Peptide conjugate molecules comprising programmable self-assembly[1] and inorganic recognition[2] motifs have proven to be powerful agents for directing the one-pot synthesis and assembly of structurally-regular and topologically-complex nanoparticle superstructures.[3] These peptide conjugate molecules serve a dual purpose: the inorganic recognition motif binds specific nanoparticles and plays a role in their synthesis, and the self-assembly motif directs the assembly of the nanoparticles into specific architectures. We recently introduced the conceptual basis of utilizing this class of molecules for nanoparticle synthesis and assembly and demonstrated that particular members of this class could be used to prepare 1-D nanoparticle superstructures,[3b, 3d] including gold nanoparticle double helices[3a] and discrete sub-100 nm spherical gold nanoparticle superstructures.[3c] One particularly useful feature of this methodology is that small changes to the composition and sequence of the peptide conjugate can dramatically impact the structure of the resulting nanoparticle assembly. It has become clear that one can potentially rationally control the structure of the nanoparticle assembly by carefully tailoring the peptide conjugate.
It is well-established that the properties of a nanoparticle superstructure can depend intrinsically on the size and shape of the superstructure and the organization of the nanoparticles within the superstructure.[4] Therefore, an ideal nanoparticle assembly methodology should allow one to carefully tune the shape and metrics of a nanoparticle superstructure. In previous work, we showed that small changes to the organic (non-peptide) component of the peptide conjugate can lead to entirely different nanoparticle superstructures. For example, we found that C12-PEPAu (C11H23CO-PEPAu; PEPAu = AYSSGAPPMPPF[5]) directs the formation of gold nanoparticle double helices[3a] while C6-A2-PEPAu (C5H11CO-A2- PEPAu; A = alanine)directs the formation of ‘hollow’ spherical gold nanoparticle assemblies (i.e. a spherical shell of gold nanoparticles).[3c] In this contribution, we show that we can use this methodology to tailor the size of a given nanoparticle superstructure. Specifically, we demonstrate that small modifications to the sequence of the peptide portion of the conjugate allow one adjust the size of spherical gold nanoparticle superstructures and ultimately enable the direct one-pot preparation of sub-50 nm spherical superstructures. Such structures may potentially exhibit useful optical and catalytic properties and possibly may serve as capsules or delivery agents.[4, 6]
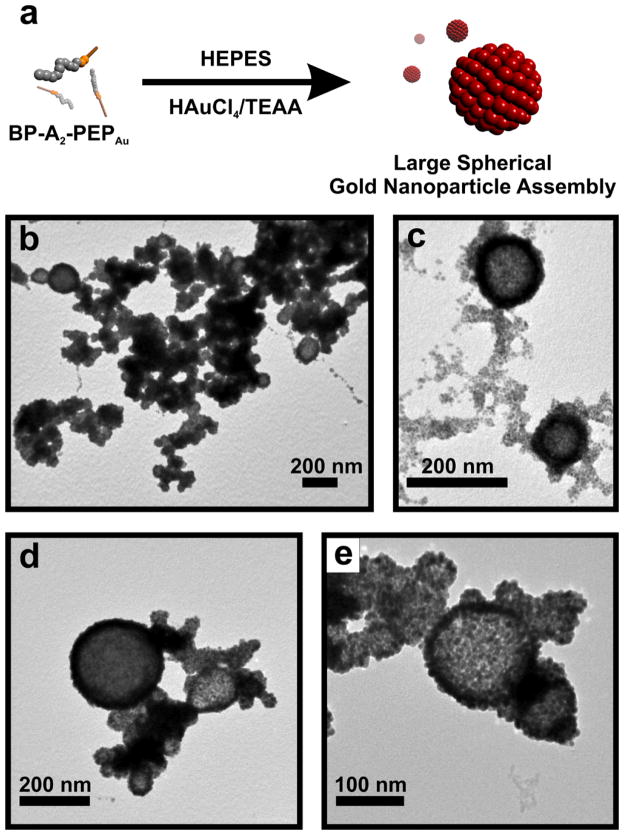
We began this work by first studying the self-assembly properties of BP-Ax-PEPAu (C12H9CO-Ax-AYSSGAPPMPPF; x = 0–3; BP = biphenyl) in HEPES buffer (0.1 M, pH 7.3±0.1; HEPES = 4-(2-hydroxyethyl)-piperazineethanesulfonic acid) both before and after the addition of a gold salt solution (0.1 M HAuCl4 in 1.0 M TEAA; TEAA = triethylammonium acetate).[7] We reasoned that the biphenyl unit would potentially lead to peptide conjugate self-assembly through π-π stacking/hydrophobic interactions. Our initial assembly studies using BP-PEPAu and BP-A-PEPAu were inconclusive under both conditions. However, once we added an additional hydrophobic alanine residue to produce BP-A2-PEPAu we observed self-assembly in the presence of the gold salt solution. Specifically, in a mixture of HEPES and HAuCl4/TEAA, BP-A2-PEPAu assembles into large spherical structures, as revealed by transmission electron microscopy (TEM) (Figure S3a, b). The size distribution (~60 nm – ~240 nm; Figure S3c) of the large spherical structures suggests a vesicular architecture.[8] We note that these structures are similar to those formed via the self-assembly of C6-A2-PEPAu.[3c] After BP-A2-PEPAu was allowed to incubate (~48 hrs) in the mixture of HEPES and HAuCl4/TEAA, large spherical nanoparticle superstructures (Figure 1a–e, S4a–f) comprising monodisperse gold nanoparticles (6.71 ± 0.24 nm; Figure S4h) formed. In general, these structures are similar to those produced using C6-A2-PEPAu,[3c] but they are significantly larger (~60 nm – ~270 nm; Figure S4g) because the peptide conjugate structures that serve as their underlying template are larger than those produced using C6-A2-PEPAu. Samples of the spherical gold nanoparticle superstructures exhibited a broad absorbance with a maximum at 656 nm (Figure S5), which, expectedly, is significantly red-shifted from that of the constituent nanoparticles (~517 nm).[9] We attribute the broad absorbance to the large size distribution of the superstructures and the presence of large aggregates of superstructures (Figure 1b).
Figure 1.
(a) Schematic illustration of the synthesis of large hollow spherical gold nanoparticle superstructures. (b–e) TEM images of the superstructures.
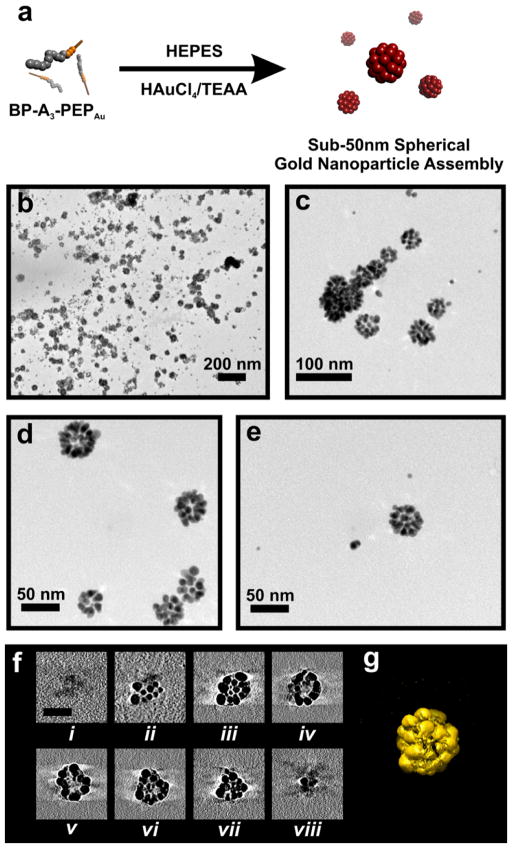
At this point, we decided to explore whether further modification of the peptide sequence would impact the structure of the resultant nanoparticle assembly. It is well-established that increasing the length of the hydrophobic component of a surfactant molecule can promote a transition from vesicular architectures to tubular micelles or discrete spherical micelles.[8] We reasoned that small spherical micelles would be an ideal underlying template for producing sub-50 nm hollow spherical nanoparticle superstructures. While sub-50 nm clusters of nanoparticles have been prepared,[10] we are not aware of any sub-50 nm hollow nanoparticle superstructures which have been prepared in a logical single-step process. In our basic conjugate, BP-Ax-PEPAu, we can systematically increase the hydrophobic character of the N-terminus by adding additional alanine residues.[11] We therefore prepared BP-A3-PEPAu and studied its self-assembly. BP-A3-PEPAu assembled into well-defined spherical structures after incubating for three days in HEPES buffer, as observed by atomic force microscopy (AFM) (Figure S6). Upon adding HAuCl4/TEAA to a solution of BP-A3-PEPAu in HEPES, we again observed, after 5 min, the formation of small spherical structures (Figure S7a). Based on their size (4.51 ± 0.06 nm), we hypothesize that these spherical structures could be micelles.[8] After prolonged incubation in the HEPES/HAuCl4/TEAA mixture, these spherical structures begin to coalesce (after 10 min; Figure S7b), ultimately yielding fibers (after 6 h; Figure S7c,d) whose diameters (4.46 ± 0.03 nm) are suggestive of tubular micelles.[8] After BP-A3-PEPAu was allowed to incubate for 2 days in the mixture of HEPES and HAuCl4/TEAA, we observed exclusively the formation of sub-50 nm spherical gold nanoparticle superstructures (Figure 2, S8a–d). These structures have uniform size (diameter = 29.43 ± 0.34 nm; Figure S8e), consist of monodisperse nanoparticles (6.15 ± 0.14 nm; Figure S8f), and exhibit a sharp absorption band at 540 nm (Figure S9), which is consistent with their size and relatively narrow size distribution. We used electron tomography to better understand the three-dimensional architecture of the superstructures. Tilted images collected at 1° tilt intervals from −70° to 70° were combined computationally to generate a three-dimensional electron density map (tomogram). The sample tomographic slices (Figure 2f) and the surface rendered 3-D tomogram (Figure 2g) reveals that the structures are roughly spherical and that their cores are hollow (devoid of nanoparticles).
Figure 2.
(a) Schematic illustration of the synthesis of sub-50 nm hollow spherical gold nanoparticle superstructures. (b–e) TEM images of the superstructures.
(f) X–Y computational slices (i–viii) of the 3-D tomographic volume containing the nanoparticle assembly (scale bar = 30 nm). (g) 3-D surface rendering of the nanoparticle superstructures.
Surprisingly, no linear nanoparticle superstructures were observed, even though tubular micelles, which could also potentially serve as templates for nanoparticle assembly, were also present (Figure S10a). We speculate that the spherical nanoparticle assemblies may be the exclusive product because spheres are the primary structures present upon the addition of the gold salt solution. Therefore, the gold nanoparticles would first grow on these structures, resulting in the sub-50 nm spherical nanoparticle superstructure products. The tubular structures which appear after prolonged incubation (vide supra) must then form from spherical stuctures which are not decorated by gold nanoparticles. Interestingly, when a msecond aliquot of the HAuCl4/TEAA solution was added six hours after adding the first aliquot, a mixture of both spherical and linear nanoparticle superstructures resulted (Figure S10b–d). We can reason that the first dose of gold salt led to the formation of the spherical nanoparticle superstructures while the second dose allowed formation of the linear structures. Thus, one can either produce exclusively spherical nanoparticle superstructures or a mixture of spherical and linear superstructures depending on the gold salt dosing regimen.
We have shown that peptide conjugate methodology for synthesizing and assembling nanoparticle superstructures can be used to prepare two different kinds of spherical structures. Importantly, we have demonstrated that small modifications to the peptide sequence, in this case addition of a single alanine residue, can significantly impact the diameter of the resulting spherical nanoparticle superstructure. In addition, we have shown that small procedural modifications, such as a second addition of gold salt, can affect nanoparticle superstructure distribution. These results point toward the versatility of this methodology and the rich structural diversity that can easily be achieved when this methodology is employed in a logical and thoughtful manner.
Experimental Section
All instrumental details and general methods are included in the Supporting Information.
Preparation of Peptide Conjugates
Ax-PEPAu (X = 0–3; PEPAu = AYSSGAPPMPPF) were synthesized and purified by New England Peptide. BP-Ax-PEPAu were synthesized and purified using established methods.[3a] Briefly, biphenyl N-Hydroxyl succinimide ester (1.69 × 10−6 mol) dissolved in dimethylformamide (DMF; 50 μL) was mixed with Ax-PEPAu (x=2, 8.797 × 10−7 mol; x=3, 7.874 × 10−7 mol) in DMF (70 μL). Triethylamine (1 μL) was added to this solution, and the resulting solution was stirred (24 h) at room temperature. Once completed, the reaction was diluted with a 1:1 mixture of water and acetonitrile (1000 μL). This solution was purified by reverse-phase HPLC using a linear gradient of 0.05% formic acid in CH3CN and 0.1% formic acid in water (Figure S1). The concentration of the purified peptide conjugates were determined using the molar extinction coefficient of tyrosine (1280 M−1cm−1) at 280 nm. Once HPLC purification was completed, the eluted product was lyophilized. A sample of the lyophilized product was deposited onto a α-cyano-4-hydroxy cinnamic acid (CHCA) matrix and its mass was determined using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Figure S2).
Preparation of Spherical Nanoparticle Superstructures
Lyophilized BP-A2-PEPAu (1.36 × 10−7 mol) or BP-A3-PEPAu (2.60 × 10−7 mol) peptide conjugate was completely dissolved in 0.1M HEPES buffer (0.5 ml; pH=7.3±0.1; Fisher Scientific) in a plastic vial. This solution was allowed to incubate at room temperature (30 min). Thereafter, a freshly prepared solution of 0.1M chloroauric acid (HAuCl4) in 1.0 M triethylammonium acetate (TEAA; pH = 7.0) buffer (2 μl) was added to the peptide conjugate solution. The resulting mixture was vortexed for a few seconds as soon as the HAuCl4 solution was added and then left undisturbed at room temperature (48 h).
Supplementary Material
Figure S1. Reverse-phase HPLC charts for product of the coupling reaction between A2- PEPAu (AAAYSSGAPPMPPF) or A3-PEPAu (AAAAYSSGAPPMPPF) with biphenyl N- hydroxyl-succinimide ester, respectively.
Note: BP-PEPAu and BP-A-PEPAu were prepared, purified and characterized in a similar fashion.
Figure S2. MALDI-TOF mass spectra of purified (a) BP-A2-PEPAu (Calcd. Mw. = 1544.2) and (b) BP-A3-PEPAu (Calcd. Mw. = 1615.2)
Figure S3. TEM images (a–b) of self-assembled BP-A2-PEPAu stained with 2% aqueous uranyl acetate. The samples used for these images were produced in the following way: 1) BP-A2-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4 solution was added to the BP-A2-PEPAu solution and the resulting mixture was allowed to incubate for 30 min. The diameter of the structures (c) ranged from ~60 nm to ~240 nm.
Figure S4. Additional TEM images of large spherical gold nanoparticle superstructures obtained 48 hrs (a–c) and 84 hrs (d–f) after adding the HAuCl4/TEAA solution to a BP-A2- PEPAu solution in HEPES. (g) Diameters of the superstructures ranged from ~60 nm to ~270 nm. (h) Size distribution of gold nanoparticles comprising the superstructures (6.71 ± 0.24 nm; based on 200 counts)
Figure S5. UV-Vis spectrum of the large spherical gold nanoparticle superstructures formed using BP-A2-PEPAu. The spectrum was collected in HEPES solution. The absorbance maximun is observed at 656 nm.
Figure S6. AFM height (a and c), phase (b), and 3-D (d) images of BP-A3-PEPAu self- assembled structures formed 3 days after incubation in HEPES buffer. (e) Height distribution of BP-A3-PEPAu structures (height = 2.87 ± 0.05 nm; based on 50 counts from AFM images).
Figure S7. TEM images (a–c) of BP-A3-PEPAu assemblies stained with 2% aqueous phosphotungstic acid. The samples used for these images were produced in the following way: 1) BP-A3-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4/TEAA solution was added to the BP-A3-PEPAu solution and the resulting mixture was allowed to incubate for (a) 5 min, (b) 10 min, (c) 6 hrs. Diameter distribution of spheres (a and b) and width distribution of fibers (c) were obtained from the TEM images: (a) 4.51 ± 0.06 nm, based on 100 counts; (b) 3.35 ± 0.05 nm, based on 120 counts; (c) 4.46 ± 0.03 nm, based on 150 counts.
Figure S8. TEM images of sub-50 nm spherical gold nanoparticle superstructures (a–d) 48 hrs after adding HAuCl4/TEAA solution to a BP-A3-PEPAu solution in HEPES. (e) Diameter distribution of superstructures (29.43 ± 0.34 nm; based on 115 counts). (f) Size distribution of gold nanoparticles comprising the superstructures (6.15 ± 0.14 nm; based on 180 counts).
Figure S9. UV-Vis spectrum of the sub-50 nm spherical gold nanoparticle superstructures formed using BP-A3-PEPAu. The spectrum was collected in HEPES solution. The absorbance maximun is observed at 540 nm.
Figure S10. TEM images of (a) spherical and (b–d) linear gold nanoparticle superstructures. The sample used for the image (a) was produced 20 hrs after adding a first aliquot of the HAuCl4/TEAA solution to a BP-A3-PEPAu solution in HEPES. This TEM grid was stained with 2% phosphotungstic acid. One can observe the sub-50 nm spherical nanoparticle assemblies as well as bare fibrillar structures in this TEM image. The samples used for the images (b–d) were produced in the following way: 1) BP-A3-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4/TEAA solution was added to the BP-A3-PEPAu solution and the resulting mixture was allowed to incubate for 6 hrs, and 3) a second aliquot of the HAuCl4/TEAA solution was added to the mixture and then allowed to incubate for (b) 16 hrs and (c–d) 20 hrs.
Footnotes
NLR acknowldedges the University of Pittsburgh and and the National Science Foundation (DMR-0954380, NLR) and the National Institutes of Health (GM085043, PZ) for funding this work. The authors also thank the Peterson NFCF and the MEMS Department for provision of access to AFM and TEM, respectively.
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
Contributor Information
Leekyoung Hwang, Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260 (USA).
Dr. Gongpu Zhao, Department of Structural Biology, University of Pittsburgh School of Medicine, 3501 Fifth Avenue, Pittsburgh, PA15260 (USA)
Prof. Peijun Zhang, Department of Structural Biology, University of Pittsburgh School of Medicine, 3501 Fifth Avenue, Pittsburgh, PA15260 (USA)
Prof. Nathaniel L. Rosi, Email: nrosi@pitt.edu, Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260 (USA)
References
- 1.Zelzer M, Ulijn RV. Chem Soc Rev. 2010;39:3351. doi: 10.1039/c0cs00035c. (and other reviews within this special issue) [DOI] [PubMed] [Google Scholar]
- 2.a) Chen CL, Rosi NL. Angew Chem. 2010;122:1968. doi: 10.1002/anie.200903572. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:1924. [Google Scholar]; b) Dickerson MB, Sandhage KH, Naik RR. Chem Rev. 2008;108:4935. doi: 10.1021/cr8002328. [DOI] [PubMed] [Google Scholar]
- 3.a) Chen CL, Zhang PJ, Rosi NL. J Am Chem Soc. 2008;130:13555. doi: 10.1021/ja805683r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen CL, Rosi NL. J Am Chem Soc. 2010;132:6902. doi: 10.1021/ja102000g. [DOI] [PubMed] [Google Scholar]; c) Song CY, Zhao GP, Zhang PJ, Rosi NL. J Am Chem Soc. 2010;132:14033. doi: 10.1021/ja106833g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hwang L, Chen CL, Rosi NL. Chem Commun. 2011;47:185. doi: 10.1039/c0cc02257h. [DOI] [PubMed] [Google Scholar]
- 4.a) Kotov NA, Stellacci F. Adv Mater. 2008;20:4221. [Google Scholar]; b) Nie ZH, Petukhova A, Kumacheva E. Nature Nanotech. 2010;5:15. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]; c) Lal S, Link S, Halas NJ. Nature Photon. 2007;1:641. [Google Scholar]
- 5.Slocik JM, Stone MO, Naik RR. Small. 2005;1:1048. doi: 10.1002/smll.200500172. [DOI] [PubMed] [Google Scholar]
- 6.a) Caruso F, Caruso RA, Mohwald H. Science. 1998;282:1111. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]; b) Boal AK, Ilhan F, DeRouchey JE, Thurn-Albrecht T, Russell TP, Rotello VM. Nature. 2000;404:746. doi: 10.1038/35008037. [DOI] [PubMed] [Google Scholar]; c) Wong MS, Cha JN, Choi KS, Deming TJ, Stucky GD. Nano Lett. 2002;2:583. [Google Scholar]; d) Park S, Lim JH, Chung SW, Mirkin CA. Science. 2004;303:348. doi: 10.1126/science.1093276. [DOI] [PubMed] [Google Scholar]; e) Liu B, Zeng HC. J Am Chem Soc. 2004;126:8124. doi: 10.1021/ja048195o. [DOI] [PubMed] [Google Scholar]; f) Liu B, Zeng HC. J Am Chem Soc. 2004;126:16744. doi: 10.1021/ja044825a. [DOI] [PubMed] [Google Scholar]; g) Rana RK, Murthy VS, Yu J, Wong MS. Adv Mater. 2005;17:1145. [Google Scholar]; h) Zubarev ER, Xu J, Sayyad A, Gibson JD. J Am Chem Soc. 2006;128:15098. doi: 10.1021/ja066708g. [DOI] [PubMed] [Google Scholar]; i) Hickey RJ, Haynes AS, Kikkawa JM, Park SJ. J Am Chem Soc. 2011;133:1517. doi: 10.1021/ja1090113. [DOI] [PubMed] [Google Scholar]; j) Chandra M, Dowgiallo AM, Knappenberger KL. J Am Chem Soc. 2010;132:15782. doi: 10.1021/ja106910x. [DOI] [PubMed] [Google Scholar]; k) Hentschel M, Saliba M, Vogelgesang R, Giessen H, Alivisatos AP, Liu N. Nano Lett. 2010;10:2721. doi: 10.1021/nl101938p. [DOI] [PubMed] [Google Scholar]; l) Vasquez Y, Sra AK, Schaak RE. J Am Chem Soc. 2005;127:12504. doi: 10.1021/ja054442s. [DOI] [PubMed] [Google Scholar]; m) Coppage R, Slocik JM, Sethi M, Pacardo DB, Naik RR, Knecht MR. Angew Chem. 2010;122:3855. doi: 10.1002/anie.200906949. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:3767. [Google Scholar]; n) Jin YD, Gao XH. J Am Chem Soc. 2009;131:17774. doi: 10.1021/ja9076765. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Wu GH, Milkhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. J Am Chem Soc. 2008;130:8175. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Note: HEPES serves as a reducing agent. Habib A, Tabata M, Wu YG. Bull Chem Soc Jpn. 2005;78:262.Xie JP, Lee JY, Wang DIC. Chem Mater. 2007;19:2823.
- 8.Shimizu T, Masuda M, Minamikawa H. Chem Rev. 2005;105:1401. doi: 10.1021/cr030072j. [DOI] [PubMed] [Google Scholar]
- 9.Link S, El-Sayed MA. J Phys Chem B. 1999;103:8410. [Google Scholar]
- 10.a) Euliss LE, Grancharov SG, O’Brien S, Deming TJ, Stucky GD, Murray CB, Held GA. Nano Lett. 2003;3:1489. [Google Scholar]; b) Kim BS, Taton TA. Langmuir. 2006;23:2198. doi: 10.1021/la062692w. [DOI] [PubMed] [Google Scholar]; c) Li Z, Sai H, Warren SC, Kamperman M, Arora H, Gruner SM, Wiesner U. Chem Mater. 2009;21:5578. doi: 10.1021/cm9020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voet D, Voet JG. Biochemistry. 2. John Wiley & Sons; New York: 1995. p. 179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Reverse-phase HPLC charts for product of the coupling reaction between A2- PEPAu (AAAYSSGAPPMPPF) or A3-PEPAu (AAAAYSSGAPPMPPF) with biphenyl N- hydroxyl-succinimide ester, respectively.
Note: BP-PEPAu and BP-A-PEPAu were prepared, purified and characterized in a similar fashion.
Figure S2. MALDI-TOF mass spectra of purified (a) BP-A2-PEPAu (Calcd. Mw. = 1544.2) and (b) BP-A3-PEPAu (Calcd. Mw. = 1615.2)
Figure S3. TEM images (a–b) of self-assembled BP-A2-PEPAu stained with 2% aqueous uranyl acetate. The samples used for these images were produced in the following way: 1) BP-A2-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4 solution was added to the BP-A2-PEPAu solution and the resulting mixture was allowed to incubate for 30 min. The diameter of the structures (c) ranged from ~60 nm to ~240 nm.
Figure S4. Additional TEM images of large spherical gold nanoparticle superstructures obtained 48 hrs (a–c) and 84 hrs (d–f) after adding the HAuCl4/TEAA solution to a BP-A2- PEPAu solution in HEPES. (g) Diameters of the superstructures ranged from ~60 nm to ~270 nm. (h) Size distribution of gold nanoparticles comprising the superstructures (6.71 ± 0.24 nm; based on 200 counts)
Figure S5. UV-Vis spectrum of the large spherical gold nanoparticle superstructures formed using BP-A2-PEPAu. The spectrum was collected in HEPES solution. The absorbance maximun is observed at 656 nm.
Figure S6. AFM height (a and c), phase (b), and 3-D (d) images of BP-A3-PEPAu self- assembled structures formed 3 days after incubation in HEPES buffer. (e) Height distribution of BP-A3-PEPAu structures (height = 2.87 ± 0.05 nm; based on 50 counts from AFM images).
Figure S7. TEM images (a–c) of BP-A3-PEPAu assemblies stained with 2% aqueous phosphotungstic acid. The samples used for these images were produced in the following way: 1) BP-A3-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4/TEAA solution was added to the BP-A3-PEPAu solution and the resulting mixture was allowed to incubate for (a) 5 min, (b) 10 min, (c) 6 hrs. Diameter distribution of spheres (a and b) and width distribution of fibers (c) were obtained from the TEM images: (a) 4.51 ± 0.06 nm, based on 100 counts; (b) 3.35 ± 0.05 nm, based on 120 counts; (c) 4.46 ± 0.03 nm, based on 150 counts.
Figure S8. TEM images of sub-50 nm spherical gold nanoparticle superstructures (a–d) 48 hrs after adding HAuCl4/TEAA solution to a BP-A3-PEPAu solution in HEPES. (e) Diameter distribution of superstructures (29.43 ± 0.34 nm; based on 115 counts). (f) Size distribution of gold nanoparticles comprising the superstructures (6.15 ± 0.14 nm; based on 180 counts).
Figure S9. UV-Vis spectrum of the sub-50 nm spherical gold nanoparticle superstructures formed using BP-A3-PEPAu. The spectrum was collected in HEPES solution. The absorbance maximun is observed at 540 nm.
Figure S10. TEM images of (a) spherical and (b–d) linear gold nanoparticle superstructures. The sample used for the image (a) was produced 20 hrs after adding a first aliquot of the HAuCl4/TEAA solution to a BP-A3-PEPAu solution in HEPES. This TEM grid was stained with 2% phosphotungstic acid. One can observe the sub-50 nm spherical nanoparticle assemblies as well as bare fibrillar structures in this TEM image. The samples used for the images (b–d) were produced in the following way: 1) BP-A3-PEPAu was incubated for 30 min in HEPES buffer and 2) HAuCl4/TEAA solution was added to the BP-A3-PEPAu solution and the resulting mixture was allowed to incubate for 6 hrs, and 3) a second aliquot of the HAuCl4/TEAA solution was added to the mixture and then allowed to incubate for (b) 16 hrs and (c–d) 20 hrs.