Abstract
Protein phosphorylation on tyrosine has emerged as a key device in the control of numerous cellular functions in bacteria. In this article, we review the structure and function of bacterial tyrosine kinases and phosphatases. Phosphorylation is catalyzed by autophosphorylating adenosine triphosphate-dependent enzymes (bacterial tyrosine (BY) kinases) that are characterized by the presence of Walker motifs. The reverse reaction is catalyzed by three classes of enzymes: the eukaryotic-like phosphatases (PTPs) and dual-specific phosphatases; the low molecular weight protein-tyrosine phosphatases (LMW-PTPs); and the polymerase–histidinol phosphatases (PHP). Many BY kinases and tyrosine phosphatases can utilize host cell proteins as substrates, thereby contributing to bacterial pathogenicity. Bacterial tyrosine phosphorylation/dephosphorylation is also involved in biofilm formation and community development. The Porphyromonas gingivalis tyrosine phosphatase Ltp1 is involved in a restraint pathway that regulates heterotypic community development with Streptococcus gordonii. Ltp1 is upregulated by contact with S. gordonii and Ltp1 activity controls adhesin expression and levels of the interspecies signal AI-2.
Keywords: oral biofilm, Porphyromonas gingivalis, Streptococcus, tyrosine phosphorylation, virulence
Introduction
Regulation of protein activity, as orchestrated by the tightly co-ordinated and balanced dynamics between kinases and phosphatases, is one of the critical determinants of normal cellular growth and development. While the addition or removal of phosphoryl groups to/from serine, threonine or tyrosine residues has long been established as one of the predominant mechanisms of post-translational protein modification in eukaryotes, it was not until the 1970s that landmark studies demonstrated its importance in prokaryotes.1 Furthermore, protein phosphorylation has now also been shown to be prevalent in archaea.2 The first characterized phosphorylation systems in prokaryotic organisms were the two component systems (TCS) and the phosphotransferase system (PTS).3 In the basic configuration of TCS, a surface-exposed sensor kinase is first autophosphorylated in response to an external signal. The phosphoryl group is then transferred to the aspartyl residue of a response regulator, which in turn can modulate gene expression. In this manner, signal transduction is effectuated by phosphate flow. TCS are widespread in bacteria, and they control the response to a wide range of environmental stimuli. Remarkably, any one organism can possess up to 50 functionally isolated systems. In the PTS system, a phosphoryl group from phosphoenol pyruvate is transferred along a chain of proteins by reversible phosphorylation of histidine residues (although HPr is phosphorylated on histidine and serine).3 The final receptor for the phosphoryl group is a sugar; hence, the PTS is involved in carbohydrate uptake rather than signal transduction.
Phosphorylation of proteins in bacteria
Serine and threonine phosphorylation
Bacterial serine/threonine specific phosphorylation was discovered more than 30 years ago, and this post-translational modification is now known to be ubiquitous and is involved in a diverse array of physiological processes including secondary metabolism, catabolite repression, oxidative stress responses and sporulation.4 Serine/threonine kinases and phosphatases are also involved in bacterial virulence, in particular through their action on host cell substrates (Table 1). For example, the YpkA/YopO kinase of Yersinia species is delivered into epithelial cells by type III secretion machinery, whereupon it disrupts actin microfilament structure.5 Two autophosphorylated Ser/Thr protein kinases, NleH1 and NleH2, in enterohemorrhagic Escherichia coli, and the OspG protein in Shigella flexneri, inhibit activation of the proinflammatory transcription factor NF-κB.6,7 The serine phosphatase SerB of Porphyromonas gingivalis, which is required for maximal invasion of the organisms into epithelial cells, can impact both the actin and tubulin cytoskeleton of host cells, and also attenuate NF-κB activation.8,9,10 The secretion of serine kinases and/or phosphatases has thus afforded bacterial pathogens the means to interfere with host signal transduction pathways.
Table 1 . Bacterial kinases and phosphatases involved in virulence through interaction with host cell proteins.
| Organism | Enzyme | Activity | Impact on host cell function | References |
|---|---|---|---|---|
| Coxiella burnetii | Acp | Tyrosine phosphatase | Inhibition of human neutrophils | 55,56 |
| Enterohemorrhagic | NleH1, NleH2 | Ser/Thr kinase | Inhibit activation of NF-kB | 6 |
| Escherichia coli | NleH1, NleH2 | Ser/Thr kinase | Inhibit activation of NF-kB | 6 |
| Listeria monocytogenes | LipA | Tyrosine phosphatase | Actin cytoskeleton disruption | 39 |
| Mycobacterium tuberculosis | MPtpA and B | Tyrosine phosphatase | Phagocytosis actin polymerization in macrophages | 35,57 |
| PknG | Ser/Thr kinase | Inhibition of phagosome-lysosome fusion | 58 | |
| Porphyromonas gingivalis | SerB | Serine phosphatase | Disruption of actin/tubulin; inhibition of NF-κB activation, intracellular persistence | 9,10 |
| Salmonella typhi | StpA | Tyrosine phosphatase | Host cytoskeleton disruption | 59 |
| Salmonella ‘typhimurium' | SptP | Tyrosine phosphatase | Actin rearrangements | 60 |
| Shigella flexneri | OspG | Ser/Thr kinase | Inhibit NF-κB activation | 6 |
| OspF | Dual specific phosphatase | Represses innate immunity | 61 | |
| Yersinia enterocolitica | YopO | Ser/Thr kinase | Disruption of actin; inhibition of phagocytosis | 62 |
| Yersinia pseudotuberculosis | YopH | Tyrosine phosphatase | Cytoskeletal rearrangements; inhibition of phagocytosis | 28 |
| Yersinia pseudotuberculosis | YpkA | Ser/Thr kinase | Disruption of actin; inhibition of phagocytosis | 5 |
| Yersinia pestis | YpkA | Ser/Thr kinase | Disruption of actin; inhibition of phagocytosis | 5 |
Tyrosine phosphorylation
The first definitive evidence of protein tyrosine kinase activity in bacteria was discovered in E. coli with the identification of phosphotyrosine in partial acid hydrolysates of proteins.11 Protein tyrosine phosphorylation subsequently was shown to direct many essential cellular processes, such as capsule production, growth, proliferation, migration, flagellin export, adaptation to stress and production of secondary metabolites (Table 2).12 Moreover, the addition of a bulky, negatively charged phosphoryl group to a protein can influence both cellular location and the overall protein interactome.13 A number of global phosphoproteome studies have now been conducted in bacteria, including E. coli, Helicobacter pylori, Bacillus subtilis, Streptomyces coelicolor, Mycoplasma pneumoniae, Streptococcus pneumoniae, Klebsiella pneumoniae, Lactococcus lactis, Campylobacter jejuni and Pseudomonas species. These databases have shown an increasing number of bacterial proteins that are phosphorylated on Ser/Thr/Tyr residues; and, moreover, these proteins are involved in a variety of important cellular functions, including virulence and cell survival.14,15,16,17,18,19,20,21,22,23,24
Table 2. Bacterial protein tyrosine kinases and phosphatases and their functional roles.
| Organism | Tyrosine Kinase | Tyrosine Phosphatase | Substrate(s) | Function | References |
|---|---|---|---|---|---|
| Acinetobacter johnsonii | Ptk | Ptp | Ptp uses Ptk as endogenous substrate | Phosphorelay reactions of inner membrane proteins | 63 |
| Acinetobacter lwoffii | Wzc | Wzb | Wzb uses Wzc as endogenous substrate | Emulsan production | 46 |
| Bacillus subtilis | YwqD, PtkA, PtkB, McsB | YwqE, YfkJ, YwlE, PtpZ | TuaD, Ugd, SsbA, McsA, CtsR, YjoA, YnfE, TvyG, YorK, Asd, YwpH | Exopolysaccharide synthesis, teichuronic acid production, DNA metabolism, heat shock response | 64,65 |
| Caulobacter crescentus | DivL | — | CtrA | Cell division | 66 |
| Erwinia amylovora | AmsA | AmsI | Lipid carrier di-/monophosphates | Amylovoran production | 67 |
| Escherichia coli K-12 | WzcCA | Wzb | Ugd; Wzb uses Wzc as endogenous substrate | Colanic acid synthesis | 40,68 |
| Escherichia coli K-12/K-30 | Etk | Etp | RpoH, RseA; Etk | Exopolysaccharide production | 69 |
| Escherichia coli K-30 | WzcCPS | Wzb | Ugd | Group 1 capsule assembly | 44 |
| Klebsiella pneumonia | Yco6, Wzc | Yor5, Wzb | Yor5 uses Yco6 as endogenous substrate | Capsule synthesis | 18,70 |
| Myxococcus xanthus | MasK | — | MgIA | Aggregation, sporulation, motility, development | 71 |
| Porphyromonas gingivalis | Ltp1 | Exopolysaccharide production, heterotypic community development | 49 | ||
| Pseudomonas aeruginosa | WaaP | Lipopolysaccharide synthesis | 72 | ||
| Pseudomonas aeruginosa | 42k | Flagellin a and b proteins; | Flagellin export | 73 | |
| Pseudomonas aeruginosa | TbpA | Diguanylate cyclase | Exopolysaccharide production, biofilm development | 47 | |
| Ralstonia solanacearum | EpsB | EpsP | Exopolysaccharide transport | 74,75 | |
| Salmonella typhimurium | PutA | — | P5C | Proline metabolism | 76 |
| Sinorhizobium meliloti | ExoP | — | Succinoglycan production | 77 | |
| Staphylococcus aureus | Cap5B2 | CapC, PtpA, PtpB | Cap5O (UDP-acetyl-mannosamine dehydrogenase) | Capsule synthesis | 78 |
| Streptococcus agalactiae | CpsD | CpsB | Polysaccharide chain length | 79 | |
| Streptococcus pneumoniae | CpsD | CpsB | Capsule synthesis | 80 | |
| Streptococcus thermophilus | EpsD | EpsB | EpsE | Exopolysaccharide biosynthesis | 81 |
| Streptomyces coelicolor A3(2) | AfsK | — | AfsR | Antibiotic production | 82,83,84 |
| SCO5717 | Cell growth |
Bacterial tyrosine kinases
Structure
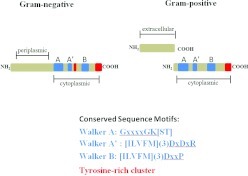
The bacterial tyrosine (BY) kinase family comprises the major group of bacterial enzymes endowed with tyrosine kinase activity. In most cases, BY kinases possess a transmembrane domain that can function both as an anchor and a sensor, as well as an intracellular catalytic domain.25 The catalytic domain lacks the distinctive eukaryotic kinase motifs, and is defined by the presence of Walker A (P-loop) and B motifs (Figure 1). In addition, some BY kinases also contain a Walker A′ motif. BY kinases autophosphorylate at a tyrosine rich cluster in the C-terminal region using adenosine triphosphate as a phosphoryl donor, and the degree of phosphorylation in this region determines the interaction strength with other proteins. Some BY kinases also autophosphorylate on a tyrosine residue in close proximity to the Walker A′ box. Recent studies have identified other bacterial tyrosine kinases including those that closely resemble eukaryotic-like kinases, and those that utilize guaidino-phosphotransferase domains. Additionally, in some cases, tyrosine can substitute for histidine in TCS.25
Figure 1.
Domain structure of BY kinases. A periplasmic (gram-negatives) or extracellular (gram-positives) sensory loop is linked to the catalytic intracellular domain, either contiguously (gram-negatives) or through protein–protein interaction (gram-negatives). The catalytic domain contains Walker A, B and A′ motifs (blue). A tyrosine-rich region (red) containing the phosphorylation sites is present in the C-terminus, and gram-negative BY kinases also possess an internal tyrosine (red) that can be autophosphorylated. Walker motifs A, A′ and B can be identified by conserved sequences motifs. BY, bacterial tyrosine.
Function
The majority of genes encoding BY kinases reside in operons responsible for regulating the synthesis and secretion of polysaccharides. The autophosphorylation state of the BY kinases exerts control over this process through phosphorylation, and activation, of UDP-sugar dehydrogenases and glucosyltransferases.13 As bacterial regulatory networks are extensively interconnected, the phosphotransfer reactions can modulate a myriad of physiological processes that include resistance to cationic peptides and polymixin, along with heat shock responses. A greater appreciation for the role of tyrosine kinases in prokaryotes has emerged from the application of global phosphoproteome technologies. For example, the PtkA BY kinase of Bacillus subtilis can phosphorylate at least nine different protein substrates.26 Several of these substrates, most notably single-stranded DNA exonuclease YorK and aspartate semialdehyde dehydrogenase Asd, are activated via phosphorylation. Yet, the activity of many others, such as enolase, YjoA, YnfE, TvyG, Ugd and SsbA, remains unaffected by phosphorylation, and rather the cellular localization of these proteins is governed by phosphorylation status. Hence, BY action can not only regulate the activity of substrates, but also ensure the correct cellular localization of specific protein targets.
Bacterial tyrosine phosphatases
Structure
Bacterial tyrosine phosphatases catalyze the dephosphorylation of tyrosyl phosphorylated proteins, which in turn can result in either the propagation or inhibition of phospho-dependent signaling. Bacterial tyrosine phosphatases can be categorized into three distinct families: (i) the eukaryotic-like phosphatases (PTPs) and dual-specific phosphatases that also display activity against phosphoserine and phosphothreonine; (ii) the low molecular weight protein-tyrosine phosphatases (LMW-PTPs), a family of small acidic enzymes also found in eukaryotes; and (iii) the polymerase–histidinol phosphatases (PHP), a family of phosphoesterases commonly found in gram-positive bacteria. The PTP, dual-specific phosphatase and LMW-PTP enzymes utilize a common catalytic mechanism that involves the conserved signature C(X)5R motif in the phosphate binding loop where cysteine, functioning as a nucleophile, attacks the phosphorus atom of the phosphotyrosine residue of the substrate. The arginine residue interacts with the phosphate moiety of the phosphotyrosine.27 This motif is flanked, more remotely, by an essential aspartic acid residue, the location of which varies among the families. Protein tyrosine phosphatases also are capable of possessing dual functions, whereby in some instances, they can stimulate actions of cognate protein tyrosine kinases, yet in other cases, they may antagonize those actions.12 In gram-negative bacteria, the gene encoding the LMW-PTP generally is upstream of the tyrosine kinase in the same operon. Conversely, in gram-positives, a PHP type phosphatase often is located in the same operon as the BY kinase alongside an adaptor protein, with the gene for the LMW-PTP at a remote site.
Function
While bacterial tyrosine phosphatases can be intimately involved in a number of cellular processes, two major themes have become apparent: involvement in polysaccharide production; and as secreted effector proteins with the potential for manipulation of host cell signal transduction pathways. Polysaccharide production, encompassing both exopolysaccharides and capsular polysaccharides, is also a key virulence determinant in many organisms and thus tyrosine phosphatase activity is emerging as a central player in the information flow that controls pathogenic activity.
The YopH protein tyrosine phosphatase of Yersinia, a member of the PTP family, is an essential virulence factor that is injected into epithelial cells by type III secretion machinery. YopH can uncouple multiple signal transduction pathways,28 and in human epithelial cells YopH dephosphorylates several focal adhesion proteins, including p130Cas (Cas), focal adhesion kinase and paxillin.29,30,31 Similarly, Salmonella ‘typhimurium' translocates the PTP tyrosine phosphatase SptP into epithelial cells where it is involved in reversing mitogen-activated protein kinase activation.32 SptP is required for full virulence in murine models of disease.33 Shigella flexneri produces a dually specific phosphatase, OspF, that dephosphorylates mitogen-activated protein kinase, which consequently prevents histone H3 phosphorylation.34 A reduction in the level of histone 3 phosphorylation impedes access of the transcription factor NF-κB to the chromosome and hence transcription of NF-κB responsive genes such as IL-8 is reduced. Thus, OspF activity allows S. flexneri to modulate host cell epigenetic information as a strategy for repressing innate immunity. Mycobacterium tuberculosis secretes two LMW-PTPs, PtpA and PtpB.35 The predicted lack of tyrosine kinases in the M. tuberculosis genome suggests a dedicated role for these phosphatases in regulation of host cell functions. Expression of ptpA in M. tuberculosis is upregulated within monocytes, and a ptpB mutant is impaired in its ability to grow in human macrophages36 and survive in a guinea pig model.37 These phosphatases appear to function by impacting actin polymerization within macrophages and thereby affecting phagocytosis of the organism.38 A recently identified phosphatase, LipA, in L. monocytogenes has a predicted structure bearing a remarkable semblance to the PtpB phosphatase.39 Moreover, both LipA and PtpB share a unique feature whereby they possess dual-function activities as phosphotyrosine and phosphoinositide phosphatases, and both harbor the potential to play pivotal roles in bacterial virulence.
In addition to physical protection, exopolysaccharide such as capsule is often poorly immunogenic and can mask protein antigens and receptors for complement and phagocytic cells. In many cases, dephosphorylation of tyrosine kinases increases the level of polysaccharide synthesis,4 as evidenced by the activity of the E. coli K-12 BY kinase Wzc-ca, which is regulated by its cognate LMW-PTP, Wzb.40 In this system, production of the capsular exopolysaccharide colonic acid is maximal when Wzc-ca is dephosphorylated by Wzb. Similarly, in Streptococcus pneumoniae autophosphorylation of the CpsD kinase, when in the presence of its cognate partner, CpsC, results in the attenuation of CpsD kinase activity, as well as a reduction in the level of encapsulation via a negative feedback regulatory loop.41 Consequently, the PHP family phosphatase CpsB can control capsule production via dephosphorylation of CpsD which functions as a reversible switch.42 The converse situation also exists. In clinical isolates of S. pneumoniae, phosphorylation of CpsD increases capsule production under anaerobic conditions,43 and in E. coli K30 the assembly of group I capsular polysaccharides is elevated by phosphorylation of Wzc-cps.44 Undoubtedly, the interplay among tyrosine kinases, phosphatases and exopolysaccharide is of a nuanced and subtle nature that may be reconfigured according to environmental conditions. Indeed, metabolic activity is one factor that has been shown to influence kinase to phosphatase ratios.45,46
Role in biofilms
A recent study in Pseudomonas aeruginosa demonstrated that tyrosine phosphatase activity is a unifying element that amalgamates polysaccharide production and biofilm formation with quorum sensing.47 The PTP family tyrosine phosphatase, TpbA, is a negative regulator of 3,5-cyclic diguanylic acid (c-di-GMP), an important second messenger which suppresses transcription across the pel operon that encodes for extracellular matrix polysaccharide. Lower levels of exopolysaccharide in turn lead to reduced biofilm formation. In addition, TpbA responds to acyl homoserine lactone, and tpbA is regulated positively by the LasR transcriptional regulator. TpbA also regulates cell lysis as a means to control extracellular DNA that is used for complex biofilm maturation.48 These findings also reveal a previously unrecognized ability for phospho-dependent signaling to intersect with other important cellular second messenger systems.
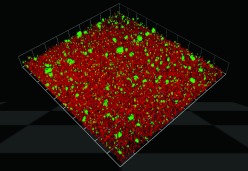
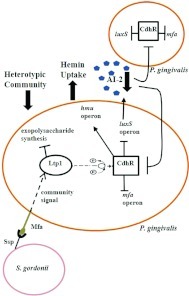
Tyrosine phosphatases can also control heterotypic biofilm formation among oral organisms. P. gingivalis accumulates into heterotypic communities with the antecedent oral biofilm colonizer S. gordonii (Figure 2). Maeda et al.49 identified a LMW-PTP, Ltp1, in P. gingivalis which functions as a negative regulator of EPS production, as well as community formation with S. gordonii. Transcription of ltp1 is increased following contact with S. gordonii,50 and Ltp1 is a component of a signaling pathway that converges on the LuxR family transcriptional regulator CdhR.51 The expression of the P. gingivalis Mfa fimbriae and of LuxS, both of which contribute to community development with S. gordonii, are negatively regulated by CdhR (Figure 3). Thus, in both P. aeruginosa and P. gingivalis, tyrosine phosphatase activity results in arrested community development which may maintain optimal biofilm architecture.
Figure 2.
Porphyromonas gingivalis (green) accumulates into a mixed species community on a substratum of Streptococcus gordonii (red). Image courtesy of Dr Christopher Wright.
Figure 3.
Model of the tyrosine phosphatase-dependent regulatory circuitry governing heterotypic community development between Porphyromonas gingivalis and Streptococcus gordonii. Initial interaction of the P. gingivalis Mfa fimbriae with S. gordonii activates the Ltp1 phosphatase and a signaling event is transduced via a cascade of phosphorylation/dephosphorylation events. Signaling converges on CdhR which represses transcription of the luxS and mfa operons in P. gingivalis, and in turn leads to constrained P. gingivalis–S. gordonii community development. Lower AI-2 levels can be sensed by neighboring planktonic P. gingivalis cells, which also upregulate CdhR, thereby propagating the original streptococcal-derived signal throughout the P. gingivalis–S. gordonii community. (Modified from Molecular Microbiology 2011; 81(2): 305–314; this material is reproduced with permission of John Wiley & Sons, Inc.)
Conclusion
It is sobering to reflect that until fairly recently, post-translational modification of tyrosine residues by phosphorylation was believed to be an indicator of the sophisticated regulatory networks characteristic of eukaryotic systems. Since then, research on bacterial tyrosine kinases and phosphatases has proceeded apace and they are now considered key contributors to bacterial cell homeostasis, virulence and even cell survival in all domains of life. Future genomics and proteomics research will decipher and dissect the underlying mechanisms of tyrosine phosphotransfer cascades in bacteria and their functional roles. Accumulating evidence reaffirms the notion that bacterial tyrosine kinases and phosphatases display exquisite substrate specificity; nevertheless, they are still capable of utilizing multiple protein substrates, both endogenous and exogenous, thereby providing versatility in phosphorelay signaling networks. Ongoing studies reveal increasing instances where bacterial kinases/phosphatases are capable of inducing post-translational modifications of host proteins and are a crucial facet of the dynamic host-pathogen relationship. Moreover, as BY kinases differ from their eukaryotic counterparts in significant biochemical and structural aspects, they provide attractive targets for specific antibacterial drugs. The crystal structures of the CapB kinase of Staphylococcus aureus and the Etk kinase of E. coli have been determined52,53 and they exhibit a high degree of structural similarity. The availability of structural and biochemical information will facilitate the rational design of compounds that can inhibit BY kinases, while concomitantly avoiding any eukaryotic kinases.54
Acknowledgments
Research in the authors' laboratory is supported by NIH/NIDCR DE12505 and DE11111.
References
- Backert S, Selbach M. Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol. 2005;13 10:476–484. doi: 10.1016/j.tim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Aivaliotis M, Macek B, Gnad F, et al. Ser/Thr/Tyr protein phosphorylation in the archaeon Halobacterium salinarum–a representative of the third domain of life. PLoS One. 2009;4 3:e4777. doi: 10.1371/journal.pone.0004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone AJ, Grangeasse C, Doublet P, et al. Protein phosphorylation on tyrosine in bacteria. Arch Microbiol. 2004;181 3:171–181. doi: 10.1007/s00203-003-0640-6. [DOI] [PubMed] [Google Scholar]
- Cozzone AJ. Role of protein phosphorylation on serine/threonine and tyrosine in the virulence of bacterial pathogens. J Mol Microbiol Biotechnol. 2005;9 3/4:198–213. doi: 10.1159/000089648. [DOI] [PubMed] [Google Scholar]
- Wiley DJ, Nordfeldth R, Rosenzweig J, et al. The Ser/Thr kinase activity of the Yersinia protein kinase A (YpkA) is necessary for full virulence in the mouse, mollifying phagocytes, and disrupting the eukaryotic cytoskeleton. Microb Pathog. 2006;40 5:234–243. doi: 10.1016/j.micpath.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Gao X, Wan F, Mateo K, et al. Bacterial effector binding to ribosomal protein s3 subverts NF-κB function. PLoS Pathog. 2009;5 12:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royan SV, Jones RM, Koutsouris A, et al. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-κB activation. Mol Microbiol. 2010;78 5:1232–1245. doi: 10.1111/j.1365-2958.2010.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge B, Verma RK, Eastman C, et al. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect Immun. 2010;78 11:4560–4569. doi: 10.1128/IAI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Tribble GD, Baker HV, et al. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76 6:2420–2427. doi: 10.1128/IAI.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble GD, Mao S, James CE, et al. A Porphyromonas gingivalis haloacid dehalogenase family phosphatase interacts with human phosphoproteins and is important for invasion. Proc Natl Acad Sci USA. 2006;103 29:11027–11032. doi: 10.1073/pnas.0509813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manai M, Cozzone AJ. Endogenous protein phosphorylation in Escherichia coli extracts. Biochem Biophys Res Commun. 1982;107 3:981–988. doi: 10.1016/0006-291x(82)90619-2. [DOI] [PubMed] [Google Scholar]
- Zhang ZY. Functional studies of protein tyrosine phosphatases with chemical approaches. Biochim Biophys Acta. 2005;1754 1/2:100–107. doi: 10.1016/j.bbapap.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Stulke J. More than just activity control: phosphorylation may control all aspects of a protein's properties. Mol Microbiol. 2010;77 2:273–275. doi: 10.1111/j.1365-2958.2010.07228.x. [DOI] [PubMed] [Google Scholar]
- Ge R, Sun X, Xiao C, et al. Phosphoproteome analysis of the pathogenic bacterium Helicobacter pylori reveals over-representation of tyrosine phosphorylation and multiply phosphorylated proteins. Proteomics. 2011;11 8:1449–1461. doi: 10.1002/pmic.201000649. [DOI] [PubMed] [Google Scholar]
- Parker JL, Jones AM, Serazetdinova L, et al. Analysis of the phosphoproteome of the multicellular bacterium Streptomyces coelicolor A3(2) by protein/peptide fractionation, phosphopeptide enrichment and high-accuracy mass spectrometry. Proteomics. 2010;10 13:2486–2497. doi: 10.1002/pmic.201000090. [DOI] [PubMed] [Google Scholar]
- Schmidl SR, Gronau K, Pietack N, et al. The phosphoproteome of the minimal bacterium Mycoplasma pneumoniae: analysis of the complete known Ser/Thr kinome suggests the existence of novel kinases. Mol Cell Proteomics. 2010;9 6:1228–1242. doi: 10.1074/mcp.M900267-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ge F, Xiao CL, et al. Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. . J Proteome Res. 2010;9 1:275–282. doi: 10.1021/pr900612v. [DOI] [PubMed] [Google Scholar]
- Lin MH, Hsu TL, Lin SY, et al. Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol Cell Proteomics. 2009;8 12:2613–2623. doi: 10.1074/mcp.M900276-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran A, Sugiyama N, Tomita M, et al. Ser/Thr/Tyr phosphoproteome analysis of pathogenic and non-pathogenic Pseudomonas species. Proteomics. 2009;9 10:2764–2775. doi: 10.1002/pmic.200800655. [DOI] [PubMed] [Google Scholar]
- Voisin S, Watson DC, Tessier L, et al. The cytoplasmic phosphoproteome of the gram-negative bacterium Campylobacter jejuni: evidence for modification by unidentified protein kinases. Proteomics. 2007;7 23:4338–4348. doi: 10.1002/pmic.200700483. [DOI] [PubMed] [Google Scholar]
- Soufi B, Gnad F, Jensen PR, et al. The Ser/Thr/Tyr phosphoproteome of Lactococcus lactis IL1403 reveals multiply phosphorylated proteins. Proteomics. 2008;8 17:3486–3493. doi: 10.1002/pmic.200800069. [DOI] [PubMed] [Google Scholar]
- Macek B, Mijakovic I, Olsen JV, et al. The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. . Mol Cell Proteomics. 2007;6 4:697–707. doi: 10.1074/mcp.M600464-MCP200. [DOI] [PubMed] [Google Scholar]
- Macek B, Gnad F, Soufi B, et al. Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol Cell Proteomics. 2008;7 2:299–307. doi: 10.1074/mcp.M700311-MCP200. [DOI] [PubMed] [Google Scholar]
- Soufi B, Jers C, Hansen ME, et al. Insights from site-specific phosphoproteomics in bacteria. Biochim Biophys Acta. 2008;1784 1:186–192. doi: 10.1016/j.bbapap.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Grangeasse C, Cozzone AJ, Deutscher J, et al. Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem Sci. 2007;32 2:86–94. doi: 10.1016/j.tibs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Jers C, Pedersen MM, Paspaliari DK, et al. Bacillus subtilis BY-kinase PtkA controls enzyme activity and localization of its protein substrates. Mol Microbiol. 2010;77 2:287–299. doi: 10.1111/j.1365-2958.2010.07227.x. [DOI] [PubMed] [Google Scholar]
- Tiganis T. Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor. IUBMB Life. 2002;53 1:3–14. doi: 10.1080/15216540210811. [DOI] [PubMed] [Google Scholar]
- Bliska JB. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 2000;8 5:205–208. doi: 10.1016/s0966-842x(00)01738-8. [DOI] [PubMed] [Google Scholar]
- Black DS, Montagna LG, Zitsmann S, et al. Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol Microbiol. 1998;29 5:1263–1274. doi: 10.1046/j.1365-2958.1998.01014.x. [DOI] [PubMed] [Google Scholar]
- Persson C, Nordfelth R, Andersson K, et al. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol Microbiol. 1999;33 4:828–838. doi: 10.1046/j.1365-2958.1999.01529.x. [DOI] [PubMed] [Google Scholar]
- Persson C, Carballeira N, Wolf-Watz H, et al. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16 9:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Le TX, Cowen DS. SptP, a Salmonella typhimurium type III-secreted protein, inhibits the mitogen-activated protein kinase pathway by inhibiting Raf activation. Cell Microbiol. 2003;5 4:267–275. doi: 10.1046/j.1462-5822.2003.t01-1-00274.x. [DOI] [PubMed] [Google Scholar]
- Kaniga K, Uralil J, Bliska JB, et al. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. . Mol Microbiol. 1996;21 3:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, et al. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8 1:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- Koul A, Choidas A, Treder M, et al. Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. . J Bacteriol. 2000;182 19:5425–5432. doi: 10.1128/jb.182.19.5425-5432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H, Papavinasasundaram KG, Wong D, et al. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe. 2008;3 5:316–322. doi: 10.1016/j.chom.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh G, Trajkovic V, et al. Intracellular expression of Mycobacterium tuberculosis-specific 10-kDa antigen down-regulates macrophage B7.1 expression and nitric oxide release. Clin Exp Immunol. 2003;134 1:70–77. doi: 10.1046/j.1365-2249.2003.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castandet J, Prost JF, Peyron P, et al. Tyrosine phosphatase MptpA of Mycobacterium tuberculosis inhibits phagocytosis and increases actin polymerization in macrophages. Res Microbiol. 2005;156 10:1005–1013. doi: 10.1016/j.resmic.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Kastner R, Dussurget O, Archambaud C, et al. LipA, a tyrosine and lipid phosphatase involved in the virulence of Listeria monocytogenes. . Infect Immun. 2011;79 6:2489–2498. doi: 10.1128/IAI.05073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C, Doublet P, Grangeasse C, et al. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J Bacteriol. 1999;181 11:3472–3477. doi: 10.1128/jb.181.11.3472-3477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona JK, Paton JC, Miller DC, et al. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. . Mol Microbiol. 2000;35 6:1431–1442. doi: 10.1046/j.1365-2958.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- Bender MH, Yother J. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. . J Biol Chem. 2001;276 51:47966–47974. doi: 10.1074/jbc.M105448200. [DOI] [PubMed] [Google Scholar]
- Weiser JN, Bae D, Epino H, et al. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. . Infect Immun. 2001;69 9:5430–5439. doi: 10.1128/IAI.69.9.5430-5439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wugeditsch T, Paiment A, Hocking J, et al. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. . J Biol Chem. 2001;276 4:2361–2371. doi: 10.1074/jbc.M009092200. [DOI] [PubMed] [Google Scholar]
- Niemeyer D, Becker A. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J Bacteriol. 2001;183 17:5163–5170. doi: 10.1128/JB.183.17.5163-5170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakar D, Gutnick DL. Involvement of a protein tyrosine kinase in production of the polymeric bioemulsifier emulsan from the oil-degrading strain Acinetobacter lwoffii RAG-1. J Bacteriol. 2003;185 3:1001–1009. doi: 10.1128/JB.185.3.1001-1009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885) PLoS Pathog. 2009;5 6:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wood TK. Tyrosine phosphatase TpbA of Pseudomonas aeruginosa controls extracellular DNA via cyclic diguanylic acid concentrations. Environ Microbiol. 2010;2 3:449–455. doi: 10.1111/j.1758-2229.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Tribble GD, Tucker CM, et al. A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Mol Microbiol. 2008;69 5:1153–1164. doi: 10.1111/j.1365-2958.2008.06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato MR, Tucker CM, Kuboniwa M, et al. Porphyromonas gingivalis genes involved in community development with Streptococcus gordonii. . Infect Immun. 2006;74 11:6419–6428. doi: 10.1128/IAI.00639-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Hirano T, Bainbridge BW, et al. Community signalling between Streptococcus gordonii and Porphyromonas gingivalis is controlled by the transcriptional regulator CdhR. Mol Microbiol. 2010;78 6:1510–1522. doi: 10.1111/j.1365-2958.2010.07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Illana V, Meyer P, Bechet E, et al. Structural basis for the regulation mechanism of the tyrosine kinase CapB from Staphylococcus aureus. . PLoS Biol. 2008;6 6:e143. doi: 10.1371/journal.pbio.0060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Zheng J, She YM, et al. Structure of Escherichia coli tyrosine kinase Etk reveals a novel activation mechanism. EMBO J. 2008;27 12:1758–1766. doi: 10.1038/emboj.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone AJ. Bacterial tyrosine kinases: novel targets for antibacterial therapy. Trends Microbiol. 2009;17 2:536–543. doi: 10.1016/j.tim.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Hill J, Samuel JE. Coxiella burnetii acid phosphatase inhibits the release of reactive oxygen intermediates in polymorphonuclear leukocytes. Infect Immun. 2011;79 1:414–420. doi: 10.1128/IAI.01011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Curley G, Lopez M, et al. Protein-tyrosine phosphatase activity of Coxiella burnetii that inhibits human neutrophils. Acta Virol. 1996;40 5/6:263–272. [PubMed] [Google Scholar]
- Cowley SC, Babakaiff R, Av-Gay Y. Expression and localization of the Mycobacterium tuberculosis protein tyrosine phosphatase PtpA. Res Microbiol. 2002;153 4:233–241. doi: 10.1016/s0923-2508(02)01309-8. [DOI] [PubMed] [Google Scholar]
- Walburger A, Koul A, Ferrari G, et al. Protein kinase G from pathogenic Mycobacteria promotes survival within macrophages. Science. 2004;304 5678:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- Arricau N, Hermant D, Waxin H, et al. Molecular characterization of the Salmonella typhi StpA protein that is related to both Yersinia YopE cytotoxin and YopH tyrosine phosphatase. Res Microbiol. 1997;148 1:21–26. doi: 10.1016/S0923-2508(97)81896-7. [DOI] [PubMed] [Google Scholar]
- Murli S, Watson RO, Galan JE. Role of tyrosine kinases and the tyrosine phosphatase SptP in the interaction of Salmonella with host cells. Cell Microbiol. 2001;3 12:795–810. doi: 10.1046/j.1462-5822.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- Reiterer V, Grossniklaus L, Tschon T, et al. Shigella flexneri type III secreted effector OspF reveals new crosstalks of proinflammatory signaling pathways during bacterial infection. Cell Signal. 2011;23 7:1188–1196. doi: 10.1016/j.cellsig.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Groves E, Rittinger K, Amstutz M, et al. Sequestering of Rac by the Yersinia effector YopO blocks Fcγ receptor-mediated phagocytosis. J Biol Chem. 2010;285 6:4087–4098. doi: 10.1074/jbc.M109.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeasse C, Doublet P, Vincent C, et al. Functional characterization of the low-molecular-mass phosphotyrosine-protein phosphatase of Acinetobacter johnsonii. . J Mol Biol. 1998;278 2:339–347. doi: 10.1006/jmbi.1998.1650. [DOI] [PubMed] [Google Scholar]
- Mijakovic I, Petranovic D, Bottini N, et al. Protein-tyrosine phosphorylation in Bacillus subtilis. . J Mol Microbiol Biotechnol. 2005;9 3/4:189–197. doi: 10.1159/000089647. [DOI] [PubMed] [Google Scholar]
- Mijakovic I, Petranovic D, Macek B, et al. Bacterial single-stranded DNA-binding proteins are phosphorylated on tyrosine. Nucleic Acids Res. 2006;34 5:1588–1596. doi: 10.1093/nar/gkj514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ohta N, Zhao JL, et al. A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc Natl Acad Sci USA. 1999;96 23:13068–13073. doi: 10.1073/pnas.96.23.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugert P, Geider K. Characterization of the amsI gene product as a low molecular weight acid phosphatase controlling exopolysaccharide synthesis of Erwinia amylovora. . FEBS Lett. 1997;400 2:252–256. doi: 10.1016/s0014-5793(96)01398-1. [DOI] [PubMed] [Google Scholar]
- Reid AN, Whitfield C. Functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J Bacteriol. 2005;187 15:5470–5481. doi: 10.1128/JB.187.15.5470-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan O, Bloch Y, Frankel G, et al. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 1999;18 12:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preneta R, Jarraud S, Vincent C, et al. Isolation and characterization of a protein-tyrosine kinase and a phosphotyrosine-protein phosphatase from Klebsiella pneumoniae. . Comp Biochem Physiol B Biochem Mol Biol. 2002;131 1:103–112. doi: 10.1016/s1096-4959(01)00490-0. [DOI] [PubMed] [Google Scholar]
- Thomasson B, Link J, Stassinopoulos AG, et al. MglA, a small GTPase, interacts with a tyrosine kinase to control type IV pili-mediated motility and development of Myxococcus xanthus. . Mol Microbiol. 2002;46 5:1399–1413. doi: 10.1046/j.1365-2958.2002.03258.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lam JS. WaaP of Pseudomonas aeruginosa is a novel eukaryotic type protein-tyrosine kinase as well as a sugar kinase essential for the biosynthesis of core lipopolysaccharide. J Biol Chem. 2002;277 7:4722–4730. doi: 10.1074/jbc.M107803200. [DOI] [PubMed] [Google Scholar]
- South SL, Nichols R, Montie TC. Tyrosine kinase activity in Pseudomonas aeruginosa. . Mol Microbiol. 1994;12 6:903–910. doi: 10.1111/j.1365-2958.1994.tb01078.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Carney BF, Denny TP, et al. A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum. . J Bacteriol. 1995;177 5:1259–1267. doi: 10.1128/jb.177.5.1259-1267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Schell M. Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol Microbiol. 1995;16 5:977–989. doi: 10.1111/j.1365-2958.1995.tb02323.x. [DOI] [PubMed] [Google Scholar]
- Ostrovsky PC, Maloy S. Protein phosphorylation on serine, threonine, and tyrosine residues modulates membrane-protein interactions and transcriptional regulation in Salmonella typhimurium. . Genes Dev. 1995;9 16:2034–2041. doi: 10.1101/gad.9.16.2034. [DOI] [PubMed] [Google Scholar]
- Jofre E, Becker A. Production of succinoglycan polymer in Sinorhizobium meliloti is affected by SMb21506 and requires the N-terminal domain of ExoP. Mol Plant Microbe Interact. 2009;22 12:1656–1668. doi: 10.1094/MPMI-22-12-1656. [DOI] [PubMed] [Google Scholar]
- Gruszczyk J, Fleurie A, Olivares-Illana V, et al. Structure analysis of the Staphylococcus aureus UDP-N-acetyl-mannosamine dehydrogenase Cap5O Involved in capsular polysaccharide biosynthesis. J Biol Chem. 2011;286 19:17112–17121. doi: 10.1074/jbc.M110.216002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens CE, Heggen LM, Haft RF, et al. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993;8 5:843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Morona JK, Morona R, Miller DC, et al. Mutational analysis of the carboxy-terminal (YGX)4 repeat domain of CpsD, an autophosphorylating tyrosine kinase required for capsule biosynthesis in Streptococcus pneumoniae. . J Bacteriol. 2003;185 10:3009–3019. doi: 10.1128/JB.185.10.3009-3019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic Z, Marie C, Delorme C, et al. Control of EpsE, the phosphoglycosyltransferase initiating exopolysaccharide synthesis in Streptococcus thermophilus, by EpsD tyrosine kinase. J Bacteriol. 2007;189 4:1351–1357. doi: 10.1128/JB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Hong SK, Ishizuka H, et al. Phosphorylation of the AfsR protein involved in secondary metabolism in Streptomyces species by a eukaryotic-type protein kinase. Gene. 1994;146 1:47–56. doi: 10.1016/0378-1119(94)90832-x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim K, Suh JW, et al. Binding study of AfsK, a Ser/Thr kinase from Streptomyces coelicolor A3(2) and S-adenosyl-L-methionine. FEMS Microbiol Lett. 2007;266 2:236–240. doi: 10.1111/j.1574-6968.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Kihara H, Watanabe W, et al. A novel tyrosine-phosphorylated protein inhibiting the growth of Streptomyces cells. Biochem Biophys Res Commun. 2009;385 4:534–538. doi: 10.1016/j.bbrc.2009.05.091. [DOI] [PubMed] [Google Scholar]