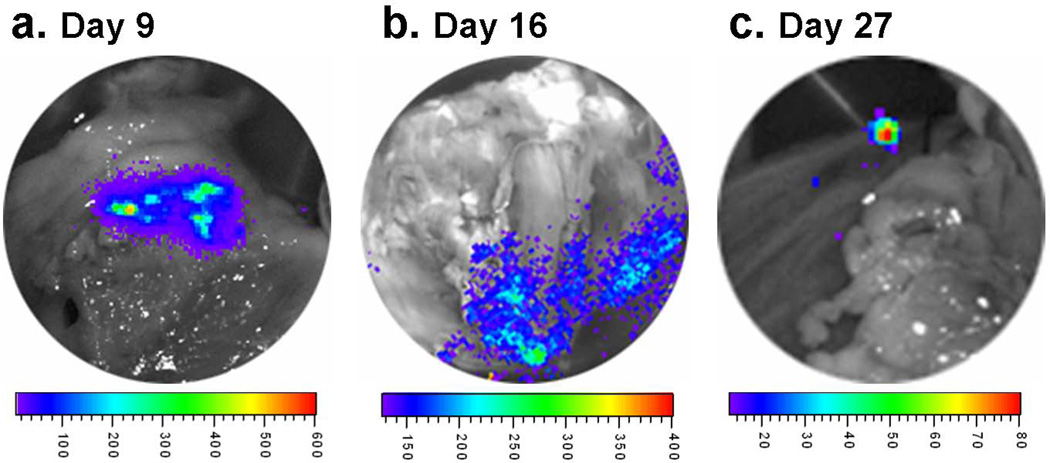
There was significant engraftment of hESC-derived endothelial cells and smooth muscle cells observed at 4 weeks after transplantation in the cell treated animals of both mouse (4 weeks, Figure 2c–e) and swine (day 9, 16 and 27, Figure 7) model of postinfarction LV remodeling as confirmed by in vivo and ex vivo BLI.
Figure 7.
Ex vivo bioluminescent imaging of excised pig heart showing substantial cell engraftments at Day 9 (panel a), 16 (panel b) and 27 (panel c) after MI.
DISCUSSION
The present study demonstrates that a fibrin patch-based hESC-derived vascular cell transplantation leads to functional improvement in a clinically relevant porcine model of postinfarction LV remodeling. Stem cell transplantation resulted in an overall improved LV function and LV remodeling following MI with an amelioration of LV scar thinning. This was associated with a reduction in scar size at 4 weeks. Cell transplantation also resulted in a significant increase of neovascularization in both infarct and peri-infarct regions. These data demonstrate that using a novel 3D porous fibrin patch-enhanced delivery, the transplantation of the equal mixture of endothelial cell and smooth muscle cells derived from the hESCs can promote the myocardial neovascularization of the recipient heart, reduce the ischemic injured myocytes from apoptosis, and improve the LV contractile function. The findings suggest a promising therapeutic potential of using this cell type and mode of delivery.
Translational Potential of hES-derived Endothelial and Smooth Muscle Cells
hESCs have been used to derive diverse cardio-vascular cell populations 24. RT-PCR and immunohistochemical studies demonstrate that hESC-derived cardiomyocytes (hESC-CMs) express early cardiac specific transcription factors, sarcomeric proteins and gap junction proteins 19. Although, electrophysiologic studies showed that most of the hESC-CMs resemble human fetal ventricular myocytes capable of propagating action potentials 25, a recent study suggested that transplantation of hESC-CMs with serum free medium to hearts with injuries resulted in a very low engraftment rate 19. On the other hand, there are reports demonstrating that beneficial effects of LV chamber function in response to the bone marrow derived progenitors transplantation therapy, is associated with the increased neovascularization 7, 9, 10, 12. Consequently, the present study was carried out to examine whether hESC derived cardiac vascular cells can be utilized to prevent LV dysfunction and postinfarction LV remodeling in hearts suffering from acute myocardial infarction. The data from the present study demonstrate that these cell types can promote the myocardial neovascularization of the recipient heart (Figure 6), which in turn, reduce the ischemic injured myocyte from apoptosis, and improve the LV chamber function.
Engraftment of hESC-derived Cells
Using the novel fibrin patch-enhanced delivery, data from both the mouse model and pig model demonstrated significant cell engraftment a few weeks after the transplantation, which was accompanied by the functional and bioenergetic improvement. Recently, there are reports suggesting that less than 50% of the cells remained in the myocardium a few hours after the transplantation, which was caused by the pulsate intramyocardial pressure changes during the continuous cardiac cycle 26. These data suggest that a significant fraction of the acute loss of delivered cells is caused by the active contraction of the LV, but is independent from the apoptosis or necrosis.
In the present study, the BLI data from both rodent and swine studies demonstrate the persistence of hESC-derived cells (Figure 2 and 7), which is in agreement with our earlier reports that applying this 3D porous biomaterials in stem cell delivery for myocardial repair is accompanied by further increase of cell engraftment rate, reduction in apoptosis in ischemia threatened myocytes, decrease of peri-infarct area fibrosis and prevention of LV scar systolic bulging 7. The reduced LV dilatation and LV scar bulging, in turn, result in significantly reduced myocardial wall stress and improved myocardial bioenergetics.
This fibrin 3D porous biomatrix is useful for several reasons. First, it is tightly patched on the surface of injured myocardium that had exposed to ischemia reperfusion. Therefore, the active myocardial contraction will not squeeze the cells out of the site of the delivery. Second, there are ample fibrinogen and thrombin proteins in the circulation that can be used for the patch biomaterial. In principle, both components can be autologous. In the present study, we try to use the autologous thrombin by scraping the surface of myocardial patch area, which effectively make the patch tightly stick on the surface at 4 weeks post transplantation. The scraping channels may also serve as routes for the stem cells get into the myocardium. Third, the fibrin patch can be chemically modified to bind any peptides such as HGF or SDF-1α, creating a progenitor cell friendly environment for the purposes such as enhancing the engraftment or controlled differentiation 7, 14–16. Fourth, we have previously demonstrated that the mesenchymal stem cell fibrin patch itself result in a remarkable increase of neovascularization into the patch material 4 weeks after the transplantation 7, 14–16, which was accompanied by a significant increase of engraftment rate and improvement of systolic thickening fraction in the patched area of myocardium. In the present study, we have found that this patch delivery is associated a significant engraftment rate (Figure 7). In some cases, the BLI signals indeed increased. Most importantly, the data from the present study demonstrate that both chamber function and regional systolic thickening fraction improved significantly (Figure 4).
Improvement in Ventricular Function and Reduction in Regional Wall Stress
The present study demonstrates that transplantation of hESC-derived vascular cells leads to improvement in ventricular function in a large animal model. We have previously reported that LV bulging at infarct zone was accompanied by a significant increase of regional LV wall stress at IZ and peri-infarct BZ 2. This particular increase of regional wall stresses and its associated severe bioenergetic abnormality were significantly ameliorated by the autologous MSC transplantation 7, 11, 12. We reasoned that the decrease of LV bulging at LV scar area (Figure 4b) resulted in reduction of the regional wall stress (Figure 5b) and energy demand, which in turn, would otherwise cause severe bioenergetic abnormality 27. In the present study, the engraftment of the hESC-derived vascular cells (Figure 7) was accompanied by increase of LV wall thickness and thickening fraction in the IZ and BZ, which in turn, resulted in a significant reduction of regional LV wall stresses (Figure 5b). By necessity, these improvements will result in the myocardial bioenergetics that has been observed using the same pig model of postinfarction LV remodeling.
During the 4 weeks follow up by cardiac MRI, we observed a significant reduction of infarct size (Figure 3b) that occurred only in the MI+P+C group. The LV dysfunction of failing hearts is associated with the oxidative stress caused by the excess of reactive oxygen species (ROS) production in the cytoplasm and the electron transport chain of mitochondria in myocytes 28. Using a similar animal model and bone marrow derived progenitor cells, we also observed that cell transplantation associated improvement in myocardial contractile function was accompanied by reduction of several different subunits of the respiratory chain oxidative enzyme, which may in turn, result in the reduction of the oxidative stress, and consequently reduce the infarct size associated with apoptosis 11.
Effect of Immunosuppression
In the current pig study, cyclosporine A was administered for immunosuppression. This approach has been utilized previously in a similar pig study with bone marrow-derived multipotent progenitor cell transplantation 12. In that study, there was no significant difference in the LV postinfarction remodeling between the cell treated hearts with and without cyclosporine A administration. From the same study, pigs (3 months of age) that received myocardial infarction and no cyclosporine A had an average body weight of 29±7 kg and a mean LV/BW ratio of 3.57±0.27. In the current study, pigs that received similar myocardial infarction and cyclosporine A had an average body weight of 35±3 kg at age 3.4 months, and mean LV/BW of 3.6±0.3. These data indicated that in pigs, the Cyclosporine A does not have an obvious influence on the LV remodeling after myocardial infarction.
Effect of Cell Transplantation on Infarct Size
Sequential MRI functional assessment allowed us to conclude that the infarct size in swine treated with hESCs decreased from 1 week to 4 weeks interval, while the scar sizes in swine without hESC treatment was maintained throughout the follow-up (Figure 3b). This reduction in scar size with stem cell treatment has been seen previously in other reports 6, 29, 30. The beneficial effects of cell transplantation could be related to the mobilization of endogenous cardiac progenitors to the injury site by paracrine effects as we and others have observed previously 6, 30. As we did not observe significant myocytes transdifferentiation at 4 weeks, the functional and structural improvements of decrease of infarct size and improved vascular density, are likely secondary to paracrine effects that include sparing of native cardiomyocytes from apoptosis as we observed recently 31. It was also observed recently that cell transplantation is accompanied by an up-regulation of MEF2A, the predominant MEF2 gene product expressed in postnatal cardiac muscle. MEFs play an important role in myogenesis 32. We postulate that cell transplantation may cause an activation of endogenous cardiac progenitor cells 6, 33, and MEF2A may play a role in their differentiation into cardiomyocytes.
In summary, the present study demonstrates that a fibrin patch based transplantation of hESC-derived vascular cells (ECs and SMCs) leads to a significant engraftment of vascular cells derived from the hESCs that is accompanied by a significant increase of neovascularization and LV contractile functional improvement, which in turn, results in a significant reduction of regional wall stress and infarct size. Taken together, these findings suggest a promising therapeutic potential of this combined approach using these vascular cell populations derived from hESC and the novel mode of delivery.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service Grants HL50470, HL67828, HL 95077, HL100407, HL077923, and the Engdahl Family Foundation.
Footnotes
Authors contribution(s) to the manuscript
Qiang Xiong: Data collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Katherine L. Hill: Data collection and/or assembly of data, data analysis and interpretation
Qinglu Li: Data collection and/or assembly of data, data analysis and interpretation
Piradeep Suntharalingam: Data collection, data analysis and interpretation
Abdul Mansoor: Data collection and/or assembly of data
Xiaohong Wang: Data collection and/or assembly of data, data analysis and interpretation
Mohammad Nurulqadr Jameel: Data collection and/or assembly of data
Pengyuan Zhang: Data collection and/or assembly of data, data analysis and interpretation
Cory Swingen: Data collection and/or assembly of data, Data analysis and interpretation
Dan S. Kaufman: Conception and design, financial support, administrative support, assembly of data, and final approval of manuscript
Jianyi Zhang: Conception and design, financial support, administrative support, assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 2.Hu Q, Wang X, Lee J, et al. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol. 2006;291:H648–H657. doi: 10.1152/ajpheart.01387.2005. [DOI] [PubMed] [Google Scholar]
- 3.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 4.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 5.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Hu Q, Nakamura Y, et al. The Role of Sca-1+/CD31- Cardiac Progenitor Cell Population in Postinfarction LV Remodeling. Stem Cells. 2006 doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Hu Q, Wang Z, et al. Autologous stem cell transplantation for myocardial repair. Am J Physiol Heart Circ Physiol. 2004;287:H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Wang X, Xu C, et al. Xenotransplantation of Long Term Cultured Swine Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hu Q, Mansoor A, et al. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290:H1393–H1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Hu Q, Nakamura Y, et al. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Jameel MN, Li Q, et al. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238–S246. doi: 10.1161/CIRCULATIONAHA.109.885236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng L, Hu Q, Wang X, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 13.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Hu Q, Braunlin EA, et al. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 15.Zhang G, Wang X, Wang Z, et al. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Nakamura Y, Wang X, et al. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 17.Hill KL, Obrtlikova P, Alvarez DF, et al. Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp Hematol. 2010;38:246–257. e241. doi: 10.1016/j.exphem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilber A, Linehan JL, Tian X, et al. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells. 2007;25:2919–2927. doi: 10.1634/stemcells.2007-0026. [DOI] [PubMed] [Google Scholar]
- 19.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 20.Tolar J, Wang X, Braunlin E, et al. The host immune response is essential for the beneficial effect of adult stem cells after myocardial ischemia. Exp Hematol. 2007;35:682–690. doi: 10.1016/j.exphem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wilke N, Wang Y, et al. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model. MRI and 31 P-MRS study. Circulation. 1996;94:1089–1100. doi: 10.1161/01.cir.94.5.1089. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–1069. doi: 10.1002/sim.4780080905. discussion 1071–1053. [DOI] [PubMed] [Google Scholar]
- 23.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. The Journal of clinical investigation. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 26.Qiao H, Surti S, Choi SR, et al. Death and proliferation time course of stem cells transplanted in the myocardium. Mol Imaging Biol. 2009;11:408–414. doi: 10.1007/s11307-009-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feygin J, Hu Q, Swingen C, et al. Relationships between regional myocardial wall stress and bioenergetics in hearts with left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2008;294:H2313–H2321. doi: 10.1152/ajpheart.01288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trachtenberg BH, Hare JM. Biomarkers of oxidative stress in heart failure. Heart Fail Clin. 2009;5:561–577. doi: 10.1016/j.hfc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 31.Jameel MN, Li Q, Mansoor A, et al. Long term functional improvement and gene expression changes after bone marrow derived multipotent progenitor cell transplantation in myocardial infarction. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.01100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.