Abstract
Background
Neuronal loss in the retina has been demonstrated pathologically in eyes of patients with multiple sclerosis (MS). In vivo, MS eyes have reduced total macular volumes by optical coherence tomography (OCT). Using high-resolution spectral-domain OCT, this pilot study used a manual method to measure ganglion cell layer (GCL) volumes and to determine the relation of these volumes to visual function in MS eyes.
Methods
Sixteen eyes of eight patients with MS and eight eyes of five disease-free control participants were studied using fast macular OCT scans performed with Spectralis OCT (Heidelberg Engineering). Visual function tests of low-contrast letter acuity and high-contrast visual acuity (VA) were administered.
Results
MS patient eyes had significantly lower GCL volumes than the control eyes (p<0.001 vs. controls, GEE regression models accounting for age and within-patient, inter-eye correlations). Within the MS group, eyes with a prior history of optic neuritis (ON, n=4) had significantly lower GCL volumes than MS eyes with no ON history (P<0.001). In contrast to measures of high-contrast VA (P=0.14), decreased GCL volumes were associated with worse performance on low-contrast letter acuity testing (P=0.003).
Conclusions
This pilot study has characterized thinning of the GCL in MS patient eyes, particularly in those with a history of acute ON, which corresponded to a reduced performance on low-contrast letter acuity testing. Studies utilizing computerized segmentation algorithms will continue to facilitate the detection of GCL loss on a larger scale and provide important information in vivo on the role and timing of neuronal vs. axonal loss in MS eyes.
Keywords: multiple sclerosis (MS), optical coherence tomography (OCT), ganglion cell layer
Neuronal loss is increasingly recognized as an important correlate of disability in multiple sclerosis (MS) (1,2). While gray matter lesions in MS have been documented pathologically, new magnetic resonance imaging (MRI) techniques (i.e., double inversion recovery imaging) have allowed us to visualize cortical lesions in the brains of MS patients in vivo. Validated and high precision methods have generated retinal metrics that reflect features of the histopathological substrate of MS. In particular, changes in retinal architecture represents a unique model for dissecting the mechanisms and temporal evolution of axonal and neuronal damage in the disease process, given that retinal nerve fiber layer (RNFL) axons are nonmyelinated (3–8). Optical coherence tomography (OCT) is a non-invasive, rapid, and highly reproducible method to image the retina, and obtain reliable measurements of macular volume (which reflects the collective thickness of axons, neurons and glia) (3). Recent studies have demonstrated a reduction in macular volume in MS patients versus age-matched controls using conventional time-domain OCT technology (5). Furthermore, reduction in volumes of the macula adjacent to the fovea (the inner macular zone), an area whose volume contains approximately 34% retinal ganglion cells, suggests that the ganglion cell layer (GCL) is thinned in MS (5,9).
While we have demonstrated that GCL and the thin adjacent inner plexiform layer (IPL) are thinned in MS vs. healthy controls (6,7), our computerized algorithm was unable to discriminate the GCL from IPL. It is of particular interest, therefore, whether thinning of the GCL can be observed in MS eyes, both with and without a prior history of acute optic neuritis (ON), and whether GCL thinning correlates with loss of visual function. The purpose of our study was to pilot a manual method for estimating retinal GCL volume by high-speed, high definition, spectral-domain OCT using Spectralis OCT (Heidelberg Engineering, Inc., Heidelberg, Germany). We also sought to compare these volumes in MS eyes with vs. without a prior history of acute ON, and to explore the relation of GCL volumes to validated patient-performed measures of visual function.
METHODS
Study Participants
Patients were enrolled as part of an ongoing prospective study of visual outcome measures in MS at the University of Pennsylvania. Subjects represent a convenience sample of patients who had undergone OCT imaging and vision testing for research purposes. Each individual was diagnosed with MS using standard criteria (10) and patients with co-morbid ocular conditions not related to MS were excluded by history, chart review, and examination. A past history of acute ON was determined by self and physician report. Disease-free controls were recruited from among staff and had no history of ocular or neurologic disease.
Optical coherence tomography (OCT)
OCT was performed for both eyes using the Spectralis OCT (Heidelberg Engineering, Inc., Heidelberg, Germany). The Spectralis OCT utilizes two medical Class I lasers to image the retina, with technology that allows for eye tracking and signal noise reduction. The fast macular scan with 25 frames per eye was used with 20°× 20° scans and an automatic real time mean value (number of scans averaged at each scan position, ART value) set at 9. OCT was performed by a trained technician following visual function testing. All scans were performed under ambient lighting and without pupil dilation to optimize patient comfort. High quality images were defined as those with individual retinal layers that could be identified (with characteristics including signal strength of approximately 26dB, uniform brightness, and crisp borders of blood vessels). In this small cohort, none of the individuals or scans were excluded for insufficient quality.
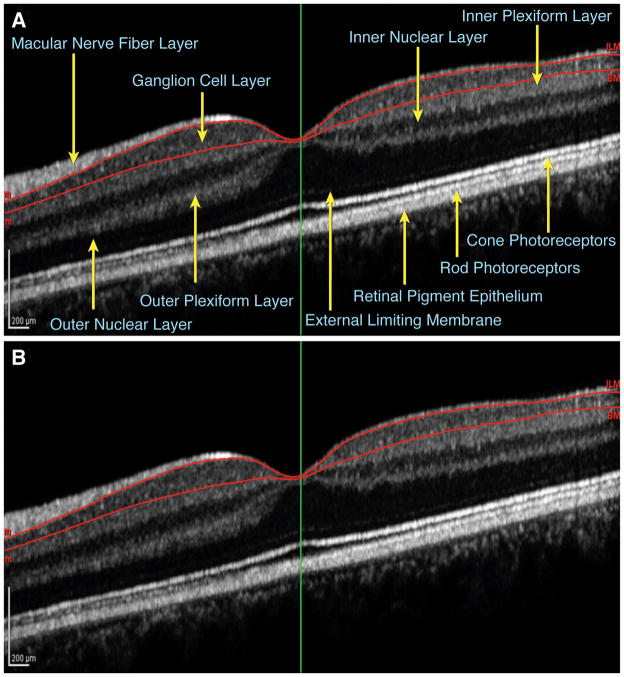
The macular scans were then manually segmented for each section (25 sections for each eye) by moving the automatic contours (outer contour along the inner limiting membrane and inner contour along the outer border of the retinal pigment epithelium) to outline the GCL. This time-intensive method involved: 1) placing individual points (>100) along the outer boundary between the GCL and the nerve fiber layer and along the inner boundary between the GCL and the inner plexiform layer (Fig. 1), 2) the computer automatically joining adjacent points with a curve to form a smooth contour using a spine algorithm, and 3) the Spectralis software estimating the total GCL volume from the cumulative frames integrated over 25 sections. Images were magnified by 400% to 800% and contrast was enhanced to maximize the accuracy of the layer delineation. Manual segmentation was performed for all scans by a single investigator who was masked to MS vs. control status of the images.
FIG. 1.
Single frame of a macular spectral-domain OCT image with A. retinal layers labeled and B. the GCL outlined in red as segmented manually for the GCL volume analysis. Labeling in Fig. 1A adapted from Werner JS, et al. (7).
Visual Function Testing
Study participants were refracted so that visual function could be measured with best corrected vision. Low-contrast letter acuity was tested for each eye individually using retro-illuminated low-contrast Sloan letter charts (2.5% and 1.25% contrast levels at 2 meters, Precision Vision, LaSalle, IL) (11). High-contrast visual acuity (VA) was determined using retro-illuminated Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 3.2 meters. Both chart types have a similar standardized format with five letters per line and were scored based on the number of letters appropriately identified (maximum score of 70 per chart). All visual function testing was performed by trained technicians.
Statistical Analysis
Analysis was performed using Stata 11.0 software (StataCorp, College Station, TX). Generalized estimating equation (GEE) models, accounting for age and within-patient, inter-eye correlations, were used to examine the GCL volume of MS patient eyes compared to control eyes. Within the MS group, the GCL volume in eyes with a history of ON was compared to the GCL volume of eyes without a history of MS. These models were also used to determine the association between GCL volume and performance on low-contrast and high-contrast VA charts. For all statistical tests, type I error for significance was set as p<0.05.
RESULTS
Patients with MS (n=8, 16 eyes) had a mean age of 50±6 years. Four of the 16 MS eyes (25%) had a history of ON. Median high-contrast VA (Snellen equivalent) for the MS patient eyes using ETDRS charts was 20/20 (range 20/32-20/12.5). Disease-free controls (n=4, 8 eyes) had a mean age of 34±11 years.
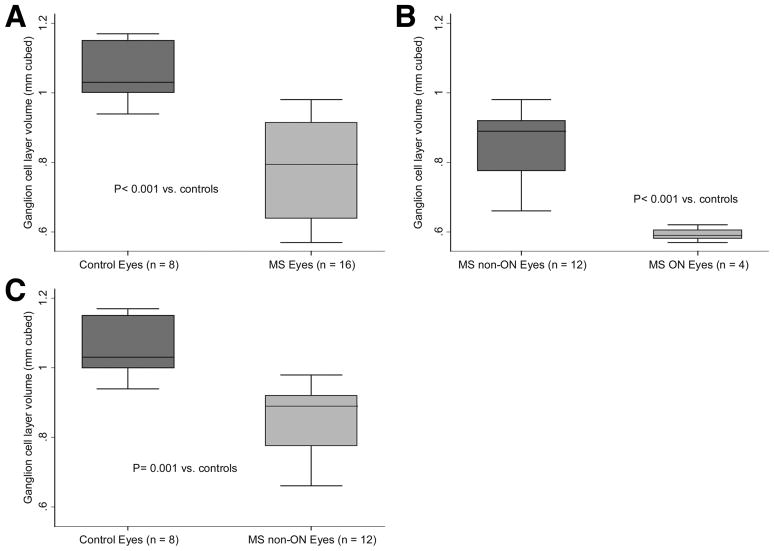
MS patient eyes were found to have significantly lower GCL volumes than control eyes (P<0.001, GEE models accounting for age and within-patient, inter-eye correlations, Fig. 2A). Within the MS group, eyes with a prior history ON had significantly lower GCL volumes than MS eyes without a prior ON history (P<0.001) (Fig. 2B). Lastly, even when MS eyes without a prior history of ON were compared to control eyes, the MS eyes had a significantly lower GCL volume than control eyes (P=0.001, Fig. 2C). Lower GCL volumes were associated with worse performance on low-contrast letter acuity tests (P=0.01 using 2.5% contrast charts and P=0.003 using 1.25% contrast charts). However, lower GCL volumes were not significantly correlated with high-contrast VA scores (P=0.14).
FIG. 2.
Box plots show ganglion cell layer volumes of A. MS patient vs. disease-free control eyes, B. MS patient eyes with prior history of ON vs. MS patient eyes without a prior ON history, and C. MS patient eyes without a prior history of ON vs. control eyes.
Abbreviations: MS = multiple sclerosis; ON = optic neuritis
* The lines in the box represent the medians, and boxes delineate the interquartile range (25th to 75th percentiles). Whiskers represent the range of observations minus outliers (no outlier values in this sample). P values are based on generalized estimating equation (GEE) models accounting for age and within-patient, inter-eye correlations.
DISCUSSION
The results of this pilot study demonstrate that GCL volumes are reduced among eyes of patients with MS compared to disease-free controls, and that MS eyes with a history of acute ON have the greatest degree of GCL neuronal loss. Although the manual method for retinal segmentation and GCL thickness measurement in this study required approximately two hours per eye to complete, this work represents an important step towards demonstrating GCL thinning in an MS cohort. Computerized segmentation algorithms are being used which will allow for automated measurement of the GCL+IPL layers and therefore examine neuronal loss on a larger scale in studies of MS and ON (6,7). While these studies may confirm that RNFL and total macular volume remain sensitive indicators of visual pathway disease, segmentation of the GCL+IPL specifically will provide important information in vivo on the role and timing of neuronal (GCL) vs. axonal loss (RNFL) in MS eyes.
This investigation provides evidence for neuronal degeneration in the anterior visual pathway in patients with MS, and suggests that larger studies should be performed in heterogeneous MS cohorts. Further, low-contrast letter acuity was a stronger correlate of GCL volume loss compared to high-contrast VA. This result supports previous findings that low-contrast acuity can detect even very subtle visual dysfunction in MS patients not captured by conventional high-contrast VA assessments (11).
The evidence of GCL volume loss in MS patients highlights the need for future research to document retinal ganglion cell loss over time in MS, thereby enabling insight to the temporal pattern of neuronal degeneration, and correspondingly has clear therapeutic implications for the development of neuro-protection strategies. In our cohort, GCL volume loss was noted even among eyes with no history of acute ON. This is similar to findings demonstrating reduced RFNL and total macular volume in MS non-ON eyes (2–5), but underscores the need to further understand the degree to which the timing and magnitude of neuronal vs. axonal loss may differ in the setting of ON vs. MS without acute visual loss. Our ongoing multicenter collaborative research initiative includes work on based on computerized segmentation techniques that measure GCL+IPL volume longitudinally in heterogeneous MS cohorts. These computerized algorithms are being used thus far successfully in MS eyes (6,7), particularly with macular predominant thinning, as well as in glaucoma (12–15), and provide important information in vivo on the role and timing of neuronal vs. axonal loss.
Acknowledgments
Funding Source: National MS Society PP1115, RG 3208-A-1, RG 3428-A/2 (Dr. Balcer), National MS Society Translational Research Partnership TR 3760-A-3 (Drs. Calabresi, Balcer), NIH/NEI K24 EY 014136, R01 EY 019473 (Dr. Balcer), McNeill Foundation, DADs Foundation, Penn Clinical Neuroscience Track Summer Fellowship (Ms. Davies), and the Foundation of the Consortium of MS Centers (Dr. Sackel).
Footnotes
Disclosure Statements: Dr. Frohman has received speaking and consulting honoraria from Biogen-Idec, Novartis, TEVA, and Acorda. Dr. Calabresi has received personal compensation for consulting and serving on scientific advisory boards from Biogen-IDEC, Teva, and Novartis; and has received research funding from companies Biogen-IDEC, Teva, EMD Serono, Vertex, Genentech, Abbott, and Bayer. Dr. Galetta has received speaking and consulting honoraria from Biogen-Idec, Novartis, and Teva. Dr. Balcer has received speaking and consulting honoraria from Biogen-Idec, Bayer, and Novartis.
References
- 1.Rudick RA, Lee JC, Nakamura K, Fisher E. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. J Neurol Sci. 2009;282:106–111. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 3.Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8:117–132. doi: 10.1007/s13311-010-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talman LS, Bisker ER, Sackel DJ, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying GS, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder BM, Osborne B, Loguidice MJ, Bisker E, Frohman TC, Conger A, Ratchford JN, Warner C, Markowitz CE, Jacobs DA, Galetta SL, Cutter GR, Maguire MG, Calabresi PA, Balcer LJ, Frohman EM. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66:1366–1372. doi: 10.1001/archneurol.2009.230. [DOI] [PubMed] [Google Scholar]
- 6.Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, Oakley JD, Durbin MK, Meyer SA, Balcer LJ, Frohman EM, Rosenzweig JM, Newsome SD, Ratchford JN, Nguyen QD, Calabresi PA. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 7.Werner JS, Keltner JL, Zawadzki RJ, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye. 2011;25:279–289. doi: 10.1038/eye.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, Enzmann V, Wolf S. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2008;50:3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 10.Polman C, Reingold S, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 11.Balcer LJ, Galetta SL, Calabresi PA, Confavreux C, Giovannoni G, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68:1299–1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–2017. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan O, Li G, Lu AT, Varma R, Huang D Advanced Imaging for Glaucoma Study Group. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115:949–956. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, Varma R, Huang D. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer MA, Hornegger J, Mardin CY, Tornow RP. Retinal nerve fiber layer segmentation on FD-OCT scans of normal subjects and glaucoma patients. Biomed Opt Express. 2010;1:1358–1383. doi: 10.1364/BOE.1.001358. [DOI] [PMC free article] [PubMed] [Google Scholar]