Abstract
Ossabaw miniswine have been naturally selected to efficiently store large amounts of lipids offering them a survival advantage. Our goal was to evaluate the myocardial response to chronic ischemia of the Ossabaw consuming a hypercaloric, high-fat/cholesterol diet with and without metformin supplementation. At 6 weeks of age animals were fed either a regular diet (OC, n = 9), a hypercaloric high-fat/cholesterol diet (OHC, n = 9), or a hypercaloric high-fat/cholesterol diet supplemented with metformin (OHCM, n = 8). At 9 weeks, all animals underwent ameroid constrictor placement to the left circumflex coronary artery to simulate chronic ischemia. Seven weeks after ameroid placement, all animals underwent hemodynamic and functional measurements followed by cardiac harvest. Both OHC and OHCM animals developed significantly greater weight gain, total cholesterol, and LDL:HDL ratio compared to OC controls. Metformin administration reversed diet-induced hypertension and glucose intolerance. There were no differences in global and regional contractility, myocardial perfusion, capillary and arteriolar density, or total protein oxidation between groups. Myocardial protein expression of VEGF, PPAR-α, γ, and δ was significantly increased in the OHC and OHCM groups. Microvessel reactivity was improved in the OHC and OHCM groups compared to controls, and correlated with increased p-eNOS expression. Overfed Ossabaw miniswine develop several components of metabolic syndrome. However, impairments of myocardial function, neovascularization and perfusion were not present, and microvessel reactivity was paradoxically improved in hypercholesterolemic animals. The observed cardioprotection despite metabolic derangements may be due to lipid-dependant upregulation of the PPAR pathway which is anti-inflammatory and governs myocardial fatty acid metabolism.
Keywords: Cardioprotection, Metabolic syndrome, Chronic ischemia, Ossabaw
Introduction
Patients with diabetes and lipid disorders have a higher mortality after surgical and percutaneous interventions than patients without these comorbidities [34], and the death rate from heart disease is up to four times higher in patients with diabetes [36]. Type 2 diabetes and related disorders not only affect the development and progression of coronary artery disease (CAD) directly, but also alter cellular function and proliferation leading to an impaired endogenous neovascular response inhibiting collateral formation [10, 15]. Thus, it is not surprising that despite successful animal studies in myopreservation, myocardial regeneration, and therapeutic neovascularization in otherwise healthy animals, the transition to successful clinical trials has been met with only modest gains, and little if any clinical impact. Indeed, type 1 diabetes [6], metabolic syndrome (MetS) [12], and hypercholesterolemia [4] are associated with diminished collateral formation and endothelial function in both animals and patients [30, 31, 36].
Understanding that models of chronic ischemia in otherwise healthy animals simply do not replicate the complex interaction of comorbid conditions seen in the human cardiac patient has lead researchers to introduce models of diabetes, metabolic syndrome, and obesity in addition to induced myocardial ischemia to better replicate the comorbid conditions seen in human cardiac patients [15]. Previous studies in swine have demonstrated impaired cardiovascular function resultant from hypercholesterolemic diets [27] as well as from induced type 1 diabetes [9], and more recent animal studies have since incorporated a model of induced endothelial dysfunction in addition to coronary ischemia [15]. However, little is known of the effects of type 2 diabetes, MetS, or obesity on neovascularization, though recent studies in knockout diabetic mice have suggested that coronary arterioles undergo different patterns of remodeling than those of macrovessels in type 2 diabetes [14], and accumulating data suggests that adipose tissue plays a critical role in the regulation of vascular function [37].
The Ossabaw miniswine were introduced to Ossabaw Island off the coast of Savannah, Georgia, by early Spanish explorers where the abundance or scarcity of food is seasonably variable. Over the past 400 years, these feral animals have adapted to the harsh conditions of the island, often with extended periods of starvation, by feeding heavily during the fall and winter months and storing a greater proportion of body fat than any other breed of swine or terrestrial mammal. Thus, the Ossabaw were selectively and genetically predisposed to obesity [11]. When fed an excess of calories these pigs develop profound obesity and every component of the metabolic syndrome, which progresses to overt type 2 diabetes evidenced by increased fasting blood glucose [7]. This abnormal lipid-storage capability has resulted in the presence of insulin resistance, yet with a survival advantage [11]. Ossabaw pigs are of research interest for their “thrifty gene” mechanism in lipid metabolism and obesity, as they are devoid of brown fat, which regulates several aspects of energy homeostasis [33]. Thus, the Ossabaw offer a unique natural animal model for MetS and CAD when fed an atherogenic diet [22].
The purpose of this study was to examine the effects of MetS with and without supplementation with the oral biguanide, metformin, on cardiac functional parameters and the neovascularization response to ischemia using this genetically unique breed of miniswine. Understanding the cardiac response to a high-fat/cholesterol diet in these animals with and without metformin treatment may provide insight into the effects of MetS on coronary revascularization. Furthermore, these studies will give us information regarding the utility of the Ossabaw miniswine as a large animal model of chronic cardiac ischemia with a genetic susceptibility to MetS and type 2 diabetes.
Methods
Animal model
Twenty-six Ossabaw miniswine (Purdue Ossabaw Facility, Indiana University, Indianapolis, IN) at 6 weeks of age were split into three groups. Control animals (OC, n = 9) were fed a 500 g/day diet of regular chow. High-fat/cholesterol animals (OHC, n = 9) were fed a diet 500 g/day diet of high-cholesterol chow consisting of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO). High-cholesterol metformin animals (OHCM, n = 8) were given the same high-cholesterol diet as the OHC group. The hypercaloric high-fat/cholesterol diet provided (24%) more calories than regular chow. All animals had unlimited access to drinking water, and were kept in a warm, non-stressful environment for the duration of the experiment. Nine weeks after diet initiation, all animals underwent ameroid constrictor placement (see “Surgical interventions”) and continued their diets as previously established. The OHCM animals began treatment with 500 mg oral metformin administration twice daily starting at the time of ameroid placement. Seven weeks after ameroid placement (16 weeks from diet initiation), all animals underwent coronary angiography, hemodynamic and functional measurements, and cardiac harvest (see “Surgical interventions”). Animals were weighed at the time of diet initiation, at ameroid placement surgery, and at cardiac harvest.
Surgical interventions
For all surgical procedures anesthesia was induced with an intramuscular injection of tiletamine HCl (Telazol, Fort Dodge Animal Health, New York, NY). Animals were endotracheally intubated, mechanically ventilated at 12–20 breaths per minute, and general anesthesia was maintained with a gas mixture of oxygen at 1.5–2 L/min and isoflurane at 0.75–3.0% concentration. A rectal probe was placed to monitor core body temperature and animals were placed on a warming mat.
Ameroid constrictor placement
General anesthesia was induced and maintained as described above. A single dose of enrofloxacin (5 mg/kg IV) was given for antibiotic prophylaxis. Animals were prepped and draped in a sterile fashion, and sterile technique was maintained throughout the procedure. Via a mini-left thoracotomy, the pericardium was opened, the left atrial appendage retracted, and the left circumflex (LCx) artery dissected in preparation for placement of a 1.5–2.25 mm internal diameter ameroid constrictor (Research Instruments SW, Escondito, CA) to the proximal LCx just after its take-off of the left main (LM) coronary artery. During a 2-min temporary occlusion of the LCx, 1.5 × 107 (5 ml) isotope-labeled gold microspheres (non-radioactive) were injected into the left atrium over a period of 30 s to determine the exact myocardial territory at risk through shadow labeling (BioPhysics Assay Laboratory, Worcester, MA). The pericardium was re-approximated followed by a layered closure of the surgical incision. Post-operative pain was controlled with a single dose of intramuscular buprenorphine (0.03 mg/kg) given at the end of the operation followed by placement of a fentanyl patch (4 µg/kg) to be continued for 72 h post-operatively. All animals received peri-operative aspirin at 325 mg/day for prophylaxis against thrombo-embolic events starting 1 day before the first operation and continuing for a total of 5 days.
Cardiac harvest
General anesthesia was induced and maintained as described above. Blood glucose measurements were taken at baseline and 30 and 60 min after an intravenous 0.5 mg/kg dextrose challenge. After a cutdown of the right femoral artery, a 5F arterial sheath was placed using the Seldinger technique. Blood was withdrawn from the femoral artery and analyzed for total cholesterol, high density lipoprotein (HDL), calculated low density lipoprotein (LDL), and triglycerides. Serum was separated by centrifugation and frozen at −80°C for enzyme-linked immunosorbent assay (ELISA) for serum insulin (Alpco Immunoassays, Salem, NH). Coronary angiography of the left and right coronary circulation was performed using <7 ml of omnipaque contrast through an Amplatz Right 1 catheter. A blinded assessment of thrombolysis in myocardial infarction (TIMI), Rentrop and Blush scores were performed by an interventional cardiologist (Roger J. Laham, MD. Beth Israel Deaconess Medical Center, Boston, MA). Median sternotomy was performed followed by functional cardiac and hemodynamic measurements as described in individual sections below. Under general anesthesia, the heart was harvested and the animals euthanized by exsanguination. Myocardial samples from both the normal left ventricle (NV) and the area at risk (AAR) in the LCx left lateral territory were collected for microvessel studies and placed in cold Krebs solution (on ice), or weighed and dried for microsphere perfusion analysis, and the remaining tissue rapidly frozen in liquid nitrogen for molecular and immunohistochemical studies.
All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital. Animals were cared for in accordance with the “Principles of Laboratory Animal Care” formulated by the national society for medical research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
Global and regional myocardial function and contractility
During the non-survival operation, four 2-mm sonomicrometer crystals (Sonometrics Corporation, Ontario, Canada) were placed into the AAR epicardium with two crystals perpendicular and two parallel to the left anterior descending coronary artery (LAD) to measure horizontal and longitudinal segmental shortening, respectively. Pressure catheters were placed in the descending aorta via right femoral sheath, and into the left ventricle (LV) via apical puncture. Measurements of longitudinal and horizontal segmental shortening, and pressure measurements were used to calculate regional contractility, global LV contractility as expressed in dP/dt, HR, and mean arterial pressure (MAP), and developed left ventricular pressure using CardioSoft Pro (Sonometrics Corporation, Ontario, Canada).
Myocardial blood flow
Prior to cardiac harvest, myocardial perfusion was measured via isotope-labeled microspheres (Biophysics Assay Laboratory) as described previously [27]. Briefly 1.5 × 107 gold microspheres (5 ml) were injected into the left atrium at the time of ameroid placement during temporary LCx occlusion to identify the AAR. Lutetium and Europium microspheres were injected during the final operation during conditions of rest and demand pacing at 150 beats/min (bpm) with an external pacing pulse generator while simultaneously withdrawing arterial blood from the femoral artery catheter. LV and blood samples were dried at 60°C for >48 h. Microsphere density in each sample was calculated with a gamma counter after exposure to neutron beam radiation (Biophysics Assay Laboratory). Myocardial blood flow to each sample was calculated using: blood flow = (withdrawal rate/tissue weight) × (tissue microsphere count/blood microsphere count).
Vessel density
Twelve micrometer thick sections of frozen myocardial tissue from the AAR were formalin-fixed and incubated with goat antibody against endothelium-specific CD31 (R&D Systems, Minneapolis, MN) followed by DyLight-conjugated anti-goat secondary antibody (Jackson ImmunoResearch, West Grove, PA) for capillary density, or with mouse antibody against smooth muscle actin (SMA, Abcam, Cambridge, MA) followed by DyLight-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch) for arteriolar density. Microscopic images of sections were digitally captured and analyzed for capillary density (CD31-positive structures between 5 and 25 µm2 in cross-sectional area) and arteriolar density (SMA-positive structures that co-stained with CD31) as previously described [8], and analyzed using Image J software (National Institutes of Health, Bethesda, MD).
Protein expression and protein oxidative stress
Sixty micrograms of total protein from the RIPA (RadioImmunoprecipitation Assay, Boston BioProducts, Ashland, MA) soluble fraction of myocardial lysates made from both the AAR and NV tissue was fractionated by SDS-PAGE using the NuPage Novex Bis-Tris Mini Gel system (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were then incubated with primary antibodies against Akt (Cell Signaling, Danvers, MA), phosphorylated endothelial nitric oxide synthase (p-eNOS, Cell Signaling), AMP-activated protein kinase-α (AMPKα, Cell Signaling), peroxisome proliferators-activated receptor-γ (PPARγ, Cell Signaling), vascular endothelial growth factor (VEGF, Abcam, Cambridge, MA), PPARδ (Abcam), and PPARα (Cayman Chemical, Ann Arbor, MI), followed by the appropriate horseradish peroxidase-linked secondary antibodies (1:2,000, Jackson ImmunoResearch, West Grove, PA). Blotting for total protein oxidation was carried out via Oxyblot Protein Oxidation Detection kit (Millipore). Immune complexes were visualized via electrochemiluminescense (ECL) and digitally captured using GeneSnap software (Syngene, Cambridge, England). Densiometry of ECL signal was performed using Image J software. Loading error for SDS-PAGE was corrected by expression of the ubiquitously expressed housekeeping gene GAPDH (Cell Signaling). Raw data collected as arbitrary light units from ECL fluorescence and Image J densiometry were averaged, and expressed in fold change (FC) as compared to OC mean.
Microvessel studies
Coronary arterioles (80–180 µm diameter) were isolated from both the NV and the AAR and placed in a microvessel chamber. Vessels were maximally preconditioned with thromboxane-A2 analog U46619 (0.1–1.0 µM) and then treated with endothelium-dependent vasodilator adenosine diphosphate (ADP, 10−9–10−4 mol/L), and the endothelium-independent vasodilator sodium nitroprusside (SNP, 10−9–10−4 mol/L). Responses were defined as % relaxation to baseline diameter after maximum vasoconstriction. All reagents were obtained from Sigma Aldrich (St. Louis, MO).
Statistical analysis
All results are reported as mean ± standard error of the mean (SEM) using one-way ANOVA followed by a post hoc Bonferroni test using GraphPad Prism 5.0 Software (GraphPad Software Inc. San Diego, CA), which was also used to create figures. Two-way ANOVA and Bonferroni post tests were used to compare blood glucose levels during glucose tolerance test at the time of the final operation as well as for microvessel relaxation response.
Results
Metabolic syndrome and hemodynamic parameters
Both groups receiving the high-fat/cholesterol diet (OHC and OHCM) developed significantly greater weight gain compared to controls (ANOVA p < 0.0001). Total cholesterol (ANOVA p < 0.0001) and LDL:HDL ratio (ANOVA p < 0.001) followed this same trend with higher levels in the animals receiving a high-fat/cholesterol diet, and an exacerbation of this effect with the addition of metformin. Mean arterial pressure (MAP) at the time of the second operation was significantly higher in the OHC group compared to the OC animals, however, this effect was normalized with the administration of metformin to the level of controls (ANOVA p < 0.001). Glucose tolerance test followed this same trend with elevated blood glucose in the high-fat/cholesterol OHC group, but normalization of the response with the addition of metformin (2-way ANOVA p < 0.01). Serum insulin as measured by ELISA at the time of cardiac harvest did not differ between groups (ANOVA p = 0.381). Refer to Fig. 1 for Bonferroni post-test comparison between groups. Developed left ventricular pressure followed the same trend as MAP, being higher in the OHC group but normalizing with the administration of metformin. Resting heart rate was lower in the OHCM group compared to controls. There was no difference in LV dp/dt max between groups (Table 1).
Fig. 1.
Manifestations of the metabolic syndrome in Ossabaw swine controls (OC), animals receiving a high-fat/cholesterol diet (OHC) and those receiving a high-fat/cholesterol diet supplemented with metformin (OHCM) including weight gain, total cholesterol, LDL:HDL ratio, serum insulin, mean arterial pressure and response to glucose challenge test at the time of cardiac harvest. *p < 0.05, †p < 0.01, ‡p < 0.001
Table 1.
Baseline hemodynamic parameters at the time of the second operation prior to cardiac harvest in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM)
| OC | OHC | OHCM | ANOVA p | Post-test p | |
|---|---|---|---|---|---|
| Heart rate (beats/min) | 103 ± 7.05 | 95 ± 5.2 | 73 ± 7.28 | 0.021 | OC vs. OHCM* |
| Developed LVP (mmHg) | 56 ± 12.9 | 98 ± 3.88 | 74 ± 2.59 | 0.009 | OC vs. OHC† |
| Mean arterial pressure (mmHg) | 85 ± 8.09 | 112 ± 4.09 | 68 ± 1.7 | 0.0002 | OC vs. OHC*, OHC vs. OHCM‡ |
| LV dp/dt max (mmHg/s) | 1,131 ± 181 | 1,322 ± 104 | 1,102 ± 49.1 | 0.495 |
Parameters expressed as Mean ± SEM
p < 0.05;
p < 0.01;
p < 0.001
Global and regional contractility
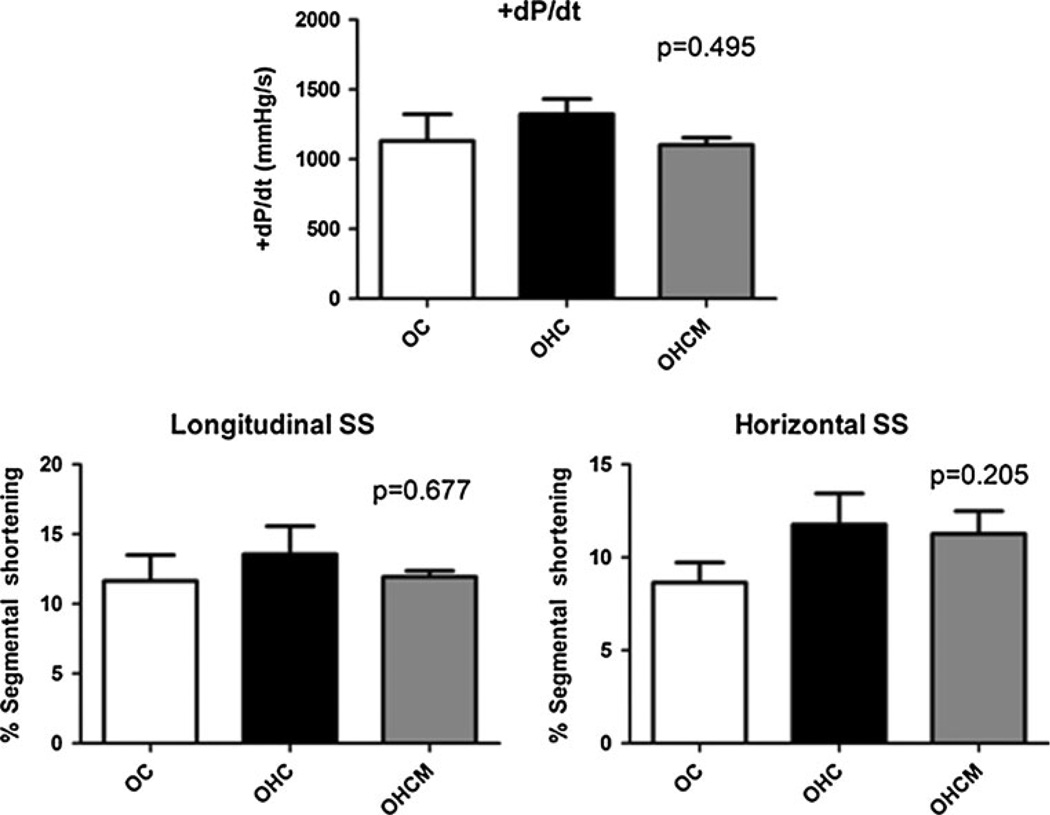
Global LV contractility at the time of the second operation did not significantly vary between any group (ANOVA p = 0.495). Regional contractility in the AAR as measured by both longitudinal and horizontal segmental shortening (SS) respective to the LAD also did not change between any of the groups (ANOVA p = 0.677 and 0.205, respectively, Fig. 2).
Fig. 2.
Global contractility (+dP/dt) and regional contractility (longitudinal and horizontal segmental shortening) of the ischemic left ventricle in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM)
Myocardial blood flow and perfusion
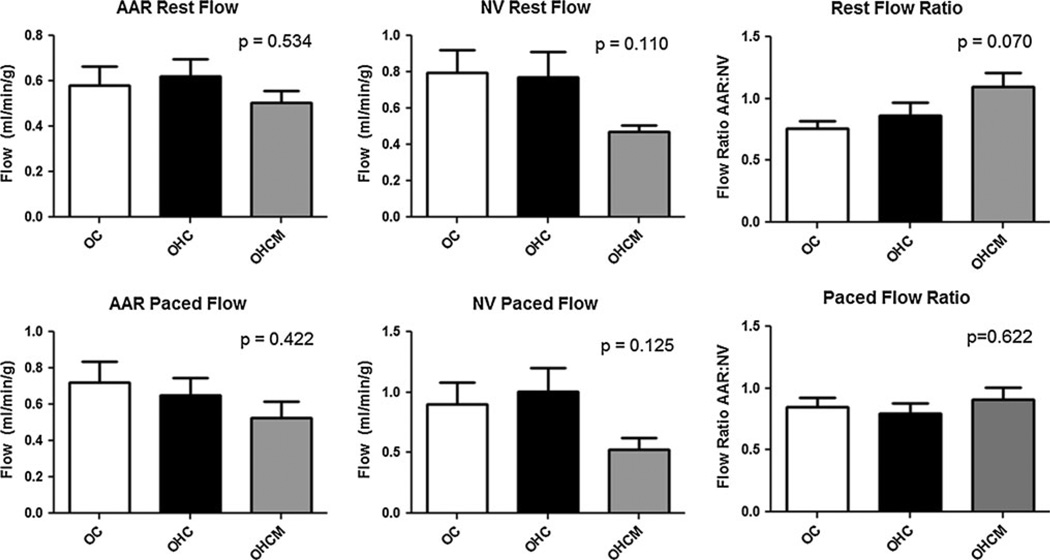
Measured at the time of the final operation both at rest and during demand pacing at 150 bpm, there were no differences between myocardial blood flow to the chronically ischemic AAR or to the remote normal ventricle (NV) between any groups (Fig. 3). The AAR:NV ratio of blood flow to the collateral dependent AAR to the remote NV was not different between groups at rest or during demand pacing.
Fig. 3.
Myocardial blood flow to the chronically ischemic area at risk (AAR) and the remote normal ventricle (NV) under conditions of rest as well as during demand pacing at 150 bpm in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM)
Complete occlusion of the left circumflex artery was confirmed angiographically in all cases. There were no differences in TIMI score, Rentrop scores of the left and right coronary circulation, or Blush scores between any of the groups (Table 2).
Table 2.
Cardiac catheterization at the time of cardiac harvest including TIMI score of coronary blood flow, Rentrop classification of collaterals in both the left and right coronary circulation, and Blush grade as a measure of myocardial perfusion in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM)
| Mean SEM | ANOVA p | |
|---|---|---|
| TIMI | ||
| OC | 0.667 ± 0.236 | |
| OHC | 0.714 ± 0.360 | 0.620 |
| OHCM | 0.333 ± 0.211 | |
| Rentrop left | ||
| OC | 0.889 ± 0.200 | |
| OHC | 0.857 ± 0.404 | 0.316 |
| OHCM | 1.50 ± 0.342 | |
| Rentrop right | ||
| OC | 1.11 ± 0.389 | |
| OHC | 1.63 ± 0.498 | 0.691 |
| OHCM | 1.50 ± 0.500 | |
| Blush | ||
| OC | 1.33 ± 0.236 | |
| OHC | 1.38 ± 0.420 | 0.317 |
| OHCM | 0.667 ± 0.333 |
Vessel density
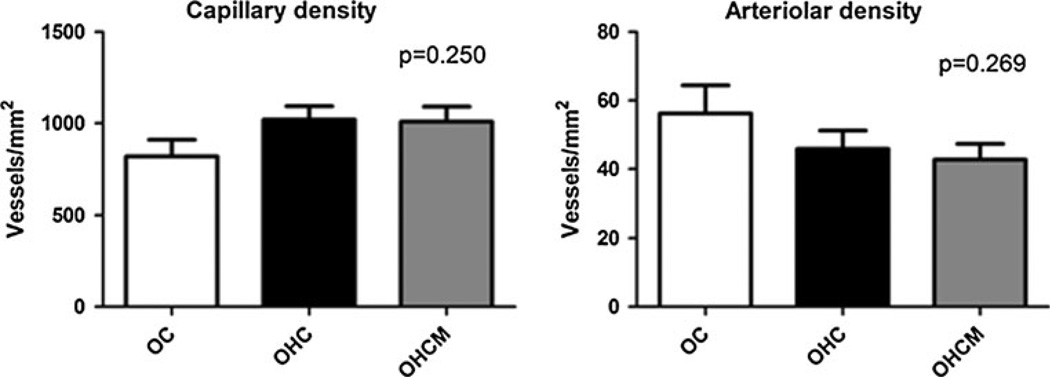
Immunohistochemical staining of the AAR myocardium for CD31 and smooth muscle actin (SMA) for measurement of capillary and arteriolar density, respectively, revealed no significant differences in vessel density between any of the groups (ANOVA p = 0.250 for CD31 and p = 0.269 for SMA, Fig. 4).
Fig. 4.
The angiogenic response to ischemia as measured by capillary density (CD31 staining) and arteriolar density (SMA staining) in the chronically ischemic myocardial territory in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM)
Protein expression and oxidative stress
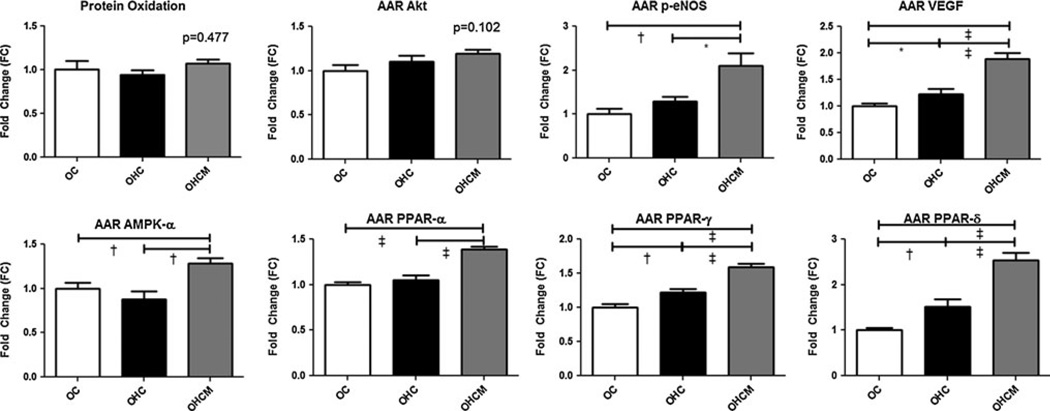
Total protein oxidative stress as measured by Oxyblot did not significantly differ between groups (ANOVA p = 0.477). Protein expression in the AAR as measured by Western immunoblot showed no significant difference between any groups for Akt (p = 0.102). However, p-eNOS expression was higher in animals fed the high-fat/cholesterol diet (OHC, OHCM) with significantly higher expression in the OHCM group (ANOVA p = 0.001). Expression of VEGF was also increased in the OHC and OHCM groups (ANOVA p < 0.0001). Expression of AMPKα (ANOVA p = 0.004) was higher in the metformin-treated animals, compared to both the high-fat/cholesterol diet alone and control groups. PPARα, γ, and δ were most highly expressed in the OHCM animals, and still elevated in the OHC group compared to controls (ANOVA p < 0.0001 for each PPARα, γ, and δ). See Fig. 5 for Bonferroni post-test analyses.
Fig. 5.
Protein expression and total protein oxidative stress in the ischemic area at risk (AAR) left ventricular myocardial tissue as measured by Western blot and Oxyblot assays in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM). *p < 0.05, †p < 0.01, ‡p < 0.001
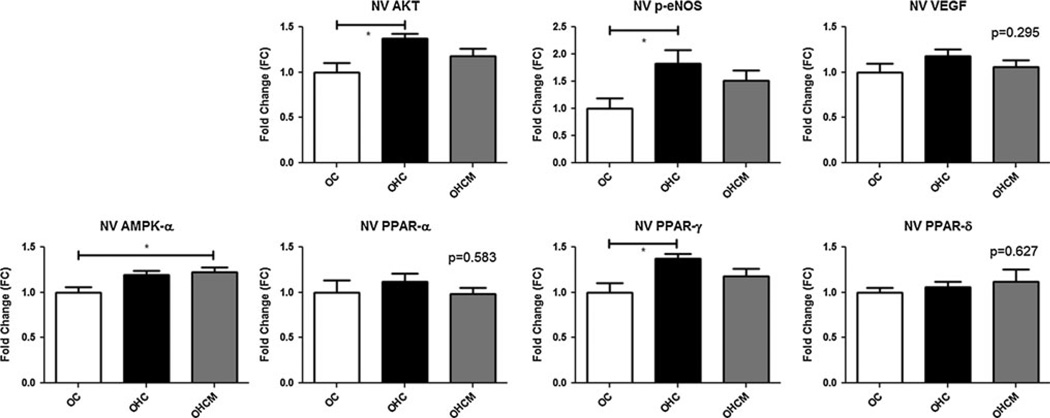
In the normal ventricle, Akt and p-eNOS were more highly expressed in the animals receiving a high-fat/cholesterol diet (ANOVA p = 0.014, and p = 0.029, respectively). Expression of VEGF was not significantly different between any groups, nor was the expression of PPARα or PPARδ. AMPKα was more highly expressed in both high/fat-cholesterol diet groups (ANOVA p = 0.018), though statistical significance on Bonferroni post-test was only reached in the metformin-treated animals. Likewise, expression of PPARγ trended higher in both high-fat/cholesterol groups (p = 0.014) with post-test significance reached in the OHC group compared to controls (Fig. 6).
Fig. 6.
Protein expression in the remote, normal ventricular (NV) myocardial tissue as measured by Western blot assay in Ossabaw controls (OC), high-fat/cholesterol diet animals (OHC), and high-fat/cholesterol diet animals supplemented with metformin (OHCM). *p < 0.05
Microvessel studies
Microvascular response to ADP was not significantly different between any groups in the AAR or the NV. Relaxation in response to SNP, however, was more robust in both the OHC and OHCM groups compared to controls in both the AAR and NV (Fig. 7).
Fig. 7.
Microvessel reactivity in both the chronically ischemic area at risk (AAR) and the remote normal ventricle (NV) to endothelium-dependent vasodilator ADP and to the endothelium-independent vasodilator SNP. *p < 0.05
Discussion
Ossabaw miniswine fed a hypercaloric high-fat/cholesterol diet developed several components of MetS compared to normal diet controls, including significantly higher weight gain, increased glucose intolerance, dyslipidemia and hypertension. Interestingly, this model of MetS had normal coronary revascularization in response to chronic myocardial ischemia, and improved endothelial function in animals receiving the high-fat/cholesterol diet. This is in contrast to previous studies in coronary collateral formation in type 1 diabetic pigs that have a profound decrease in collateral formation associated with a marked reduction in endothelium-dependent relaxation to agents that operate predominantly through the release of nitric oxide [5, 6]. Murine studies, however, have demonstrated that obesity-induced insulin resistance and endothelial dysfunction alone are not sufficient to alter the angiogenic response to ischemia [1], which is in contrast to type 1 diabetic murine models which demonstrate long-term deterioration of stem and hematopoietic progenitor cells [24].
Insulin levels did trend higher in the high-fat/cholesterol fed animals, and the highest insulin levels were seen in the animals supplemented with metformin. This may explain two findings: first, the markedly increased weight gain may be due to the anabolic effects of high insulin levels, as seen in MetS patients; and second, the improved glucose tolerance seen in the metformin-treated animals may also be explained by elevated insulin levels. Finally, the well established pro-angiogenic properties of insulin [5, 13] may explain why the high-fat/cholesterol group (OHC) demonstrated a preserved angiogenic response despite impairments in glucose tolerance. Previous studies in a type 1 diabetic swine model have demonstrated increased collateral formation with the administration of insulin [5]. These findings suggest that insulin itself may play a greater role in preserving the angiogenic response than that of maintaining normoglycemia, at least in the short term.
Protein expression and oxidative stress studies did offer a possible mechanism for cardioprotection in the setting of MetS, as the peroxisome-proliferator-activated receptor (PPAR) pathways governing myocardial fatty acid metabolism were upregulated in the animals fed a high-fat/cholesterol diet. This effect was further induced with higher total cholesterol and a higher LDL:HDL profile seen in the high-fat/cholesterol diet animals supplemented with metformin. It appears that the upregulation of the PPAR pathway is activated in a dose–response manner with escalating total cholesterol and a worsening LDL:HDL profile. However, it needs to be noted that pigs were fed a modified diet for only 16 weeks, which is relatively early stage MetS in large animals [2, 16]. Despite this, other models of type 1 diabetes demonstrated marked endothelial dysfunction and diminished collateral formation after a much shorter time period.
One surprising and counterintuitive finding in this study was the absence of impaired endothelial-dependent relaxation to ADP in the high-fat/cholesterol fed animals. In fact, endothelial-independent microvessel relaxation to SNP was actually increased in animals receiving the high-fat/cholesterol diet. One possible explanation for the apparent improvement of the endothelial-dependent relaxation to ADP in both the NV and AAR in animals receiving a high-fat/cholesterol diet as compared to controls is the concomitant increase in expression of p-eNOS in the OHC and OHCM groups. In other words, despite the administration of a diet that one would expect to induce endothelial dysfunction, overexpression of p-eNOS seems to abrogate this effect. Metformin had no effect on vascular reactivity when administered with the high-fat/cholesterol diet, despite an even higher increase in p-eNOS expression in the AAR of the OHCM group. Previous animal studies have demonstrated that MetS impairs myocardial oxygen delivery via impairments in coronary circulation [29] and vasomotor function [18]. Though several studies of type 2 diabetes and MetS in mice have demonstrated normal SNP-induced endothelium-dependent vasodilation [23, 25], an overt improvement of the vasomotor response remains counterintuitive. Thus, this unexpected improvement in microvascular relaxation coupled with an increased expression of p-eNOS offers insight into the cardioprotective mechanisms of the Ossabaw during early metabolic syndrome. Whether this improvement in the microvascular response is maintained in later stages of MetS is yet to be determined and warrants further investigation.
Previous animal experiments have identified PPARα agonism as playing a key role in maintaining cardiac function in the setting of lipid disorders [35], and PPARγ activation to prevent unfavorable cardiac remodeling through a reduction in myocardial hypertrophy and fibrosis after myocardial infarction [20]. Furthermore, increased myocardial PPARα activity has been associated with improved adaptation to hyperglycemia and hyperlipidemia-induced metabolic disturbances [17]. In our experiment, increases in the expression of PPARα, γ, and δ in the AAR correlated with rising total cholesterol and LDL:HDL ratio. This may explain how cardiac function in the Ossabaw is preserved despite derangements in lipid metabolism. Alternatively, the 16-week duration of this study is a relatively early stage of MetS, perhaps similar to a 9-week study in which coronary flow responses to adenosine were not impaired and there was no increase in atherosclerosis [2]. Renal microvascular proliferation has also been noted in early stage MetS Ossabaw swine [27]. The fact that porcine cardiovascular physiology is a much closer simulator of human cardiac physiology than murine models holds promise for the development of cardioprotective strategies in humans with diabetes and lipid derangements. It is worth mentioning that the increased expression of PPAR subtypes in the MetS animals was more robust in the AAR as compared to the NV. This suggests that diet-induced MetS alone may not upregulate PPAR, but when coupled with myocardial stress such as chronic ischemia, augmentation of this protective pathway is clearly manifested.
Though the oral biguanide metformin did normalize glucose tolerance and hypertension in this experiment, its administration exacerbated dyslipidemia compared to both the normal diet control group as well as the high-fat/cholesterol diet animals. It is not clear why the lipid profiles in the animals receiving a high-fat/cholesterol diet was made worse with the addition of metformin, as metformin has been shown to decrease the expression of lipogenic genes while increasing AMPK activation [21], as well as protect ischemic myocardium via the upregulation of Akt-mediated pathways [3]. Both AMPK (in the NV and AAR), and Akt (in the NV) upregulation was demonstrated in this experiment. Furthermore, metformin has conclusively been shown to prevent adverse cardiovascular events in overweight patients with diabetes [26]. One possible explanation for these derangements in the cholesterol profile is that the Ossabaw lack brown adipose [32] which may significantly alter their lipid-metabolism response to metformin as compared to humans and other mammals. Alternatively, the mutation in the gamma regulatory subunit of AMPK in the Ossabaw [19] may have resulted in impaired AMPK activity, despite the increased expression of AMPKα.
Interestingly, though there were no differences in the angiogenic response to chronic ischemia as measured by capillary and arteriolar density in the AAR, the VEGF pathway was significantly upregulated in the AAR of the groups with a worsening cholesterol profile, as was the expression of p-eNOS. Expression of NOS isoforms and endothelial VEGF secretion is essential for arteriogenesis and new vascular smooth muscle formation [28]. One explanation for the observed increase in pro-angiogenic protein expression without a corresponding angiogenic response is that the experiment duration was only 16 weeks total, thus perhaps not sufficient time for the sequelae of the VEGF pathway to manifest in new blood vessel growth.
The unique ability of the Ossabaw miniswine to maintain a survival advantage with the overt manifestation of the metabolic syndrome while sparing the negative cardiovascular effects of the disorder that would otherwise be seen in humans sheds light not only on the novel mechanisms that these animals have developed over four centuries, but may give insight into the relationships and causality of diabetes, hyperlipidemia, cardiovascular health, and the neovascularization response to ischemia. A better understanding of the cardioprotective effects of PPAR activation, and mounting evidence of this relationship can help shape future pharmacologic and gene therapies in overweight and diabetic patients with cardiovascular disease. It is interesting to speculate that patients with MetS may have improved survival in part due to a relatively preserved neovascularization response to chronic ischemia, as opposed to that observed in patients with type 1 diabetes. However, this will need to be demonstrated in clinical studies.
Acknowledgments
Funding was provided by grants from the National Heart, Lung, and Blood Institute (R01HL46716, R01HL 69024, and R01HL85647, Dr. Sellke), NIH Training grant 5T32-HL076134 (Dr. Lassaletta), NIH Training grant 5T32-HL094300 (Dr. Chu, Dr. Elmadhun), NIH Training grant T32HL007734 (Dr. Robich), and through the Thoracic Surgery Foundation for Research and Education Fellowship (Dr. Lassaletta). We would like to thank the animal facility staff at the Rhode Island Hospital.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
Antonio D. Lassaletta, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA
Louis M. Chu, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA
Michael P. Robich, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA
Nassrene Y. Elmadhun, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA
Jun Feng, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA.
Thomas A. Burgess, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA
Roger J. Laham, Division of Cardiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Michael Sturek, Department of Cellular and Integrative Physiology, Indiana University School of Medicine, Indianapolis, IN, USA.
Frank W. Sellke, Email: fsellke@lifespan.org, Division of Cardiothoracic Surgery, Cardiovascular Research Center, Warren Alpert Medical School of Brown University, 2 Dudley Street, MOC 360, Providence, RI 02905, USA.
References
- 1.Belin de Chantemele EJ, Irfan Ali M, Mintz J, Stepp DW. Obesity-induced insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic Res Cardiol. 2009;104:707–717. doi: 10.1007/s00395-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood) 2009;234:683–692. doi: 10.3181/0812-RM-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 5.Boodhwani M, Sodha NR, Mieno S, Ramlawi B, Xu SH, Feng J, Clements RT, Ruel M, Sellke FW. Insulin treatment enhances the myocardial angiogenic response in diabetes. J Thorac Cardiovasc Surg. 2007;134:1453–1460. doi: 10.1016/j.jtcvs.2007.08.025. (discussion 1460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BK(Ca) channels to local metabolic coronary vasodilation: effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu LM, Robich MP, Lassaletta AD, Feng J, Laham RJ, Burgess T, Clements RT, Sellke FW. Resveratrol supplementation abrogates pro-arteriogenic effects of intramyocardial vascular endothelial growth factor in a hypercholesterolemic swine model of chronic ischemia. Surgery. 2011;150:390–399. doi: 10.1016/j.surg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements RT, Sodha NR, Feng J, Boodhwani M, Liu Y, Mieno S, Khabbaz KR, Bianchi C, Sellke FW. Impaired coronary microvascular dilation correlates with enhanced vascular smooth muscle MLC phosphorylation in diabetes. Microcirculation. 2009;16:193–206. doi: 10.1080/10739680802461950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot D, Pasterkamp G, Hoefer IE. Cardiovascular risk factors and collateral artery formation. Eur J Clin Invest. 2009;39:1036–1047. doi: 10.1111/j.1365-2362.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- 11.Dohner JW. The encyclopedia of historic and endangered livestock and poultry breeds. Yale University Press; 2001. [Google Scholar]
- 12.Hattan N, Chilian WM, Park F, Rocic P. Restoration of coronary collateral growth in the Zucker obese rat: impact of VEGF and ecSOD. Basic Res Cardiol. 2007;102:217–223. doi: 10.1007/s00395-007-0646-3. [DOI] [PubMed] [Google Scholar]
- 13.Iliadis F, Kadoglou N, Didangelos T. Insulin and the heart. Diabetes Res Clin Pract. 2011;93(Suppl 1):S86–S91. doi: 10.1016/S0168-8227(11)70019-5. [DOI] [PubMed] [Google Scholar]
- 14.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA, Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol. 2011;106:1123–1134. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Woollard JR, Wang S, Korsmo MJ, Ebrahimi B, Grande JP, Textor SC, Lerman A, Lerman LO. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol. 2011;301:F1078–F1087. doi: 10.1152/ajprenal.00333.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liepinsh E, Skapare E, Svalbe B, Makrecka M, Cirule H, Dambrova M. Anti-diabetic effects of mildronate alone or in combination with metformin in obese Zucker rats. Eur J Pharmacol. 2011;658:277–283. doi: 10.1016/j.ejphar.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd PG, Fang M, Brisbin ILJ, Anderson L, Sturek M. AMP kinase gene mutation is consistent with a thrifty phenotype (metabolic syndrome) in a population of feral swine. FASEB J. 2006;20:A299. [Google Scholar]
- 20.Maejima Y, Okada H, Haraguchi G, Onai Y, Kosuge H, Suzuki J, Isobe M. Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Lab Invest. 2011;91:932–944. doi: 10.1038/labinvest.2011.45. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Navarrete JM, Ortega FJ, Rodriguez-Hermosa JI, Sabater M, Pardo G, Ricart W, Fernandez-Real JM. OCT1 expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes. 2011;60:168–176. doi: 10.2337/db10-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med. 2010;60:300–315. [PMC free article] [PubMed] [Google Scholar]
- 23.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780–H1787. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 24.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–712. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–123. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84:183–190. [PubMed] [Google Scholar]
- 27.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–S149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol. 2003;98:416–423. doi: 10.1007/s00395-003-0418-7. [DOI] [PubMed] [Google Scholar]
- 30.Sodha NR, Boodhwani M, Clements RT, Xu SH, Khabbaz KR, Sellke FW. Increased antiangiogenic protein expression in the skeletal muscle of diabetic swine and patients. Arch Surg. 2008;143:463–470. doi: 10.1001/archsurg.143.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 33.Varga O, Harangi M, Olsson IA, Hansen AK. Contribution of animal models to the understanding of the metabolic syndrome: a systematic overview. Obes Rev. 2010;11:792–807. doi: 10.1111/j.1467-789X.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 34.Volzke H, Henzler J, Menzel D, Robinson DM, Hoffmann W, Vogelgesang D, John U, Motz W, Rettig R. Outcome after coronary artery bypass graft surgery, coronary angioplasty and stenting. Int J Cardiol. 2007;116:46–52. doi: 10.1016/j.ijcard.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Wolkart G, Schrammel A, Dorffel K, Haemmerle G, Zechner R, Mayer B. Cardiac dysfunction in adipose triglyceride lipase deficiency: treatment with a PPARalpha agonist. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01490.x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Y, Perez-Polo JR, Aguilar D, Birnbaum Y. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2011;106:925–952. doi: 10.1007/s00395-011-0216-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Potter BJ, Cao JM, Zhang C. Interferon-gamma induced adipose tissue inflammation is linked to endothelial dysfunction in type 2 diabetic mice. Basic Res Cardiol. 2011;106:1135–1145. doi: 10.1007/s00395-011-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]