Abstract
Histone octamers are the basic building blocks of chromatin and platforms for diverse genetic mechanisms. We report a simple method for preparing recombinant histone octamers by overexpressing all four histones from a single polycistronic vector followed by standard chromatography under native conditions. This approach reduces the time needed for the octamer preparation to a single day and should be applicable to making a variety of unmodified and modified histone octamers.
Keywords: Histones, chromatin, nucleosome, polycistronic vector
75 - 90% of eucaryotic genomes are packaged in the repeating units of nucleosomes, each of which contains ~147-bp of DNA tightly wrapped around an octameric histone complex [1; 2]. Histone octamers in nucleosomes set the stages for genetic transactions such as replication, transcription and repair, and they are often the direct targets of protein machineries controlling these processes. Compositional and post-translational variations in histones also sculpt the epigenetic landscape for gene regulation [3].
Studies of detailed genetic and epigenetic mechanisms mandate the preparation of purified homogeneous histone octamers to reconstitute nucleosomes and chromatin structures in vitro. Currently, there are two major ways to prepare histones to reconstitute nucleosomes. First, endogenous histones can be isolated from sources such as HeLa cells or calf thymus [4; 5]. However, these endogenous histone complexes are inhomogeneous in their composition and post-translational modifications. In addition, they are not amenable to molecular cloning and engineering. A second method overcomes this limitation by using recombinant histones. In this method, four histone core subunits (H2A, H2B, H3 and H4) are individually overexpressed and purified from insoluble, inclusion bodies from bacteria under denaturing conditions. Octameric complexes are then formed by refolding the denatured histones together [6; 7]. The procedure, however, is labor-intensive and lengthy; it involves chromatography procedures under high concentrations of denaturants (e.g., 6 M guanidinium chloride) and multiple rounds of dialysis and lyophilization, which typically takes over a month to complete. The required amount of work can be exacerbated when generating octamers with various histone mutants.
To overcome the limitations of the existing methods, we have developed a simple and fast way to express and purify recombinant histone octamers, which can reduce the time to purify histone octamers to one day. In this method, all four core histones (H2A, H2B, H3 and H4) are expressed together from a single polycistronic vector, lysed and purified with two standard chromatography steps under non-denaturing conditions (Fig. 1A). To verify our results, we have characterized the resulting histones by size-exclusion chromatography and gel electrophoresis, as well as, the resulting reconstituted nucleosomes by salt dissociation and Förster resonance energy transfer (FRET). We show that the histones prepared by this method form octamers and nucleosomes that are virtually identical (by the methods used) to those formed by histones prepared by conventional methods.
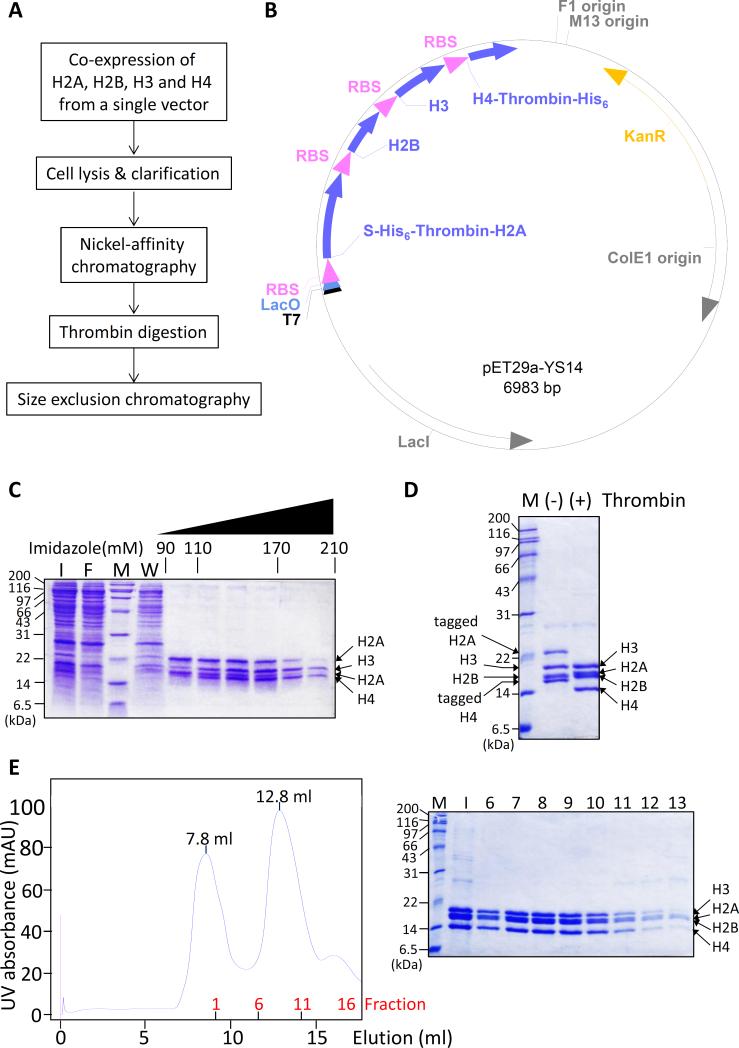
Fig. 1.
Four core histones are co-expressed and purified in simple steps. (A) Histones are expressed from a single vector and purified under non-denaturing conditions. This method allows histone octamers to be purified in a single day. (B) Schematic map of the histone coexpressing polycistronic vector. Each histone genes are preceded by a ribosome-binding sequence (RBS). Both H2A and H4 contain His6-tags which can be removed by thrombin digestion. Full sequence of the plasmid is included in Supplementary Information. (C) Cell supernatant from histone-overexpressing bacteria was purified over a nickel affinity column. SDS-PAGE gel shows that histones that contain stoichiometric ratio of histones eluted at 110-170 mM imidazole. M: molecular weight marker, I: input, F: flow-through, W: wash. (D) The affinity tags on H2A and H4 were removed by thrombin digestion. (-) and (+) thrombin indicates histone samples before and after thrombin digestion, respectively. (E) Thrombin-digested histones were finally purified over a Superdex 200 column. The chromatogram (left) and SDS-PAGE gel (right) show that stoichiometric histone complexes elute at 12.8 ml. M: molecular weight marker, I: input, 6-14 indicate fraction numbers.
The polycistronic vector to co-express all four histone genes was constructed by incorporating the genes into the multiple cloning site of a pET vector under the control of a single T7 promoter (Fig. 1B). Each histone-encoding sequence was preceded by a ribosome-binding site to enable the co-expression of the proteins. To facilitate the purification, H2A and H4 were engineered to contain a hexahistidine (His6)-tag and a thrombin site in the N- and C-termini, respectively. The resulting polycistronic vector indeed showed co-expression of all four histones from bacteria when induced by isopropyl -D-1-thiogalactopyranoside (Fig. S1A). Overexpression of histones was optimal when the culture was induced at OD600 0.4 and grown for overnight.
The induced cells were subsequently collected and lysed in a high salt buffer containing 2 M NaCl. Under this condition, histones can dissociate from bulk DNA in cells [1], and we observed that the histones remained mostly in solution instead of inclusion bodies as they do when expressed by themselves (Fig. S1B). This allowed us to bypass denaturation and refolding procedures used in conventional methods [7; 8]. Instead, histones could be purified under native conditions using the following two chromatography steps. First, the histone-containing cell extract was loaded onto a nickel affinity chromatography column in the presence of 2 M NaCl and 30 mM imidazole. The column was subsequently washed with 20 column volumes of buffer, which effectively removed most other proteins and the majority of cellular DNA from histones. His6-tags on both H2A and H4 enabled such extensive washing as the interaction between H2AH2B and H3-H4 can be labile under these conditions. The bound proteins were then eluted over a linear imidazole gradient. Fractions eluted at 110–170 mM imidazole contained clean histones of roughly stoichiometric ratio, while complexes mainly consisting of H2A-H2B eluted before 110 mM and those consisting of H3-H4 eluted after 170 mM of imidazole (Fig. 1C). We pooled the fractions containing stoichiometric ratio of the four histones and concentrated them using ultrafiltration. The concentrated histones were subsequently digested with thrombin to remove the affinity tags on H2A and H4. Concentrating histones before digestion significantly accelerates the speed of digestion, and the digestion could be completed in 3 hours (Fig. 1D & S1C).
Thrombin-digested samples were subsequently purified over a Superdex 200 size exclusion column. All histones eluted as a single peak at 12.8 ml elution volume (Fig. 1E). This corresponded to 111 kDa, closely matching the calculated molecular weight of the octameric complex (109 kDa) (Fig. S2A). SDS-PAGE analysis showed stoichiometric ratios of the histones across the histone peak fractions. Together with the molecular weight of the complex, the result indicates that the peak mainly consists of histone octamers. This elution profile is similar to the profile of octamers prepared in a conventional method [8] including the fact that the histone peak was preceded by a void volume peak at 7.8 ml, which may contain non-specific high molecular weight aggregates. When we subjected the purified histones to a second round of size-exclusion chromatography, the 7.8 ml peak was absent, and the histone peak remained at 12.8 ml with a stoichiometric ratio of the subunits (Fig. S2B, C). This result indicates that one round of size-exclusion chromatography can efficiently purify the histone octamer and that it remains intact over these procedures. Finally, SDS-PAGE analysis of the new histones and histones from a conventional method confirmed their equivalence in the composition and sizes (Fig. S2D).
To test whether the histones obtained from this method could form nucleosomes, we compared these histones with histones prepared by a conventional method in a nucleosome reconstitution assay [7; 8]. In this assay, histone samples were first mixed with a 147-bp double-stranded DNA containing a strong nucleosome-positioning “601” sequence [9] in 1.1:1 ratio in a buffer containing 2 M NaCl and dialyzed in a step-wise fashion to gradually remove the salt. The reconstitution products were analyzed on a native PAGE gel. The products of the co-purified histone sample migrated similarly to those formed by the control histones (Fig. 2A). This result indicates the co-purified histones can form nucleosome core particles (NCPs) similarly to the histones from conventional methods.
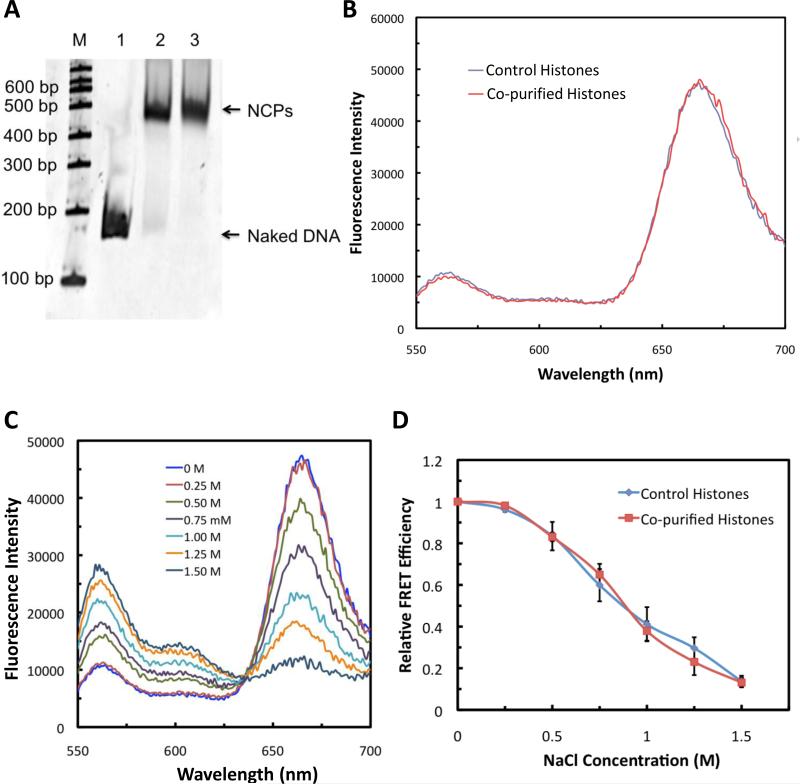
Fig. 2.
Co-purified histones can form nucleosome core particles (NCPs). (A) NCPs reconstituted using histone octamers prepared as in Fig. 1A run as one band in a native gel analysis at a position equivalent to that of NCPs formed by commercially available histone octamers. (B) The formation of NCPs with the purified histones was confirmed by FRET. The emission spectra of NCPs (ex= 515 nm) reconstituted with the co-purified histones show robust FRET peak at 670 nm as with the control NCPs. Schematic diagrams of the locations of donor (Cy3) and acceptor (Cy5) on a naked DNA and a nucleosome are shown in Fig. S3. (C) Emission spectra of NCPs reconstituted with the co-purified histones show salt-induced NCP dissociation upon increase in the salt concentration. (D) Salt-induced NCP dissociation profile of the co-purified histones is also similar to that of control histones.
To further validate our results, we reconstituted NCPs using a 601 DNA sequence labeled with a fluorescence donor (Cy3) and an acceptor (Cy5) at specific positions (Fig. S3A) [10]. The 601 DNA sequence has a strong propensity to bind to histone octamers in a specific orientation [11] and shortens the distance between the dye pairs from ~26 nm to ~3 nm (Fig. S3A, B). Thus a dramatic increase in the FRET signal is expected upon donor excitation when the DNA is specifically assembled into NCPs but not if the binding occurs in a non-specific manner. The histones prepared in the abovementioned method show robust FRET signal similarly to that of the control histones, which further supports that these new histone-DNA complexes form canonical NCP structures (Fig. 2B).
We have also measured the fluorescence of the labeled DNA as the salt concentration decreased. This can also serve as a diagnostic of the nucleosome structure as nucleosomes show increased dissociation with increased salt concentration [12]. The results show identical salt-induced dissociation profiles between the NCPs reconstituted using the new histones and histones from a conventional preparation (Fig. 2C). After normalization of the FRET signal to the lowest salt concentration (0 mM), we did not see any difference between the control histones and co-purified histones (Fig. 2D).
Based on these three criteria (native gel migration, FRET and salt dissociation profiles), we conclude that our histones reconstitute NCPs similar (or identical) to the control histones.
It has been known for many years that histones can form multimeric complexes containing H2A-H2B dimers and H3-H4 tetramers [13]. Polycistronic vectors have also been developed and used extensively to make protein complexes [14]. Recently, it has also been reported that H2A-H2B dimers and H3-H4 tetramers can each be co-expressed and purified under non-denaturing conditions [15]. Here, we took one step further of these results and successfully prepared histone octamers in the native state using much simplified protocol. Using this protocol, obtaining pure histone proteins that can be readily used for NCP reconstitution can be accomplished in a single day, saving weeks of time required in the conventional methods. We anticipate that this new method will greatly facilitate endeavours to make various unmodified and modified histone complexes and be used to study biological systems that work on chromatin in vitro.
Supplementary Material
Acknowledgments
We thank Professors B. Bartholomew and K. Luger for the histone-encoding plasmids. This study was supported by grants from the State of Illinois and the Chicago Biomedical Consortium Catalyst Award (to JM) and by NIH grant ES004106 from the National Institute of Environmental Health Sciences (to MJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary information Supplementary information associated with this article can be found in the online version.
References
- 1.Van Holde KE. Chromatin. Springer-Verlag; New York: 1989. [Google Scholar]
- 2.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 3.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–99. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzler GR. Isolation of histones and nucleosome cores from mammalian cells. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb2105s50. Chapter 21. Unit 21 5. [DOI] [PubMed] [Google Scholar]
- 5.Shechter D, Dormann HL, Allis CD, Hake SB. Extraction, purification and analysis of histones. Nat Protoc. 2007;2:1445–57. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 6.Luger K, Rechsteiner TJ, Richmond TJ. Expression and purification of recombinant histones and nucleosome reconstitution. Methods Mol Biol. 1999;119:1–16. doi: 10.1385/1-59259-681-9:1. [DOI] [PubMed] [Google Scholar]
- 7.Dyer PN, Edayathumangalam RS, White CL, Bao Y, Chakravarthy S, Muthurajan UM, Luger K. Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 2004;375:23–44. doi: 10.1016/s0076-6879(03)75002-2. [DOI] [PubMed] [Google Scholar]
- 8.Luger K, Rechsteiner TJ, Richmond TJ. Preparation of nucleosome core particle from recombinant histones. Methods Enzymol. 1999;304:3–19. doi: 10.1016/s0076-6879(99)04003-3. [DOI] [PubMed] [Google Scholar]
- 9.Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- 10.Duan MR, Smerdon MJ. UV Damage in DNA Promotes Nucleosome Unwrapping. J Biol Chem. 2010;285:26295–303. doi: 10.1074/jbc.M110.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makde RD, England JR, Yennawar HP, Tan S. Structure of RCC1 chromatin factor bound to the nucleosome core particle. Nature. 2010;467:562–6. doi: 10.1038/nature09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Widom J. Nucleosomes facilitate their own invasion. Nat Struct Mol Biol. 2004;11:763–9. doi: 10.1038/nsmb801. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub H, Palter K, Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975;6:85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]
- 14.Selleck W, Tan S. Recombinant protein complex expression in E. coli. Curr Protoc Protein Sci. 2008 doi: 10.1002/0471140864.ps0521s52. Chapter 5. Unit 5 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson M, Huh JH, Ngo T, Lee A, Hernandez G, Pang J, Perkins J, Dutnall RN. Co-expression as a convenient method for the production and purification of core histones in bacteria. Protein Expr Purif. 2010;72:194–204. doi: 10.1016/j.pep.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Russell DW. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2001. pp. 10.14–10.15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.