Summary
Recent insight into the N-glycosylation pathway of the haloarchaeon, Haloferax volcanii, is helping to bridge the gap between our limited understanding of the archaeal version of this universal post-translational modification and the better-described eukaryal and bacterial processes. To delineate as yet undefined steps of the Hfx. volcanii N-glycosylation pathway, a comparative approach was taken with the initial characterization of N-glycosylation in Haloarcula marismortui, a second haloarchaeon also originating from the Dead Sea. While both species decorate the reporter glycoprotein, the S-layer glycoprotein, with the same N-linked pentasaccharide and employ dolichol phosphate as lipid glycan carrier, species-specific differences in the two N-glycosylation pathways exist. Specifically, Har. marismortui first assembles the complete pentasaccharide on dolichol phosphate and only then transfers the glycan to the target protein, as in the bacterial N-glycosylation pathway. In contrast, Hfx. volcanii initially transfers the first four pentasaccharide subunits from a common dolichol phosphate carrier to the target protein and only then delivers the final pentasaccharide subunit from a distinct dolichol phosphate to the N-linked tetrasaccharide, reminiscent of what occurs in eukaryal N-glycosylation. This study further indicates the extraordinary diversity of N-glycosylation pathways in Archaea, as compared with the relatively conserved parallel processes in Eukarya and Bacteria.
Introduction
Proteins from all three domains of life, i.e. Eukarya, Bacteria and Archaea, can experience N-glycosylation, namely the covalent attachment of glycans to select Asn residues (Helenius and Aebi, 2004; Eichler and Adams, 2005; Szymanski and Wren, 2005; Weerapana and Imperiali, 2006; Abu-Qarn et al., 2008a; Calo et al., 2010a). At present, the archaeal version of this universal post-translational modification is not as well understood as the parallel processes in Eukarya or Bacteria. Recent studies on the halophilic archaeon, Haloferax volcanii, have, however, begun to close this gap.
Haloferax volcanii cells are surrounded by a proteinaceous S-layer comprising a single component, the S-layer glycoprotein (Kessel et al., 1988; Sumper et al., 1990; Trachtenberg et al., 2000). In addition to other post-translational processing events, such as signal sequence cleavage (Fine et al., 2006) and lipid modification (Konrad and Eichler, 2002), the Hfx. volcanii S-layer glycoprotein experiences both N- and O-glycosylation (Sumper et al., 1990). N-glycosylation of the S-layer glycoprotein is catalysed by a series of clustered agl genes involved in the biosynthesis and attachment of a pentasaccharide comprising a hexose, two hexuronic acids, a methyl ester of hexuronic acid and a mannose to at least two Asn residues of the protein. AglJ, AglG, AglI, AglE are glycosyltransferases that sequentially add the first four pentasaccharide residues to a common dolichol phosphate carrier, while the dolichol phosphate mannose synthase, AglD, adds the final pentasaccharide residue to a distinct dolichol phosphate (Abu-Qarn et al., 2007; 2008b; Yurist-Doutsch et al., 2008; 2010; Guan et al., 2010; Kaminski et al., 2010; Magidovich et al., 2010). AglB, the archaeal oligosaccharyl-transferase (Abu-Qarn and Eichler, 2006; Chaban et al., 2006; Igura et al., 2008), subsequently delivers glycans to selected S-layer glycoprotein Asn residues (Abu-Qarn et al., 2007). AglF, AglM and AglP also contribute to the N-glycosylation process, respectively, acting as a glucose-1-phosphate uridyltransferase, as a UDP-glucose dehydrogenase and as a S-adenosyl-L-methionine-dependent methyltransferase (Magidovich et al., 2010; Yurist-Doutsch et al., 2010).
While significant advances have been made in delineating the Hfx. volcanii N-glycosylation pathway, much is still unknown. For instance, the route taken by the final pentasaccharide residue, mannose, in reaching the S-layer glycoprotein remains unclear. Two possibilities can be entertained. In the first, the mannose residue is transferred from its dedicated dolichol phosphate carrier to the glycan comprising the first four pentasaccharide subunits already loaded onto a distinct dolichol phosphate. The resulting pentasaccharide would then be delivered to the S-layer glycoprotein en bloc via the actions of AglB. Alternatively, the final mannose residue could be added to the tetrasaccharide already N-linked to the S-layer glycoprotein.
Answering this and other outstanding questions related to Hfx. volcanii N-glycosylation may benefit from efforts addressing how other Archaea perform this post-translational modification. Also originating from the Dead Sea (Oren et al., 1990), Haloarcula marismortui encodes for a predicted S-layer glycoprotein. Like its Hfx. volcanii and Halobacterium salinarum counterparts (cf. Calo et al., 2010a), the Har. marismortui S-layer glycoprotein is also likely to be N-glycosylated, given the presence of sequons, namely the Asn-X-Ser/Thr (X ≠ Pro) motif that serves the site of archaeal N-glycosylation (Abu-Qarn and Eichler, 2007; Igura and Kohda, 2011). Such expected similarities in the Hfx. volcanii and Har. marismortui S-layer glycoproteins, as well as the availability of complete genome sequences for both organisms (Baliga et al., 2004; Hartman et al., 2010), invite comparison of N-glycosylation in the two geographically and phylogenetically proximal archaeal species in the hope of shedding new light on the archaeal version of this post-translational modification.
In the following, N-glycosylation of the Har. marismortui S-layer glycoprotein is addressed in terms of the glycan N-linked to the proteins, the genes encoding putative enzymes involved in N-glycosylation in this species and the dolichol phosphate carriers upon which the N-linked glycan is assembled, relative to the parallel aspects of the Hfx. volcanii N-glycosylation pathway. The results reveal that while the Har. marismortui S-layer glycoprotein is modified by the identical N-linked glycan that decorates its Hfx. volcanii counterpart, differences in the two N-glycosylation pathways exist. Thus, these findings serve not only to further delineate steps of the Hfx. volcanii N-glycosylation pathway but also reveal diversity in N-glycosylation pathways across Archaea.
Results
Initial characterization of the Har. marismortui S-layer glycoprotein
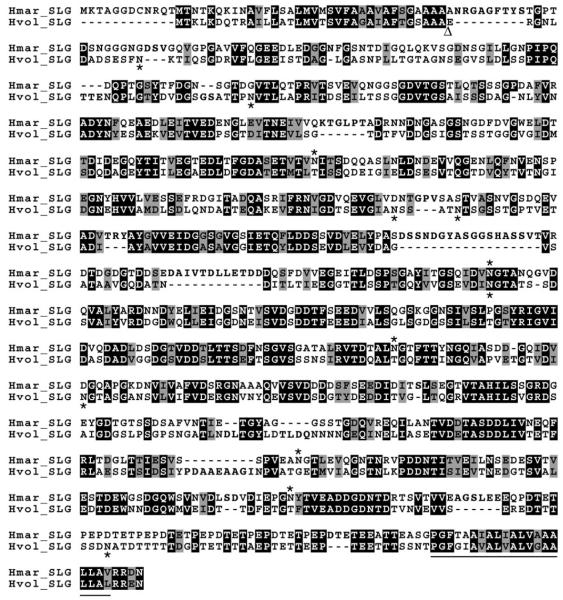
The S-layer glycoprotein is the sole component of the surface layer surrounding Hfx. volcanii cells (Kessel et al., 1988; Sumper et al., 1990; Trachtenberg et al., 2000). Har. marismortui encodes for a protein annotated as a S-layer glycoprotein (pNG5138) that is 45% identical to its Hfx. volcanii homologue (Fig. 1). The amino acid sequence of the Har. marismortui S-layer glycoprotein includes five putative N-glycosylation sites, i.e. N265, N467, N575, N727 and N786 (amino acid numbering before signal peptide cleavage). One of these residues, N467, coincides with a putative N-glycosylation site present but not modified in the Hfx. volcanii S-layer glycoprotein, i.e. N370 (Abu-Qarn et al., 2007). Otherwise, there is little resemblance in the distribution of putative N-glycosylation sites in the two proteins. Like the Hfx. volcanii S-layer glycoprotein, the Har. marismortui S-layer glycoprotein also presents a substantial number of Thr residues near the C-terminal end of the protein. In the Hfx. volcanii S-layer glycoprotein, many of these Thr residues are thought to undergo O-glycosylation (Sumper et al., 1990). Finally, both S-layer glycoproteins contain identical putative trans-membrane domains predicted to terminate four residues from the C-terminus, assigned to the cell interior.
Fig. 1.
Alignment of the Har. marismortui and Hfx. volcanii S-layer glycoprotein amino acid sequences. The sequences of the Har. marismortui and Hfx. volcanii S-layer glycoproteins [AAV44550 (Hmar_SLG) and ADE05144 (Hvol_SLG) respectively] were aligned using CLUSTALW and drawn using Boxshade, programs both found at the Biology Workbench (http://workbench.sdsc.edu/). In the figure, identical positions are drawn on a black background, while similar residues are drawn on a grey background. Verified or predicted N-glycosylation sites are identified by asterisks either above (Har. marismortui ) or below (Hfx. volcanii ) the sequences. The predicted trans-membrane domain of each sequence is underlined, while the known site of signal sequence cleavage in the Hfx. volcanii S-layer glycoprotein is depicted by an open triangle.
Biochemical characterization of the Har. marismortui S-layer glycoprotein began with separation of the protein content of Har. marismortui cells by SDS-PAGE. Sub-sequent Coomassie staining revealed a major protein species that migrates to a similar position as does the Hfx. volcanii S-layer glycoprotein (Fig. 2, left panel, arrow). Glycosylation of the Har. marismortui S-layer glycoprotein was demonstrated by PAS glycostaining (Fig. 2, middle panel) and upon incubation of Har. marismortui cells with [2-3H]mannose. It was previously been shown that Hfx. volcanii cells incubated with [2-3H]mannose incorporate the radiolabelled saccharide into the S-layer glycoprotein, reflecting that mannose is the final residue of the pentasaccharide N-linked to the Hfx. volcanii protein (Calo et al., 2011). As with the Hfx. volcanii S-layer glycoprotein, radiolabel was also incorporated into the S-layer glycoprotein of Har. marismortui cells incubated with [2-3H]mannose (Fig. 2, right panel).
Fig. 2.
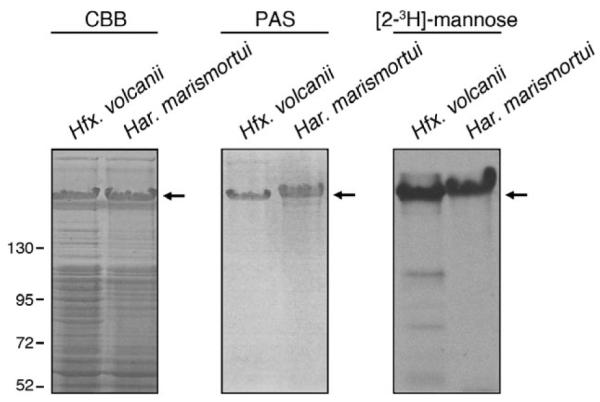
Like its Hfx. volcanii counterpart, the Har. marismortui S-layer protein is glycosylated. The protein contents of Hfx. volcanii (left) and Har. marismortui (right) cells grown to identical OD550 values separated by 7.5% SDS-PAGE and either Coomassieor PAS-stained [left (CBB) and middle panels respectively]. In addition, the protein contents of Hfx. volcanii (left) and Har. marismortui (right) cells challenged with [2-3H]-mannose were separated by 7.5% SDS-PAGE and processed for fluorography (right panel). In each panel, the Hfx. volcanii and Har. marismortui S-layer glycoproteins are indicated by an arrow. Molecular mass markers are shown on the left of the figure.
Har. marismortui contains pentasaccharide-linked dolichol phosphate
Guan et al. (2010) previously reported that the first four residues of the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein are derived from a tetrasaccharide-charged dolichol phosphate carrier, while the final pentasaccharide residue, mannose, is derived from a distinct dolichol phosphate carrier. To assess whether dolichol phosphate also serves as the lipid glycan carrier in Har. marismortui N-glycosylation, a total Har. marismortui lipid extract was examined by normal-phase liquidchromatography-electrospray ionization/mass spectrometry (LC-ESI/MS) performed in the negative ion mode.
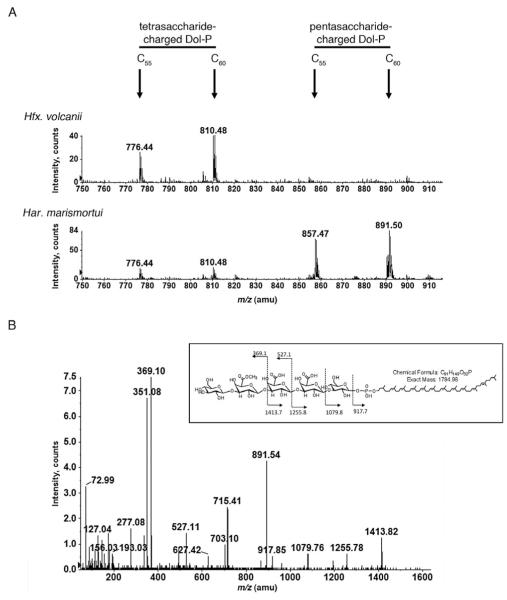
As observed upon examination of a total Hfx. volcanii lipid extract (Fig. 3A, upper panel), mass spectrometric analysis of a Har. marismortui lipid extract also revealed [M–2H]2− ion peaks at m/z 776.44 (this and all subsequently reported m/z values given are for the monoisotopic peak) and 810.48, respectively, corresponding to C55 and C60 dolichol phosphate species modified by a hexose, two hexuronic acids and a methyl ester of hexuronic acid (Fig. 3A, lower panel), namely the first four residues of the pentasaccharide N-linked to the Hfx. volcanii S-layer glycoprotein. However, in contrast to what was seen in the Hfx. volcanii glycan-charged dolichol phosphate pool, the Har. marismortui sample additionally included [M–2H]2− ion peaks at m/z 857.47 and 891.5 (Fig. 3A, lower panel), representing C55 and C60 dolichol phosphate modified by the same tetrasaccharide plus an additional 162 Da hexose residue.
Fig. 3.
Pentasaccharide-modified dolichol phosphate is detected in a total lipid extract from Har. marismortui but not from Hfx. volcanii. A. LC-ESI/MS analysis of the total lipid extract from Hfx. volcanii (upper panel) and Har. marismortui (lower panel) reveals the presence of tetrasaccharide-modified C55 and C60 dolichol phosphate in both extracts. In contrast, pentasaccharide-modified dolichol phosphate is only seen in the Har. marismortui sample. The peaks shown correspond to [M–2H]2− ions. The positions of the different glycan-modified dolichol phosphates are indicated. B. MS/MS analysis of Har. marismortui pentasaccharide-modified C60 dolichol phosphate. The fragmentation profile is schematically represented in the inset.
Analysis of the tandem mass spectrometry (MS/MS) fragmentation pattern obtained from the m/z 891.5 species confirmed its assignment as pentasaccharide-modified C60 dolichol phosphate. Such analysis revealed product ions of m/z 1079.760, corresponding to C60 dolichol phosphate modified by a hexose (calculated monoisotopic mass of 1079.8 Da, as shown in the fragmentation scheme of Fig. 3B, inset), m/z 1255.78, corresponding to C60 dolichol phosphate modified by a hexose-hexuronic acid disaccharide (calculated monoisotopic mass of 1255.8 Da), m/z 527.11, corresponding to a hexose-methyl ester of hexuronic acid-hexuronic acid trisaccharide (calculated monoisotopic mass of 527.1 Da), and m/z 369.10, corresponding to a hexosemethyl ester of hexuronic acid disaccharide (calculated monoisotopic mass of 369.1 Da) (Fig. 3B). Hence, the monoisotopic [M–2H]2− ion peak detected at m/z 891.50 (monoisotopic mass of 1785.02 Da; Fig. 3A, lower panel) is in good agreement with calculated mass of C60 dolichol phosphate modified by a pentasaccharide comprising a hexose, two hexuronic acids, the methyl ester of hexuronic acid and a final hexose (1784.98 Da).
The Har. marismortui and Hfx. volcanii S-layer glycoproteins are N-glycosylated by the same pentasaccharide
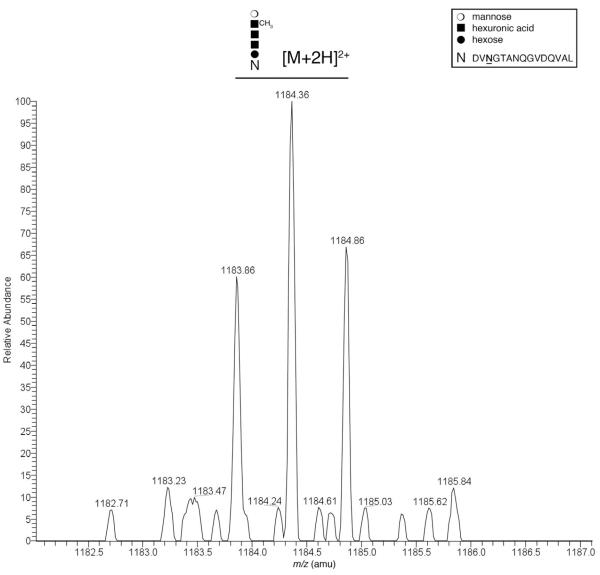
To determine whether the Har. marismortui S-layer glycoprotein is N-glycosylated by the same pentasaccharide bound to dolichol phosphate, chymotrypsingenerated fragments of the S-layer glycoprotein were examined by nano-flow, reverse-phase LC-ESI/MS. Such analysis revealed a peak of m/z 1183.86, corresponding to the [M+2H]2+ ion of a S-layer glycoproteinderived peptide containing the N-glycosylation site, Asn-467 [465DVNGTANQGVDQVAL478 (amino acid numbering before signal peptide cleavage)], and modified by a pentasaccharide comprising two hexoses, two hexuronic acids and the methyl ester of hexuronic acid (Fig. 4). This value is in good agreement with calculated mass of the pentasaccharide-modified peptide (1183.8608 Da). As such, it can be concluded that not only does dolichol phosphate serve as the glycan lipid carrier in Har. marismortui N-glycosylation, as is the case in Hfx. volcanii (Guan et al., 2010; Kaminski et al., 2010), but that the S-layer glycoproteins of both species are modified by the same N-linked pentasaccharide. However, in contrast to what has been observed in Hfx. volcanii, where S-layer glycoprotein modified by pentasaccharide precursors are detected, in Har. marismortui, only S-layer glycoprotein modified by the intact pentasaccharide was detected.
Fig. 4.
The Har. marismortui S-layer protein is modified by the same N-linked pentasaccharide as bound to dolichol phosphate and the Hfx. volcanii S-layer protein. A monoisotopic [M+2H]2+ ion peak corresponding to a Har. marismortui S-layer glycoprotein Asn-467 (amino acid numbering before signal peptide cleavage)-containing chymotrypsin-generated fragment, as detected by LC-ESI/MS. The mass-to-charge ratio of the doubly charged peak observed (1183.86 Da) is in good agreement with calculated mass of the peptide modified by a pentasaccharide comprising two hexoses, two hexuronic acids and the methyl ester of hexuronic acid (1183.8608 Da), schematically depicted above the peak. The inset depicts the symbols used for each sugar residue as well as the peptide sequence represented by ‘N’. The modified Asn residue in the peptide is indicated in bold and underlined.
agl genes are clustered in the Hfx. volcanii and the Har. marismortui genomes
In Hfx. volcanii, all but one of agl genes involved in the assembly and attachment of the pentasaccharide N-linked to the S-layer glycoprotein are sequestered in a region of the genome between HVO_1517 (aglJ) and HVO_1531 (aglM); aglD (HVO_0798) lies elsewhere in the genome (Yurist-Doutsch and Eichler, 2009; Yurist-Doutsch et al., 2010). Given the similarity in N-linked glycosylation, the existence of similar clustering of putative N-glycosylation genes in Har. marismortui was considered. Accordingly, the region surrounding rrnAC0431, encoding the homologue of the oligosaccharyltransferase, AglB, was analysed. When Har. marismortui rrnAC0419, rrnAC0427 and rrnAC0430 were used to query the Hfx. volcanii genome in a BLAST search, these were revealed to respectively encode homologues of Hfx. volcanii AglJ, AglR and AglG. Likewise, when the reverse was performed and Hfx. volcanii agl sequences were used to query the Har. marismortui genome in a BLAST search, it was revealed that Har. marismortui rrnAC0443, annotated as a hypothetical protein, is a homologue of Hfx. volcanii AglP (e-value of 8e−21). The Har. marismortui gene cluster encoding AglB, AglG, AglJ and AglR homologues also includes rrnAC0421 and rrnAC0429, encoding proteins annotated as glycosyltransferases. Using the same bioinformatics strategy, it was further revealed that Har. marismortui also encodes for homologues of Hfx. volcanii AglE (pNG7005, e-value of 1e−15), AglI (pNG7005, e-value of 1e−11), AglF (rrnAC3238, e-value of 3e−119) and AglM (rrnAC3239, e-value of 9e−159) (best hits, according to e-values, are listed). Finally, no Har. marismortui homologue of Hfx. volcanii aglQ was detected. Figure 5A shows a schematic comparison of the distribution and orientation of agl genes and other putative N-glycosylation sequences near aglB and elsewhere in the two genomes.
Fig. 5.
agl genes in Hfx. volcanii and their putative Har. marismortui homologues are clustered to different degrees.
A. Regions of the Hfx. volcanii genome encoding Agl proteins and of the Har. marismortui genome encoding predicted Agl protein homologues are schematically presented. In addition, genes encoding predicted glycosyltransferases (GTase) near Har. marismortui aglB are shown. In each case, the genome identifier is provided above the gene while the known (Hfx. volcanii ) or predicted (Har. marismortui ) product is given below.
B. Homologous genes in the immediate vicinity of Hfx. volcanii aglD and its Har. marismortui homologue, rrnAC1873, are schematically shown. Below each gene, the current annotation is provided. In both (A) and (B), the full identifier is provided for only the first gene of a group of adjacent genes; only numbers are provided for the subsequent genes. Otherwise, the full identifier is provided. The size of each gene is considered to be equal in the scheme, as are the distances between proximal yet non-adjacent genes. Genes encoding the known (Hfx. volcanii ) or predicted (Har. marismortui ) oligosaccharyltransferase, AglB, are hatched, genes encoding known or predicted glycosyltransferases are shown in black and genes encoding proteins known or predicted to contribute to N-glycosylation are shown in dark grey. Genes encoding proteins apparently not related to N-glycosylation are shown in light grey.
As noted above, aglD (HVO_0798) does not cluster with genes encoding the other known Hfx. volcanii Agl proteins (Yurist-Doutsch and Eichler, 2009). The Har. marismortui homologue of AglD, rrnAC1873 (Calo et al., 2010b), is also found in a region of the genome distinct from the aglB-based cluster or other agl homologues. Moreover, comparing the sequences near Hfx. volcanii aglD and Har. marismortui rrnAC1873 reveals that homologous genes are found clustered in the immediate vicinities of the two, albeit in opposing orientations. Specifically, Hfx. volcanii HVO_0787, HVO_0788, HVO_0789, HVO_0790, HVO_0792, HVO_0794 and HVO_0795 correspond to Har. marismortui rrnAC1886, rrnAC1885, rrnAC1884, rrnAC1881, rrnAC1879, rrnAC1876 and rrnAC1875 (Fig. 5B).
Differences exist in the Har. marismortui and Hfx. volcanii N-glycosylation processes
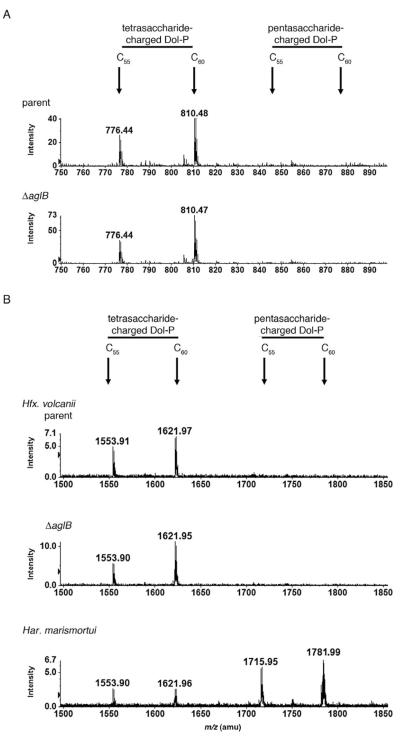
Although pentasaccharide-modified dolichol phosphate was readily detected in Har. marismortui, no such species was detected in Hfx. volcanii (Fig. 3 and Guan et al., 2010), even when a fivefold larger aliquot of the total Hfx. volcanii lipid extract was considered (not shown). To test whether this was due to the rapid delivery of lipid-bound pentasaccharide to the S-layer glycoprotein in Hfx. volcanii, the glycan-charged dolichol phosphate poolwas considered in Hfx. volcanii cells deleted of aglB. In the absence of the oligosaccharyltransferase, AglB, N-glycosylation of the Hfx. volcanii S-layer glycoprotein does not transpire (Abu-Qarn et al., 2007), presumably because the complete pentasaccharide or its tetrasaccharide precursor is not delivered to the protein from a dolichol phosphate carrier. Hence, any pentasaccharide-charged dolichol phosphate formed in Hfx. volcanii would be expected to accumulate in cells deleted of aglB. However, when total lipid extracts were prepared from Hfx. volcanii ΔaglB cells and analysed by normal-phase LC-ESI/MS, it was noted that as with the parent strain, only tetrasaccharide-modified C55 and C60 dolichol phosphate species were detected (Fig. 6A).
Fig. 6.
No pentasaccharide-modified dolichol phosphate accumulates in Hfx. volcanii cells lacking AglB nor is detected using aminopropyl resin for liquid chromatography.
A. Tetrasaccharide-modified C55 and C60 dolichol phosphate are detected upon LC-ESI/MS analysis of the total lipid extract from Hfx. volcanii parent strain (upper panel) and ΔaglB cells (lower panel). In neither case is pentasaccharide-modified dolichol phosphate detected. The peaks shown correspond to doubly charged [M–2H]2− ions, with the positions of the different glycan-modified dolichol phosphates indicated.
B. Tetrasaccharide-modified C55 and C60 dolichol phosphate are detected upon LC-ESI/MS analysis of the total lipid extract from Hfx. volcanii parent strain (upper panel) and ΔaglB cells (middle panel) using aminopropyl resin for the liquid chromatography step. In neither case is pentasaccharide-modified dolichol phosphate detected. In contrast, pentasaccharide-modified C55 and C60 dolichol phosphates are detected upon similar analysis of the total lipid extract from Har. marismortui cells (lower panel). The peaks shown correspond to [M–H]− ions, with the positions of the different glycan-modified dolichol phosphates indicated.
To eliminate the possibility that no pentasaccharide-modified Hfx. volcanii dolichol phosphate was detected as this entity simply could not elute from the silica resin used for the liquid chromatography step, LC-ESI/MS was repeated, this time using less hydrophilic aminopropyl resin for the initial separation step. While such analysis of the total Hfx. volcanii lipid extract yielded singly charged [M–H]− ion peaks at m/z 1553.91 and 1621.97, respectively, corresponding to tetrasaccharide-charged C55 and C60 dolichol phosphate, no pentasaccharide-modified lipid carriers were detected (Fig. 6B, top panel). Likewise, no pentasaccharide-modified dolichol phosphate was detected when the total lipid extract prepared from ΔaglB cells was similarly examined (Fig. 6B, middle panel). In contrast, a total Har. marismortui lipid extract analysed by aminopropyl resin-based LC-ESI/MS not only contained the tetrasaccharide-charged species but also singly charged [M–H]− ion peaks at m/z 1715.95 and 1781.91, corresponding to pentasaccharide-charged C55 and C60 dolichol phosphate respectively (Fig. 6B, bottom panel).
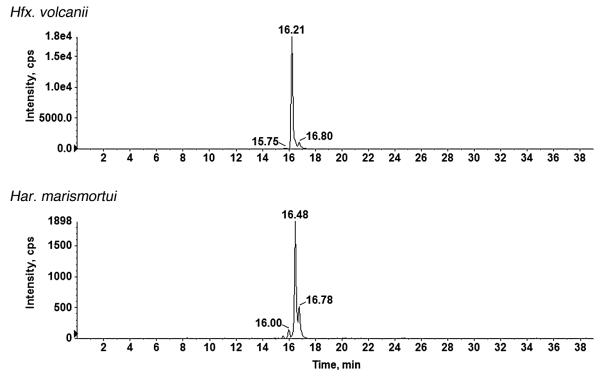
Finally, to determine whether the final sugar residue of the pentasaccharide-modified dolichol phosphate detected in Har. marismortui, i.e. mannose, is derived from a soluble nucleoside-charged species or from a dolichol phosphate-bound precursor, the monosaccharide-charged C60 dolichol phosphate ion peak was detected in the Har. marismortui and Hfx. volcanii lipid extracts during a single LC/MS run, over a 40 min span. As reflected in Fig. 7, the single m/z 1079.8 ion peak from each strain could be resolved into three distinct species, distinguished by their slightly different LC retention times. Earlier efforts (Guan et al., 2010; Kaminski et al., 2010) had shown the third of these Hfx. volcanii peaks as corresponding to mannose-charged dolichol phosphate. As such, it is likely that the 16.80 min peak in the Har. marismortui sample also corresponds to mannosecharged dolichol phosphate.
Fig. 7.
Har. marismortui contains mannose-charged dolichol phosphate. Normal-phase LC extracted ion chromatograms (EIC) of the dolichylphosphate-hexose [M–H]− ion at m/z 1079.8 from Hfx. volcanii (upper panel) and from Har. marismortui (lower panel) are shown. The Hfx. volcanii peak retained at 16.80 min was previously shown to correspond to mannose-charged dolichol phosphate (Guan et al., 2010).
Discussion
In the present study, it was revealed that like Hfx. volcanii and Hbt. salinarum (cf. Calo et al., 2010a), Har. marismortui is also capable of performing N-glycosylation. Specifically, examination of the Hfx. volcanii and Har. marismortui S-layer glycoproteins showed that both reporter glycoproteins are modified by the same N-linked pentasaccharide comprising two hexoses, two hexuronic acids and the methyl ester of hexuronic acid. Of course, more detailed future analysis of each glycan may reveal the use of distinct hexoses or hexuronic acids in each case, although both N-linked pentasaccharides were shown to include at least one mannose.
In Hfx. volcanii, a series of clustered agl gene products are involved in the assembly and attachment of the N-linked pentasaccharide (cf. Calo et al., 2010a). While the Har. marismortui genes involved in N-glycosylation remain to be defined, it appears that Har. marismortui has clustered genes encoding homologues of several Agl proteins in the immediate region of the oligosaccharyltransferase-encoding aglB sequence. Such clustering of agl genes allows for more co-ordinated control of N-glycosylation gene expression in response to changes in growth environment, as suggested by earlier quantitative PCR analysis assessing the transcription of a subset of Hfx. volcanii agl genes in response to changing growth conditions (Yurist-Doutsch et al., 2008; 2010). Previous efforts have also shown that Hfx. volcanii strains lacking either aglD or aglJ can be functionally complemented by introduction of their respective Har. marismortui homologues, i.e. rrnAC1873 and rrnAC0149 (Calo et al., 2010b; 2011; D. Calo, unpublished), pointing to the common activities of these proteins in each organism. Moreover, the fact that those genes encoding Har. marismortui homologues of AglF and AglM are found adjacent to each other is of note, given in vitro studies showing that Hfx. volcanii AglF and AglM work in concert (Yurist-Doutsch et al., 2010). Still, the number of putative Har. marismortui N-glycosylation genes in the vicinity of aglB does not match the number of clustered agl sequences in Hfx. volcanii. In fact, the Har. marismortui genome contains sequences encoding homologues of other Agl proteins scattered throughout the genome.
Despite the various N-glycosylation-related similarities of Hfx. volcanii and Har. marismortui, significant species-specific differences nonetheless exist. The most striking difference is seen at the level of the dolichol phosphates that serve as lipid glycan carriers in archaeal N-glycosylation (Mescher et al., 1976; Lechner et al., 1985; Hartmann and Konig, 1989; Guan et al., 2010). The present study finds that the pentasaccharide N-linked to the Har. marismortui S-layer glycoprotein is first fully assembled on a single dolichol phosphate and only then transferred to the protein target. This strategy differs from what is seen in Hfx. volcanii, where the same S-layer glycoprotein N-linked pentasaccharide is derived from a tetrasaccharide sequentially assembled on a single dolichol phosphate and a final mannose residue derived from a distinct dolichol phosphate carrier (Guan et al., 2010). As such, Har. marismortui N-glycosylation is reminiscent of the parallel bacterial process, where a heptasaccharide is assembled through the sequential addition of seven soluble nucleotide-activated sugars onto a common undecaprenol phosphate carrier (Szymanski and Wren, 2005; Weerapana and Imperiali, 2006; Abu-Qarn et al., 2008a). In contrast, the Hfx. volcanii N-glycosylation process, involving multiple glycan-charged dolichol phosphates, is reminiscent of the parallel eukaryal process. In higher Eukarya, the first seven sugar subunits of the 14-member oligosaccharide eventually formed in the endoplasmic reticulum are sequentially added to a common dolichol phosphate carrier, while the second set of seven sugar subunits are derived from individual hexose-charged doli-chol phosphates (Burda and Aebi, 1999; Helenius and Aebi, 2004). However, despite these pathway differences, the final mannose subunit of the pentasaccharide N-linked to the Har. marismortui S-layer glycoprotein is derived from a distinct dolichol phosphate carrier, as in Hfx. volcanii.
The Hfx. volcanii N-glycosylation process further relies on a degree of sophistication until now only seen in Eukarya. The fact that tetrasaccharide-modified but no pentasaccharide-modified dolichol phosphate could be detected in Hfx. volcanii cells, even in cells lacking the oligosaccharyltransferase, AglB, points to the final pentasaccharide subunit, mannose, as being added to the tetrasaccharide already N-linked to the S-layer glycoprotein. This same general strategy is employed in Eukarya, where in the Golgi, additional sugar subunits are attached to oligosaccharides already N-linked to the target polypeptide. However, since no S-layer glycoprotein modified by pentasaccharide precursors was detected in Har. marismortui, and since significantly more tetrasaccharide- than pentasaccharide-modified S-layer glycoprotein is seen in Hfx. volcanii (Abu-Qarn et al., 2007), it would appear that Archaea pay a price in N-glycosylation efficiency for adopting the eukaryal strategy of adding sugar subunits to protein-linked glycans. This could explain why Eukarya have spatially separated the two N-glycosylation strategies, assigning transfer of the dolichol phosphate-bound oligosaccharide to a protein target to the endoplasmic reticulum, and then having the subsequent introduction of more protein-specific sugar subunits occur in the Golgi (Helenius and Aebi, 2004). Of course, the inability to detect Har. marismortui S-layer glycoprotein-derived glycopeptides modified by pentasaccharide precursors could be due to other considerations, such as AglB specificity or differing efficiencies in modifying the different N-glycosylation sites in the protein.
In a recent analysis of 56 archaeal genome sequences, aglB, encoding the archaeal oligosaccharyltransferase, was detected in all but two species (Magidovich and Eichler, 2009). Although experimental verification for N-glycosylation is still lacking for the majority of these species, it would appear that this post-translational modification is common in Archaea. Continued analysis of the archaeal version of N-glycosylation will thus not only provide additional insight into this universal proteinprocessing event but will also elucidate strategies adopted by extremophiles to cope with the physically challenging environments in which they live.
Experimental procedures
Strains and growth conditions
Haloferax volcanii WR536 (H53) cells were grown in medium containing 3.4 M NaCl, 0.15 M MgSO4·7H2O, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3% (w/v) yeast extract, 0.5% (w/v) tryptone, 50 mM Tris-HCl, pH 7.2, at 42°C (Mevarech and Werczberger, 1985). Har. marismortui cells were grown in 3.5 M NaCl, 0.19 M MgSO4·7H2O, 3 mM CaCl2, 1 mM MnCl2 and 1% (w/v) yeast extract (Mevarech et al., 1976).
[2-3H]-mannose radiolabelling
Haloferax volcanii and Har. marismortui cells grown to midexponential phase were incubated with 6 μl of [2-3H]-mannose (23.8 mCi mmol−1; PerkinElmer, Boston, MA) in a volume of 100 μl. Sixty minutes later, the samples were precipitated with 15% (w/v) trichloroacetic acid and separated by SDS-PAGE. The S-layer glycoprotein was identified by Coomassie staining and fluorography and exposure to film.
LC/MS and MS/MS
LC-ESI/MS/MS analysis of total Hfx. volcanii and Har. marismortui lipid extracts was performed as described previously (Kaminski et al., 2010), with one modification. In some cases, liquid chromatographic separation was achieved using a Unison UK-Amino column (250 × 2 mm; Imtakt, Philadelphia, PA).
For LC-ESI/MS analysis of Hfx. volcanii and Har. marismortui S-layer glycoproteins, the protein contents of Hfx. volcanii and Har. marismortui cells were separated on 7.5% polyacrylamide gels and stained with Coomassie R-250 (Fluka, St. Louis, MO). For in-gel digestion of the Hfx. volcanii S-layer glycoprotein, the relevant bands (identified via the unique SDS-PAGE migration and staining pattern of the protein) were excised, destained in 400 μl of 50% (v/v) acetonitrile (Sigma, St Louis, MO) in 40 mM NH4HCO3, pH 8.4, dehydrated with 100% acetonitrile and dried using a Speed-Vac drying apparatus. The S-layer glycoprotein was reduced with 10 mM dithiothreitol (Sigma) in 40 mM NH4HCO3 at 56°C for 60 min and then alkylated for 45 min at room temperature with 55 mM iodoacetamide in 40 mM NH4HCO3. The gel pieces were washed with 40 mM NH4HCO3 for 15 min, dehydrated with 100% acetonitrile, and SpeedVac dried. The gel slices were rehydrated with 12.5 ng μl−1 mass spectrometry (MS)-grade Trypsin Gold (Promega, Madison, WI) in 40 mM NH4HCO3. In the case of the Har. marismortui S-layer glycoprotein, the same protocol was followed except that the protein was digested with 12.5 ng μl−1 sequencing-grade chymotrypsin (Promega). In both cases, protease-generated peptides were extracted with 0.1% (v/v) formic acid in 20 mM NH4HCO3, followed by sonication for 20 min at room temperature, dehydration with 50% (v/v) acetonitrile and additional sonication. After three rounds of extraction, the gel pieces were dehydrated with 100% acetonitrile, dried completely with a SpeedVac, resuspended in 5% (v/v) acetonitrile containing 1% formic acid (v/v) and infused into the mass spectrometer using static nanospray Econotips (New Objective, Woburn, MA). The protein digests were separated on-line by nano-flow reverse-phase liquid chromatography (LC) by loading onto a 150 mm by 75 μm (internal diameter) by 365 μm (external diameter) Jupifer prepacked fused silica 5 μm C18 300 Å reverse-phase column (Thermo Fisher Scientific, Bremen, Germany). The sample was eluted into the LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scien-tific) using a 60 min linear gradient of 0.1% formic acid (v/v) in acetonitrile/0.1% formic acid (1:19, by volume) to 0.1% formic acid in acetonitrile/0.1% formic acid (4:1, by volume) at a flow rate of 300 nl min−1.
Other methods
Glycostaining of the S-layer glycoprotein by periodic acidSchiff reagent was performed as previously described (Dubray and Bezard, 1982).
Acknowledgements
J.E. is supported by the Israel Science Foundation (Grant 30/07). The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant No. GM-069338 from NIH. S.N. is the recipient of a Negev-Zin Associates Scholarship.
References
- Abu-Qarn M, Eichler J. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol Microbiol. 2006;61:511–525. doi: 10.1111/j.1365-2958.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Eichler J. An analysis of amino acid sequences surrounding archaeal glycoprotein sequons. Archaea. 2007;2:73–81. doi: 10.1155/2006/510578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, et al. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J Mol Biol. 2007;14:1224–1236. doi: 10.1016/j.jmb.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Eichler J, Sharon N. Not just for Eukarya anymore: N-glycosylation in Bacteria and Archaea. Curr Opin Struct Biol. 2008a;18:544–550. doi: 10.1016/j.sbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Abu-Qarn M, Giordano A, Battaglia F, Trauner A, Morris HR, Hitchen P, et al. Identification of AglE, a second glycosyltransferase involved in N-glycosylation of the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2008b;190:3140–3146. doi: 10.1128/JB.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga NS, Bonneau R, Facciotti MT, Pan M, Glusman G, Deutsch EW, et al. Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea. Genome Res. 2004;14:2221–2234. doi: 10.1101/gr.2700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- Calo D, Kaminski L, Eichler J. Protein glycosylation in Archaea: sweet and extreme. Glycobiology. 2010a;20:1065–1079. doi: 10.1093/glycob/cwq055. [DOI] [PubMed] [Google Scholar]
- Calo D, Eilam Y, Lichtenstein RG, Eichler J. Towards glyco-engineering in Archaea: replacing Haloferax volcanii AglD with homologous glycosyltransferases from other halophilic archaea. Appl Environ Microbiol. 2010b;76:5684–5692. doi: 10.1128/AEM.00681-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo D, Guan Z, Eichler J. Glyco-engineering in Archaea: differential N-glycosylation of the S-layer glycoprotein in a transformed Haloferax volcanii strain. Microbiol Biotechnol. 2011;4:461–470. doi: 10.1111/j.1751-7915.2011.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF. Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea. Mol Microbiol. 2006;61:259–268. doi: 10.1111/j.1365-2958.2006.05226.x. [DOI] [PubMed] [Google Scholar]
- Dubray G, Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Eichler J, Adams MWW. Post-translational protein modification in Archaea. Microbiol Mol Biol Rev. 2005;69:393–425. doi: 10.1128/MMBR.69.3.393-425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A, Irihimovitch V, Dahan I, Konrad Z, Eichler J. Cloning, expression and purification of functional Sec11a and Sec11b, type I signal peptidases of the archaeon Haloferax volcanii. J Bacteriol. 2006;188:1911–1919. doi: 10.1128/JB.188.5.1911-1919.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Norais C, Badger JH, Delmas S, Haldenby S, Madupu R, et al. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS ONE. 2010;5:e9605. doi: 10.1371/journal.pone.0009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Konig H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch Microbiol. 1989;151:274–281. [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Igura M, Kohda D. Quantitative assessment of the preferences for the amino acid residues flanking archaeal N-linked glycosylation sites. Glycobiology. 2011;21:575–583. doi: 10.1093/glycob/cwq196. [DOI] [PubMed] [Google Scholar]
- Igura M, Maita N, Kamishikiryo J, Yamada M, Obita T, Maenaka K, Kohda D. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2008;27:234–243. doi: 10.1038/sj.emboj.7601940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, Raetz CRH, et al. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2010;192:5572–5579. doi: 10.1128/JB.00705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M, Wildhaber I, Cohen S, Baumeister W. Three-dimensional structure of the regular surface glycoprotein layer of Halobacterium volcanii from the Dead Sea. EMBO J. 1988;7:1549–1554. doi: 10.1002/j.1460-2075.1988.tb02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad Z, Eichler J. Lipid modification of proteins in Archaea: attachment of a mevalonic acid-based lipid moiety to the surface-layer glycoprotein of Haloferax volcanii follows protein translocation. Biochem J. 2002;366:959–964. doi: 10.1042/BJ20020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J, Wieland F, Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985;260:860–866. [PubMed] [Google Scholar]
- Magidovich H, Eichler J. Glycosyltransferasesand oligosaccharyltransferases in Archaea: putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol Lett. 2009;300:122–130. doi: 10.1111/j.1574-6968.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- Magidovich H, Yurist-Doutsch S, Konrad Z, Ventura VV, Hitchen PG, Dell A, Eichler J. AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol Microbiol. 2010;76:190–199. doi: 10.1111/j.1365-2958.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- Mescher MF, Hansen U, Strominger JL. Formation of lipid-linked sugar compounds in Halobacterium salinarium. Presumed intermediates in glycoprotein synthesis. J Biol Chem. 1976;251:7289–7294. [PubMed] [Google Scholar]
- Mevarech M, Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985;162:461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M, Leicht W, Werber MM. Hydrophobic chromatography and fractionation of enzymes from extremely halophilic bacteria using decreasing concentration gradients of ammonium sulfate. Biochemistry. 1976;15:2383–2387. doi: 10.1021/bi00656a021. [DOI] [PubMed] [Google Scholar]
- Oren A, Ginzburg M, Ginzburg BZ, Hochstein LI, Volcani BE. Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely halophilic bacterium from the Dead Sea. Int J Syst Bacteriol. 1990;40:209–210. doi: 10.1099/00207713-40-2-209. [DOI] [PubMed] [Google Scholar]
- Sumper M, Berg E, Mengele R, Strobel I. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J Bacteriol. 1990;172:7111–7118. doi: 10.1128/jb.172.12.7111-7118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S, Pinnick B, Kessel M. The cell surface glycoprotein layer of the extreme halophile Halobacterium salinarum and its relation to Haloferax volcanii: cryo-electron tomography of freeze-substituted cells and projection studies of negatively stained envelopes. J Struct Biol. 2000;130:10–26. doi: 10.1006/jsbi.2000.4215. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Eichler J. Manual annotation, transcriptional analysis and protein expression studies reveal novel genes in the agl cluster responsible for N-glycosylation in the halophilic archaeon Haloferax volcanii. J Bacteriol. 2009;191:3068–3075. doi: 10.1128/JB.01838-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Abu-Qarn M, Battaglia F, Morris HR, Hitchen PG, Dell A, Eichler J. aglF, aglG and aglI, novel members of a gene cluster involved in the N-glycosylation of the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2008;69:1234–1245. doi: 10.1111/j.1365-2958.2008.06352.x. [DOI] [PubMed] [Google Scholar]
- Yurist-Doutsch S, Magidovich H, Ventura VV, Hitchen PG, Dell A, Eichler J. N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol Microbiol. 2010;75:1047–1058. doi: 10.1111/j.1365-2958.2009.07045.x. [DOI] [PubMed] [Google Scholar]