Abstract
Background
In contrast to many observational studies, women in the Women’s Health Initiative (WHI) trial randomised to oestrogen-alone had lower invasive breast cancer incidence than those assigned placebo. Influence of oestrogen use on breast cancer mortality has not been reported.
Methods
Between 1993 and 1998, the WHI enrolled 10,739 postmenopausal women from 40 US centres into a randomized, double-masked, placebo-controlled trial evaluating oral conjugated equine oestrogen (0·625 mg/d). Women aged 50–79 years with prior hysterectomy, anticipated 3-year survival, and mammography clearance were randomized by a computerized, permuted block algorithm, stratified by age group and centre, to receive oestrogen or matching placebo. The trial was terminated early, in 2004, for an adverse effect on stroke. In extended follow-up through August 2009, we assessed long-term effects of oestrogen use on invasive breast cancer incidence, tumor characteristics, and mortality. Cox regression models were used to estimate intention-to-treat hazard ratios [HRs].
Findings
After a median 11.8 (interquartile range [IQR], 9·1 to 12·9) years of follow-up, conjugated equine oestrogen-alone use for a median of 5·9 (IQR, 2·5 to 7·3) years was associated with lower invasive breast cancer incidence compared to placebo (151 vs. 199 breast cancers; annualized rates, 0·27% vs. 0·35%; HR, 0·77; 95% confidence interval [CI], 0·62 to 0·95; P=0·02) with no difference (P=0·76) between intervention-phase (HR, 0·79; 95% CI, 0·61 to 1·02) and post-intervention effects (HR, 0·75; 95% CI: 0·51 to 1·09) ). Potential effect modification by benign breast disease (P=0·01) and family history of breast cancer (P=0·02) was observed. In the oestrogen-alone group fewer women died from breast cancer (6 vs.16 deaths; annualized rates 0·009% vs. 0·024%; HR, 0·37; 95% CI, 0·13 to 0·91; P=0.03) and fewer died from all causes after a breast cancer diagnosis (30 vs. 50 deaths; annualized rates, 0·046% vs. 0·076%; HR, 0·62; 95% CI, 0·39 to 0·9;, P=0·04).
Interpretation
Women with hysterectomy seeking relief of climacteric symptoms may be given reassurance regarding breast cancer influence of oestrogen use consistent with durations observed in this trial. However, these findings do not support oestrogen use for breast cancer risk reduction since this benefit may not apply to populations at higher risk.
Funding
US National Heart, Lung and Blood Institute. Wyeth provided study medications.
Keywords: menopausal hormone therapy, breast neoplasms, breast cancer mortality, prevention trial
INTRODUCTION
Endogenous oestrogen levels have been consistently associated with increased breast cancer risk.1 Exogenous oestrogen use has also been associated with higher breast cancer incidence in many2–5 but not all6,7 observational studies, particularly in leaner women3–5,8 and those with longer duration of use.4,5,8,9 Oestrogen use has been linked to hormone receptor positive and earlier stage disease,3,5 suggesting better prognosis10 although associations with breast cancer mortality are mixed.2,9,10–17
During intervention in the Women’s Health Initiative (WHI) randomised trial in 10,739 menopausal women with prior hysterectomy, a non-significant breast cancer risk reduction with conjugated equine oestrogens compared to placebo was observed (hazard ratio [HR], 0·79; 95% confidence interval [CI], 0·61 to 1·02).17 After trial intervention was stopped in February 2004 for an adverse effect on stroke,18 follow-up continued through the planned termination in 2005 and annually thereafter for those consenting to extended surveillance. Results of a pre-specified analysis in 2009 indicated that most estimates of oestrogen influence on chronic disease risk were attenuated. The lower breast cancer risk in the oestrogen group persisted (HR 0.77; 95% CI, 0.62–0.95) however, reaching statistical significance.19 Here we provide additional details on effects of oestrogen use on invasive breast cancer incidence during and after intervention by tumor characteristics and previously identified effect modifiers. For the first time we present results on breast cancer-related mortality.
METHODS
Study population, randomisation, and masking
Menopausal women aged 50–79 years with prior hysterectomy were recruited into the WHI randomised, double-masked, placebo-controlled trial of oestrogen-alone at 40 US centres between 1993 and 1998. Prior breast cancer was an exclusion criterion. Baseline mammograms and clinical breast exams were required. Current hormone users required a three-month washout. Eligible women were randomised (1:1) to oral conjugated equine oestrogen (0·625mg/d) or matching placebo using a computerized, permuted-block algorithm, stratified by age group and clinical centre. Randomization and medication dispensing was supported through a secure database system developed and implemented by the WHI Clinical Coordinating Center. Clinical centre staff entered the eligibility data into the database and executed a database function which confirmed eligibility and performed the randomisation. Double-masking was implemented through an associated database medication dispensing system. The study was approved by each centre’s Institutional Review Board. All women provided written informed consent. Details of study design, eligibility and implementation have been published.18,19 The trial is registered with ClinicalTrials.gov (NCT00000611).
Data collection
Prior hormone use was ascertained at baseline by an interviewer-administered questionnaire. Medical, reproductive and family histories were obtained by self-administered questionnaires. Height and weight were measured by study staff. All medication use was assessed by intervention-administered questionnaire at baseline and follow-up years 1, 3, 6, and 9. Adherence was assessed primarily by pill counts or weighing returned bottles; self-report was used only rarely.
Until 2005, vital and health status was assessed twice a year. Mammography was required annually throughout the trial. When the intervention ended on 29 February, 2004 (mean (SD) follow-up of 7·1(1·6) years), all participants were unmasked and asked to stop taking study medications. Follow-up continued according to the original protocol until the planned study termination (March 2005). During study close-out, 7645 participants (79% of the 9671 living participants in active follow-up at that time) consented to extended follow-up.19 Beginning in 2005, vital and health status updates have been obtained yearly. This report presents data through 14 August, 2009 (overall median 11·8 (IQR, 9·1 to 12·9) years of follow-up including median 4.7 (IQR, 3·5 to 5·1) years of post-intervention follow-up).
Breast cancers were documented by medical and pathology record review by centrally-trained physician adjudicators. Tumor characteristics were coded at the WHI Clinical Coordinating Center using Surveillance, Epidemiology, and End Results guidelines.20 Deaths were documented with death certificates and medical records. Periodic searches of the National Death Index were conducted, most recently in 2010, to identify deaths in participants lost to follow-up through 2008. Central physician adjudicators reviewed medical records to determine cause of death. All adjudicators were masked to randomisation assignment.
Statistical analysis
Contrasts of breast cancer incidence rates by treatment group were made using failure-time methods under the intention-to-treat principle. Hazard ratios and 95% confidence intervals were estimated from Cox regression models, stratified by age group and randomisation assignment in the concurrent WHI dietary modification trial. Analyses considered three time periods: the intervention phase representing randomisation through 29 February, 2004; the post-intervention phase beginning 1 March, 2004 until the database snapshot on 14 August, 2009; and overall results. Event times during the intervention phase were censored at date of death, last follow-up, or intervention phase termination (29 February, 2004) whichever occurred first. Participants were included in post-intervention phase incidence analyses if they were alive, in follow-up and had no prior breast cancer as of 1 March, 2004. Censoring was defined by the earlier of death or last follow-up date. Analyses of overall results began at randomisation with censoring as above. Analyses of breast cancer subtypes incorporated censoring at diagnosis of any other breast cancer subtype. Heterogeneity of hazard ratios across tumor subtypes was tested using competing risk models. Analyses of breast cancer mortality included all participants using the timeframes defined above. Women in active follow-up were censored at last contact date. Women in passive follow-up were censored on 31 December, 2008, the last date covered by the National Death Index linkage. To examine oestrogen effects over time, we fit linear, time-varying hazard ratios for randomization assignment over the intervention and post-intervention phases separately. We compared slopes for each phase using Wald tests.
We examined the influence of non-adherence to protocol-assigned treatment by censoring events six months after participants first became non-adherent (consumed <80% of study medications or initiated non-study hormone therapy). In addition, time-varying weights, inversely proportional to the estimated probability of continued adherence, were used in these proportional-hazards models to adjust for changes in the distribution of sample characteristics during follow-up.21
Secondary analyses tested interactions between randomisation assignment and 16 baseline characteristics within the primary Cox model, expanded to include the designated baseline factor, randomisation assignment, and interaction term(s). Participants with missing values were omitted from the corresponding analysis. At most one interaction was expected to be significant at the 0·05 level by chance alone.
All analyses were conducted using SAS version 9·1·3 (SAS Institute). Figures were created using R 2·11 (R Development Core Team). All P-values are two-sided.
Role of the Sponsor
The WHI Project Office at the US National Heart, Lung, and Blood Institute (NHLBI), the Sponsor, reviewed and approved the final manuscript but had no other role in the preparation of this report. Decisions concerning study design, data collection and analysis, result interpretation, manuscript preparation, or the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives. AA had access to the raw data. GA had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Baseline breast cancer risk factors among women who consented to extended follow-up were similar to those of the original cohort18 and comparable across randomisation groups, except for a small imbalance in bilateral oophorectomy and benign breast disease (Table 1). During the intervention phase, 80%–90% of participants had yearly mammograms with comparable frequencies in the two treatment groups.17,22 Post-intervention 81·2% (3894/4794) of oestrogen group and 81·3% (3965/4877)of placebo group participants had at least one mammogram.
Table 1.
Baseline characteristics of WHI trial of oestrogen-alone participants who consented to extended follow-up by randomised treatment assignment.
| Oestrogen | Placebo | |||
|---|---|---|---|---|
| N | % | N | % | |
| Age group at screening | ||||
| 50–59 | 1223 | 32·4 | 1232 | 31·9 |
| 60–69 | 1740 | 46·1 | 1799 | 46·5 |
| 70–79 | 815 | 21·6 | 836 | 21·6 |
| Race/ethnicity | ||||
| White | 2945 | 78·0 | 3001 | 77·6 |
| Black | 514 | 13·6 | 565 | 14·6 |
| Hispanic | 189 | 5·0 | 181 | 4·7 |
| American Indian | 31 | 0·8 | 18 | 0·5 |
| Asian/Pacific Islander | 54 | 1·4 | 49 | 1·3 |
| Unknown | 45 | 1·2 | 53 | 1·4 |
| Education | ||||
| ≤ High school/GED | 1167 | 31·2 | 1137 | 29·6 |
| Post-high school | 1630 | 43·6 | 1704 | 44·4 |
| College degree or higher | 945 | 25·3 | 998 | 26·0 |
| Family income ($) | ||||
| <20K | 891 | 24·8 | 913 | 24·9 |
| 20–<35K | 1070 | 29·8 | 1084 | 29·6 |
| 35–<50K | 741 | 20·6 | 758 | 20·7 |
| 50–<75K | 550 | 15·3 | 555 | 15·2 |
| ≥75K | 340 | 9·5 | 352 | 9·6 |
| Marital status | ||||
| Never married | 123 | 3·3 | 111 | 2·9 |
| Divorced / Separated | 709 | 18·8 | 687 | 17·9 |
| Widowed | 777 | 20·7 | 785 | 20·4 |
| Presently married/Living as married | 2153 | 57·2 | 2264 | 58·9 |
| Body-mass index (kg/m2) | ||||
| <25 | 785 | 20·9 | 771 | 20·1 |
| 25 – <30 | 1289 | 34·3 | 1391 | 36·2 |
| ≥30 | 1687 | 44·9 | 1683 | 43·8 |
| Smoking status | ||||
| Never | 1988 | 53·1 | 1972 | 51·5 |
| Past | 1417 | 37·9 | 1489 | 38·9 |
| Current | 336 | 9·0 | 370 | 9·7 |
| Age at menarche | ||||
| ≤11 | 890 | 23·7 | 929 | 24·1 |
| 12–13 | 2030 | 54·1 | 2013 | 52·3 |
| ≥14 | 833 | 22·2 | 908 | 23·6 |
| Number of term pregnancies | ||||
| Never pregnant/No term pregnancy | 350 | 9·3 | 307 | 8·0 |
| 1–2 | 1033 | 27·5 | 1099 | 28·6 |
| 3–4 | 1527 | 40·7 | 1605 | 41·7 |
| ≥5 | 840 | 22·4 | 835 | 21·7 |
| Age at first birth, years | ||||
| Never pregnant/No term pregnancy | 350 | 10·3 | 307 | 8·8 |
| <20 | 822 | 24·2 | 872 | 24·9 |
| 20 – 29 | 2060 | 60·7 | 2128 | 60·9 |
| 30+ | 163 | 4·8 | 190 | 5·4 |
| Number of months breastfed | ||||
| Never breastfed | 1775 | 47·8 | 1739 | 45·8 |
| Breastfed ≤1 year | 1412 | 38·0 | 1525 | 40·1 |
| Breastfed >1 year | 525 | 14·1 | 535 | 14·1 |
| Benign breast disease | ||||
| No | 2758 | 80·2 | 2693 | 77·7 |
| Yes, 1 biopsy | 500 | 14·5 | 550 | 15·9 |
| Yes, 2+ biopsies | 183 | 5·3 | 222 | 6·4 |
| First degree female relatives with breast cancer | ||||
| None | 2987 | 85·5 | 3084 | 86·0 |
| 1 | 459 | 13·1 | 453 | 12·6 |
| 2 or more | 48 | 1·4 | 49 | 1·4 |
| Gail model 5-year risk score, | ||||
| <1.25% | 1456 | 38·5 | 1505 | 38·9 |
| [1.25%, 1.75%) | 1185 | 31·4 | 1220 | 31·5 |
| ≥1.75% | 1137 | 30·1 | 1142 | 29·5 |
| Age at hysterectomy | ||||
| <40 | 1495 | 39·8 | 1501 | 39·0 |
| 40–49 | 1643 | 43·7 | 1662 | 43·2 |
| 50–54 | 345 | 9·2 | 412 | 10·7 |
| 55+ | 275 | 7·3 | 271 | 7·0 |
| Bilateral oophorectomy | ||||
| No | 2143 | 61·0 | 2094 | 58·2 |
| Yes | 1370 | 39·0 | 1507 | 41·8 |
| Years since menopause | ||||
| <10 | 636 | 19·9 | 623 | 18·8 |
| 10 - <20 years | 1025 | 32·1 | 1104 | 33·3 |
| ≥20 | 1535 | 48·0 | 1586 | 47·9 |
| Hormone therapy use | ||||
| Never | 1929 | 51·1 | 1916 | 49·6 |
| Past | 1304 | 34·5 | 1373 | 35·5 |
| Current | 544 | 14·4 | 575 | 14·9 |
| Unopposed oestrogen use | ||||
| Non-user | 2006 | 53·1 | 2007 | 51·9 |
| <5 years | 938 | 24·8 | 1008 | 26·1 |
| ≥5 years | 834 | 22·1 | 852 | 22·0 |
| Oestrogen + Progestin use | ||||
| Non-user | 3613 | 95·6 | 3668 | 94·9 |
| <5 years | 108 | 2·9 | 123 | 3·2 |
| ≥5 years | 57 | 1·5 | 76 | 2·0 |
We previously reported that 53·8% of participants had stopped study medications at trial termination with frequencies closely comparable between randomization groups. By that time 5·7% of oestrogen-alone and 9·1% of placebo group participants initiated hormone use from their own healthcare providers.18 Among 7472 participants with extended follow-up, 604 (8·1%) reported using hormones post-intervention, with slightly more in the oestrogen group than the placebo group (9·0% (334/3699) vs. 7·2% (270/3773); P=0·003).
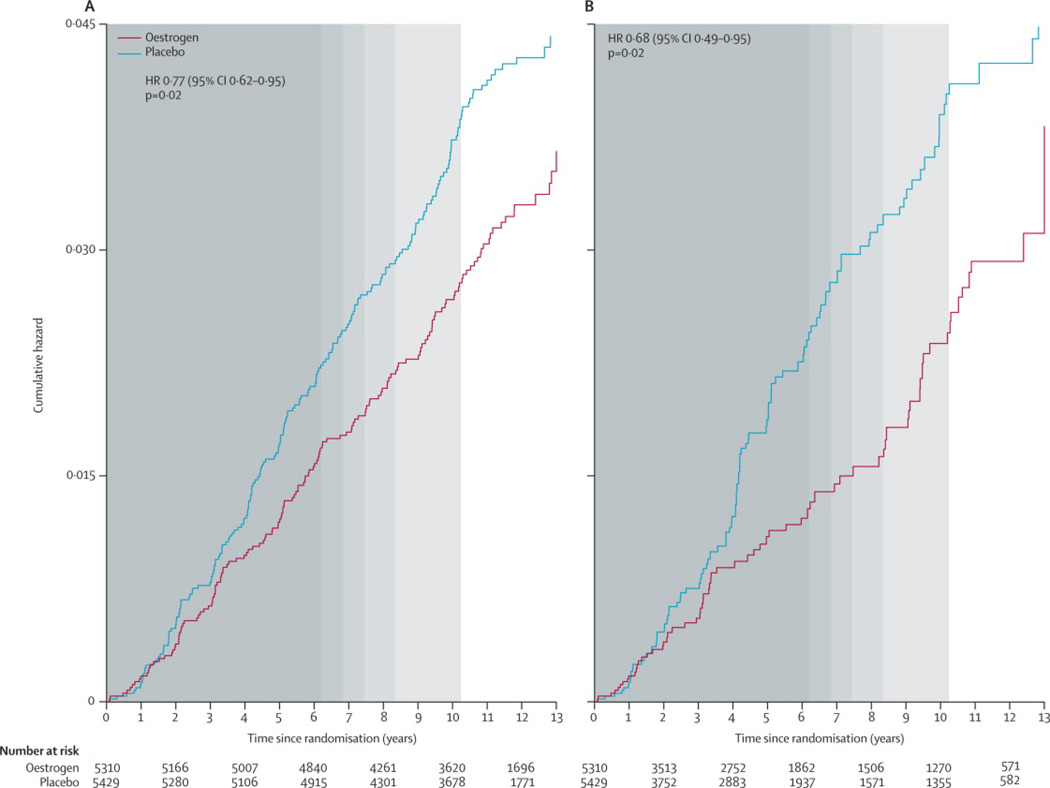
In intention-to-treat analyses reflecting a median 5·9 years (IQR, 2·7 to 7·3) from randomization until the early termination of the trial intervention and a median 11·8 (IQR, 9.1 to 12·9) years of follow-up, conjugated equine oestrogen use was associated with lower overall invasive breast cancer incidence compared to placebo (151 versus 199 events; annualized rates, 0·27% vs. 0·35%; HR, 0·77; 95% CI, 0·62 to 0·95; P=0·02)19 (Table 2 and Figure 1), with no difference between intervention and post-intervention hazard ratios (P for interaction=0·76). Adjustment for the small imbalances observed in the characteristics of extended follow-up participants had no appreciable effect on the post-intervention results. The estimated trends in oestrogen hazard ratios by time since randomisation and by time since trial cessation were both positive but were not statistically significant (P for slope, 0·19 and 0·32, respectively).
Table 2.
Associations between conjugated equine oestrogen and breast cancer incidence and mortality during and after the intervention in the Women's Health Initiative randomised trial
| Intervention Phase, N=10,739 (Events on or Before 29 Feb, 2004) |
Post-Intervention Phase1,N=9,671 (Events occurring 1 March, 2004–14 Aug, 2009) |
Overall (Events through 14 August, 2009) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oestrogen (n = 5,310) |
Placebo (n=5,429) |
Oestrogen (n=4,794) |
Placebo (n=4,877) |
Oestrogen (n=5,310) |
Placebo (n=5,429) |
||||||
| Follow-up time (months) | 85·5 | 85·9 | 47·1 | 47·2 | 128·2 | 128·5 | |||||
| Outcomes | N (%) | N (%) | HR2,3 (95% CI) | N (%) | N (%) | HR2,4 (95% CI) | P-diff5 | N (%) | N (%) | HR2,3 (95% CI) | P-het6 |
| Invasive Breast Cancer | 104 (0·28%) | 135 (0·35%) | 0·79 (0·61,1·02) | 47 (0·26%) | 64 (0·34%) | 0·75(0·51,1·09) | 0·76 | 151 (0·27%) | 199 (0·35%) | 0·77 (0·62,0·95) | |
| Histology7 | 0·33 | ||||||||||
| Ductal | 60 (0·16%) | 88 (0·23%) | 0·70 (0·51,0·98) | 26 (0·14%) | 43 (0·23%) | 0·62(0·38,1·01) | 0·63 | 86 (0·15%) | 131 (0·23%) | 0·67 (0·51,0·88) | |
| Lobular | 18(0·048%) | 12(0·031%) | 1·56 (0·75,3·24) | 2(0·011%) | 7 (0·038%) | 0·28(0·06,1·34) | 0·06 | 20 (0·036%) | 19 (0·033%) | 1·09 (0·58,2·04) | |
| Ductal and Lobular | 12 (0·032%) | 13 (0·034%) | 0·93 (0·42,2·03) | 7 (0·038%) | 5 (0·027%) | 1·38(0·44,4·34) | 0·55 | 19 (0·034%) | 18 (0·032%) | 1·06 (0·55,2·01) | |
| Other | 14 (0·037%) | 21 (0·055%) | 0·68 (0·34,1·33) | 12 (0·065%) | 9 (0·048%) | 1·37(0·58,3·25) | 0·22 | 26 (0·047%) | 30 (0·053%) | 0·88 (0·52,1·49) | |
| ER status7 | 0·78 | ||||||||||
| ER+ | 72 (0·19%) | 96 (0·25%) | 0·7 (0·57,1·05) | 38 (0·21%) | 53 (0·29%) | 0·72(0·48,1·10) | 0·79 | 110 (0·20%) | 149 (0·26%) | 0·75 (0·59,0·96) | |
| ER− | 19(0·051%) | 22 (0·058%) | 0·88 (0·48,1·63) | 6 (0·033%) | 9 (0·048%) | 0·70(0·25,1·96) | 0·68 | 25 (0·045%) | 31 (0·055%) | 0·81 (0·48,1·38) | |
| PR status7 | 0·34 | ||||||||||
| PR+ | 57 (0·15%) | 71 (0·19%) | 0·83 (0·58,1·17) | 35 (0·19%) | 41 (0·22%) | 0·86(0·55,1·36) | 0·91 | 92 (0·17%) | 112 (0·20%) | 0·84 (0·63,1·10) | |
| PR− | 32 (0·086%) | 43 (0·11%) | 0·76 (0·48,1·20) | 9 (0·049%) | 20 (0·11%) | 0·46(0·21,1·02) | 0·27 | 41 (0·074%) | 63 (0·11%) | 0·66 (0·45,0·98) | |
| HER2 overexpression7 | 0·045 | ||||||||||
| Yes | 18(0·048%) | 12(0·031%) | 1·58 (0·76,3·27) | 5 (0·027%) | 4 (0·022%) | 1·32(0·35,4·94) | 0·84 | 23 (0·041%) | 16 (0·028%) | 1·50 (0·79,2·83) | |
| No | 53 (0·14%) | 67 (0·18%) | 0·81 (0·56,1·16) | 36 (0·20%) | 54 (0·29%) | 0·67(0·44,1·03) | 0·50 | 89 (0·16%) | 121 (0·21%) | 0·74 (0·56,0·97) | |
| Triple-negative tumor7 | 0·26 | ||||||||||
| Yes | 12 (0·032%) | 8 (0·021%) | 1·54 (0·63,3·79) | 4 (0·022%) | 6 (0·032%) | 0·69(0·19,2·44) | 0·30 | 16 (0·029%) | 14 (0·025%) | 1·14 (0·56,2·34) | |
| No | 92 (0·25%) | 127 (0·33%) | 0·74 (0·57,0·97) | 43 (0·23%) | 58 (0·31%) | 0·75(0·51,1·12) | >0·99 | 135 (0·24%) | 185 (0·33%) | 0·74 (0·60,0·93) | |
| Stage7 | 0·19 | ||||||||||
| Local | 67 (0·18%) | 100 (0·26%) | 0·69 (0·51,0·94) | 34 (0·18%) | 44 (0·24%) | 0·79(0·50,1·24) | 0·67 | 101 (0·18%) | 144 (0·25%) | 0·72 (0·56,0·92) | |
| Regional or distant | 35 (0·094%) | 31(0·081%) | 1·15 (0·71, 1·86) | 13 (0·071%) | 18 (0·097%) | 0·72(0·36, 1·48) | 0·30 | 48 (0·086%) | 49 (0·086%) | 0·98 (0·66, 1·46) | |
| Grade7 | 0·56 | ||||||||||
| Well differentiated | 19(0·051%) | 26 (0·068%) | 0·74(0·41, 1·33) | 11 (0·060%) | 16 (0·086%) | 0·70 (0·32,1·51) | 0·91 | 30 (0·054%) | 42 (0·074%) | 0·72(0·45, 1·16) | |
| Moderately differentiated | 31 (0·083%) | 52 (0·14%) | 0·61(0·39,0·96) | 20 (0·11%) | 31 (0·17%) | 0·65 (0·37,1·14) | 0·91 | 51 (0·092%) | 83 (0·15%) | 0·62 (0·44, 0·88) | |
| Poorly differentiated | 29 (0·078%) | 40 (0·10%) | 0·75(0·46, 1·21) | 14 (0·076%) | 13 (0·070%) | 1·11 (0·52,2·37) | 0·40 | 43 (0·077%) | 53 (0·093%) | 0·83 (0·55, 1·24) | |
| Tumor size7 | 0·06 | ||||||||||
| ≤1 cm | 31 (0·083%) | 51 (0·13%) | 0·63 (0·40, 0·98) | 11 (0·060%) | 21 (0·11%) | 0·53 (0·26,1·11) | 0·70 | 42 (0·075%) | 72 (0·13%) | 0·60 (0·41, 0·87) | |
| 1 <2 cm | 35 (0·094%) | 52 (0·14%) | 0·69(0·45, 1·06) | 16 (0·087%) | 21 (0·11%) | 0·78 (0·41,1·50) | 0·77 | 51 (0·092%) | 73 (0·13%) | 0·71 (0·50, 1·02) | |
| 2+ cm | 25 (0·067%) | 23 (0·060%) | 1·10(0·63, 1·95) | 16 (0·087%) | 16 (0·086%) | 0·99 (0·49,1·97) | 0·79 | 41 (0·074%) | 39 (0·069%) | 1·05 (0·68, 1·63) | |
| Positive lymph nodes7 | 0·14 | ||||||||||
| No | 61 (0·16%) | 93 (0·24%) | 0·68 (0·49, 0·94) | 27 (0·15%) | 41 (0·22%) | 0·67 (0·41,1·09) | 0·94 | 88 (0·16%) | 134 (0·24%) | 0·67(0·51,0·88) | |
| Yes | 32 (0·086%) | 28 (0·073%) | 1·16(0·70, 1·92) | 11 (0·060%) | 16 (0·086%) | 0·69 (0·32,1·49) | 0·27 | 43 (0·077%) | 44 (0·077%) | 0·98(0·64, 1·49) | |
| All-Cause Mortality after | 7 (0·018%) | 12 (0·031%) | 0·60(0·22, 1·48) | 23 (0·087%) | 38 (0·14%) | 0·62 (0·36,1·03) | 0·92 | 30 (0·046%) | 50 (0·076%) | 0·62 (0·39, 0·97) | |
| Breast Cancer | |||||||||||
| Breast Cancer Deaths | 4 (0·011%) | 9 (0·023%) | 0·45 (0·12, 1·37) | 2 (0·008%) | 7 (0·026%) | 0·29 (0·04, 1·21) | 0·66 | 6 (0·009%) | 16 (0·024%) | 0·37(0·13, 0·91) | |
HR, hazard ratio; CI, confidence interval; ER, oestrogen receptor; PR, progesterone receptor;
Post-intervention phase includes data collected under extended follow-up (after 31 March, 2005). 3778(77·9%) of 4851 eligible participants randomised to conjugated equine oestrogen, and 3867(78·4%) of 4935 eligible participants randomized to placebo consented to extended follow-up.
From a proportional hazards model stratified by age group (50–54, 55–59, 60–69, 70–79), dietary modification randomisation arm.
Time to event measured from date of randomisation.
Time to event measured from 1 March, 2004.
P-diff based on tests for equality of the intervention and post-intervention phase hazard ratios in a proportional hazards model stratified by age group (50–54, 55–59, 60–69, 70–79), dietary modification randomisation arm, and trial phase (time-dependent) with time to event measured from date of randomisation.
P-het from a competing risks analysis that tests whether hazard ratios differ between tumor types.
Hazard ratios for a specific tumor characteristic are from proportional hazards model where incidence of tumor with alternate characteristics are censored. Tumor characteristics were missing on the following numbers of cases (active vs. placebo) for histology (0 vs. 1), ER status (16 vs. 19), PR status (18 vs. 24), HER2 over expression (39 vs. 62), stage (2 vs. 6), grade (27 vs. 21), tumor size (10 vs. 11), and positive lymph nodes (20 vs. 21). Eleven tumors of indeterminate size were found (7 vs. 4).
Figure 1.
Kaplan-Meier estimates of cumulative hazards for invasive breast cancer by randomization assignment in the Women’s Health Initiative randomised trial of conjugated equine oestrogen-alone (CEE) (a) under the intention to treat principle and (b) with adherence adjustments. Background shading represents the distribution of the duration of intervention (in quintiles).
Median time to first becoming non-adherent (consuming <80% of study pills) was 3·5 (IQR, 1·5 to 6·5) years. Sensitivity analyses adjusting for non-adherence yielded a stronger association between oestrogen use and lower breast cancer risk overall (HR, 0·68; 95% CI, 0·49 to 0·95) (Figure 1) and was somewhat greater still when limited to the intervention period (HR, 0·58; 95% CI, 0·39 to 0·84).
In analyses of tumor characteristics (Table 2), conjugated equine oestrogen use was associated with a lower risk of infiltrating ductal carcinoma (HR, 0·67; 95% CI, 0·51 to 0·88) but not infiltrating lobular cancers but the test for heterogeneity was not statistically significant (P=0.33). Hazard ratios for receptor positive and negative tumors were comparable. There were fewer HER2 negative (HR, 0·74; 95% CI, 0·56 to 0·97) but not fewer HER2 positive tumors in women taking oestrogen compared to placebo (P for heterogeneity=0·045), although missing HER2 data were not infrequent. There were fewer small and node negative tumors but not fewer tumors ≥2 cm or node positive tumors in the oestrogen-alone group but these comparisons between subtypes were not statistically significant.
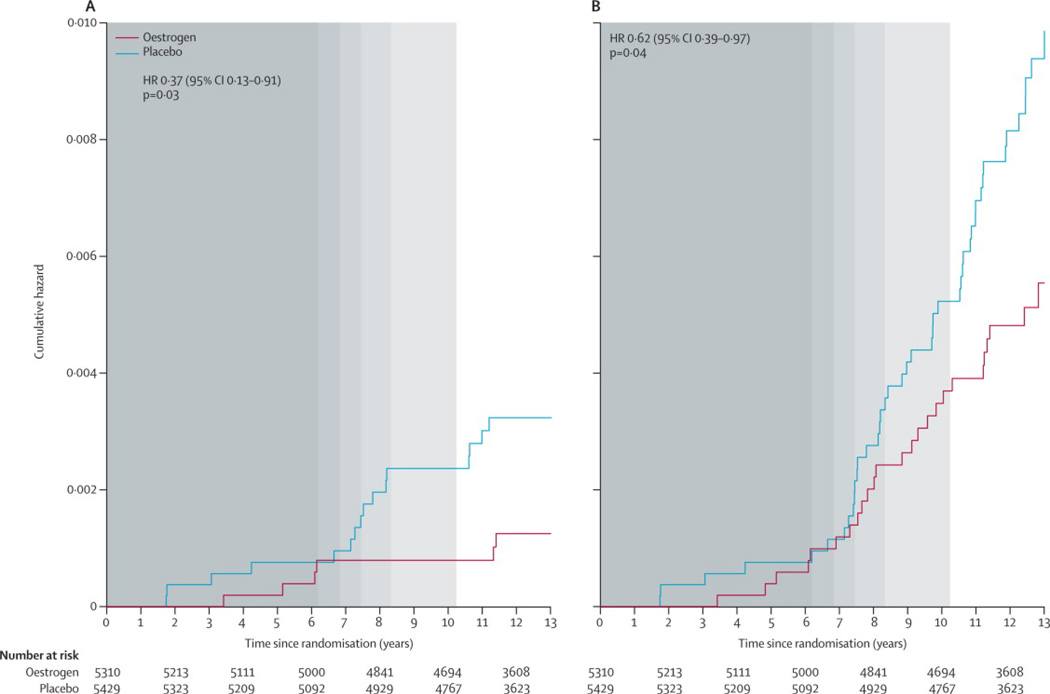
In analyses beginning at randomization (Table 2 and Figure 2), fewer women diagnosed with breast cancer died in the oestrogen group compared with the placebo group (30 vs. 50 deaths; annualized rates, 0·046% vs. 0·076%; HR, 0·62; 95% CI, 0·39 to 0·97; P=0·04). Of these, six deaths were directly attributed to breast cancer in the oestrogen group compared with 16 in the placebo group (annualized rates, 0·009% vs. 0·024%; HR, 0·37; 95% CI, 0·13 to 0·91; P=0·03).
Figure 2.
Kaplan-Meier estimates of cumulative hazards by randomisation assignment in the Women’s Health Initiative randomised trial of conjugated equine oestrogen-alone (CEE) for (a) breast cancer deaths, and (b) all-cause mortality following breast cancer. Background shading represents the distribution of the duration of intervention (in quintiles).
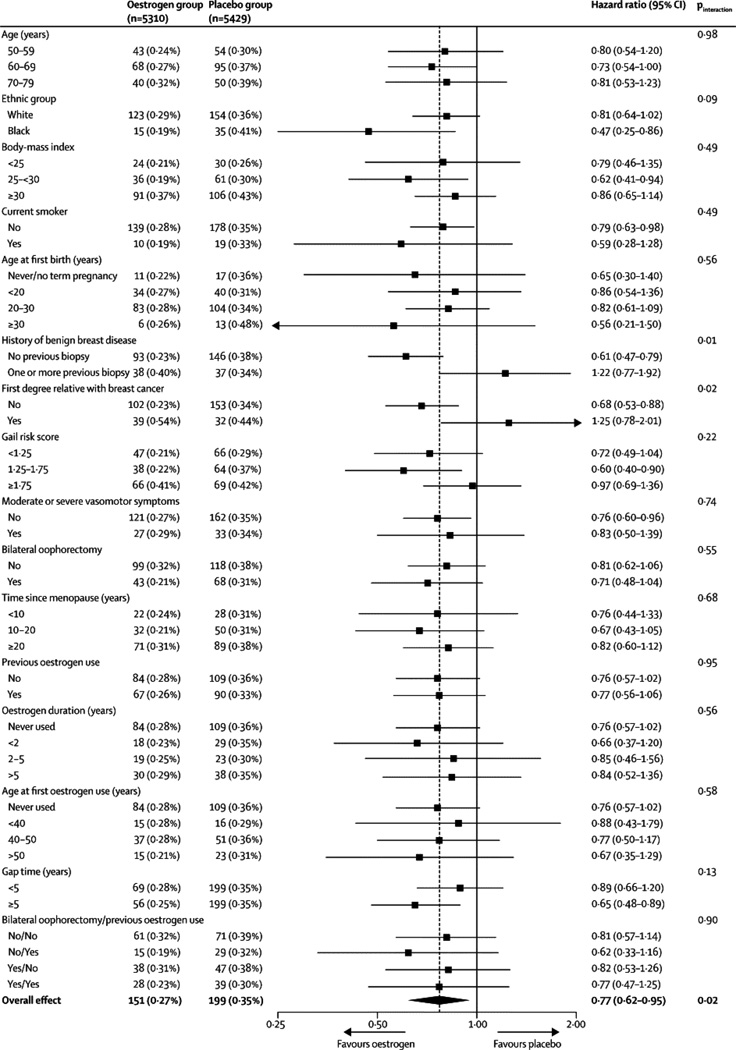
Subgroup analyses provide a mostly consistent pattern of lower breast cancer incidence with oestrogen use (Figure 3). Among 16 interactions tested, two were nominally statistically significant: history of benign breast disease (P=0·01) and first degree family history of breast cancer (P=0·02). In both of these analyses, evidence for lower breast cancer incidence was restricted to women without these risk factors. No interactions were observed with age (P=0·98), body mass index (P=0·49), Gail model risk score (P=0·22), oophorectomy status (P=0·55), years since menopause onset (P=0·68), prior oestrogen use (P=0·95), or vasomotor symptoms (P=0·74).
Figure 3.
Conjugated equine oestrogen (CEE) hazard ratios (95% CIs) for invasive breast cancer incidence by baseline characteristics in the Women’s Health Initiative trial of oestrogen-alone.
Among women who first used hormone therapy five or more years after menopause (i.e., gap time ≥5 years), the estimated effect of oestrogen (HR, 0·65; 95% CI, 0·48 to 0·89) was lower than that for women who initiated hormone therapy closer to menopause (HR, 0·89; 95% CI, 0·66 to 1·20) but the interaction was not statistically significant (P=0·13).
DISCUSSION
Conjugated equine oestrogen use (0·625mg/d) for a median of 5·9 years in postmenopausal women with hysterectomy in the WHI randomised trial was associated with a statistically significant 23% (95% CI, 5% to 38%) reduction in invasive breast cancer incidence, a reduction that continued over the median 4.7 years of post-intervention follow-up. Adjustment for adherence suggested somewhat stronger protective effects. Potential effect modification with benign breast disease (P=0.01) and family history of breast cancer (P=0·02) suggest these reductions may not pertain to women at increased risk. A statistically significant reduction in breast cancer-related mortality and all-cause mortality after breast cancer diagnosis was observed with oestrogen use, but the number of such deaths was limited.
Although many observational studies have reported an increased risk of breast cancer with oestrogen use,2–5 some have observed lower risks.6,23 Among previous studies reporting an adverse effect on breast cancer, most2–5,8 but not all,5,9 found an increased risk only after prolonged (>5 years) oestrogen use. In this trial, with substantial variation in exposure length (median duration of the intervention, 5·9 years, range <1 to 10 years; median adherent time 3·5 years, and 21% (2291/10,739) of participants reporting prior oestrogen use for >5 years at baseline), no time-trends with duration or time since cessation of use nor interactions with prior oestrogen use were observed, although power for interaction tests was limited. The continued, post-intervention effect of oestrogen on breast cancer incidence is similar to that seen for other hormone-targeted agents demonstrated to reduce breast cancer incidence.32,36
Many observational studies report increased breast cancer risk with oestrogen-alone in normal weight but not heavier women.4,5,8 There was no evidence in this trial for effect modification with BMI; oestrogen hazard ratios were less than one in normal weight, overweight, and obese women.
Mammography use remains a potential source of confounding in observational studies.24 In clinical practice, hormone therapy users have mammograms more regularly than non-users25 and screened populations have more breast cancers detected,26,27 potentially explaining the increased breast cancer risk among oestrogen users seen in previous studies that did not assess screening. Controlling for ongoing screening in observational studies has not usually been done nor is it straightforward since it depends on adequate data collection and modeling, as well as the assumption that mammography use is not an intermediate variable of exposure and disease.28,29
Detection bias is an unlikely explanation for the current results. Mammography rates were protocol-defined and closely comparable between randomisation groups.17,22 Further, oestrogen use has little effect on breast density30 or breast cancer detection.22 Finally, the statistically significant reduction in breast cancer mortality seen with oestrogen use provides strong evidence against the possibility that the risk reduction seen is an artifact of oestrogen effects on screening,29 as a delay in detection would be expected to increase mortality.
Favorable effects of oestrogen-alone use on breast cancer survival, measured from cancer diagnosis, have been seen in many,10–12 but not all2,7,13–16 reports. Measuring survival by time since diagnosis may introduce lead-time bias in hormone therapy studies if screening rates vary between hormone users and non-users. Studies of mostly oestrogen-alone use on breast cancer mortality, measured from hormone therapy initiation have provided mixed results with favorable,10–12 neutral,13–15 and unfavorable2,7 associations seen. The current results, measured from randomisation, although still imprecise, provide important new evidence that oestrogen use for approximately five years reduces breast cancer mortality, supporting the favorable association with breast cancer incidence.
A reduction in breast cancer incidence with conjugated equine oestrogen is biologically plausible. While oestrogen is a recognized mitogen that usually stimulates mammary cell proliferation and inhibits apoptosis through activation of the oestrogen receptor,1 both preclinical38–42 and clinical41 findings suggest that after long-term oestrogen deprivation, adaptive changes in mammary tumor gene expression profiles render tumors paradoxically susceptible to oestrogen-induced apoptosis.39,40 While the mechanisms are complex and not completely understood,42 preclinical studies suggest involvement of the Fas/Fas L extrinsic (receptor-mediated) death regulatory pathway43 and the intrinsic (mitochondrial) apoptotic pathway, mediated via increased expression of several pro-apoptotic proteins including PUMA (p53-unregulated modulator of apoptosis).44,45
Efforts to reconcile original trial findings17 with observational study results found that time-from menopause to first hormone use (gap time) is a potential modulator of hormone therapy influence on breast cancer risk.46 In the parallel WHI randomized clinical trial47 and the observational Million Women Study,5 women initiating oestrogen plus progestin use closer to menopause were at greater breast cancer risk. For oestrogen-alone use, Million Women Study investigators reported no breast cancer increase with oestrogen use beginning >5 years from menopause but increased risk with shorter gap time.5 In the current analyses, oestrogen hazard ratios were <1 for both early (gap-time <5 years) and later initiators. Somewhat greater influence was seen among those initiating oestrogen use ≥5 years after menopause, but the interaction with gap-time was not statistically significant.
While breast cancer incidence and related mortality were lower for oestrogen-alone users, the findings do not support oestrogen use for breast cancer risk reduction since subgroup analyses suggest the benefit may not apply to populations at increased breast cancer risk. In addition, other hormone-targeted agents have substantially greater influence on breast cancer.31–33,36 However, the current findings, together with a relatively balanced risk-benefit profile for clinical events18,48 provide reassurance regarding breast cancer safety for postmenopausal women with prior hysterectomy using unopposed oestrogen to limit climacteric symptoms for duration similar to those in this trial.
Tamoxifen, raloxifene, and exemestane all provide greater breast cancer risk reduction than oestrogen but have other important limitations. The selective oestrogen receptor modulators tamoxifen and raloxifene reduce risk of breast cancer and fractures but they increase climacteric symptoms and their adverse effects on stroke, blood clots and endometrial cancer provide an unfavorable risk/benefit profile in most postmenopausal women.32,35 Exemestane, an aromatase inhibitor which lowers oestrogen levels, also substantially reduces breast cancer risk.36 However, aromatase inhibitors cause bone loss and increase climacteric symptoms and arthralgias,49 with greater influence when initiated closer to menopause.37 The mechanisms by which exogenous oestrogens, tamoxifen, raloxifene and aromatase inhibitors all reduce breast cancer risk are not known but clearly warrant further study.
Study strengths include the randomised, double-masked, placebo-controlled prospective design with breast cancer as the designated primary safety outcome, a large sample size, high quality outcomes ascertainment, and protocol-required mammography throughout most of the follow-up period. Median time to first becoming non-adherent to intervention was only 3·5 years, considerably shorter than studies reporting increased risks. However, any bias arising from poor adherence is likely to dilute the differences between randomization groups over the observed follow-up, as the adherence-adjusted analyses confirmed. Limited numbers of breast cancer deaths, some attrition associated with re-consenting for extended follow-up, and a median of only 4·7 years of post-intervention follow-up must also be noted. The trial evaluated only a single dose and schedule of oral conjugated equine oestrogens; whether these findings apply to lower doses, other oestrogen preparations, or longer durations of use is not known.
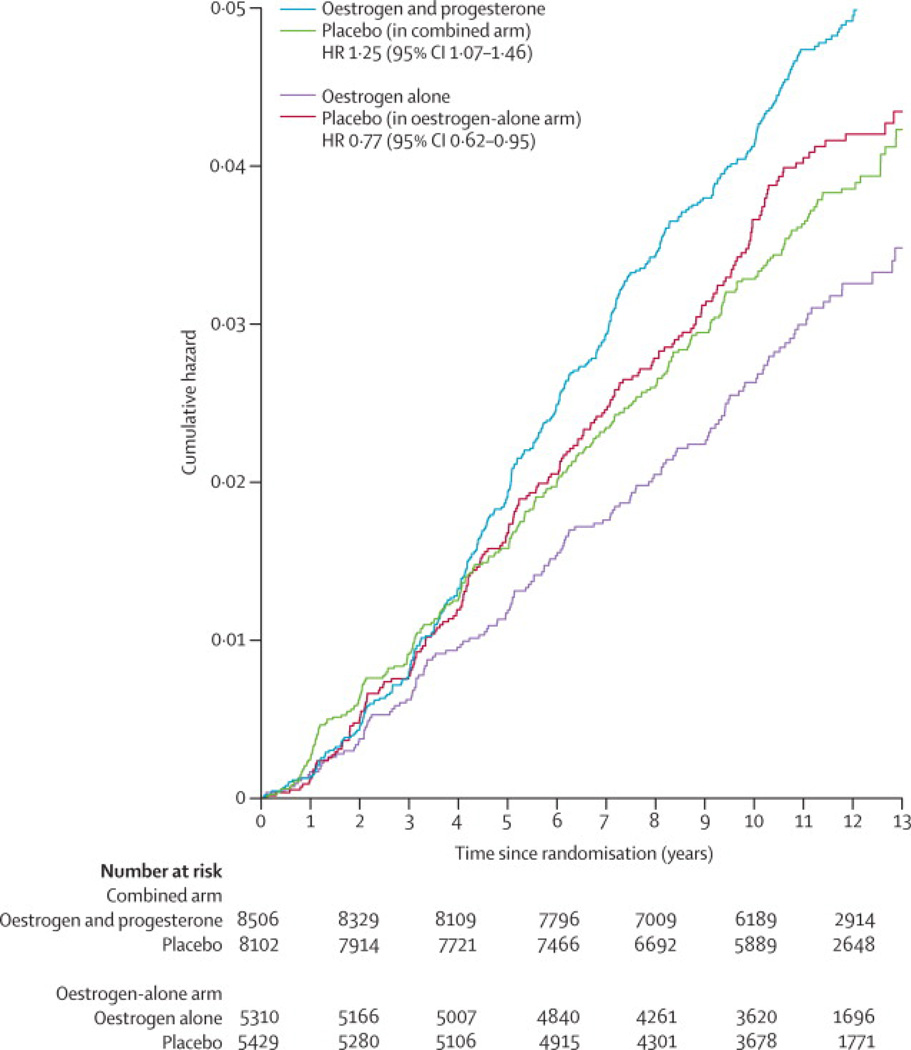
There are major differences in WHI trial findings regarding oestrogen-alone use in women with prior hysterectomy and those of the parallel WHI randomised trial of oestrogen plus medroxyprogesterone acetate among women with an intact uterus. While oestrogen-alone is associated with lower breast cancer incidence and lower breast cancer mortality, combined hormone therapy increased breast cancer incidence,47,48 delayed breast cancer diagnosis43 and increased breast cancer mortality.44 The biologic basis for this difference is unknown. The comparability of breast cancer incidence rates for the placebo groups in the two trials (Figure 4) suggests that differences in hormone therapy, rather than hysterectomy, is the primary determinant. Changes in the serum proteome in response to oestrogen and combined hormones are generally quite similar but differences have been identified that could influence breast cancer risk, including differences in NOTCH2 and some IGF binding proteins.51
Figure 4.
Cumulative hazards, adjusted for age and race/ethnicity, for invasive breast cancer by randomisation assignment in the Women’s Health Initiative trials of conjugated oestrogen-alone (CEE) and conjugated equine oestrogen plus medroxyprogesterone acetate (CEE+MPA), derived from Chlebowski et al.53
In conclusion, in this trial oestrogen-alone use for 5·9 years was associated with decreased invasive breast cancer incidence and breast cancer mortality over a median 11·8 years of follow-up in postmenopausal women with prior hysterectomy. Further research to elucidate these relationships is warranted.
Research in Context
Conjugated equine oestrogen was approved for climacteric symptom management in several countries in the early 1940’s. By the 1990’s about 40% of postmenopausal women in the USA were using hormone therapy (oestrogen-alone or oestrogen plus progestin)55 with comparable frequency of use in the UK.56,57 However, the risks and benefits of this commonly used therapy had never been established in a clinical trial setting. Against this background, scientists at the US National Institutes of Health, working in conjunction with experts in a number of disciplines, developed the Women’s Health Initiative (WHI) hormone therapy program to meet this critical unmet need with potential implications for a large number of postmenopausal women in the United States and around the world.58
The WHI hormone therapy program included two full-scale randomized, placebo-controlled clinical trials to separately evaluate oestrogen-alone and oestrogen plus progestin influence on chronic disease in postmenopausal women with and without prior hysterectomy, respectively, at 40 US clinical sites. When the WHI trials were developed, observational study evidence suggested that oestrogen, alone or with progestin, would modestly increase breast cancer risks2–5 but the cancers would have a favorable prognosis.3–5
The results from the WHI randomized, placebo-controlled trial of oestrogen-alone contradicts most prior observational studies since oestrogen use was associated with reduced breast cancer incidence and reduced breast cancer-associated mortality. Previous efforts to reconcile these e results have pointed to the issue of timing of first hormone therapy use.46,47 Additionally, some of the differences between prior observational studies and the current randomized clinical trial results may reflect variation in mammography frequency in observational study populations. In that setting, oestrogen users more frequently have mammography leading to more common breast cancer diagnosis at earlier stage. The WHI oestrogen-alone findings also differ from those seen in the WHI randomized trial evaluating oestrogen plus progestin in women with no prior hysterectomy since combined hormone therapy statistically significantly increased breast cancer incidence and breast cancer mortality.50,51,53
Interpretation
These findings provide reassuring evidence regarding breast safety of oestrogen-alone use for climacteric symptom management in postmenopausal women with hysterectomy for durations consistent with those observed in this trial. Although a reduced risk of breast cancer incidence and mortality was observed in oestrogen users, these findings do not support its use for breast cancer risk reduction in light of the lack of benefit for populations at higher risk, the adverse effects on stroke and venous thromboembolism18 and the greater risk reduction available with other agents.
Acknowledgments
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: GA, RC and AA were responsible for the original conception of the study. AA performed the statistical analysis. GA and RC drafted the initial manuscript. GA, RC, LK, JM, MG, EB, FH, DL, LM, JO, RS, and JW were responsible for data collection, data interpretation, and acquired funding. All authors participated in critical review of the manuscript and approved the final draft. GA and AA had full access to the data.
CONFLICTS OF INTEREST:
Rowan Chlebowski reported being a consultant for AstraZeneca, Novartis, Amgen, and Pfizer; receiving funding support from Amgen; and serving on speaker’s bureaus for AstraZeneca and Novartis. No other authors reported any other potential conflicts of interest.
REFERENCES
- 1.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 4.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 5.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103:1–10. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss LK, Berkman RT, Cushing-Haugen KL, et al. Hormone replacement therapy regimens and breast cancer risk. Obstet Gynecol. 2002;100:1148–1158. doi: 10.1016/s0029-7844(02)02502-4. [DOI] [PubMed] [Google Scholar]
- 7.Li CI, Malone KE, Porter PL, et al. Relationships between long durations and different regiments of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Richesson D, Leitzmann MF, et al. Menopausal hormone therapy and breast cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:3150–3160. doi: 10.1158/1055-9965.EPI-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beral V. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg LU, Granath F, Dickman PW, et al. Menopausal hormone therapy in relation to breast cancer characteristics and prognosis: a cohort study. Breast Cancer Res. 2008;10:R78. doi: 10.1186/bcr2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schairer C, Gail M, Byrne C, et al. Estrogen replacement therapy and breast cancer survival in a large screening study. J Natl Cancer Inst. 1999;91:264–270. doi: 10.1093/jnci/91.3.264. [DOI] [PubMed] [Google Scholar]
- 12.Willis DB, Calle EE, Miracle-McMahill HL, Heath CW., Jr Estrogen replacement therapy and risk of fatal breast cancer is a prospective cohort of postmenopausal women in the United States. Cancer Causes Control. 1996;7:449–457. doi: 10.1007/BF00052671. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Petitti DB, Geiger AM. Mortality following development of breast cancer while using oestrogen or oestrogen plus progestin: a computer record-linkage study. BMC. 2005;93:392–398. doi: 10.1038/sj.bjc.6602701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcomb PA, Egan KM, Trentham-Dietz A, et al. Prediagnostic use of hormone therapy and mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:864–871. doi: 10.1158/1055-9965.EPI-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman SA, Weber AL, Localio AR, et al. Hormone therapy and fata breast cancer. Pharmacoepidemiol Drug Saf. 2010;19:440–447. doi: 10.1002/pds.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reding KW, Doody DR, McTiernan A, et al. Age-related variation in the relationship between menopausal hormone therapy and the risk of dying from breast cancer. Breast Cancer Res Treat. 2011;126:749–761. doi: 10.1007/s10549-010-1174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 19.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute. [Accessed April 11, 2011];Surveillance, Epidemiology and End Results program. http://seer.cancer.gov/
- 21.Robbins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–781. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Chlebowski RT, Anderson GL, Manson JE, et al. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. J Clin Oncol. 2010;28:2690–2697. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerlikowske K, Miglioretti DL. Effects of estrogen-only treatment in postmenopausal women. JAMA. 2004;292:684–685. doi: 10.1001/jama.292.6.684-c. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR, Rosner BA, Hankinson SE, Colditz GA. Mammographic screening and risk factors for breast cancer. Am J Epidemiol. 2009;170:1422–1432. doi: 10.1093/aje/kwp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening and breast cancer characterization and control of a bias. Epidemiology. 2001;12:429–438. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Althius MD, Dozier JM, Anderson WF, Devessa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 27.Waller M, Moss S, Watson Moller H. The effect of mammographic screening and hormone replacement therapy use on breast cancer incidence in England and Wales. Cancer Epidemiol Biomarkers Prev. 2007;16:2257–2261. doi: 10.1158/1055-9965.EPI-07-0262. [DOI] [PubMed] [Google Scholar]
- 28.Weiss NS. Adjusting for screening history in epidemiologic studies of cancer: why, when, and how to do it. Am J Epidemiol. 2003;157:957–961. doi: 10.1093/aje/kwg062. [DOI] [PubMed] [Google Scholar]
- 29.Joffe MM. Invited Commentary: Screening as a nuisance variable in cancer epidemiology: methodological considerations. Am J Epidemiol. 2003;157:962–964. doi: 10.1093/aje/kwg063. [DOI] [PubMed] [Google Scholar]
- 30.McTiernan A, Chlebowski RT, Martin C, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a sub-study of the Women’s Health Initiative randomized trial. J Clin Oncol. 2009;27:6135–6143. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzick J, Powled T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 32.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology 2008 clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beral V, Bull D, Reeves G. Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 35.Freedman AN, Yu B, Gail MN, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goss PE, Ingle JN, Ales-Martines JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 37.Kanematsu M, Morimoto N, Honda J, et al. The time since last menstrual period is important as a clinical predictor for non-steroidal aromatase inhibitor on related arthralgia. BMC Cancer. 2011;11:436. doi: 10.1186/1471-2407-11-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- 39.Jordan VC. The 38th David A Karnofsky Lecture: the paradoxical actions of estrogen in breast cancer – survival or death? J Clin Oncol. 2008;26:3073–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 40.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs. high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunbier AK, Martin LA, Dowsett M. New and translational perspectives of oestrogen deprivation in breast cancer. Mol Cell Endocrinol. 2011;340:137–144. doi: 10.1016/j.mce.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 43.Song RXD, Mor G, Naftolin F, et al. Effect of long term estrogen deprivation on apoptotic responses of breast cancer cells to 17B-estradiol. J Natl Cancer Inst. 2001;93:1414–1423. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JS, Keeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Lee ES, Gajdos C, et al. Apoptosis action of 17 beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2008;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 46.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167:1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prentice RL, Chlebowski RT, Stefanick SL, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167:1207–1216. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidmiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 51.Chlebowski RT, Kuller L, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chlebowski RT, Anderson GL, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–377. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 53.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitteri SJ, Hanash SM, Aragaki A, et al. Postmenopausal estrogen and progestin effects on the serum proteome. Genome Med. 2009;1:121. doi: 10.1186/gm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greendale GA, Judd HL. The menopause: health implications and clinical management. J Am Geriatr Soc. 1993;41:426–436. doi: 10.1111/j.1532-5415.1993.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 56.Iversen L, Delaney EK, Hannaford PC, Black C. Menopause-related workload in general practice 1996–2005: a retrospective study in the UK. Family Practice. 2010;27:499–506. doi: 10.1093/fampra/cmq038. [DOI] [PubMed] [Google Scholar]
- 57.Million Women Study Collaborators. Patterns of use of hormone replacement therapy in one million women in Britain, 1996–2000. BJOG. 2002;109:1319–1330. doi: 10.1016/s1470-0328(02)02914-2. [DOI] [PubMed] [Google Scholar]
- 58.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]