Abstract
Essential tremor (ET) is a widespread late-life neurological disease. Genetic and environmental factors are likely to play important etiological roles. Harmane (1-methyl-9H-pyrido[3,4-b]indole) is a potent tremor-producing neurotoxin. Previously, elevated blood harmane concentrations were demonstrated in ET cases compared to controls, but these observations were all been cross-sectional, assessing each subject at only one time point. Thus, no one has ever repeat-assayed blood harmane in the same subjects twice. Whether the observed case-control difference persists at a second time point, years later, is unknown. The current goal was to re-assess a sample of our ET cases and controls to determine whether blood harmane concentration remained elevated in ET at a second time point. Blood harmane concentrations were quantified by a well-established high performance liquid chromatography method in 63 ET cases and 70 controls. A mean of approximately 6 years elapsed between the initial and this subsequent blood harmane determination. The mean log blood harmane concentration was significantly higher in cases than controls (0.30 ± 0.61 g−10/ml vs. 0.08 ± 0.55 g−10/ml), and the median value in cases was double that of controls: 0.22 g−10/ml vs. 0.11 g−10/ml. The log blood harmane concentration was highest in cases with a family history of ET. Blood harmane concentration was elevated in ET cases compared to controls when re-assessed at a second time point several years later, indicating what seems to be a stable association between this environmental toxin and ET.
Keywords: essential tremor, epidemiology, β-carboline alkaloid, harmane, toxin, environmental risk factors
Introduction
Essential tremor (ET) is a widespread, predominantly late-life neurological disease that affects 4% of individuals aged 40 years and older, and an estimated 20% or more of individuals in their 90s and older (Benito-Leon et al., 2003; Dogu et al., 2003; Louis and Ferreira, 2010; Louis et al., 2009). As such, it is one of the most common neurological diseases (Louis et al., 1998, 2010). In addition to action tremor, which may range from mild to severe, ET patients may exhibit other neuropsychiatric signs, including gait ataxia and incoordination (Louis et al., 2010; Rao et al., 2011; Singer et al., 1994) and various levels of cognitive impairment (Benito-Leon et al., 2006). Both genetic (Gulcher et al., 1997; Higgins et al., 1997) and non-genetic (environmental) factors (Jiménez-Jiménez et al., 2007; Louis, 2001, 2008; Salemi et al., 1998) are likely to play a role in disease etiology.
The β-carboline alkaloids are a group of neurotoxins that produce tremor. Lab animals injected with high doses acutely exhibit action tremor that resembles ET (Fuentes et al., 1971; Zetler et al., 1972). Human volunteers exposed to high doses display a coarse, reversible action tremor (Lewin, 1928).
Harmane (1-methyl-9H-pyrido[3,4-b]indole) is among the most potent tremor-producing β-carboline alkaloids; 38 mg/kg of subcutaneously administered harmane produces tremor in mice (McKenna, 1996). Harmane is also lipid soluble (Zetler et al., 1972), and broadly distributed within the rat brain (Anderson et al., 2006; Matsubara et al., 1993; Moncrieff, 1989). Indeed, brain concentrations are several fold higher than those in the blood in both control lab animals as well as harmane-injected lab animals (Anderson et al., 2006; Zetler et al., 1972). Although harmane is produced endogenously, it is also present in the diet and exogenous exposure is postulated to be the main source of bodily exposure to harmane (Pfau et al., 2004). Humans may be exposed to low levels of harmane through a variety of foods, including vegetables and meat, with concentrations in animal protein considered to be among the highest. Harmane and other β-carboline alkaloids are found in particularly high ng/gm concentrations in beef, chicken and pork, and cooking leads to furthermore increased concentrations (Louis et al., 2008). The formation of β-carboline alkaloids in cooked meat is a function of cooking temperature and time, with β-carboline alkaloid concentrations increasing most rapidly with time at higher temperatures (Louis et al., 2008). Pan frying and grill/barbequing produce the highest concentrations of β-carboline alkaloids (Louis et al., 2008).
In 2000, it was proposed that this neurotoxin could play a role in the etiology of ET, and in 2002 it was demonstrated that blood harmane concentration was elevated in 100 ET patients compared with 100 controls (“sample 1 at time point 1”) (Louis et al., 2002). In 2008, Louis et al. demonstrated that blood harmane concentration was elevated in a new sample of 150 ET cases and 135 controls (“sample 2 at time point 1”). The 2000 and 2008 studies enrolled completely different individuals; there was no overlap and no one was assessed twice. Thus, in our studies, the finding of an elevated harmane concentration remains a cross-sectional observation, sampling blood from each individual (a combined sample of 250 ET cases and 235 controls) at only a single time point (time point 1), thereby raising the possibility that the finding is spurious. Since no one has ever repeat-assayed blood harmane in the same subject twice, it is not known whether the case-control difference (observed at time point 1) persists at a second time point now many years later. Thus, the current goal (2009 – 2011) was to re-assess a subsample of our ET cases and controls (i.e., “sample 1 and 2 at time point 2”) to determine whether blood harmane concentration is still elevated in ET.
Methods
Participants
Our initial published sample of cases and controls was enrolled between 2000 and 2007 (Louis et al., 2002, 2008). Beginning in 2009, a subsample of the participants initially enrolled between 2000 and 2007 was re-enrolled. Sixty-three cases and 70 controls have been re-enrolled thus far.
Initial recruitment (2000 – 2007, Time Point 1)
As described in two prior studies of blood harmane in ET (Louis et al., 2002, 2008), between 2000 and 2007, 250 ET cases were enrolled in a study of the environmental epidemiology of ET at Columbia University Medical Center (CUMC). The large majority of cases were derived from two sources: (1) a computerized billing database of ET patients at the Neurological Institute of New York, CUMC, and (2) advertisements to members of the International Essential Tremor Foundation (Louis et al., 2008). All cases lived within two hrs driving distance of CUMC in New York, New Jersey, and Connecticut. The case finding process included screening questionnaires for alternative neurological diagnoses and, when available, an examination of clinical records (E.D.L.); patients with diagnoses of dystonia, Parkinson’s disease (PD), or spinocerebellar ataxia were excluded. Control subjects (n = 235) were recruited during the same time period (2000 – 2007). Controls, identified using random digit telephone dialing within a defined set of telephone area codes that were represented by the ET cases (e.g., 212, 201, 203, 516, 718, 914) within the New York Metropolitan area, were frequency-matched to cases based on gender, race, and current age (5 year intervals). The CUMC Internal Review Board approved of all study procedures; written informed consent was obtained upon enrollment.
Subsequent Recruitment (2009 – 2011, Time Point 2)
As described above, 250 ET cases and 235 controls were enrolled between 2000 and 2007 (samples 1 and 2 at time point 1). In April 2009, follow-up assessments of ET cases and controls began (samples 1 and 2, time point 2), starting with the oldest because these had the highest likelihood of loss to follow-up due to mortality. Cases were frequency-matched to controls based on age and gender. All cases and controls signed informed consent approved by the CUMC Internal Review Board. Sixty-three cases and 70 controls have been re-enrolled thus far. Aside from age, these 63 ET cases and 70 controls were similar in demographic and clinical features (gender, race, education, body mass index, smoking habits, and medical co-morbidity) to the base sample of 250 ET cases and 235 controls from which they were drawn. As expected, they had aged during the intervening time between initial and subsequent assessments.
Clinical Evaluation
At the time of the initial (time point 1) and subsequent (time point 2) evaluations, all case and control subjects were evaluated in person by a trained tester who administered clinical questionnaires and performed a videotaped examination (Louis et al., 2002, 2008). Most evaluations required significant travel either to the subjects’ homes or vice versa, with subjects coming in to the medical center. Therefore, evaluations were performed in the late morning or early afternoon, making fasting blood harmane concentrations impractical. Some data suggest that plasma concentrations of harmane do not change significantly during the day; indeed, in one study (Rommelspacher et al., 1991), human subjects ingested food or ethanol, and plasma harmane concentrations were measured hourly for 8 hrs. The concentration remained stable.
The trained tester collected demographic, clinical and family history information using a structured questionnaire. ET cases and controls were classified as having a family history of tremor if they reported at least one first-degree relative with tremor (i.e., ET or any unspecified tremor), and were classified as having a family history of ET if they reported at least one first-degree relative with ET. Current smoking status was assessed in each subject, as was past cigarette use, which allowed us to calculate cigarette pack-years. Medical co-morbidity was assessed using the Cumulative Illness Rating Scale, in which the severity of medical problems (0 [none] - 3 [severe]) was rated in 14 body systems (e.g., cardiac, respiratory, renal) and a Cumulative Illness Rating Scale score was assigned (range = 0 – 42 [maximal co-morbidity]) to each subject (Linn et al., 1968).
Weight and height were assessed using a balance scale designed for field surveys (Scale-Tronix 5600, White Plains, NY) and a movable anthropometer (GPM Martin Type, Pfister Inc, Carlstadt, NJ), and body mass index (weight/height2) was calculated and expressed as kg/m2.
The tester videotaped a tremor examination in all subjects (Louis et al., 1997; 2002), and each of 12 videotaped action tremor items was rated by Dr. Louis, resulting in a total tremor score (range = 0 – 36 [maximum]) (Louis et al., 2001). These ratings were assigned blinded to data on blood harmane concentration. The diagnosis of ET was confirmed by Dr. Louis using published diagnostic criteria (moderate or greater amplitude tremor during three activities or a head tremor in the absence of PD, dystonia or another neurological disorder) (Louis et al., 1997). None of the cases or control subjects had PD or dystonia.
Blood Harmane Concentrations
As at time point 1, at time point 2, phlebotomy was performed. At each time point, blood concentrations of harmane were measured blinded to all demographic, clinical and diagnostic information; 63 ET cases and 70 controls had phlebotomy at both time points 1 and 2, and it is their time point 2 blood harmane concentration that is the focus of these analyses.
Harmane concentrations in blood were quantified by a well-established high performance liquid chromatography (HPLC) method in this group and used in our previous studies (Louis et al., 2002, 2008; Zheng et al., 2000). In short, one volume (9 – 12 ml) of whole blood was mixed with half-a-volume (5 – 6 ml) of 1 M NaOH. Following vortex for 30 sec., the samples were placed on a horizontal rotator and shaken at room temperature for 30 min. An aliquot (15 ml) of the extraction solution consisting of ethyl acetate and methyl-t-butyl ether (2:98, V:V) was added to the tube. The tube was then vigorously shaken by hand for 1–2 min, followed by shaking on a horizontal rotor at room temperature for 45 min. After centrifugation at 3000 × g for 10 min, the upper organic phase was separated. The extraction procedure was repeated two additional times. The organic phase was combined and evaporated under nitrogen to dryness. The samples were reconstructed in 0.25 ml of methanol. After centrifugation at 3000 × g for 10 min, the supernatant was transferred to autosampler vials with sealed caps for HPLC analysis.
A Waters Model 2695XE complete HPLC system including autosampler, temperature control module, seal wash and degasser, and a Waters Model 2475 Multi-channel fluorescent detector was used for separation and quantification. Separation was accomplished using an ion-interaction, reversed-phase Econosphere C18 column (ODS2, 5 µm, 250 × 4.6 mm) attached to a Spherisorb guard column (ODS2, 5 µm, 10 × 4.6 mm). Both analytical and guard columns were purchased from Alltech (Deerfield, IL). An isocratic mobile phase consisted of 17.5 mM potassium phosphate buffer, pH 6.5 (equal molar concentration of both monobasic and dibasic potassium salts) and methanol (30:70, V:V). A 50-µl aliquot of sample extracts was injected and the separation performed at room temperature at a flow rate of 1 ml/min. The detector was set at an excitation wavelength of 300 nm and an emission wavelength of 435 nm. A Dell Window-based computer equipped with Waters data analysis package was used to collect and analyze the data. The identity of harmane on HPLC chromatographs previously has been clarified (Guan et al., 2001; Zheng et al., 2000). The intraday precision, measured as a coefficient of variation at 25 ng/ml, was 6.7% for harmane. The interday precision was 7.3% for harmane (Zheng et al., 2000).
Statistical Analyses
Statistical analyses were performed in SPSS (Version 19.0). Chi-square tests (Χ2) and Fisher’s Exact tests were used to analyze proportions, and Student’s t tests were used to examine group differences in continuous variables. Pearson (r) correlation coefficients were used to assess correlations between continuous variables.
The empirical distribution of harmane was positively skewed. Therefore, harmane concentrations were logarithmically transformed; the log-transformed blood harmane values were normally distributed. Case-control differences in log blood harmane concentrations were assessed using Student’s t tests. In confirmatory analyses, a non-parametric (Mann Whitney U) test also was performed on harmane data that were not logarithmically transformed. Log blood harmane concentrations were also stratified based on the median concentration (0.25 g−10/ml) and then based on tertiles, and cases and controls were compared with respect to proportion within each stratum. The total tremor score was also stratified into a high score (≥ 25) vs. low (<25) score category, as in prior studies (Louis et al., 2008).
To assess the null hypothesis that blood harmane concentration was not a predictor of diagnostic group (ET vs. control), logistic regression analysis was performed using diagnostic group as the outcome, and log blood harmane concentration as the primary independent variable, resulting in odds ratios (OR) with 95% confidence intervals (CI) As in prior studies (Louis et al., 2002, 2008), a number of potential confounders were considered (age in years, gender, race, years of education, body mass index, Cumulative Illness Rating scale score, current cigarette smoker, ever cigarette smoker, cigarette pack years among current cigarette smokers, cigarette pack years among ever cigarette smokers, number of cigarettes smoked on the day of the evaluation, answered “yes” to the question “have you had something to drink today”, and answered “yes” to the question “have you had something to eat today”), and were included in the adjusted logistic regression analyses if they were associated with either ET or blood harmane concentration in the current dataset or were consistently associated with blood harmane concentration in prior publications (Louis et al., 2002).
In three analyses, which tested for trend, log blood harmane concentration was the dependent variable in a linear regression analysis. In the first analysis, the ordinally-distributed independent variable was as follows: ET cases with a family history of tremor, ET cases without a family history of tremor, controls. In the second analysis, the independent variable was: ET cases with a family history of ET, ET cases without a family history of ET, controls. In the third analysis, the independent variable was: ET cases with high total tremor score (≥25), ET cases with lower total tremor score, controls.
A correlation coefficient was used to assess the association between initial and subsequent log blood harmane concentration. Then, in a linear regression model in which subsequent log blood harmane concentration was the dependent variable and initial log blood harmane concentration was the independent variable, the role of confounding factors at the time of the subsequent visit was assessed (see list of confounders above).
Results
The 63 ET cases and 70 controls had phlebotomy at both time points 1 and 2, and it is their time point 2 blood harmane concentrations that is the focus of these analyses. The 63 ET cases and 70 controls were similar with respect to demographic and clinical variables, including age, gender, race, education, body mass index, smoking habits, and medical co-morbidity (Table 1). A numerically larger but not significant % of controls had something to drink on the morning of the evaluation but a similar % had something to eat (Table 1). ET cases had a mean age of tremor onset of 42.1 ± 21.6 years, and their mean tremor duration was 30.7 ± 18.8 years. Forty (63.5%) ET cases had a family history of tremor and 16 (25.4%) had a family history of ET. Thirty-one (49.2%) ET cases took daily medication to treat tremor. The mean total tremor score in ET cases was 21.7 ± 5.4.
Table 1.
Characteristics of 63 ET cases vs. 70 controls
| Characteristic | ET Cases (N = 63) | Controls (N = 70) | Significance |
|---|---|---|---|
| Age in years | 71.7 ± 11.6 | 70.0 ± 10.0 | t = 0.89, p = 0.38 |
| Female gender | 31 (49.2) | 43 (61.4) | Χ2 = 2.01, p = 0.16 |
| Non-Hispanic white race | 59 (93.7) | 63 (90.0) | Χ2 = 0.58, p = 0.45 |
| Years of education | 15.8 ± 2.4 | 16.3 ± 2.2 | t = 1.11, p = 0.27 |
| Body mass index in kg/m2 | 25.1 ± 4.3 | 25.9 ± 4.7 | t = 1.02, p = 0.31 |
| Current cigarette smoker | 4 (6.3) | 5 (7.1) | Fisher’s Exact Test p = 1.00 |
| Pack-years (among current cigarette smokers) | 12.0 ± 8.5 | 35.6 ± 17.3 | t = 1.77, p = 0.14 |
| Ever cigarette smoker | 34 (54.0) | 35 (50.0) | Χ2 = 0.21, p = 0.65 |
| Pack-years (among ever cigarette smokers) | 14.5 ± 16.5 | 22.6 ± 23.7 | t = 1.61, p = 0.11 |
| Number of cigarettes smoked on the day of the evaluation | 0.08 ± 0.50 | 0.09 ± 0.50 | t = 0.09, p = 0.93 |
| Answered “yes” to the question “have you had something to eat today”. | 39 (67.2) | 53 (76.8) | Χ2 = 1.45, p = 0.23 |
| Answered “yes” to the question “have you had something to drink today”. | 32 (50.8) | 46 (65.7) | Χ2 = 3.04, p = 0.08 |
| Cumulative Illness Rating Scale score | 7.1 ± 3.1 | 6.2 ± 3.7 | t = 1.44, p = 0.15 |
Values are mean ± standard deviation, or numbers (percentages).
A mean of 5.8 ± 2.4 (range = 1.6 – 10.1) years had elapsed between the initial and the subsequent blood harmane determinations, and this was similar in cases and controls (5.5 ± 2.4 vs. 6.1 ± 2.4 years).
Using our control sample, the correlates of log blood harmane concentration were assessed. Log blood harmane concentration was higher in women than men (0.19 ± 0.49 g−10/ml vs. −0.11 ± 0.60 g−10/ml). However, log blood harmane concentration was not associated with age in years (r = −0.16), years of education (r = −0.005), body mass index (r = −0.14) or Cumulative Illness Rating Scale score (r = −0.05). Log blood harmane did not differ by race (0.08 ± 0.57 g−10/ml for non-Hispanic whites vs. −0.004 ± 0.33 g−10/ml in others). There was no difference between current cigarette smokers and nonsmokers (−0.26 ± 0.73 g−10/ml vs. 0.10 ± 0.53 g−10/ml respectively), between ever cigarette smokers vs. never cigarette smokers (0.11 ± 0.50 g−10/ml vs. 0.04 ± 0.60 g−10/ml respectively), and no association with number of pack years among current cigarette smokers (r = −0.64), number of pack years among ever cigarette smokers (r = −0.04), or number of cigarettes smoked on the day of the evaluation (r = −0.09). Controls who had answered “yes” to the question “have you had something to drink today” had significantly higher log blood harmane concentrations than did those who had answered “no”: 0.18 ± 0.53 g−10/ml vs. −0.13 ± 0.55 g−10/ml respectively. Controls who had answered “yes” to the question “have you had something to eat today” had numerically higher log blood harmane concentrations than those who had answered “no”: 0.40 ± 0.53 g−10/ml vs. 0.14 ± 0.69 g−10/ml respectively.
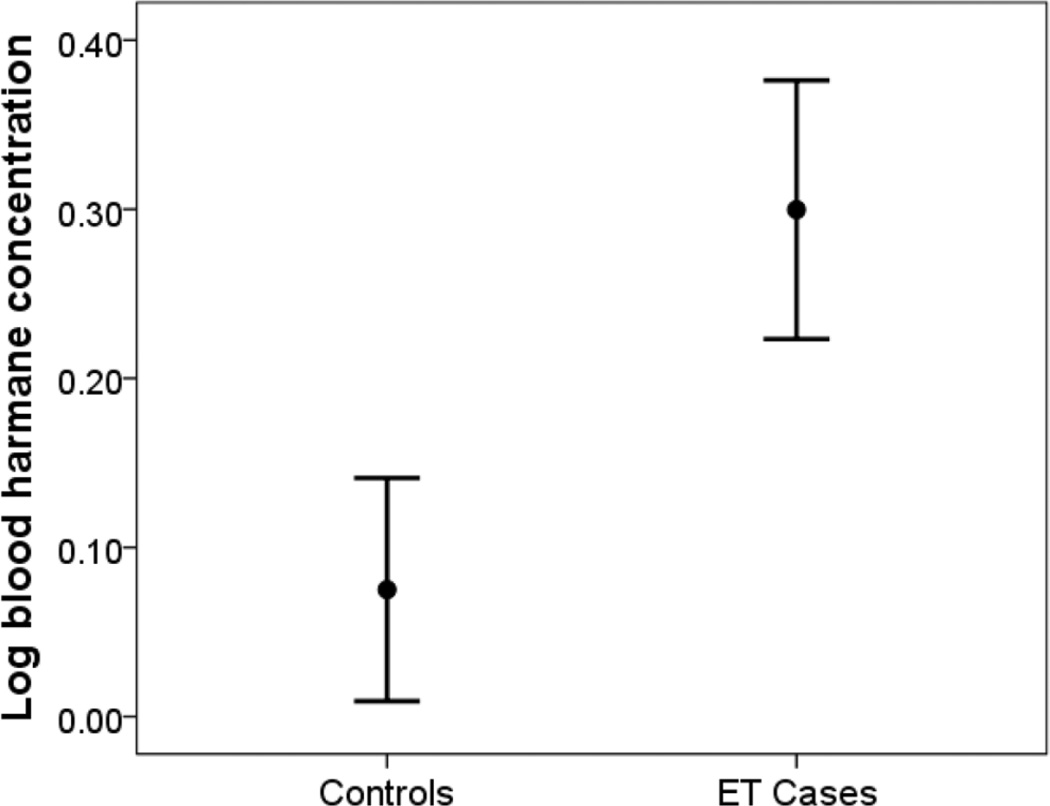
The mean log blood harmane concentration was several-fold significantly higher in our 63 cases than 70 controls (0.30 ± 0.61 g−10/ml vs. 0.08 ± 0.55 g−10/ml) (Figure 1). In a confirmatory analysis, a non-parametric (Mann Whitney test) test also was performed on harmane data that were not logarithmically transformed, and the median blood harmane value in cases was twice as high as that of controls: 0.22 g−10/ml vs. 0.11 g−10/ml. Strata were created based on the median log blood harmane concentration (0.25 g−10/ml); a significantly larger % of cases vs. controls (39 or 61.9% vs. 28 or 40%) had a high log blood harmane concentration based on this median split. Strata were also created based on the tertiles of log blood harmane concentration, and 27 (42.9%) cases vs. 17 (24.3) controls were in the highest log blood harmane tertile (>0.43 g−10/ml), a difference which was significant.
Figure 1.
The mean log blood harmane concentration was several-fold higher in cases than controls. The mean ± 1 standard error are shown.
In an unadjusted logistic regression analysis, log blood harmane concentration was significantly associated with the outcome (diagnosis of ET vs. control) (OR = 1.98, 95% CI = 1.07 – 3.68) (i.e., for every doubling of the harmane concentration, the odds of ET increased by 98%). In a logistic regression analysis that adjusted for age in years, gender, non-Hispanic white race, years of education, current smoker, ever cigarette smoker, number of cigarette pack years, number of cigarettes smoked on the day of the evaluation, answered “yes” to the question “have you had something to eat today”, and answered “yes” to the question “have you had something to drink today”, the OR increased to 2.72, 95% CI = 1.34 – 5.55, indicating that these factors did not account for the observed association between log blood harmane concentration and diagnosis, and may indeed have enhanced the association.
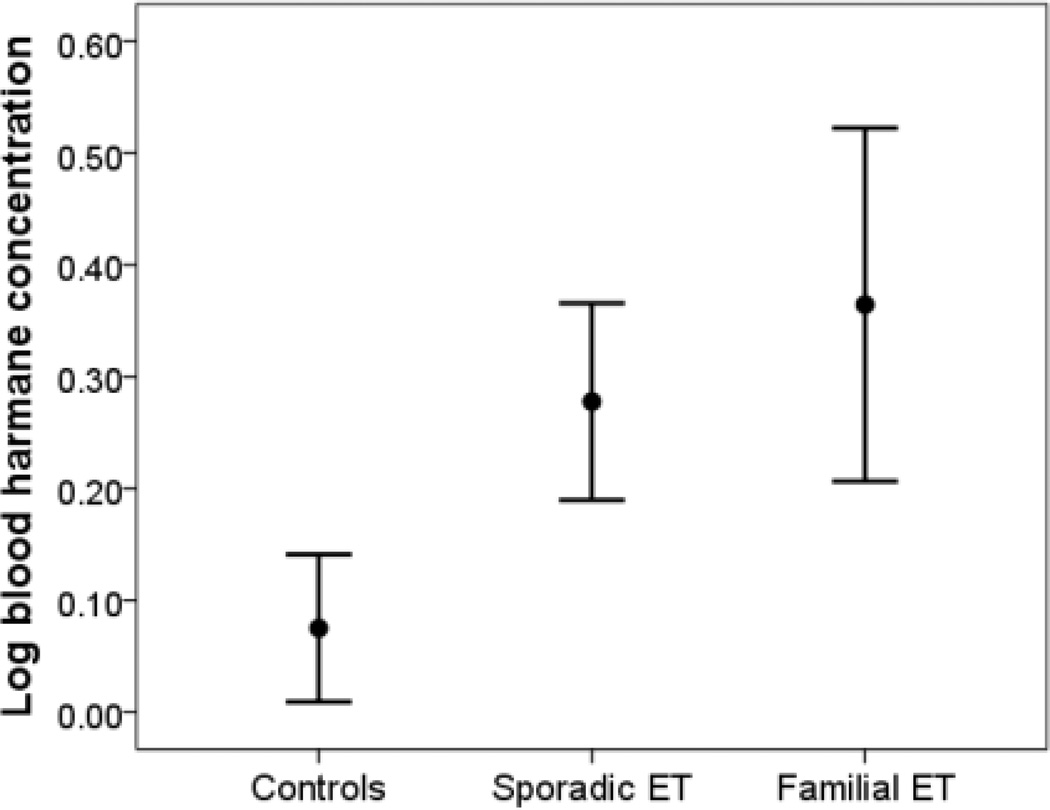
After stratifying by family history, and the log blood harmane concentration was highest in ET cases with a family history of ET (Table 2) (Figure 2). The log blood harmane concentration was highest in the 15 ET cases with high total tremor scores (≥25), intermediate in 48 ET cases with lower total tremor scores and lowest in the 70 controls (0.39 ± 0.54 g−10/ml vs. 0.22 ± 0.59 g−10/ml vs. 0.08 ± 0.55 g−10/ml), a trend which was significant. ET cases who took daily medication to treat tremor had a log blood harmane concentration that was similar to ET cases that did not take such medication (0.26 ± 0.66 g−10/ml vs. 0.34 ± 0.56 g−10/ml).
Table 2.
Log blood harmane concentration by group
| Group | Log blood harmane concentration |
Significance |
|---|---|---|
| Controls | 0.08 ± 0.55 | Beta = 0.12, p = 0.03a |
| ET cases with no family history of tremor | 0.28 ± 0.75 | |
| ET cases with family history of tremor | 0.31 ± 0.40 | |
| Controls | 0.08 ± 0.55 | Beta = 0.16, p = 0.027b |
| ET cases with no family history of ET | 0.28 ± 0.60 | |
| ET cases with family history of ET | 0.36 ± 0.63 | |
Test for trend by treating log blood harmane concentration as the dependent variable in a linear regression analysis, and examining the association with an ordinally-distributed independent variable (ET cases with a family history of tremor, ET cases without a family history of tremor, controls).
Test for trend by treating log blood harmane concentration as the dependent variable in a linear regression analysis, and examining the association with an ordinally-distributed independent variable (ET cases with a family history of ET, ET cases without a family history of ET, controls).
Figure 2.
The log blood harmane concentration was highest in ET cases with a family history of ET, intermediate in sporadic ET cases, and lowest in controls. The mean ± 1 standard error are shown.
Strata were also created based on the tertiles of log blood harmane concentration; 1 case (1.6%) was in the lowest tertile at time point 1 and the highest tertile at time point 2, and 9 cases (14.3%) were in the highest tertile at time point 1 and the lowest tertile at time point 2; otherwise the time point 1 and 2 values were similar. Yet there was not a significant linear correlation between the initial (time point 1) log blood harmane concentration and the subsequent (time point 2) log blood harmane concentration. In a linear regression model in which subsequent log blood harmane concentration was the dependent variable and initial log blood harmane concentration was the independent variable, the role of confounding factors was assessed. Confounding factors that were associated significantly with subsequent log blood harmane concentration in the final model were: fewer years of education, non-Hispanic white race, number of cigarettes smoked on the day of the subsequent evaluation, and having answered “yes” to the question “have you had something to drink today”. In the final model, there was no correlation between the initial and subsequent log blood harmane concentrations.
Discussion
In this study, blood harmane concentration was elevated in ET cases compared to controls re-assessed at a second time point many years later, indicating what seems to be a stable association between this environmental toxin and ET.
β-carboline alkaloids such as harmane are an obvious choice for investigation in the search for possible toxic environmental causes of ET. These toxins are structurally similar to MPTP, a neurotoxin which serves as one of the main animal models for PD (Langston et al., 1984; Smeyne et al., 2005). For more than 100 years, it has been known that the administration of β-carboline alkaloids to a broad range of lab animals (e.g., mice, cats, monkeys) produces a form of action tremor that shares clinical and drug-response features with ET (Cross et al., 1993; Du et al., 1997; Fuentes et al., 1971; Milner et al., 1995; Rappaport et al., 1984; Sinton et al., 1989; Trouvin et al., 1987). Indeed, β-carboline alkaloid administration is the main animal model for ET, and new pharmacotherapies are tested using exposed animals (Handforth et al., 2001; Martin et al., 2005, 2006).
In our earlier studies, blood harmane concentrations seemed to be highest both among ET cases with a family history of ET and in ET cases with more marked tremor (Louis et al., 2008). These results were observed again in cases re-sampled at a second time point. The higher concentration in familial ET cases suggests that the mechanism for this elevated concentration may be at least partly genetic. ET itself is a highly familial disorder. Many kindreds with autosomal dominant inheritance of ET have been described, and linkage has been demonstrated to regions on chromosomes 2p, 3q, and 6p, although at present the genes that are responsible for ET have not been identified (Deng et al., 2007).
There was no correlation between the initial log blood harmane concentration and the subsequent log blood harmane concentration. This may reflect differences in dietary structure over time. Other lifestyle factors (number of cigarettes smoked on the day of the evaluation, consumption of food or liquids on the day of the evaluation) may have influenced the continuity of blood harmane concentrations over time as well. Finally, harmane in the body is known to metabolize to harmine in the liver (Guan et al., 2001), and changes in subjects’ metabolic activities over the time period of this study, either due to endogenous or extraneous induction/inhibition of the harmane metabolic pathways, may also contribute to the variation of blood harmane concentrations in each individual participating in this study.
This study had limitations. Fasting blood harmane concentrations were not assessed. Therefore, it was difficult to assess the extent to which case-control differences reflect a difference in dietary intake of harmane. While a similar proportion of cases and controls had had something to eat on the morning of the evaluation, a larger proportion of controls had had something to drink on the morning of the evaluation, yet consideration of the possible confounding effects of these data in our models only served to increase the magnitude of the association between log blood harmane concentration and ET diagnosis. Also, liver function, variability in the cytochrome P450 system, or renal function were not assessed to see whether factors that might influence the metabolism of harmane differed between cases and controls. This study also had several strengths. To our knowledge, this is the only repeat-measure study of the association between blood levels of this toxin and ET. Blood harmane levels were measured in the same lab using identical analytic methods at both time points. Participants were re-evaluated up to 10 years after their initial assessment, and all blood harmane determinations were performed blinded to clinical information and vice versa. In the analyses, the potential confounding effects of multiple relevant covariates were assessed. Finally, data on tremor severity and familial occurrence of tremor complemented the primary data on case vs. control levels of blood harmane.
In summary, both genetic and environmental factors are likely to play a role in the etiology of ET. The contribution of environmental risk factors to disease etiology has been examined in detail in epidemiological studies of PD as well as Alzheimer’s disease and amyotrophic lateral sclerosis (Baldereschi et al., 2008; Dick, 2006; Gorell et al., 1997, 19988; Morahan et al., 2007; Racette et al., 2001; Shcherbatykh et al., 2007). Yet the study of such toxins in ET lags far behind. It is commonly stated that approximately 50% of ET cases are sporadic (Lambert et al., 1999; Louis et al., 1996). With a population prevalence for ET of 4% (age 40 and older) (Dogu et al., 2003), this suggests that approximately 2% of the population aged ≥ 40 years has a nonfamilial form of ET, yet the environmental determinants for this tremor are only just beginning to be explored. Blood harmane has been associated with ET in cross-sectional studies. The present study reveals a persistent case-control difference after a multi-year lapse, indicating what seems to be a stable association between this environmental toxin and ET.
Acknowledgments
Elan D. Louis was funded by R01 NS39422, P30 ES09089, and CTSA grant number UL1 RR024156 from the National Institutes of Health (Bethesda, MD and Research Triangle, NC). Pam Factor-Litvak was funded by R01 ES12231, R01 ES017024 from the National Institutes of Health (Research Triangle, NC). Wei Zheng was funded by R01 NS39422, R01 ES008146 and R21 ES017055 from the National Institutes of Health (Research Triangle, NC). The National Institutes of Health played no role in the study design, the collection of data, the analysis and interpretation of data, the writing of the paper or in the decision to submit the paper for publication. The authors were free to design, conduct, interpret, and publish research and this was not compromised by the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Anderson NJ, Tyacke RJ, Husbands SM, Nutt DJ, Hudson AL, Robinson ES. In vitro and ex vivo distribution of [3H]harmane, an endogenous beta-carboline, in rat brain. Neuropharmacology. 2006;50:269–276. doi: 10.1016/j.neuropharm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Inzitari M, Vanni P, Di Carlo A, Inzitari D. Pesticide exposure might be a strong risk factor for Parkinson's disease. Ann Neurol. 2008;63:128. doi: 10.1002/ana.21049. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Misra A, Sandilands A, Taylor MJ, Green AR. Effect of chlormethiazole, dizocilpine and pentobarbital on harmaline-induced increase of cerebellar cyclic GMP and tremor. Psychopharmacology (Berl) 1993;111:96–98. doi: 10.1007/BF02257413. [DOI] [PubMed] [Google Scholar]
- Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain. 2007;130:1456–1464. doi: 10.1093/brain/awm018. [DOI] [PubMed] [Google Scholar]
- Dick FD. Parkinson's disease and pesticide exposures. Br Med Bull. 2006;79–80:219–231. doi: 10.1093/bmb/ldl018. [DOI] [PubMed] [Google Scholar]
- Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. 2003. [DOI] [PubMed] [Google Scholar]
- Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. doi: 10.1016/s0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- Fuentes JA, Longo VG. An investigation on the central effects of harmine, harmaline and related beta-carbolines. Neuropharmacology. 1971;10:15–23. doi: 10.1016/0028-3908(71)90004-9. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. 1997. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Guan Y, Louis ED, Zheng W. Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. J Toxicol Environ Health A. 2001;64:645–660. doi: 10.1080/152873901753246241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcher JR, Jónsson P, Kong A, Kristjánsson K, Frigge ML, Kárason A, Einarsdóttir IE, Stefánsson H, Einarsdóttir AS, Sigurthoardóttir S, Baldursson S, Björnsdóttir S, Hrafnkelsdóttir SM, Jakobsson F, Benedickz J, Stefánsson K. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat Genet. 1997;17:84–87. doi: 10.1038/ng0997-84. [DOI] [PubMed] [Google Scholar]
- Handforth A, Krahl SE. Suppression of harmaline-induced tremor in rats by vagus nerve stimulation. Mov Disord. 2001;16:84–88. doi: 10.1002/1531-8257(200101)16:1<84::aid-mds1010>3.0.co;2-s. 2001. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Pho LT, Nee LE. A gene (ETM) for essential tremor maps to chromosome 2p22-p25. Mov Disord. 1997;12:859–864. doi: 10.1002/mds.870120605. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, de Toledo-Heras M, Alonso-Navarro H, Ayuso-Peralta L, Arévalo-Serrano J, Ballesteros-Barranco A, Puertas I, Jabbour-Wadih T, Barcenilla B. Environmental risk factors for essential tremor. Eur Neurol. 2007;58:106–113. doi: 10.1159/000103646. [DOI] [PubMed] [Google Scholar]
- Lambert D, Waters CH. Essential tremor. Curr Treat Options Neurol. 1999;1:6–13. doi: 10.1007/s11940-999-0027-3. [DOI] [PubMed] [Google Scholar]
- Langston JW, Langston EB, Irwin I. MPTP-induced Parkinsonism in human and non-human primates--clinical and experimental aspects. Acta Neurol Scand Suppl. 1984;100:49–54. [PubMed] [Google Scholar]
- Lewin L. Untersuchungen Uber Banisteria caapi Sp. Arch Exp Pathol Pharmacol. 1928;129:133–149. [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Louis ED. Etiology of essential tremor: Should we be searching for environmental causes? Mov Disord. 2001;16:822–829. doi: 10.1002/mds.1183. [DOI] [PubMed] [Google Scholar]
- Louis ED. Environmental epidemiology of essential tremor. Neuroepidemiology. 2008;31:139–149. doi: 10.1159/000151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Barnes L, Wendt KJ, Ford B, Sangiorgio M, Tabbal S, Lewis L, Kaufmann P, Moskowitz C, Comella CL, Goetz CC, Lang AE. A teaching videotape for the assessment of essential tremor. Mov Disord. 2001;16:89–93. doi: 10.1002/1531-8257(200101)16:1<89::aid-mds1001>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- Louis ED, Jiang W, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, Zheng W. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008;29:294–300. doi: 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R. How familial is familial tremor? The genetic epidemiology of essential tremor. Neurology. 1996;46:1200–1205. doi: 10.1212/wnl.46.5.1200. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, Hauser WA. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010;25:1633–1638. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32:208–214. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, Parides M. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59:1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FC, Handforth A. Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor. Mov Disord. 2006;21:1641–1649. doi: 10.1002/mds.20940. [DOI] [PubMed] [Google Scholar]
- Martin FC, Thu LeA, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Collins MA, Akane A, Ikebuchi J, Neafsey EJ, Kagawa M, Shiono H. Potential bioactivated neurotoxicants, N-methylated beta-carbolinium ions, are present in human brain. Brain Res. 1993;610:90–96. doi: 10.1016/0006-8993(93)91221-d. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Plant hallucinogens: springboards for psychotherapeutic drug discovery. Behav Brain Res. 1996;73:109–116. doi: 10.1016/0166-4328(96)00079-4. [DOI] [PubMed] [Google Scholar]
- Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol. 1995;73:2568–2577. doi: 10.1152/jn.1995.73.6.2568. [DOI] [PubMed] [Google Scholar]
- Moncrieff J. Determination of pharmacological levels of harmane, harmine and harmaline in mammalian brain tissue, cerebrospinal fluid and plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr. 1989;496:269–278. doi: 10.1016/s0378-4347(00)82576-1. [DOI] [PubMed] [Google Scholar]
- Morahan JM, Yu B, Trent RJ, Pamphlett R. Genetic susceptibility to environmental toxicants in ALS. Am J Med Genet B Neuropsychiatr Genet. 2007;144:885–890. doi: 10.1002/ajmg.b.30543. [DOI] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to beta-carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related Parkinsonism: Clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Rao AK, Gillman A, Louis ED. Quantitative gait analysis in elderly essential tremor cases and age-matched controls. Gait Posture. 2011;34:65–70. doi: 10.1016/j.gaitpost.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport MS, Gentry RT, Schneider DR, Dole VP. Ethanol effects on harmaline-induced tremor and increase of cerebellar cyclic GMP. Life Sci. 1984;34:49–56. doi: 10.1016/0024-3205(84)90329-1. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Schmidt LG, May T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol Clin Exp Res. 1991;15:553–559. doi: 10.1111/j.1530-0277.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Salemi G, Aridon P, Calagna G, Monte M, Savettieri G. Population-based case-control study of essential tremor. Ital J Neurol Sci. 1998;19:301–305. doi: 10.1007/BF00713856. [DOI] [PubMed] [Google Scholar]
- Shcherbatykh I, Carpenter DO. The role of metals in the etiology of Alzheimer's disease. J Alzheimer's Dis. 2007;11:191–205. doi: 10.3233/jad-2007-11207. [DOI] [PubMed] [Google Scholar]
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–196. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Krosser BI, Walton KD, Llinas RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflugers Arch. 1989;414:31–36. doi: 10.1007/BF00585623. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson's disease. Brain Res Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Trouvin JH, Jacqmin P, Rouch C, Lesne M, Jacquot C. Benzodiazepine receptors are involved in tabernanthine-induced tremor: in vitro and in vivo evidence. Eur J Pharmacol. 1987;140:303–309. doi: 10.1016/0014-2999(87)90287-1. [DOI] [PubMed] [Google Scholar]
- Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Barnes LF, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem. 2000;279:125–129. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]