Abstract
Fibrosis is a hallmark histologic event of chronic liver diseases and is characterized by the excessive accumulation and reorganization of the extracellular matrix (ECM). The gold standard for assessment of fibrosis is liver biopsy. As this procedure has various limitations, including risk of patient injury and sampling error, a non-invasive serum marker for liver fibrosis is desirable. The increasing understanding of the pathogenesis of hepatic fibrosis has suggested several markers which could be useful indicators of hepatic fibrogenesis and fibrosis. These markers include serum markers of liver function, ECM synthesis, fibrolytic processes, ECM degradation and fibrogenesis related cytokines. Recently, neo-epitopes, which are post-translational modifications of proteins, have been successfully used in bone and cartilage diseases which are characterized by extensive ECM remodeling. Increasing numbers of studies are being undertaken to identify neo-epitopes generated during liver fibrosis, and which ultimately might be useful for diagnosing and monitoring fibrogenesis. To date, the metalloproteinases generated fragment of collagen I, III, IV and VI have been proven to be elevated in two rat models of fibrosis. This review summarizes the recent efforts that have been made to identify potentially reliable non-invasive serum markers. We used the recently proposed BIPED (Burden of disease, Investigative, Prognostic, Efficacy and Diagnostic) system to characterize potential serum markers and neo-epitope markers that have been identified to date.
Keywords: serum marker, liver fibrosis, extracellular matrix, neo-epitope
Introduction
Chronic liver diseases are major global health problems causing approximately 800,000 deaths per year worldwide.1,2 Liver fibrosis is the common pathologic process of all chronic liver diseases, regardless of the cause, which results from excessive accumulation of extracellular matrix.3,4 Liver fibrosis may progress to cirrhosis and eventually death. However, increasing evidence suggests that even advanced fibrosis is reversible,5 although end-stage cirrhosis is irreversible and affected patients can only survive with a liver transplant. Estimating the current degree of fibrosis is crucial for determining whether the fibrosis could be reversed with treatment.
Liver fibrosis evaluation methods can be divided into those that are invasive and those that are non-invasive.6 Liver biopsy is an invasive method that has long been regarded as the ‘gold standard’ for staging liver fibrosis. Biopsy allows physicians to obtain diagnostic information not only on fibrosis, but also on many other liver-injuring processes, such as inflammation, necrosis, steatosis and hepatic deposits of iron or copper.7 However, several issues prevent the routine use of liver biopsy as a clinical tool, including risk of injury to the patient, variable accessibility of the damaged section of the liver, high cost, sampling errors and inaccuracy due to inter- and intra-observer variability of pathologic interpretations.8
Non-invasive methods include serum and genetic tests, and imaging techniques. In recent years, interest in identifying and describing liver fibrosis using molecular serum markers has been on the rise. Serum markers offer a cost effective alternative to liver biopsy for both patients and clinicians. In addition to being less invasive, there is a low risk of sampling error and small observer-related variability. Moreover, measurements may be performed repeatedly over time, allowing for ongoing monitoring of fibrosis.9 However, there are many limitations for serum biomarkers. They are not liver-specific and have a tendency to be more elevated in the presence of inflammation. In addition, serum marker readings may be falsely high due to their low clearance rates, which are influenced by dysfunction of endothelial cells, impaired biliary excretion or renal function. Until now, most serum biomarkers have only been used as investigative, rather than diagnostic, parameters in the clinic.10 This review describes major molecular serum markers of liver fibrosis and their limitations.
Classifications of Serum Markers
No biomarkers are currently available to replace liver biopsy in the evaluation of liver fibrosis. One possible reason is an imprecise and confusing classification of potential biomarkers. Most commonly, fibrosis biomarkers are classified in one of two classes. Class I fibrosis markers are direct serum markers, which are molecules derived from ECM turnover reflecting the activity of the fibrotic process, and are thought to indicate the extent of connective tissue deposition. Class II biomarkers are indirect serum fibrosis biomarkers that have been used in clinical practice, and have been identified from retrospective studies. They are calculated by mathematical algorithms, and do not necessarily reflect ECM turnover and/or fibrogenic cell changes.11 A disadvantage of this second classification is that it does not provide information about the potential clinical use of the individual serum biomarkers that were used in the algorithm, nor does it go far enough in terms of recognizing, differentiating and understanding them.12
Hepatic Fibrogenesis and its Molecular Serum Markers
Mechanisms of hepatic fibrogenesis
Liver fibrosis is the final common stage of most chronic liver diseases, which is triggered by chronic liver injury and develops from a series of events including apoptosis or necrosis, inflammation, tissue remodeling and repair processes. Hepatocytes are the most abundant cells in liver. Their apoptosis is prominent in liver injury and can be identified in virtually all forms of liver injury.13–19 Some proteins are released from damaged hepatocytes and their levels in serum can reflect liver function. After liver injury, the repair process occurs, which can take either of two distinct paths: a regenerative path, in which injured cells are replaced by the same type of cells; or a path known as fibroplasias or fibrosis, in which connective tissue replaces normal parenchymal tissue in an uncontrolled fashion. Repeated injury and uncontrolled repair processes result in substantial deposition of extracellular matrix (ECM) components in which normal tissue is replaced by scar tissue.20–23
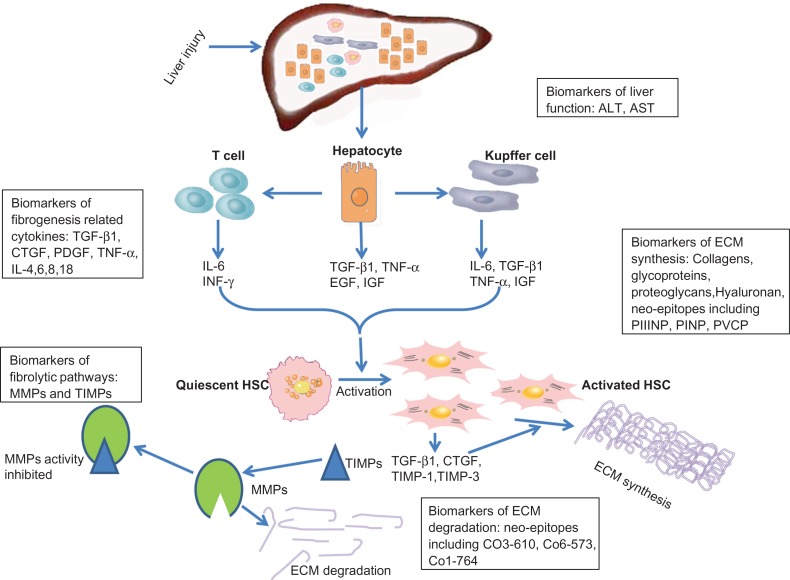
Basic research has explored the mechanisms of hepatic fibrogenesis, as shown in Figure 1. Hepatic stellate cells (HSCs) are the key fibrogenic cells and their ‘activation’ is the dominant event in fibrogenesis. Activation of HSCs refers to the conversion of quiescent, vitamin A-storing cells into proliferative, fibrogenic and contractile myofibroblasts which can synthesize and secrete large amounts of fibril-forming collagens, particularly collagen type I and III.5,24 The activation of HSCs is a complex but tightly programmed response to liver injury. The earliest changes in HSCs reflect paracrine stimulation by all neighboring cells, including Kupffer cells, hepatocytes and leukocytes, while autocrine cytokines (including transforming growth factor β (TGF-β) and connective tissue growth factor (CTGF)) play vital roles in regulating and maintaining their activation.25,26 Damaged hepatocytes release cytokines (TGF-β, tumor necrosis factor α (TNF-α), epidermal growth factor (EGF) and insulin-like growth factor (IGF)) responsible for the activation of Kupffer cells and the recruitment of activated T-cells. Activated Kupffer cells, T-cells and damaged hepatocytes also release the inflammatory cytokines (TNF-α, interferon γ (INF-γ), IL-6), free radicals and growth factors (platelet-derived growth factor (PDGF), CTGF) which further promote HSC activation and proliferation. Since the cytokines are closely related with fibrogenesis, they possibly could be used as biomarkers for liver fibrosis.
Figure 1.
Mechanisms of hepatic fibrogenesis and possible molecular serum biomarkers. Some molecular serum biomarkers may reflect the pathogenesis of liver fibrosis: neo-epitopes, are related to basement membrane degradation; pro-collagen, is related to extracellular matrix (ECM) synthesis; MMPs and TIMPs are relate to ECM fibrolytic processes; ALT and AST are related to liver function and injury; other serum markers are fibrogenesis-related cytokines.
Liver fibrosis is characterized by excessive ECM accumulation which results from both increased synthesis and decreased degradation of ECM. Initiating events in stellate cell activation occur in the background while progressive changes are taking place in the surrounding ECM within the sub-endothelial space of Disse. Over time, the sub-endothelial matrix composition changes from one comprised of type IV collagen and laminin to one rich in fibril-forming collagens, especially collagen type I and III.4 The pro-peptide or the mature protein of collagen types I and III can be used as biomarkers for liver fibrosis. There are two kinds of ECM degradation during hepatic fibrogenesis. One involves the basement membrane (comprised of collagen type IV) and is called pathologic matrix degradation.5 Thus, the protein fragment (a neo-epitope) of matrix degradation could be used as a biomarker for liver fibrosis. The other type involves excess fibril-forming collagen and is called restorative matrix degradation.5 In the extracellular space, matrix degradation occurs as a consequence of the action of a family of enzymes called matrix metalloproteinases (MMPs). The active forms of these MMPs can be inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs), which are important regulatory molecules in tissue remodeling and repair and act by binding with MMPs. Through this combination of mechanisms, extracellular matrix degradation is closely regulated.27 Activated HSCs also produce MMP-2, MMP-9 and MMP-3, which disrupt the basement membrane, allowing inflammatory cells to be easily recruited to the site of injury.23,28–32 The levels of MMPs and TIMPs are closely related to liver fibrosis and could possibly be used as biomarkers for liver fibrosis.
These events of pathogenesis of hepatic fibrosis have indicated some potential serum markers of hepatic fibrogenesis and fibrosis (Fig. 1 and Table 2).
Table 2.
Molecular serum markers of liver fibrosis.
| Marker | Function | References | BIPED |
|---|---|---|---|
| Liver function | |||
| ALT | Metabolic enzymes in the liver | 33–36 | I |
| AST | Metabolic enzymes in the liver | 37,38 | I |
| ECM formation | |||
| PIIINP | Propeptide of collagen type III | 46–48,50,55,57 | I,D |
| PINP | Propeptide of collagen type I | 62 | I |
| Type IV collagen | Basement membrane formation | 58–60 | I,D |
| P4NP 7S | N-terminal pro-peptides of type IV collagen 7S domain | 61 | I |
| PVCP | Propeptide of collagen type V | 64 | I |
| HA | Component of ECM | 66–68 | I,D |
| YKL-40 | Glycoprotein involved in ECM turnover | 72–75 | I,D |
| MFAP | Glycoprotein involved in ECM turnover | 76 | I,D |
| Fibrolytic process | Neo-epitope | ||
| MMP-1/MMP-13 | Degrade fibrotic matrix | 77,78 | I |
| MMP-2 | Degrades basal membranes and fibrotic matrix | 80 | I |
| MMP-9 | Degrades basal membranes | 77 | I |
| TIMP-1 | Inhibits MMP-1 activity | 77,78,81 | I |
| ECM degradation | |||
| CO3-610 | Collagen type III fragment generated by MMP-9 | 85–87 | I |
| CO6-MMP | Collagen type VI fragment generated by MMP-2,9 | 89 | I |
| CO1-764 | Collagen type I fragment generated by MMP-2,9,13 | 90 | I |
| C4M | Collagen type IV fragment generated by MMP-9, | 88 | I |
| Cytokines | |||
| TGF-β | Growth factor stimulates production of ECM by HSC | 92–94 | I |
| CTGF | Potent pro-fibrogenic factor | 101,103 | I |
| PDGF | Growth factor stimulates proliferation of HSC | 98,99 | I |
| TNF-α | Inflammatory cytokine involved in fibrogenesis | 95,96 | I |
| IL-4,6,8,18 | Inflammatory cytokine involved in fibrogenesis | 95,96 | I |
Potential molecular serum markers of liver fibrosis
Serum markers of liver function
Since fibrosis is the result of liver injury, serum markers of liver fibrosis could indicate the degree of liver damage and function. These markers are easy to measure as they are based on routine laboratory tests conducted in a hospital. Serum alanine aminotransferases (ALT) are released from liver tissue into the circulation in proportion to the degree of hepatocellular damage,33 and their level is thought to be one of the most sensitive markers of liver injury and liver disease progression.34–36 Serum aspartate aminotransferases (AST) levels are even more important predictors of histological activity than ALT,37,38 and the ratio of AST/ALT > 1 (AAR) has been proposed as a test of cirrhosis.39,40 However, the diagnostic accuracy of this ratio is highly variable among different studies.41–43
Serum markers of ECM synthesis
Liver fibrosis is associated with major alterations in both quantity and composition of ECM.44 In advanced stages, the liver contains approximately 6 times more ECM components than normal, including collagen type I, III, and IV, fibronectin, undulin, elastin, laminin, hyaluronan, and proteoglycans.1 Therefore, some parameters related to elevated ECM synthesis could be used as markers of liver fibrosis.
Collagens are synthesized by HSCs as precursor molecules with large pro-peptide extensions at both the N- and C-terminal ends.45 The mature pro-peptide are cleaved from pro-collagen by N- and C-terminal proteinases, and the mature collagen is then integrated into the ECM. Both the pro-collagen and the pro-peptide reflect the synthesis of ECM. The N-terminal pro-peptide of collagen type III (PIIINP) is the most widely studied marker of liver fibrosis.46,47 It is useful to detect cirrhosis with a sensitivity of about 94% and specificity of about 81%, which can be increased up to 93% if combined with additional serum markers.48 PIIINP has achieved a limited clinical application, but not widespread acceptance.49 In chronic hepatitis C (CHC) patients, PIIINP levels have not been shown to correlate with the degree of fibrosis but do correlate with scores for necrosis.50,51 PIIINP levels are known to be elevated in acute and chronic active hepatitis and correlate with aminotransferase levels.52,53 However PIIINP is not specific for liver fibrosis as its levels are also elevated in lung fibrosis, chronic pancreatitis and rheumatoid arthritis (RA).54–57 Therefore PIIINP is more likely a marker of inflammation than of fibrosis.
Type IV collagen is regarded as a putative marker of basement membrane formation and sinusoids capillarization, which are important pathological processes in fibrosis disease. The serum levels of type IV collagen can be used for predicting the state of liver fibrosis,58,59 and they are also increased in alcoholic liver diseases and in hepatocellular carcinoma.60 P4NP 7S, the N-terminal pro-peptide of type IV collagen 7S domain, is significantly elevated in rats with liver fibrosis detected by histology in the bile duct ligation (BDL) and carbon tetrachloride (CCl4)-induced liver fibrosis models, and is correlated with increased hepatic type IV collagen expression in BDL rats.61 The N-terminal pro-peptide of collagen type I (PINP) has been shown to be associated with the development of liver fibrosis, but not bone formation, in adult rats subjected to BDL.62 Thus, PINP may be useful in studying the pathogenesis of liver fibrosis. However, caution should be applied when interpreting PINP levels in other disease states such as inflammation.63
Increased serum levels of PVCP-1230, the pro-peptide of collagen type V, have been demonstrated to be associated with the extent of collagen deposition in two different models of fibrotic processes in the liver. The data indicate that formation of type V collagen may be of value as a disease-specific diagnostic biomarker that reflects the total burden of liver disease.64
Hyaluronan (HA) is a glycosaminoglycan synthesized by HSCs and it is a component of the ECM.65 High levels of HA in serum may reflect increased synthesis of ECM by HSCs, and it appears to be the best individual test that reflects ECM concentration.66–68 Since the negative value of HA in serum at a cut-off value of 60 μg/ml is much higher (98%–100%) than the positive value (61%), it can be used to exclude advanced fibrosis and cirrhosis.54,69 However, HA levels may be elevated from non-hepatic sources such as chronic inflammatory processes, as in rheumatoid arthritis, and after meal or glucose drink.70,71
YKL-40 is a 39-kilodalton glycoprotein that is involved in remodeling of the ECM.72 It is claimed that the serum level of YKL-40 is closely related to the degree of liver fibrosis.73 YKL-40 has been tested in HCV-patients showing a sensitivity and specificity of around 80% and an AUROC of 0,81 for fibrosis.74 In those with alcoholic liver disease, a specificity of 88% and sensitivity of 51% have been reported.72 A study of YKL-40 in alcoholic liver disease has suggested that it could function as a marker of clinical outcomes.46 The limitations of YKL-40 persist largely due to its ubiquitous presence and therefore it cannot be considered a liver-specific marker.75
Microfibrillar-associated protein 4 (MFAP-4) is a ubiquitous protein which is a ligand for integrins and plays a potential role in ECM turnover during fibrogenesis. The serum levels of MFAP-4 were detected in a large number of patients including 139 patients with different hepatic fibrosis stages on HCV infection. The results showed that MFAP-4 could be a novel candidate biomarker due to its high accuracy in distinguishing healthy versus cirrhotic livers (AUC = 0.97, P < 0.0001) as well as stage 0 versus stage 4 fibrosis (AUC= 0.84, P < 0.0001), and stages 0 to 3 versus stage 4 fibrosis (AUC = 0.76, P < 0.0001).76 However, as with YKL-40, ubiquitous presence of MFAP-4 excludes its possible use as a liver-specific marker, unless changes in other related diseases are investigated and excluded.
Serum markers of fibrolytic processes
In the fibrotic liver there is a net deposition of fibrillar matrix, predominantly of collagen type I and III. Interstitial collagenases (MMP-1 in human and MMP-13 in rat) are the main enzymes which degrade collagen types I and III through cleaving the α-chain at a specific Gly-Ile/Leu site. Circulating MMP-1 concentrations are significantly reduced, while TIMP-1 levels are higher, as fibrosis grades increase in hepatitis C in humans.77 However, a study performed on a rat fibrosis model showed that the level of MMP-13 did not change but remained at a constant level throughout the fibrosis regression phase, while the level of TIMP-1 decreased rapidly and significantly, indicating that TIMP-1 reduction is associated with apoptosis of active HSCs.78
In the early stage of fibrosis, MMPs can degrade normal basal membranes and this may contribute to the pathogenesis of liver fibrosis.27 The two most relevant MMPs are gelatinase A (MMP-2) and gelatinase B (MMP-9). MMP-2 is secreted by activated HSCs, and MMP-9 is mainly secreted by activated Kupffer cells. In the progression of liver fibrosis, MMP-2 is also involved in the degradation of fibrotic matrix.79 Both MMP-2 and MMP-9 are correlated with fibrosis,77 but some studies examining the correlation of MMP-2 with chronic HCV have yielded contradictory results.80 The study by Boeker and co-workers81 shows that TIMP-1 and MMP-2 levels are accurate in detecting cirrhosis in patients with HCV (sensitivity of TIMP-1 levels, 100%; specificity, 56%–75%; AUC for MMP-2 levels, 0.97). However they are not capable of differentiating between mild and moderate fibrosis (AUC of 0.71 for TIMP-1 and 0.59 for MMP-2), therefore their clinical utility has been demonstrated only in advanced stages of liver fibrosis.
Serum markers of ECM degradation (neo-epitopes)
ECM degradation mediated by MMPs can occur at different stages of fibrosis. In the early stage of liver fibrosis the degradation of basal membranes occurs, while the degradation of fibrotic matrix characterizes the progression of the disease.27 The products of degradation of the ECM, the so-called neo-epitopes, may reflect different stages of the fibrosis and thus may be used as markers.
Neo-epitopes are post-translational modifications (PTMs) of proteins generated by protease cleavage, citrullination, nitrosylation, glycosylation and isomerization. Each modification results from a specific local physiological or pathobiological process.82 A range of protease-generated neo-epitopes has already been described in the literature, but they have not yet been used in applied science to develop quantifiable methods of disease assessment. In the context of bone and cartilage diseases, neo-epitopes of collagen types I and II as well as aggrecan have been well described.83,84 Preliminary neo-epitopes generated during the process of liver fibrogenesis have been investigated, and have been proved to be elevated in CCl4-rats and BDL-rats.85–89 The levels of the MMP-9 generated fragment of collagen type III, CO3-610, have been shown to correlate with the degree of liver fibrosis in rats during the progression phase of fibrosis, but not with the levels of total collagen during regression, indicating CO3-610 is a potential marker of progression rather than regression. The steep elevation of CO3-610 levels appeared as early as 4 weeks after initiating treatment with CCl4 in the rat model of liver fibrosis, following a progressive increase in total collagen and collagen type III levels.87 In addition, raised CO3-610 levels closely reflect portal hypertension in experimental liver fibrosis in rats.86 These findings underline the potential prognostic capacity of this novel marker for liver fibrosis.85,87 CO6-MMP, a collagen type VI fragment generated by MMP-2 and -9, was demonstrated to be elevated in both BDL and CCl4 rat models.89 A specific peptide sequence, 1438’GTPSVDHGFL’1447 (CO4-MMP), in the α 1 chain of type IV collagen generated by MMP-9, was significantly increased in the serum of all BDL rats compared with baseline, with a maximum increase of 248% seen two weeks after BDL.88 In the CCl4 model, levels of CO4-MMP were significantly elevated at weeks 12, 16 and 20 compared to baseline levels, with a maximum increase of 88% after 20 weeks. CO4-MMP levels correlated to Sirius red staining results of CCl4 induced liver fibrosis.88 CO1-764, a type I collagen fragment generated by MMP-2, -9 and -13 cleavage, was elevated in liver fibrosis but not in patients with prostate-, lung- or breast cancer with skeletal metastases, and appears not to be derived from bone breakdown.90 These data further indicate the high potential for the use of neo-epitope biomarkers specific for liver fibrosis.
Since many ECM components, as well as enzymes responsible for remodeling, are present in different tissues, further identification of liver- and fibrosis-specific neo-epitopes is needed for their optimal application in liver fibrosis monitoring, assessment and in the characterization of the pathogenesis. The proteolytic action of MMPs results in the generation of specific cleavage fragments, and different MMPs have different functions at different stages of fibrosis. Therefore, the combination of MMPs and specific cleavage products of the ECM could enhance the sensitivity and specificity of neo-epitopes82 and produce a more specific indication of the specific stage of liver fibrosis. The combination of serum markers of ECM synthesis and degradation, such as CO3-610 and PIIINP, CO4-MMP and PIVCP, PINP and CO1-764, can also be used to investigate the pathogenesis of different stages.
Serum markers of fibrogenesis-related cytokines
Unregulated cytokine synthesis and release contribute to the initiation, progression and maintenance of fibrosis.91 Some cytokines thought to mediate hepatic fibrogenesis have been studied as potential markers of fibrosis. However, only a few studies have addressed the diagnostic accuracy of fibrosis-associated cytokines, and showed that they are less valuable markers than ECM components.
TGF-β is the major stimulus for HSCs to synthesize ECM. TGF-β concentrations in plasma are elevated in, and correlate with the severity of, liver disease and were suggested as non-invasive biomarkers of fibrosis. TGF-β has been shown to correlate well with the presence of liver fibrosis in patients with alcoholic liver disease (ALD) and HCV.92 However, its release is injury-dependent and correlates with ALT and AST.93,94 Therefore it was suggested as a more appropriate marker of necrosis rather than fibrogenesis. TNF-α and its induction of IL-6, -8 and -18 are associated with alcoholic fibrosis and they may also predict clinical outcome.95 TNF-α and IL-4 levels correlated more closely in chronic hepatitis B patients than in the controls.96 PDGF is a potent fibrogenic growth factor known to synergize with TGF-β.97 PDGF, mainly produced by Kupffer cells, is the predominant mitogen inducing migration and proliferation of mesenchymal cells including HSCs to the site of injury.98 Serum levels of PDGF have shown high potential as markers for fibrosis progression.99
CTGF is another important fibrogenic factor which is synthesized by both HSCs and hepatocytes and is strongly dependent on TGF-β presence.100,101 CTGF is a general mediator of fibre-fibre, fibre-matrix, as well as matrix-matrix interaction. It is proposed as a fibrogenic ‘master switch’ in the epithelial-to-mesenchymal transition which plays a key role in the increase of ECM-producing fibroblasts during liver fibrosis.101 A recent preliminary study reported not only a significant elevation in circulating CTGF in patients with fibrosis, but also a correlation with fibrogenesis. This study showed CTGF levels decreased in fully developed, end-stage cirrhosis, in which the process of fibrogenesis is almost terminated. Thus, CTGF has been suggested as a valuable biomarker of active fibrogenesis. Serum CTGF is also suitable for determining hepatic fibrosis and it is a powerful marker in patients with chronic HCV infection.102,103
Serum Marker Model of Liver Fibrosis
Currently, no efficient and accurate markers of fibrosis diagnosis, staging and prognosis exist. Numerous attempts have been made to identify non-invasive markers that are capable of providing accurate information about fibrogenesis and the extent of fibrosis in the liver, and others have examined the combination of several parameters indicating fibrogenesis. The most widely known combined parameters are discussed below.
AST/ALT
AST and ALT are hepatic enzymes elevated in the blood before the clinical signs and symptoms of liver diseases appear. The ratio of these two enzymes was first mentioned by Naiki M in the study of Tyzzer’s disease,104 and has been found useful in evaluation of chronic viral hepatitis.35 The ratio of AST to ALT tends to increase with advancing stages of fibrosis from approximately 0.8 in healthy subjects. The greatest value of this ratio is that it is suitable for the non-invasive diagnosis of cirrhosis, where a ratio of more than 1 suggests the presence of cirrhosis.105,106 However, the AST/ALT ratio is confounded when used in alcoholic and many other acute and chronic fatty infiltrating liver diseases.107 For this reason, the ratio is not recommended for evaluating the stage of fibrosis.
APRI
The AST-to-platelet ratio index (APRI) is calculated as (AST/upper limit of normal range)/platelet count (109/L) × 100. This index was first put forward by Wai and co-workers108 using to identify CHC patients with significant fibrosis and cirrhosis with a high degree of accuracy. Application of this index may decrease the need for staging liver biopsy specimens among CHC patients. Several later studies in CHC showed the major strength of the APRI is that it is able to exclude significant HCV-related fibrosis,109 but a recent large meta-analysis suggested that APRI can identify hepatitis C-related fibrosis with a moderate degree of accuracy.110
Fibrotest
The fibrotest is a composite of five serum biochemical markers (alpha-2-macroglobulin, apolipoprotein A1, haptoglobin, γ-glutamyl transpeptidase, and bilirubin) associated with hepatic fibrosis developed by Posynard and co-workers.111 It generates a score that is correlated with the degree of liver damage in people with a variety of liver diseases. Due to the variability of components of assays and analyzers, fibrotest assays can only be performed in validated laboratories.112 The test has been validated by multiple groups in several liver diseases, including chronic hepatitis.113–116 Although preliminary results are encouraging, frequently cited limitations include the assay cost, failed external validation, difficulty in differentiating intermediate fibrosis stages, and the inability to exclude other conditions such as steatosis.117
Fibrometer
Fibrometer is an algorithm combining a number of parameters including number of platelets, pro-thrombin time, AST, α2-macroglobulin, hyaluronate, urea and age.118 The performance of Fibrometer for the detection of late fibrosis was compared to five other well described algorithms in 180 hepatitis C patients.119 The overall diagnostic scores were evaluated by the AUROCs ranging from 0.86 for Fibrometer to 0.78 for Forns’ score (not significant) for the discrimination of F0F1 from F2F3F4. For the discrimination of F0F1F2 from F3F4, the AUROC’s ranged from 0.91 for Fibrometer to 0.78 for Forns’ score. Furthermore, extensive fibrosis was predicted in 10%–86% of patients.
ELF
The Enhanced Liver Fibrosis (ELF) algorithm includes hyaluronic acid, the N-terminal pro-peptide of type III collagen, and tissue inhibitors of matrix metalloproteinase. The algorithm detected fibrosis (sensitivity, 90%) and accurately detected the absence of fibrosis (negative predictive value for significant fibrosis, 92%; AUROC, 0.804). Performance was excellent for alcoholic liver disease and nonalcoholic fatty liver disease.47 In a study of patients with primary biliary cirrhosis, the event-free survival was significantly lower in those with a high baseline ELF than those with a low baseline. Each 1-point increase in ELF was associated with a 3-fold increase in future complications. The researchers concluded that the ELF algorithm is a highly accurate non-invasive measure of the severity of primary biliary cirrhosis which also provides useful long-term prognostic information.120,121 In pediatric patients with non-alcoholic fatty liver disease (NAFLD), the ELF test predicted liver fibrosis stage with a high degree of sensitivity and specificity (AUROC of 0.92 for fibrosis stage 1, 0.98 for stage 2, and 0.99 for stage 3, respectively); results were superior to those reported for adults.122 The key components of the ELF algorithm are expressed during early stages of collagen deposition in the liver, and this is most likely the reason why ELF retains its prognostic ability even in early stages of the disease process (AUROC 0.737–0.863 at all time points). However, because the test uses direct markers of fibrogenesis (HA and TIMP-1), the results will be unreliable in patients with chronic diseases characterized by fibrogenesis in organs other than the liver.123
BIPED Classification
The Burden of disease, Investigative, Prognostic, Efficacy of intervention and Diagnostic (BIPED) classification (Table 1) has been recently proposed to assess biomarkers used in clinical trials and in research and development.124 Classification by the BIPED system may enable biomarker researchers working in different disease areas to communicate and compare results in a robust framework. This should lead to consistency in the assessment of sensitivity and specificity of different biomarkers.
Table 1.
The BIPED classification.
| Burden of disease (B) | Burden-of-disease markers assess the severity or extent of disease, typically at a single point in time, among individuals with a certain disease. |
| Investigative (I) | An investigative marker lacks sufficient information to allow its inclusion in one of the existing biomarker categories. |
| Prognostic (P) | The key feature of a prognostic marker is the ability to predict the future onset of a disease. |
| Efficacy of intervention (E) | Information about the efficacy of treatment among persons with a certain disease or those at high risk for its development. |
| Diagnostic (D) | Diagnostic markers are defined by the ability to classify individuals as either having or not having a disease. |
Extracted from Bauer et al.124
In Table 2, the serum markers described in this review are classified following the BIPED methods.
Discussion
At present, the most studied biomarkers for liver fibrosis are represented by the products of extracellular matrix synthesis and degradation, and by the enzymes that regulate their production or modification, such as hyaluronic acid, matrix metalloproteinases, their inhibitors TIMPs and cytokines such as TGF-β.
Some of the biomarkers discussed in this paper reflect fibrosis progression and others fibrosis regression, and so could evaluate different stages of liver fibrosis. Nevertheless none of these molecules completely fulfill the requirements of an ideal biomarker, mainly because of their lack of sensitivity in identifying patients with a mild degree of fibrosis but who are at risk of progression. Moreover these markers are often not specific, because they can be detected in organs other than liver and can be affected by other pathological conditions, such as renal or liver failure.125
Liver biopsy is currently the ‘gold standard’ for assessing liver disease and fibrosis. However, it is invasive, has the potential to cause side-effects, it is subject to sample variations, and is seldom a first choice in the clinic. Use of this test could be reduced with a combination of routine laboratory tests and fibrosis biomarkers capable of accurately detecting the presence of cirrhosis. However most markers identified so far have been evaluated in only small cohorts of patients. Some assays for measuring the markers have included some subjective variables or laboratory tests that are costly and not readily available, and very few molecules have been satisfactorily validated. Therefore, neither single biomarker nor parameter can at present substitute liver biopsy.
In the clinic, most patients with chronic liver disease will progress to cirrhosis if they are not treated correctly and at the appropriate time. Cirrhosis is the end-stage of chronic liver diseases, causing increased morbidity and complications such as portal hypertension, development of esophageal varices, ascites, encephalopathy, variceal hemorrhage and hepatocellular carcinoma,5,23 and ultimately death, which only can be avoided by liver transplantation.126
Routine clinical tests and inspections, such as liver function evaluation, coagulation index, blood cell counts, abdominal ultrasound and Fibroscan, are normally used to monitor and assess the state of chronic liver diseases. However, a most helpful diagnostic tool would be the one that enables the clinician to identify patients who are at risk of developing cirrhosis and cirrhotic decompensation, and need immediate treatment. The ideal tool would be a biomarker that can discriminate between the presence of mild disease, active fibrogenesis, advanced fibrosis (cirrhosis) and decompensation. Tests for the biomarker measurement should be non-invasive, easy to administer, and used repeatedly with little intra- or inter-user variation from diagnosis throughout the progression of chronic liver diseases. The protein fingerprint technology,127 based on measurement of neo epitopes, which are special pathologically generated fragments of proteins released during extracellular remodeling, may assist in isolating the ideal biomarker, which may be used either alone or in combination with other validated biochemical markers.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: TL, XW. Contributed to the writing of the manuscript: FG, DJL. Jointly developed the structure and arguments for the paper: MAK, TL, XW, FG. Made critical revisions and approved final version: FG, MAK, DJL. All authors reviewed and approved the final manuscript.
Funding
The authors received funding from the Danish Research Foundation for this work.
Competing Interests
MAK, DJL and FG are full-time employees at Nordic Bioscience. All other authors have no competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005 Feb;115(2):209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brundtland GH. Reducing risks to health, promoting healthy life. JAMA. 2002 Oct 23;288(16):1974. doi: 10.1001/jama.288.16.1974. From the World Health Organization. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000 Jan 28;275(4):2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008 May;134(6):1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad W, Ijaz B, Gull S, et al. A brief review on molecular, genetic and imaging techniques for HCV fibrosis evaluation. Virol J. 2011;8(1):53. doi: 10.1186/1743-422X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006 Jun 21;12(23):3682–94. doi: 10.3748/wjg.v12.i23.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi E, Adams LA, Bulsara M, Jeffrey GP. Assessing liver fibrosis with serum marker models. Clin Biochem Rev. 2007 Feb;28(1):3–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou K, Lu LG. Assessment of fibrosis in chronic liver diseases. J Dig Dis. 2009 Feb;10(1):7–14. doi: 10.1111/j.1751-2980.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis. 2006 Jun;15(2):149–59. [PubMed] [Google Scholar]
- 11.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J Cell Mol Med. 2007 Sep;11(5):1031–51. doi: 10.1111/j.1582-4934.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veidal SS, Bay-Jensen AC, Tougas G, Karsdal MA, Vainer B. Serum markers of liver fibrosis: combining the BIPED classification and the neo-epitope approach in the development of new biomarkers. Dis Markers. 2010;28(1):15–28. doi: 10.3233/DMA-2010-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003 Aug;125(2):437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 14.Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia reperfusion injury in the rat liver. Transplantation. 1999 Apr 27;67(8):1099–105. doi: 10.1097/00007890-199904270-00003. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Selzner M, Valentino KL, et al. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase dependent mechanism. Transplantation. 1999 Jul 15;68(1):89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- 16.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001 Feb;34(2):248–53. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 17.Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN 6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003 Mar;9(3):278–84. doi: 10.1053/jlts.2003.50019. [DOI] [PubMed] [Google Scholar]
- 18.Papakyriakou P, Tzardi M, Valatas V, et al. Apoptosis and apoptosis related proteins in chronic viral liver disease. Apoptosis. 2002 Apr;7(2):133–41. doi: 10.1023/a:1014472430976. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro PS, Cortez Pinto H, Sola S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004 Sep;99(9):1708–17. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004 Dec;1(2):98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 21.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002 May;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007 Mar;117(3):524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008 Jan;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001 Aug;21(3):351–72. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19(2):129–40. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 26.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001 Aug;21(3):397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 27.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000 Aug;279(2):G245–9. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 28.Arthur MJ, Stanley A, Iredale JP, Rafferty JA, Hembry RM, Friedman SL. Secretion of 72 kDa type IV collagenase/gelatinase by cultured human lipocytes. Analysis of gene expression, protein synthesis and proteinase activity. Biochem J. 1992 Nov 1;287(Pt 3):701–7. doi: 10.1042/bj2870701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Han YP, Yan C, Zhou L, Qin L, Tsukamoto H. A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix. J Biol Chem. 2007 Apr 27;282(17):12928–39. doi: 10.1074/jbc.M700554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008 Feb;233(2):109–22. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 32.Vyas SK, Leyland H, Gentry J, Arthur MJ. Rat hepatic lipocytes synthesize and secrete transin (stromelysin) in early primary culture. Gastroenterology. 1995 Sep;109(3):889–98. doi: 10.1016/0016-5085(95)90399-2. [DOI] [PubMed] [Google Scholar]
- 33.Daxboeck F, Gattringer R, Mustafa S, Bauer C, Assadian O. Elevated serum alanine aminotransferase (ALT) levels in patients with serologically verified Mycoplasma pneumoniae pneumonia. Clin Microbiol Infect. 2005 Jun;11(6):507–10. doi: 10.1111/j.1469-0691.2005.01154.x. [DOI] [PubMed] [Google Scholar]
- 34.Akkaya O, Kiyici M, Yilmaz Y, Ulukaya E, Yerci O. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J Gastroenterol. 2007 Nov 7;13(41):5481–5. doi: 10.3748/wjg.v13.i41.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000 Dec;46(12):2027–49. [PubMed] [Google Scholar]
- 36.Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991 Feb;151(2):260–5. [PubMed] [Google Scholar]
- 37.Shiffman ML, Diago M, Tran A, et al. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol. 2006 May;4(5):645–52. doi: 10.1016/j.cgh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Zechini B, Pasquazzi C, Aceti A. Correlation of serum aminotransferases with HCV RNA levels and histological findings in patients with chronic hepatitis C: the role of serum aspartate transaminase in the evaluation of disease progression. Eur J Gastroenterol Hepatol. 2004 Sep;16(9):891–6. doi: 10.1097/00042737-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003 Jan 27;163(2):218–24. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 40.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998 Jan;93(1):44–8. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 41.Fujii H, Enomoto M, Fukushima W, et al. Noninvasive laboratory tests proposed for predicting cirrhosis in patients with chronic hepatitis C are also useful in patients with non-alcoholic steatohepatitis. J Gastroenterol. 2009;44(6):608–14. doi: 10.1007/s00535-009-0046-6. [DOI] [PubMed] [Google Scholar]
- 42.Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005 Jun;41(6):1376–82. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 43.Reedy DW, Loo AT, Levine RA. AST/ALT ratio > or = 1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci. 1998 Sep;43(9):2156–9. doi: 10.1023/a:1018888021118. [DOI] [PubMed] [Google Scholar]
- 44.Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000 Apr;46(4):443–6. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gelse K, Poschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003 Nov 28;55(12):1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Nojgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003 Aug;39(2):179–86. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004 Dec;127(6):1704–13. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 48.Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993 Oct 9;342(8876):895–8. doi: 10.1016/0140-6736(93)91946-j. [DOI] [PubMed] [Google Scholar]
- 49.Collazos J, Diaz F. Role of the measurement of serum procollagen type III N-terminal peptide in the evaluation of liver diseases. Clin Chim Acta. 1994 Jun;227(1–2):37–43. doi: 10.1016/0009-8981(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 50.Gabrielli GB, Capra F, Casaril M, et al. Serum laminin and type III procollagen in chronic hepatitis C. Diagnostic value in the assessment of disease activity and fibrosis. Clin Chim Acta. 1997 Sep 8;265(1):21–31. doi: 10.1016/s0009-8981(97)00103-4. [DOI] [PubMed] [Google Scholar]
- 51.Giannini E, Caglieris S, Ceppa P, Risso D, Lantieri PB, Testa R. Serum pro-collagen III peptide levels are related to lobular necrosis in untreated patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2001 Feb;13(2):137–41. doi: 10.1097/00042737-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996 Apr;42(4):558–63. [PubMed] [Google Scholar]
- 53.Montalto G, Soresi M, Aragona F, et al. Procollagen III and laminin in chronic viral hepatopathies. Presse Med. 1996 Jan 20;25(2):59–62. [PubMed] [Google Scholar]
- 54.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007 Jun;381(2):107–13. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 55.Hiwatari N, Shimura S, Yamauchi K, Nara M, Hida W, Shirato K. Significance of elevated procollagen-III-peptide and transforming growth factor-beta levels of bronchoalveolar lavage fluids from idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1997 Feb;181(2):285–95. doi: 10.1620/tjem.181.285. [DOI] [PubMed] [Google Scholar]
- 56.Lohr M, Hummel F, Martus P, et al. Serum levels of extracellular matrix in acute pancreatitis. Hepatogastroenterology. 1999 Nov;46(30):3263–70. [PubMed] [Google Scholar]
- 57.Tebib JG, Viguier P, Noel E, Colson F, Barbier Y, Bouvier M. Serum N terminal procollagen III fragment: a predictive marker of joint destruction in rheumatoid arthritis? Clin Rheumatol. 1992 Dec;11(4):502–7. doi: 10.1007/BF02283106. [DOI] [PubMed] [Google Scholar]
- 58.Gabrielli GB, Corrocher R. Hepatic fibrosis and its serum markers: a review. Dig Dis. 1991;9(5):303–16. doi: 10.1159/000171314. [DOI] [PubMed] [Google Scholar]
- 59.Maruyama K, Okazaki I, Takagi T, Ishii H. Formation and degradation of basement membrane collagen. Alcohol Alcohol Suppl. 1991;1:369–74. [PubMed] [Google Scholar]
- 60.Hirayama C, Suzuki H, Takada A, Fujisawa K, Tanikawa K, Igarashi S. Serum type IV collagen in various liver diseases in comparison with serum 7S collagen, laminin, and type III procollagen peptide. J Gastroenterol. 1996 Apr;31(2):242–8. doi: 10.1007/BF02389524. [DOI] [PubMed] [Google Scholar]
- 61.Leeming DJ, Nielsen MJ, Dai Y, et al. Enzyme-linked immunosorbent serum assay specific for the 7S domain of Collagen Type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res. 2012 Jan 5; doi: 10.1111/j.1872-034X.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 62.Veidal SS, Vassiliadis E, Bay-Jensen AC, Tougas G, Vainer B, Karsdal MA. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrogenesis Tissue Repair. 2010;3(1):5. doi: 10.1186/1755-1536-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schytte S, Hansen M, Moller S, et al. Hepatic and renal extraction of circulating type I procollagen aminopropeptide in patients with normal liver function and in patients with alcoholic cirrhosis. Scand J Clin Lab Invest. 1999 Dec;59(8):627–33. doi: 10.1080/00365519950185120. [DOI] [PubMed] [Google Scholar]
- 64.Vassiliadis E, Veidal SS, Simonsen H, et al. Immunological detection of the type V collagen propeptide fragment, PVCP-1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers. 2011 Aug;16(5):426–33. doi: 10.3109/1354750X.2011.584131. [DOI] [PubMed] [Google Scholar]
- 65.McGary CT, Raja RH, Weigel PH. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989 Feb 1;257(3):875–84. doi: 10.1042/bj2570875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McHutchison JG, Blatt LM, De Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000 Aug;15(8):945–51. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 67.Stickel F, Poeschl G, Schuppan D, et al. Serum hyaluronate correlates with histological progression in alcoholic liver disease. Eur J Gastroenterol Hepatol. 2003 Sep;15(9):945–50. doi: 10.1097/00042737-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Wong VS, Hughes V, Trull A, Wight DG, Petrik J, Alexander GJ. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepat. 1998 May;5(3):187–92. doi: 10.1046/j.1365-2893.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 69.Gressner AM, Gao CF, Gressner OA. Non-invasive biomarkers for monitoring the fibrogenic process in liver: a short survey. World J Gastroenterol. 2009 May 28;15(20):2433–40. doi: 10.3748/wjg.15.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emlen W, Niebur J, Flanders G, Rutledge J. Measurement of serum hyaluronic acid in patients with rheumatoid arthritis: correlation with disease activity. J Rheumatol. 1996 Jun;23(6):974–8. [PubMed] [Google Scholar]
- 71.Fraser JR, Gibson PR. Mechanisms by which food intake elevates circulating levels of hyaluronan in humans. J Intern Med. 2005 Nov;258(5):460–6. doi: 10.1111/j.1365-2796.2005.01564.x. [DOI] [PubMed] [Google Scholar]
- 72.Tran A, Benzaken S, Saint-Paul MC, et al. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000 Sep;12(9):989–93. doi: 10.1097/00042737-200012090-00004. [DOI] [PubMed] [Google Scholar]
- 73.Berres ML, Papen S, Pauels K, et al. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol. 2009 Feb;50(2):370–6. doi: 10.1016/j.jhep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Saitou Y, Shiraki K, Yamanaka Y, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005 Jan 28;11(4):476–81. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006 May;53(2):172–209. [PubMed] [Google Scholar]
- 76.Molleken C, Sitek B, Henkel C, et al. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology. 2009 Apr;49(4):1257–66. doi: 10.1002/hep.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leroy V, Monier F, Bottari S, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004 Feb;99(2):271–9. doi: 10.1111/j.1572-0241.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 78.Murphy FR, Issa R, Zhou X, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002 Mar 29;277(13):11069–76. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 79.Kerkvliet EH, Docherty AJ, Beertsen W, Everts V. Collagen breakdown in soft connective tissue explants is associated with the level of active gelatinase A (MMP-2) but not with collagenase. Matrix Biol. 1999 Aug;18(4):373–80. doi: 10.1016/s0945-053x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 80.Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases-1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999 Mar;44(3):624–30. doi: 10.1023/a:1026630129025. [DOI] [PubMed] [Google Scholar]
- 81.Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002 Feb;316(1–2):71–81. doi: 10.1016/s0009-8981(01)00730-6. [DOI] [PubMed] [Google Scholar]
- 82.Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin Biochem. 2010 Jul;43(10–11):793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Fosang AJ, Stanton H, Little CB, Atley LM. Neoepitopes as biomarkers of cartilage catabolism. Inflamm Res. 2003 Jun;52(7):277–82. doi: 10.1007/s00011-003-1177-5. [DOI] [PubMed] [Google Scholar]
- 84.Karsdal MA, Henriksen K, Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009 May;14(3):181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 85.Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010 Jul;43(10–11):899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 86.Segovia-Silvestre T, Reichenbach V, Fernandez-Varo G, et al. Circulating CO3-610, a degradation product of collagen III, closely reflects liver collagen and portal pressure in rats with fibrosis. Fibrogenesis Tissue Repair. 2011;4:19. doi: 10.1186/1755-1536-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vassiliadis E, Larsen DV, Clausen RE, et al. Measurement of CO3-610, a Potential Liver Biomarker Derived from Matrix Metalloproteinase-9 Degradation of Collagen Type III, in a Rat Model of Reversible Carbon-Tetrachloride-Induced Fibrosis. Biomark Insights. 2011;6:49–58. doi: 10.4137/BMI.S6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veidal SS, Karsdal MA, Vassiliadis E, et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis—identification and validation of a novel biochemical marker assay. PLoS One. 2011;6(9):e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leeming D, He Y, Veidal S, et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011 Nov;16(7):616–28. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 91.Moreno M, Bataller R. Cytokines and renin-angiotensin system signaling in hepatic fibrosis. Clin Liver Dis. 2008 Nov;12(4):825–52. ix. doi: 10.1016/j.cld.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Kanzler S, Baumann M, Schirmacher P, et al. Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-beta. J Viral Hepat. 2001 Nov;8(6):430–7. doi: 10.1046/j.1365-2893.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- 93.Flisiak R, Maxwell P, Prokopowicz D, Timms PM, Panasiuk A. Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta 1-possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepatogastroenterology. 2002 Sep;49(47):1369–72. [PubMed] [Google Scholar]
- 94.Roth S, Michel K, Gressner AM. (Latent) transforming growth factor beta in liver parenchymal cells, its injury-dependent release, and paracrine effects on rat hepatic stellate cells. Hepatology. 1998 Apr;27(4):1003–12. doi: 10.1002/hep.510270416. [DOI] [PubMed] [Google Scholar]
- 95.McClain C, Barve S, Joshi-Barve S, et al. Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease. Alcohol Clin Exp Res. 2005 Nov;29(Suppl 11):180S–8S. doi: 10.1097/01.alc.0000189276.34230.f5. [DOI] [PubMed] [Google Scholar]
- 96.Akpolat N, Yahsi S, Godekmerdan A, Demirbag K, Yalniz M. Relationship between serum cytokine levels and histopathological changes of liver in patients with hepatitis B. World J Gastroenterol. 2005 Jun 7;11(21):3260–3. doi: 10.3748/wjg.v11.i21.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006 Jan;44(1):57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- 98.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004 Aug;15(4):255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Zhang BB, Cai WM, Weng HL, et al. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003 Nov;9(11):2490–6. doi: 10.3748/wjg.v9.i11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gressner OA, Lahme B, Demirci I, Gressner AM, Weiskirchen R. Differential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007 Nov;47(5):699–710. doi: 10.1016/j.jhep.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008 Sep;28(8):1065–79. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 102.Gressner AM, Yagmur E, Lahme B, Gressner O, Stanzel S. Connective tissue growth factor in serum as a new candidate test for assessment of hepatic fibrosis. Clin Chem. 2006 Sep;52(9):1815–7. doi: 10.1373/clinchem.2006.070466. [DOI] [PubMed] [Google Scholar]
- 103.Kovalenko E, Tacke F, Gressner OA, et al. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat. 2009 Sep;16(9):612–20. doi: 10.1111/j.1365-2893.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 104.Naiki M, Takagaki Y, Fujiwara K. Note on the change of transaminases in the liver and the significance of the transaminase ratio in experimental Tyzzer’s disease of mice 1. Jpn J Exp Med. 1965 Aug;35(4):305–9. [PubMed] [Google Scholar]
- 105.Imperiale TF, Said AT, Cummings OW, Born LJ. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C 1. Am J Gastroenterol. 2000 Sep;95(9):2328–32. doi: 10.1111/j.1572-0241.2000.02322.x. [DOI] [PubMed] [Google Scholar]
- 106.Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? 1. J Gastroenterol Hepatol. 2000 Apr;15(4):386–90. doi: 10.1046/j.1440-1746.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 107.Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005 Mar 15;71(6):1105–10. [PubMed] [Google Scholar]
- 108.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C 1. Hepatology. 2003 Aug;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 109.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review 5. Hepatology. 2007 Sep;46(3):912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 110.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis 1. Hepatology. 2011 Mar;53(3):726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 111.Poynard T, Imbert-Bismut F, Munteanu M. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004 Sep;3(1):8. doi: 10.1186/1476-5926-3-8. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castera L, Foucher J, Bertet J, Couzigou P, de Ledinghen V. sFibroScan and FibroTest to assess liver fibrosis in HCV with normal aminotransferases 1. Hepatology. 2006 Feb;43(2):373–4. doi: 10.1002/hep.21019. [DOI] [PubMed] [Google Scholar]
- 113.Myers RP, Benhamou Y, Imbert-Bismut F, et al. Serum biochemical markers accurately predict liver fibrosis in HIV and hepatitis C virus co-infected patients 4. AIDS. 2003 Mar 28;17(5):721–5. doi: 10.1097/00002030-200303280-00010. [DOI] [PubMed] [Google Scholar]
- 114.Rossi E, Adams L, Prins A, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients 1. Clin Chem. 2003 Mar;49(3):450–4. doi: 10.1373/49.3.450. [DOI] [PubMed] [Google Scholar]
- 115.Varaut A, Fontaine H, Serpaggi J, et al. Diagnostic accuracy of the fibrotest in hemodialysis and renal transplant patients with chronic hepatitis C virus 1. Transplantation. 2005 Dec 15;80(11):1550–5. doi: 10.1097/01.tp.0000183399.85804.02. [DOI] [PubMed] [Google Scholar]
- 116.Wilson LE, Torbenson M, Astemborski J, et al. Progression of liver fibrosis among injection drug users with chronic hepatitis C 1. Hepatology. 2006 Apr;43(4):788–95. doi: 10.1002/hep.21091. [DOI] [PubMed] [Google Scholar]
- 117.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis 4. Hepatology. 2006 Feb;43(2 Suppl 1):S113–20. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 118.Cales P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005 Dec;42(6):1373–81. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 119.Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007 May;46(5):775–82. doi: 10.1016/j.jhep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 120.Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis 1. BMC Gastroenterol. 2010;10:103. doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayo MJ, Parkes J, ms-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay 1. Hepatology. 2008 Nov;48(5):1549–57. doi: 10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009 Jan;136(1):160–7. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 123.Stevenson M, Lloyd-Jones M, Morgan MY, Wong R. Non-invasive diagnostic assessment tools for the detection of liver fibrosis in patients with suspected alcohol-related liver disease: a systematic review and economic evaluation. Health Technol Assess. 2012 Feb;16(4):1–174. doi: 10.3310/hta16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bauer DC, Hunter DJ, Abramson SB, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006 Aug;14(8):723–7. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008 May;134(6):1670–81. doi: 10.1053/j.gastro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 126.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008 Mar 8;371(9615):838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uto H, Kanmura S, Takami Y, Tsubouchi H. Clinical proteomics for liver disease: a promising approach for discovery of novel biomarkers. Proteome Sci. 2010;(8):70. doi: 10.1186/1477-5956-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]