Abstract
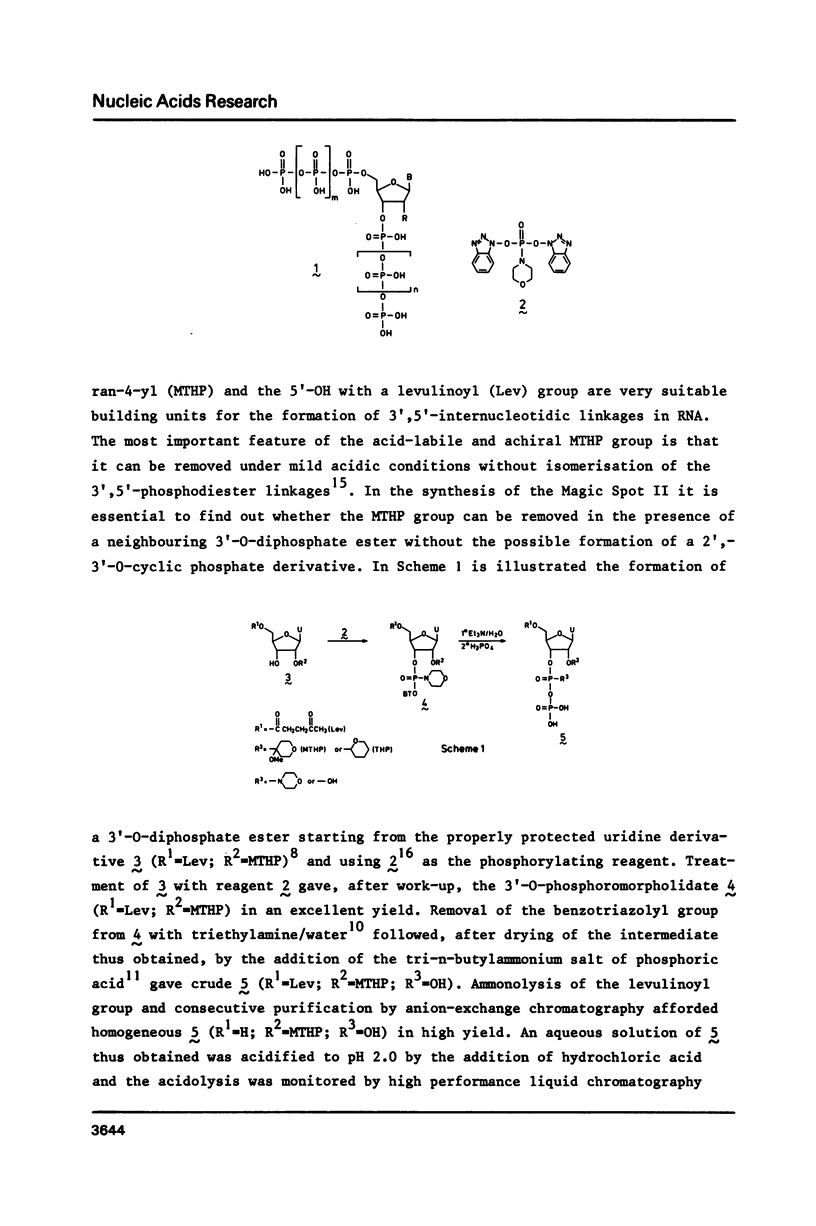
The bifunctional phosphorylating reagent O,O-bis[1-benzotriazolyl]phosphoromorpholidate was used to introduce a 5'-O-triphosphate and a 3'-O-diphosphate function in a partially protected riboguanosine. Pilot studies indicated that protection of the 2'-OH of ribonucleosides with the acid-labile tetrahydropyranyl group, instead of the more labile 4-methoxytetrahydropyran-4-yl group, was most satisfactory for the preparation of the Magic Spot II.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. N., Gough G. R., Gilham P. T. Guanosine tetraphyosphate and its analogues. Chemical synthesis of guanosine 3',5'-dipyrophosphate, deoxyguanosine 3',5'-dipyrophosphate, guanosine 2',5'-bis(methylenediphosphonate), and guanosine 3',5'-bis(methylenediphosphonate). Biochemistry. 1976 Oct 19;15(21):4623–4628. doi: 10.1021/bi00666a012. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem. 1974 Jan;57(1):100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B. The synthesis of oligoribonucleotides. IV. Preparation of dinucleoside phosphates from 2',5'-protected ribonucleoside derivatives. Tetrahedron. 1968 Jan;24(2):639–662. doi: 10.1016/0040-4020(68)88015-9. [DOI] [PubMed] [Google Scholar]
- Hamel E., Heimer E. P., Nussbaum A. L. Synthesis of deoxyguanosine polyphosphates and their interactions with the guanosine 5'-triphosphate requiring protein synthetic enzymes of Escherichia coli. Biochemistry. 1975 Nov 18;14(23):5055–5060. doi: 10.1021/bi00694a004. [DOI] [PubMed] [Google Scholar]
- Hassner A., Strand G., Rubenstein M., Patchornik A. Letter: Levulinic esters. An alcohol protecting group applicable to some nucleosides. J Am Chem Soc. 1975 Mar 19;97(6):1614–1615. doi: 10.1021/ja00839a077. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Chinault A. C., Hecht S. M. Ribonucleoside 3'-di- and -triphosphates. Synthesis of guanosine tetraphosphate (ppGpp). Biochemistry. 1975 Mar 11;14(5):981–988. doi: 10.1021/bi00676a017. [DOI] [PubMed] [Google Scholar]
- Schattenkerk C., Visser G. M., van der Marel G. A., van Boom J. H. Synthesis of ppTppp via phosphotriester intermediates. Nucleic Acids Res. 1983 Nov 11;11(21):7545–7554. doi: 10.1093/nar/11.21.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Tomasz J. Chemical synthesis of ppGpp. Biochim Biophys Acta. 1974 Apr 10;340(4):509–515. doi: 10.1016/0005-2787(74)90071-9. [DOI] [PubMed] [Google Scholar]
- den Hartog J. A., Wille G., Scheublin R. A., van Boom J. H. Chemical synthesis of a messenger ribonucleic acid fragment: AUGUUCUUCUUCUUCUUC. Biochemistry. 1982 Mar 2;21(5):1009–1018. doi: 10.1021/bi00534a028. [DOI] [PubMed] [Google Scholar]
- van der Marel G. A., van Boeckel C. A., Wille G., van Boom J. H. A general method for the synthesis of 5'-monophosphates of DNA fragments via phosphotriester intermediates. Nucleic Acids Res. 1982 Apr 10;10(7):2337–2351. doi: 10.1093/nar/10.7.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]



