Abstract
Objective
Activation of the toll-like receptor (TLR) signaling pathway exacerbates ischemic brain damage. The present study tested the hypothesis that combination treatment with VELCADE and tissue plasminogen activator (tPA) modulates the TLR signaling pathway on cerebral vasculature, which leads to neuroprotection in aged rats after stroke.
Methods and Results
Focal cerebral ischemia acutely increased TLR2, TLR4, and interleukin-1 receptor activated kinases 1 (IRAK1) immunoreactivity on fibrin/fibrinogen positive vessels in aged rats. Monotherapy of tPA further amplified these signals. However, VELCADE in combination with tPA blocked stroke- and tPA-potentiated vascular TLR signals, leading to robust reduction of infarct volume compared with respective monotherapies. Quantitative RT-PCR analysis of cerebral endothelial cells isolated by laser capture microdissection revealed that the combination treatment increased miR-l46a levels, which was inversely associated with the reduction of vascular IRAK1 immunoreactivity. In vitro, fibrin upregulated IRAK1 and TLR4 expression and downregulated miR-146a on primary human cerebral endothelial cells. VELCADE elevated miR-146 levels and abolished fibrin-increased IRAK1 proteins.
Conclusions
Stroke acutely activates the TLR signaling pathway on cerebral vasculature. Upregulation of miR-146a and inactivation of ischemia and tPA-potentiated TLR signaling pathway by VELCADE may play an important role in the neuroprotective effect of the combination therapy of VELCADE and tPA for acute stroke.
Keywords: ischemia, endothelium, microRNA, thrombolysis, toll-like receptors
Toll-like receptors (TLRs) are pattern-recognition receptors that are key players of the innate immune responses 1, 2. In the CNS, TLRs are widely distributed in neurons, microglia, and astrocytes, and to a lesser extent in cerebral endothelial cells3–6. Although the functional relevance of TLR signaling in these cells remains to be elucidated, emerging evidence shows that the activation of TLR signaling pathway contributes to the pathophysiology of brain ischemia 6–10. In experimental stroke, TLR2 and TLR4 are upregulated in neurons as early as 1h after stroke onset whereas the upregulation of TLR2 in microglia appeared in a relative delayed fashion (24–48h) in the mouse brain 8, 11. In addition, mice with defective TLR2 or TLR4 genes are less susceptible to ischemic brain injury 6–8. Most importantly, upregulation of TLR2 and TLR4 has been found in stroke patients, and is associated with poor stroke outcome. Thus, these studies establish a direct linkage between TLR signaling and the pathogenesis of stroke. However, the effects of TLR signaling on cerebral endothelium – the major regulatory component of blood–brain barrier (BBB) and cerebral inflammation in the ischemic brain have not been studied in vivo, although studies in vitro show that oxidative stress upregulates TLR expression on cerebral endothelial cells, causing downregulation of tight junction proteins 9.
VELCADE, a potent proteasome inhibitor, has been approved for clinical use in patients with multiple myeloma 12. In addition to its well established anti-tumor actions, evidence from experimental stroke demonstrates that VELCADE provides potent neuroprotection for the ischemic brain 13, 14. We have demonstrated that VELCADE extends the therapeutic window of thrombolytic therapy for stroke by enhancement of cerebral vascular patency and integrity 14. Accumulating evidence indicates that VELCADE possesses an immuno-modulatory property via suppressing TLR signaling in the treatment of inflammatory related diseases 15, 16. Given the observation that cerebral endothelial cells express TLRs 6, 17, the present study tested the hypothesis that adjuvant treatment of VELCADE and tPA suppresses the endothelial TLR signaling pathway triggered by stroke and tPA, leading to neuroprotection in aged rats after stroke.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. All outcome measurements were performed by observers blinded to the treatments.
Embolic stroke model
Male Wistar rats at the age of 16–18 months, weighing 450–700g were subjected to embolic middle cerebral artery (MCA) occlusion, as previously described 18.
Experimental groups
Ischemic rats were randomly divided into the following groups: 1) VELCADE (Millenium Pharmaceuticals) at a dose of 0.1mg/kg was given intravenously at 2h after stroke onset (n=16); 2) tPA at a dose of 5mg/kg (generously provided by Genentech) was given intravenously (10% bolus and the remainder at a continuous infusion over a 30 min interval using a syringe infusion pump) at 2h after stroke onset (n=17); 3) combination of VELCADE with tPA (n=17); 4) The control rats were treated with the same volume of saline (1ml) at 2h after MCA occlusion (n=18).
Measurement of lesion volume
Animals were sacrificed at 7 days after MCAo. Infarct volume was measured on hematoxylin and eosin (H&E) stained seven coronal sections using the microcomputer imaging device system (MCID, Imaging Research), as described previously 18.
Functional Outcome
Neurological functional deficits were graded with the modified Neurological Severity Score (mNSS) at 1 and 7days after stroke onset, as previously described 19.
Immunohistochemistry
Immunohistochemistry was performed on coronal sections obtained from rats sacrificed 1d after MCA occlusion. To detect TLRs and associated signaling molecules, a goat anti-TLR2 (1:100, Santa Cruz), a rabbit TLR4 (1:100, Santa Cruz), a mouse anti-NF-κB (P65, 1:150, Chemicon), which is specific for the detection of activated NF-κB 20, and a mouse anti-interleukin-1 receptor-associated kinase-1 (IRAK1, 1:50, Santa Cruz) antibodies were used. Double immunofluorescence labeling with mAb anti-fibrinogen/fibrin (1:1000, Accurate Chemical & Scientific) and mAb anti-endothelial barrier antigen (EBA, Sternberger Monoclonals Inc; 1:1000) was performed to detect fibrin deposition. Double immunofluorescence stainings were performed to visualize cellular co-localization of TLR2 with neuron-specific marker-Neuronal nuclear antigen NeuN (1:200, Millipore), glial fibrillary acidic protein (GFAP, 1:400, DAKO), and with a pericyte marker-platelet derived growth factor-β (PBGFrβ, 1:100, R&D). For quantification, TLR2, TLR4, IRAK1, and EBA immunoreactive area were measured throughout the territory supplied by the right MCA. Data are presented as the percentage area relative to the total EBA immunoreactive vessel area. The numbers of vessels with fibrin/fibrinogen and NF-κB immunoreactivity were counted throughout the territory supplied by the right MCA and are presented as the density of immunoreactive vessels relative to the scan area (mm2).
Laser Capture Microdissection (LCM)
LCM was performed according to our published protocol 21. See Supplemental Methods for details.
Quantification of mRNA by real-time RT-PCR
All measurements of mRNAs and miRNAs were performed on an ABI 7000 PCR instrument (Applied Biosystems), as previously described 21. To verify the purity of endothelial cells isolated by LCM, mRNA levels of platelet endothelial cell adhesion molecule-1 (PECAM-1) and GFAP were measured using TaqMan probes specific for rat PECAM-1 and GFAP, After the verification, we then examined mRNA expression of matrix metalloproteinase 9 (MMP-9) and intracellular adhesion molecule-1 (ICAM-1) in cerebral endothelial cells. Relative quantities were calculated using the 2−ΔΔ CT method with GAPDH as the endogenous normalization control.
Quantification of mature and primary miRNAs by real-time RT-PCR
For evaluation of miR-146a and primary miR-146a (pri-miR-146a) levels, individual reverse transcription and TaqMan® microRNA assays were performed. See Supplemental Methods for details.
Cell Culture
Primary human brain microvascular endothelial cells (HBECs) obtained from Applied Cell Biology Research Institute (Kirkland, WA) were cultured in endothelial cell growth media according to the protocols provided by the manufacturer. To investigate the effect of fibrin and fibrinogen on TLR genes, HBECs were treated with fibrin (0.15 and 1.5µg/ml; Sigma) or fibrinogen (15, 150, and 300µg/ml; Sigma). To examine whether VELCADE blocks fibrin induced TLR signaling, HBECs were treated with fibrin (1.5µg/ml) and VELCADE (10ng/ml). At the end of experiments, the HBECs were collected for Western blot or RT-PCR assay.
Transfection of miRNA mimics and inhibitors
To examine the effect of miR-146a on TLR4 and IRAK1 expression, the cerebral endothelial cells were transfected with miR-146a mimic or inhibitor (Dharmacon). Briefly, ten microliters (20uM) of miR-146a mimic, a control miRNA mimic (cel-miR-67), miR-146a inhibitor, or a negative control miRNA inhibitor (Dharmacon) were diluted into OPTIMEM (Life Technologies) and mixed with 4 µl of oligofectamine™ (Life Technologies) according to supplier’s protocol. Prior to transfection, the growth medium was replaced with 2 ml of OPTIMEM. DNA- Oligofectamine™ complexes were added to the cells and incubated for 6 h. The transfection medium was then replaced by growth medium and cells were incubated for an additional 18 h.
Western blot analysis
Protein levels of total IRAK1, phosphorylated IRAK1 (p-IRAK1), and TLR4 on cultured cerebral endothelial cells and brain tissue isolated from the ischemic boundary zone was measured by Western blot. The following antibodies were used: IRAK1 (1:1000, Santa Cruz Biotechnology Inc), p-IRAK1 (for brain tissue, 1:100, Santa Cruz Biotechnology Inc), p-IRAK1 (for HBECs, 1:250, Abcam), TLR4 (1:500, Santa Cruz Biotechnology Inc,), and β-actin (1:10,000, Abcam). Signal bands were visualized by the ECL system (Amersham). The relative densities among the blot lanes were analyzed using the MCID system (Imaging Research).
Statistical analysis
Data are presented as means ± SE. Statistical comparisons were made using an analysis of variance (ANOVA) followed by Bonferroni’s post hoc multiple comparisons. Comparisons between two groups were evaluated by the Student’s t test. P < 0.05 was considered statistically significant.
Results
VELCADE attenuates the TLR signaling pathway on cerebral vasculature
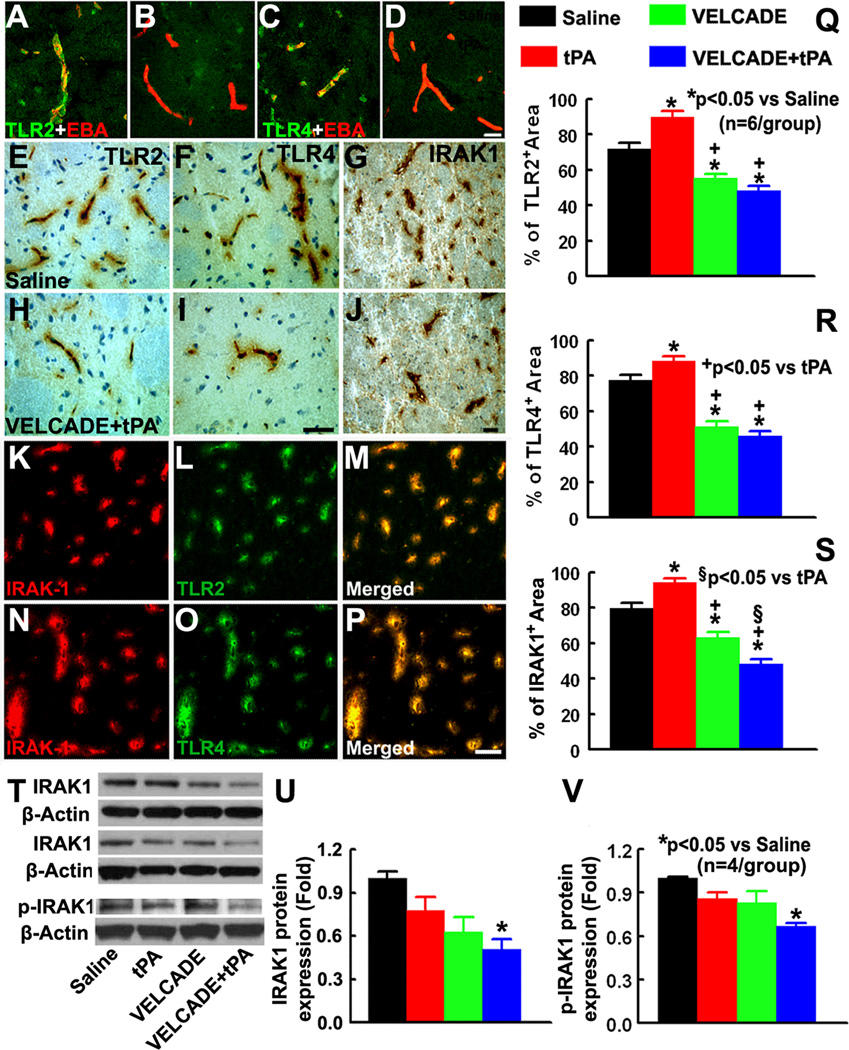
To examine the effect of cerebral ischemia on TLR expression on cerebral vasculature in vivo, double immunofluorescent staining of TLR2, TLR4, and IRAK1 with EBA were performed. Our data showed that 24h after MCAo, EBA immunoreactive cerebral vessels were TLR2 and TLR4 positive throughout the ipsilateral MCA territory, whereas TLR2 and TLR4 immunoreactivity was not detected on EBA positive vessels in the non-ischemic hemisphere 24h after stroke (Fig. 1A–D). In addition, TLR2 and TLR4 positive vessels were IRAK1 immunoreactive (Fig. 1K–P), a key downstream kinase in the signaling pathway of TLRs 22. Furthermore, double immunofluorescent staining revealed that TLR2 positive vasculature was not GFAP and NeuN positive (Supplement Fig.1). However, some of TLR2 positive vessels were PDGFRβ positive, a marker of pericytes, (Supplement Fig.1). These data suggest that stroke acutely activates TLR signaling on cerebral vasculature. Monotherapy with tPA further increased the percentage of TLR2, TLR4, and IRAK1 immunoreactive area compared with saline treated rats (Fig. 1Q–S). In contrast, treatment with VELCADE significantly reduced the percentage area of TLR2, TLR4, and IRAK1 immunoreactive vessels compared with saline-treated rats (Fig. 1Q–S). In addition, VELCADE in combination with tPA significantly reduced TLR2, TLR4, and IRAK1 immunoreactive vasculature compared with tPA treated or saline treated rats (Fig. 1Q–S). The combination therapy also significantly reduced the percentage of IRAK1 immunoreactive area compared with rats treated with monotherapy of VELCADE (Fig. 1S). Western blot analysis of brain tissue isolated from ischemic boundary zone showed reductions of IRAK1 and p-IRAK1 levels by the combination therapy (Fig. 1T–V). These data suggest that exogenous tPA amplifies TLR signals, whereas VELCADE attenuates this signaling pathway triggered by ischemia and tPA.
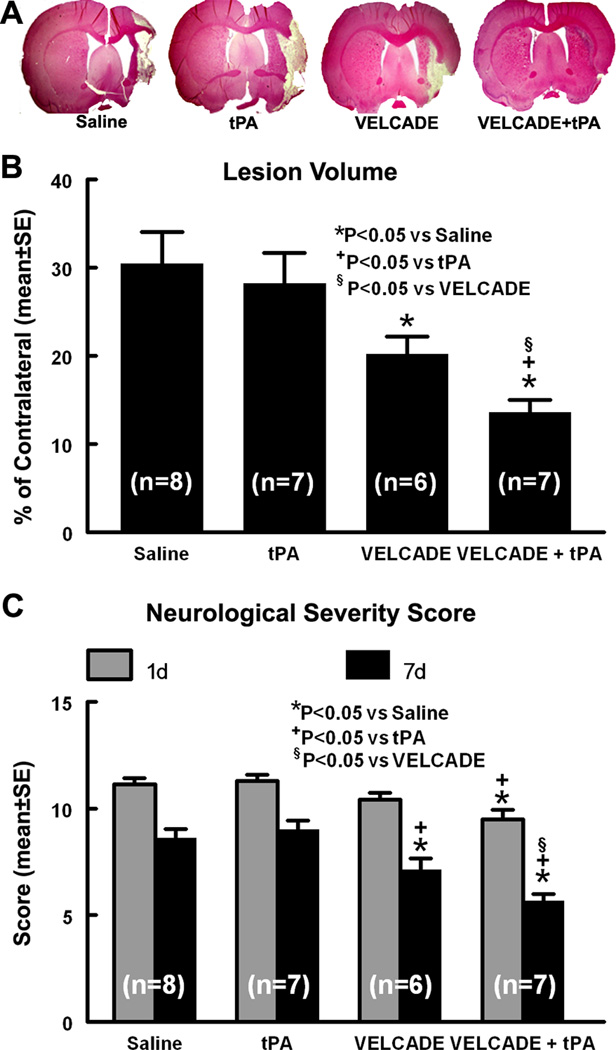
Figure 1. Expression of TLR2, TLR4, and IRAK1.
Panels A through D show double immunofluorescent stainings of TLR2 (A, B green) or TLR4 (C, D; green) with EBA (red) acquired from ipsilateral (A, C) and contralateral hemisphere (B, D) 24h after stroke onset. Panels E through J are TLR2 (E, H), TLR4 (F, I), and IRAK1 (G, J) immunostaining in representative rats treated with saline (E–G) and the combination of VELCADE and tPA (H–J). Panels K-M, and N-P show the colocalization of IRAK1(red) with TLR2 and TLR4 (green) imunoreactive vessels, respectively. Panels Q to S show quantitative data of TLR2 (Q), TLR4 (L), and IRAK1 (S) immunoreactivies in saline, tPA, VELCADE, and VELCADE with tPA groups. Panel T shows 3representative Western blot analyses (T) of IRAK1 and p-IRAK1. Panels U and V are quantitative data (U) of IRAK1 and p-IRAK1 levels on brain tissue isolated from ischemic boundary zone. Values are mean ± SE. Bar=20µm in panels A-D. Bars =50µm in panels E-P.
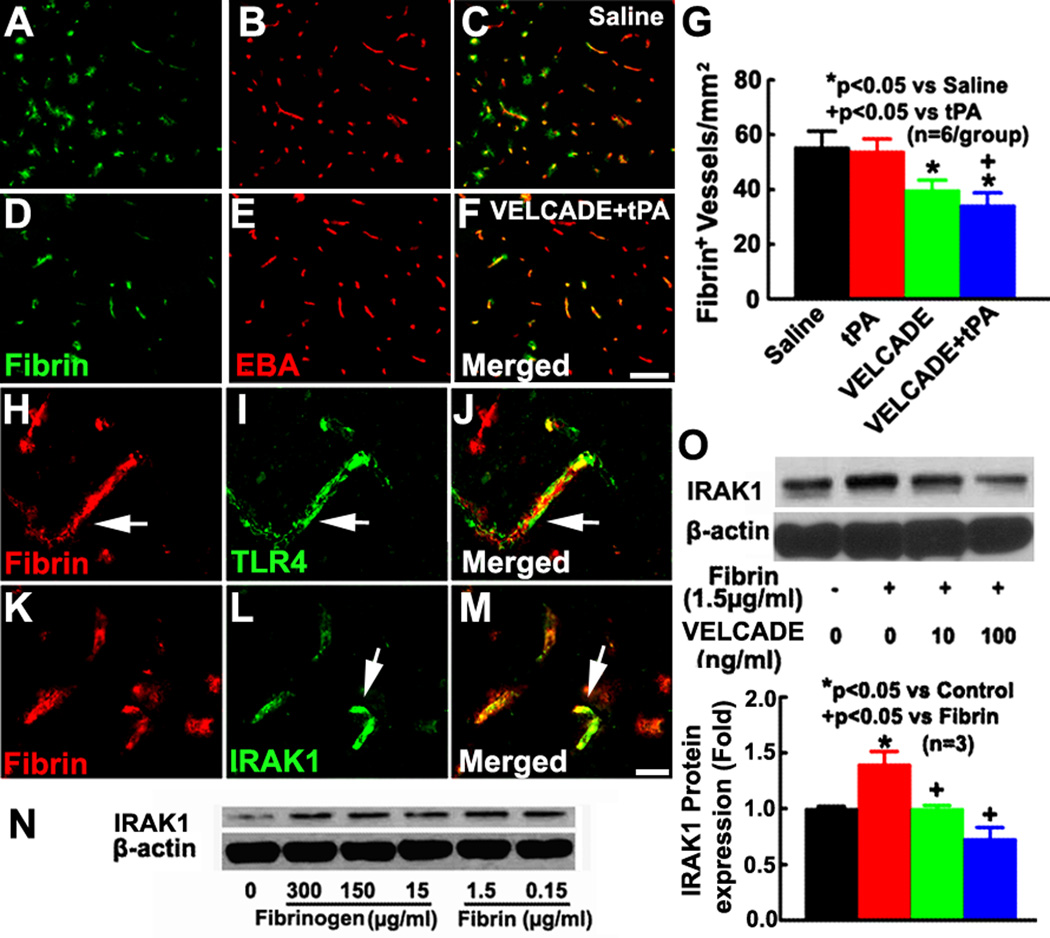
Fibrinogen activates TLR4 23. Interestingly, we found that TLR2, TLR4, and IRAK1 immunoreactive vessels were fibrin/fibrinogen positive and that VELCADE alone or in combination with tPA substantially reduced the number of fibrin/fibrinogen positive vessels (Fig. 2A–M). These data suggest that stroke-induced fibrin/fibrinogen deposition on cerebral vessels could trigger TLR expression. To further examine this possibility, we performed in vitro experiments using primary HBECs. Western blot analysis shows that incubation of the HBECs with fibrin and fibrinogen increased IRAK1, but fibrin elevated IRAK1 at a concentration of ten times less than the dose of fibrinogen (Fig. 2N), suggesting that fibrin and fibrinogen activate TLR signaling on cerebral endothelial cells. We therefore selected fibrin at 1.5µg/ml for subsequent in vitro experiments. VELCADE suppressed fibrin-elevated IRAK1 (Fig. 2O), but failed to reduce fibrin elevated TLR4 levels in HBECs (Supplemental Fig.2).
Figure 2. Fibrin/fibrinogen upregulates IRAK1 in cerebral endothelial cells.
Panels A through F show double immunofluorescent staining of fibrin/fibrinogen (green) with EBA (green) from representative rats treated with saline (A–C) and the combination of VELCADE and tPA (D–F). Panel G shows the quantitative data of fibrin/fibrinogen immunoreactive vessels in saline, VELCADE, tPA, and VELCADE with tPA groups. Double immunofluorescence staining (H to M) shows co-localization of fibrin/fibrinogen (red) with TLR4 or IRAK1 (green) from representative rats treated with saline. Panel N showing IRAK1 levels on HBECs treated with different concentrations of fibrin or fibrinogen. Panel O shows Western blot and quantitative data of IRAK1 protein levels on HBECs in the presence and absences of VELCADE. Values are mean ± SE. Bar=100µm in panels A-F. Bar=20 µm in H-M.
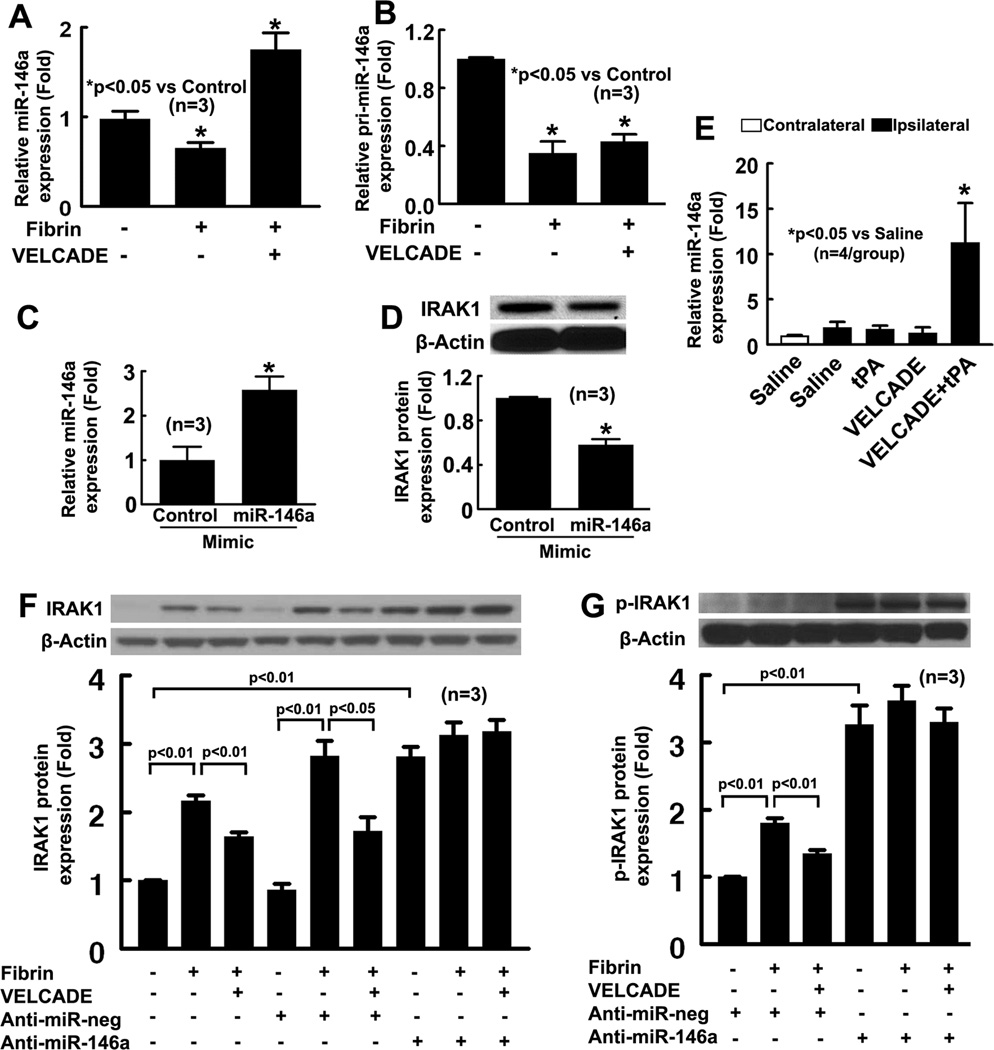
Aforementioned in vitro data indicate that VELCADE blocks IRAK1, but not TLR4 on the endothelial cells. miR-146a targets IRAK1 24. We then asked whether miR-146a is involved in fibrin-induced activation of the TLR signaling pathway and whether VELCADE affects miR-146a levels. HBECs were treated with fibrin in the presence and absence of VELCADE. RT-PCR analysis revealed that fibrin alone significantly reduced mature miR-146a and pri-miR-146a levels, whereas VELCADE abolished fibrin induced reduction of mature miR-146a, but failed to reverse fibrin induced reduction of pri-miR-146a in HBECs (Fig. 3A, B). To examine the cause effects of miR-146a on IRAK1 expression, the endothelial cells were transfected with miR-146a-mimic or miR-146a inhibitor. Using real-time RT-PCR analysis, we first examined the efficacy of transfection and found that transfection of the miR-146a mimic elevated miR-146a levels by 3 fold (Fig 3C), whereas miR-146 inhibitor reduced endogenous miR-146a by 52% in naïve HBECs (Supplement Fig. 3). Western blot analysis shows that an increase in exogenous miR-146a attenuated IRAK1 protein levels by 50% (Fig. 3D). Interestingly, attenuation of endogenous miR-146a by the miR-146a inhibitor significantly increased IRAK1 and p-IRAK1 protein levels compared to the negative control miRNA (Fig. 3F–G). Addition of fibrin or fibrin along with VELCADE did not significantly alter both IRAK1 levels in HBECs transfected with the miR-146a inhibitor (Fig. 3F–G). Collectively, our data suggest that endogenous miR-146a represses endothelial IRAK1 , that the reduction of fibrin-increased IRAK1 in HBECs by VELCADE is mediated by miR-146a, and that derepression of endogenous miR-146a increases IRAK1, leading to concealing the effect of fibrin and VELCADE on IRAK1 in HBECs.
Figure 3. Endothelial miR-146a expression.
Panels A and B are the real-time RT-PCR analyses of miR-146a (A) and pri-miR-146a (B) levels on HBECs treated with fibrin in the presence and absence of VELCADE. Transfection of miR-146a mimic increased miR-146a levels on HBECs (C) and reduced IRAK1 protein levels on HBECs (D). Quantitative RT-PCR data (E) show miR-146a levels on cerebral endothelial cells isolated by LCM in ischemic hemisphere of rats treated with saline, tPA, VELCADE, and VELCADE with tPA. Panels F and G are the representative Western blot and quantitative data of IRAK1 (F) and p-IRAK1(G) expression in HEBCs with or without anti-miR-146a transfection.
To verify our in vitro findings, we measured miR-146a levels on cerebral endothelial cells isolated from brain coronal sections by LCM. Real-time RT-PCR analysis shows the presence of PECAM mRNA (Supplemental Fig. 4), but not GFAP transcripts in the cells (data not shown), indicating that these cells are cerebral endothelial cells. Rats subjected to the combination treatment with VELCADE and tPA exhibited significantly increased miR-146a compared with saline treated rats (Fig. 3E). Monotherapy with VELCADE did not significantly change miR-146a expression compared with saline treated rats (Fig. 3E). These data suggest that miR-146a is involved in the effect of VELCADE on the TLR signaling pathway.
Treatment effects on inflammatory responses
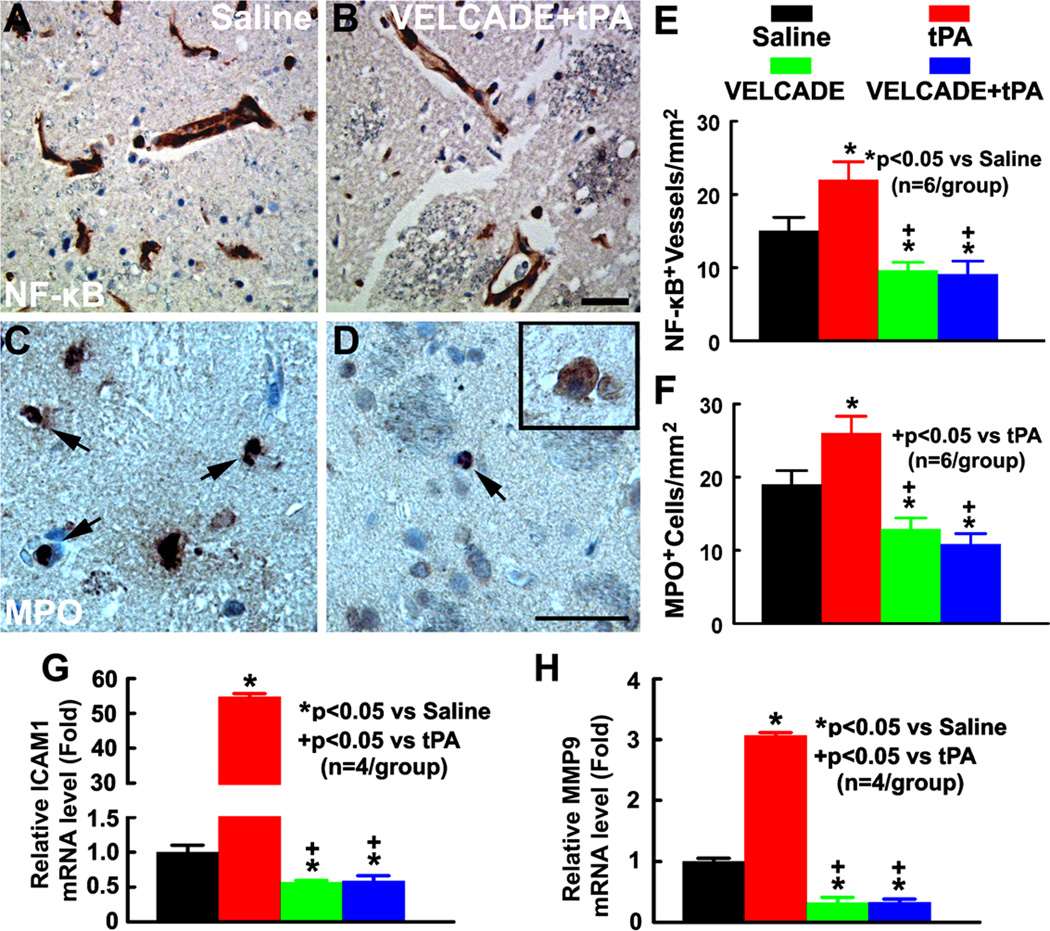
Activation of the TLR signaling triggers NF-κB activation through recruitment of IRAK family members, which initiates an inflammatory cascade via proinflammatory mediator production 25. We examined the effect of VELCADE on NF-κB activation, and proinflammatory gene expression, ICAM-1 and MMP9, on cerebral vessels, and parenchymal neutrophil infiltration. tPA monotherapy significantly increased the number of NF-κB immunoreactive vessels compared with saline treated rats (Fig. 4E). In contrast, treatment with VELCADE alone and in combination with tPA significantly reduced the number of NF-κB immunoreactive vessels compared with saline or tPA monotherapy treated rats (Fig. 4E). Concurrently, while rats treated with tPA alone exhibited a robust increase in endothelial ICAM-1 and MMP9 gene expression, treatment with VELCADE alone significantly reduced endothelial ICAM1 and MMP9 gene expression compared with saline treated rats (Fig. 4G–H). In addition, VELCADE in combination with tPA completely blocked tPA induced upregulation of endothelial ICAM1 and MMP9 gene expression, which was associated with a significant reduction of the parenchymal neutrophil infiltration compared with rats treated with saline or tPA (Fig. 4F). Thus, our data suggest that VELCADE alone and in combination with tPA reduces NF-κB activation and inflammatory response.
Figure 4. Treatment effects on proinflammatory gene expression and neutrophil accumulation.
Panels A and B show p65 (NF-κB) immunoreactive cerebral vessels from representative rats treated with saline (A) and the combination of VELCADE and tPA (B). Panels C and D show MPO immunoreactive cells from representative rats treated with saline (C), and the combination of VELCADE and tPA (D). MPO-positive cells having the typical polymorphonucleated neutrophil morphology(arrows, C, D) which can be easily distinguished from macrophages by their large size and apparent cytoplasmic inclusions (insert in D). Quantitative data (E, F) show of the number of NF-κB immunoreactive vessels (E) and MPO immunoreactive cells (F) in ischemic brain of the rat treated with saline, tPA, VELCADE, and VELCADE with tPA. Quantitative RT-PCR data show mRNA levels of ICAM1 (G) and MMP9 (H) on cerebral endothelial cells isolated by LCM in the ischemic hemisphere of the rat treated with saline, tPA, VELCADE, and VELCADE with tPA. Bars=50µm.
Infarct volume and functional outcome
We then examined the neuroprotective effect of VELCADE by measurement of infarct volume and functional outcome in ischemic rats treated with VELCADE at a dose of 0.1 mg/kg of VELCADE, since this concentration has minimal toxic effects and produces a comparable level of proteasome inhibition as its clinical usage in cancer patients 26. Treatment with VELCADE significantly reduced lesion volume and mNSS score compared with saline-treated rats 7d after stroke onset. In addition, ischemic rats treated with combination of VELCADE and tPA exhibited a significant improvement of neurological outcome as early as one day after stroke and had significantly reduced ischemic lesion volume compared with rats treated with monotherapies of VELCADE, tPA and saline at 7 days after stroke (Fig. 5). These data indicate that VELCADE is beneficial in aged rats after stroke and that the combination treatment with VELCADE and tPA attains synergistic neuroprotective effects.
Figure 5. Stroke outcome.
Panel A is the H&E stained coronal sections from representative rats treated saline, tPA, VELCADE, and the combination of VELCADE and tPA. Panel B shows infarct volume assessed 7 days after stroke onset. Panel C shows the modified neurological severity score measured at 1 and 7d after stroke onset.
Discussion
The present study demonstrated that stroke acutely activated the TLR signaling pathway on cerebral vasculature in aged rats. Treatment with VELCADE either alone or in combination with tPA substantially attenuated activation of TLR signaling, which was associated with amelioration of proinflammatory responses and ischemic brain injury. In vitro, fibrin upregulated IRAK1 and TLR4 on primary cerebral endothelial cells, whereas VELCADE suppressed fibrin-elevated IRAK1, which was associated with upregulation of miR-146a. These data suggest that the cerebral vascular TLR signaling pathway plays an important role in stroke of the aged rat, while the neuroprotective effect of VELCADE is at least in part mediated via inactivation of TLR signaling pathway.
In cerebral endothelial cells, a recent in vitro study indicated that the activation of TLRs provokes BBB permeability triggered by oxidative stress 9. In addition, preconditioning with various TLR ligands, such as Pam3CSK4 and lipopolysaccharide, induce tolerance to cerebral ischemia via attenuation of BBB disruption and microvascular perfusion deficits 27, 28. These data implicate the importance of TLR signaling in regulating cerebral vasculature homeostasis following stroke. In the present study, a robust increase in TLR2 and TLR4 immunoractivity in cerebral vessels 24h after stroke, suggesting an acute upregulation of TLR2 and TLR4 on the cerebral vasculature following stroke. Moreover, as TLRs exert their effects by signaling through downstream adaptors, we found that the expression of TLRs on the cerebral vessels was closely associated with the upregulation of IRAK1 following stroke. Thus, our data provide direct evidence indicating that cerebral ischemia evokes the activation of TLR signaling on cerebral vasculature in the aged brain. Previous studies indicated that cerebral ischemia evokes the activation of TLR2 and TLR4 in neurons and glia, which orchestrates the activation of proinflammatory and proapoptotic pathways, and subsequently contributes to ischemic brain damage 8, 10, 11, 29. The discrepancy of cellular sources of TLR2 and TLR4 in the ischemic brain between the present study and published data may be due to differences among species (mouse vs rat), age (young adult vs aged), and models (transient vs embolic MCA occlusion). Nevertheless, the present results along with the published data indicate that the TLR signaling pathway plays an important role in acute stroke development.
In the CNS, several endogenous ligands of TLRs have been identified 30. Among these, TLR signaling has been shown to respond to fibrin/fibrinogen stimulation which induces proinflammatory responses 23. However, the interaction between fibrin/fibrinogen and TLRs signaling has not been established in the ischemic brain. The present study shows that stroke-induced activation of TLRs occurred at the site of vascular fibrin/fibrinogen accumulation. In addition, incubation of the cerebral endothelial cells with fibrin increased IRAK1 and TLR4 expression. Thus, we contend that stroke triggers vascular fibrin accumulation to function as an endogenous ligand, which evokes the activation of TLR signaling on the cerebral endothelial cells. As an important pathological characteristic of vascular dysfunction, the acute accumulation of fibrin has been tightly intertwined with inflammatory response as well as BBB disruption after ischemic insult 31, 32. The activation of TLRs may exacerbate these detrimental vascular events, and therefore plays a causative role in ischemic brain damage.
The activation of IRAK1 is a key event involved in downstream signaling of TLRs 22. Interestingly, in the present study, while treatment with VELCADE alone and in combination with tPA both exhibited a moderate reduction of TLR expression, the combination treatment resulted in a further reduction of IRAK1 expression compared with VELCADE monotherapy. In addition, our in vitro data show that VELCADE suppressed fibrin-elevated IRAK1, but failed to reduce TLR4 levels on endothelial cells. These data imply that additional regulatory mechanisms may be involved in VELCADE mediated TLR signaling via targeting the IRAK1. In this context, miR-146a is reported to be an important regulator of TLR signaling through IRAK1 repression 24, 33. The profound effects of VELCADE and tPA on TLR signaling prompted us to investigate the involvement of endothelial miR-146a following stroke. Here we show, for the first time, that miR-146a was upregulated on the cerebral vasculature in aged rats after stroke. The combination treatment with VELCADE and tPA further substantially increased miR-146a levels on the cerebral vessels. As a number of experiments demonstrate that elevated miR-146 levels block TLR signaling pathway through targeting IRAK1 and tumor necrosis factor receptor-associated factor 6 (TRAF6) genes 24, 33, we found that the elevated miR-146a levels after the combination treatment was inversely associated with a robust reduction of IRAK1 expression. A direct effect of VELCADE on miR-146a was demonstrated by our in vitro data showing that VELCADE upregulated miR-146a on cerebral endothelial cells, which completely abolished fibrin-elevated IRAK1 proteins. Moreover, transfection of miR-146a mimics decreased IRAK1 expression, whereas attenuation of endogenous miR-146a in cerebral endothelial cells by the miR-146 inhibitor substantially increased IRAK1, suggesting that miR-146 represses IRAK1 in cerebral endothelial cells. These data are consistent with in vivo findings from miR-146a knockout mice showing that miR-146a directly targets IRAK1 and knockout of miR-146a derepresses IRAK1 34. IRAK1 is one of the signal transducers in activation of the NF-κB pathway 22. By repressing IRAK1, miR-146a acts as a negative regulator of the NF-κB pathway 35. We speculate that miR-146a upregulated by VELCADE represses IRAK1, which results in attenuation of NF-κB activation, consequently leading to diminishing inflammatory responses (please see Fig, 6 for a simplified schematic diagram illustrating the effects of VELCADE on miR-146a and TLR signaling pathway).
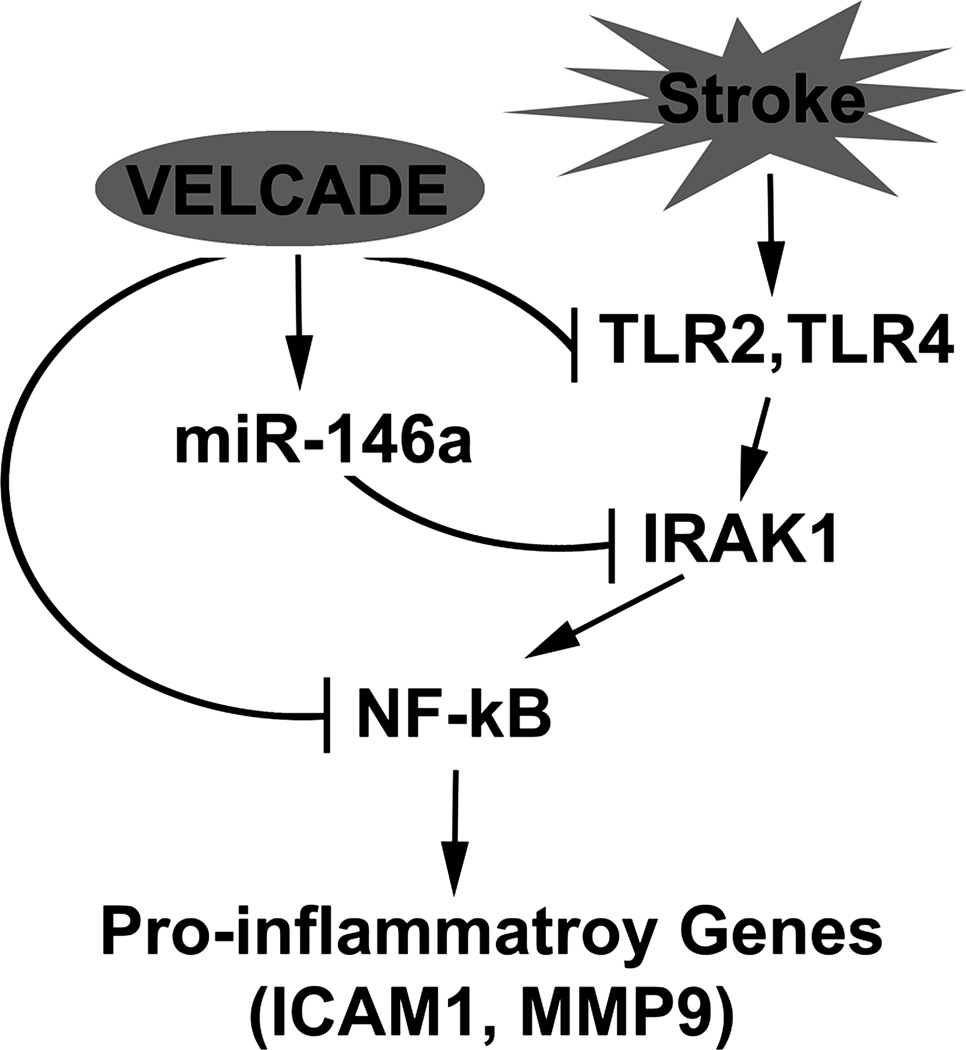
Figure 6. Schematic representation of potential effects of VELCADE on TLR signaling pathway.
Stroke induces the activation of vascular TLR signal, which leads to upregulation of proinflammatory genes via the activation of NF-κB. VELCADE inactivates NF-kB through either directly blocking stroke-activated TLR2 and TLR4 or indirectly repressing IRAK1 by VELCADE upregulated miR-146a, which consequently downregulates proinflammatory gene expression. Thus, VELCADE acts on a multi-level inhibition of TLR signaling in ischemic cerebral vessels.
miRNA genes are transcribed into pri-miRNA that generate pre-miRNA by a Drosha. The pre-miRNA is then cleaved to produce the mature by a Dicer 36. Our data suggest that that fibrin suppresses the biosynthesis of miR-146a at both pri-miRNA and mature miRNA levels, while VELCADE likely acts on mature miR-146a because VECALDE significantly increased the miR-146a levels in fibrin-treated cerebral endothelial cells, but failed to reverse fibrin-induced reduction of pri-miR-146a. Currently, we do not know mechanisms underlying upregulation of mature miR-146a by VELCADE. Nevertheless, it is noteworthy that proteasome inhibitors stabilize Smads and the tumor-suppressor protein p53 37, 38, all of which has been shown to interactive with miRNA processing enzymes, such as Drosha and Dicer, and thereby, regulates miRNA biogenesis at the post-transcriptional level 39, 40.
Interestingly, while in vivo monotherapy of VELCADE significantly reduced TLR2, TLR4, and IRAK1 expression and ischemic lesion volume, it did not substantially elevate miR-146a levels on cerebral vasculature. These data suggests that VELCADE may directly modulate TLR signaling, and exerts the neuroprotective effect independent of miR-146a regulation. Proteasome inhibitors target the ubiquitin-proteasome pathway, which mediates a variety of cellular functions through multiple mechanisms. Given the large variety of substrates in the ubiquitin-proteasome pathway, we propose that the upregulation of miR-146a following the combination treatment represents another potential mechanism for turning down the stroke induced activation of TLR signaling, which in addition to the direct effects of VELCADE on TLR signaling, may offer greater neuroprotective effects in the treatment of stroke. Further in vivo experiments are warranted to address a cause-effect of miR-146a on VELCADE-mediated neuroprotection in stroke.
In conclusion, we demonstrated that treatment of acute stroke with VELCADE alone and in combination with tPA facilitates a multi-level inhibition of cerebral vascular TLR signaling in the ischemic brain, leading to attenuation of ischemic brain damage. miR-146a functions as a negative regulator of endothelial TLR signaling, and likely participates in VELCADE and tPA mediated neuroprotection through targeting IRAK1 .
Supplementary Material
Acknowledgments
The authors wish to thank Cynthia Roberts, Yisheng Cui, Min Wei, Qing-e Lu, and Sutapa Santra for technical assistance.
Funding Sources: This work was supported by NIH grants RO1 NS62832, RO1 AG037506, and RO1 NS75156.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 3.Constantin D, Cordenier A, Robinson K, Ala'Aldeen DA, Murphy S. Neisseria meningitidis-induced death of cerebrovascular endothelium: Mechanisms triggering transcriptional activation of inducible nitric oxide synthase. J Neurochem. 2004;89:1166–1174. doi: 10.1111/j.1471-4159.2004.02393.x. [DOI] [PubMed] [Google Scholar]
- 4.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains cns inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. Tlr2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 7.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemiareperfusion injury in toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 8.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates cns injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int. 2010;57:556–564. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 11.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voorhees PM, Dees EC, O'Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- 13.Henninger N, Sicard KM, Bouley J, Fisher M, Stagliano NE. The proteasome inhibitor velcade reduces infarction in rat models of focal cerebral ischemia. Neurosci Lett. 2006;398:300–305. doi: 10.1016/j.neulet.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Zhang ZG, Buller B, Jiang J, Jiang Y, Zhao D, Liu X, Morris D, Chopp M. Combination treatment with velcade and low-dose tissue plasminogen activator provides potent neuroprotection in aged rats after embolic focal ischemia. Stroke. 2010;41:1001–1007. doi: 10.1161/STROKEAHA.109.577288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grunebach F, Brossart P. Proteasome inhibitor bortezomib modulates tlr4-induced dendritic cell activation. Blood. 2006;108:551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 16.Yannaki E, Papadopoulou A, Athanasiou E, Kaloyannidis P, Paraskeva A, Bougiouklis D, Palladas P, Yiangou M, Anagnostopoulos A. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010;62:3277–3288. doi: 10.1002/art.27690. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Nishio M, Peters SC, Tschernatsch M, Walberer M, Weidemann S, Heidenreich R, Couraud PO, Weksler BB, Romero IA, Gerriets T, Preissner KT. Signaling mechanism of extracellular rna in endothelial cells. Faseb J. 2009;23:2100–2109. doi: 10.1096/fj.08-121608. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 20.Kaltschmidt C, Kaltschmidt B, Henkel T, Stockinger H, Baeuerle PA. Selective recognition of the activated form of transcription factor nf-kappa b by a monoclonal antibody. Biol Chem Hoppe Seyler. 1995;376:9–16. doi: 10.1515/bchm3.1995.376.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 22.Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of toll-like receptor-dependent responses by irak1 and irak2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 23.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 24.Taganov KD, Boldin MP, Chang KJ, Baltimore D. Nf-kappab-dependent induction of microrna mir-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Gao X, Li S, Cao Z. Recruitment of irak to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci U S A. 1997;94:12829–12832. doi: 10.1073/pnas.94.24.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott PJ, Ross JS. The proteasome: A new target for novel drug therapies. Am J Clin Pathol. 2001;116:637–646. doi: 10.1309/44HW-5YCJ-FLLP-3R56. [DOI] [PubMed] [Google Scholar]
- 27.Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a tlr2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson DA, Furuya K, Gotoh J, Nakao Y, Hallenbeck JM. Cerebrovascular hemodynamics and ischemic tolerance: Lipopolysaccharide-induced resistance to focal cerebral ischemia is not due to changes in severity of the initial ischemic insult, but is associated with preservation of microvascular perfusion. J Cereb Blood Flow Metab. 1999;19:616–623. doi: 10.1097/00004647-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 31.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 32.Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost. 2009;7 Suppl 1:151–154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. Microrna-146a feedback inhibits rig-i-dependent type i ifn production in macrophages by targeting traf6, irak1, and irak2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 34.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. Mir- 146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boldin MP, Baltimore D. Micrornas, new effectors and regulators of nf-kappab. Immunol Rev. 2012;246:205–220. doi: 10.1111/j.1600-065X.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 36.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: Microrna biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 37.Izzi L, Attisano L. Regulation of the tgfbeta signalling pathway by ubiquitinmediated degradation. Oncogene. 2004;23:2071–2078. doi: 10.1038/sj.onc.1207412. [DOI] [PubMed] [Google Scholar]
- 38.Lo RS, Massague J. Ubiquitin-dependent degradation of tgf-beta-activated smad2. Nat Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- 39.Boominathan L. The tumor suppressors p53, p63, and p73 are regulators of microrna processing complex. PLoS One. 2010;5:e10615. doi: 10.1371/journal.pone.0010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis BN, Hilyard AC, Lagna G, Hata A. Smad proteins control drosha-mediated microrna maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.