Abstract
OBJECTIVES:
Increased colonic bile acids can cause chronic diarrhea. Bile acid diarrhea (BAD) is treatable by sequestrants, and may be secondary to ileal disease or primary BAD. It is underdiagnosed, partly because the selenium-75-homocholic acid taurine (SeHCAT) retention test is not available in many countries, and is underutilized in others. Serum 7α-hydroxy-4-cholesten-3-one (C4), a measure of bile acid synthesis, is available for diagnosis in specialist centers. Recently, deficiency of the ileal hormone fibroblast growth factor 19 (FGF19) has been shown in BAD. Our aim is to evaluate the diagnostic value of FGF19 in a large and prospective group of patients with chronic diarrhea, previously investigated with C4.
METHODS:
Patients undergoing routine investigation provided fasting blood samples. C4 was determined by high-performance liquid chromatography, and used to stratify two groups: group 1 (n=119), consisting of patients with normal C4 (≤ 28 ng/ml), and group 2 (n=139), consisting of patients with high C4 (>28 ng/ml), including any of the possible causes of BAD. Serum FGF19 was measured in stored samples by enzyme-linked immunosorbent assay.
RESULTS:
FGF19 and C4 were significantly inversely related (rs=−0.64, P<0.001). Patients with raised C4 had significantly lower median FGF19 values. Both of these were more marked when secondary to ileal disease, in particular ileal resection, than in primary BAD. The sensitivity and specificity of FGF19 at 145 pg/ml for detecting a C4 level >28 ng/ml were 58% and 79%, respectively. For C4 >60 ng/ml, these were 74% and 72% on receiver-operating characteristic analysis, the area under the curve was 0.80 (95% confidence interval 0.74–0.87).
CONCLUSIONS:
Serum FGF19 could be developed as a simple blood test to increase the diagnostic rates of BAD.
INTRODUCTION
Bile acid diarrhea (BAD) is a syndrome of chronic watery diarrhea where there is an excessive fecal loss of bile acids.1 Colonic bile acids become deconjugated and dehydroxylated, producing diarrhea from a number of processes, including inducing secretion of sodium and water, increasing colonic motility thereby stimulating defecation, and causing damage to the mucosa by increasing the mucosal permeability.2, 3, 4, 5, 6 Bile acid sequestrants such as cholestyramine, colestipol, or colesevelam are specific treatments for this condition, but these are often poorly tolerated and require titration to achieve maximum benefit.7, 8 The condition can have a significant impact on a patient′s lifestyle as the increased frequency of bowel motions, urgency, and fear of incontinence affects daily activities, and limits travel or the ability to leave the house.
Bile acids undergo an enterohepatic circulation, with over 95% of secreted bile acids normally being reabsorbed in the terminal ileum.9 Bile acid malabsorption (BAM) was first recognized as a cause of diarrhea secondary to disruption of the enterohepatic circulation of bile acids following surgical resection or disease of the terminal ileum.10 This has been classified as type 1 BAM (or BAD).11 A similar picture, where no obvious disease can be identified, was first described by Van Thaysen12 and has been called idiopathic BAM, type 2 BAM/BAD, or primary BAD. As described below, primary BAD is due to an overproduction of bile acids rather than malabsorption. Type 3 BAM/BAD occurs when miscellaneous conditions affect bile acid absorption; these include cholecystectomy, vagotomy, and small-bowel bacterial overgrowth.
Although BAM is commonly listed as a possible cause of persistent chronic diarrhea, it is usually regarded as rare, and so is of little importance in routine clinical practice. It is often considered only when conditions such as colonic cancer, inflammatory bowel disease, celiac disease, and colonic infections have been excluded.13, 14 Investigations to diagnose BAD may not be routinely performed, or an attempt at diagnosis is made from the response to a trial of treatment, which is not always successful.8, 15 It is frequently misdiagnosed as diarrhea-predominant irritable bowel syndrome (IBS) and these patients do not then receive specific therapy.
In fact, BAD, particularly the primary form (type 2), is common.16, 17 IBS patients are the largest group of patients seen in a general gastroenterology clinic. Our systematic review of many studies indicates that over 30% of patients with diarrhea-predominant IBS or chronic diarrhea in fact have BAD. The population prevalence is around 1%, approaching 10 million people in the West.16 There is a large undiagnosed population because of poor acceptance of the condition or owing to difficulties with the available tests.
In many countries, the selenium-75-homocholic acid taurine (SeHCAT) test is the standard way to diagnose BAD and has been available since 1983.18 (Measurement of fecal bile acids is considered to be unpleasant and difficult for both the patient and the laboratory and is now rarely performed.) 75SeHCAT 7-day retention is determined with a gamma-camera. Most centers report a 7-day retention of <5% as indicative of severe BAM, <5–10% as moderate, and 10–15% as mild malabsorption. These values correlate well with the response to treatment.16 The SeHCAT test is highly reliable and reproducible19 but, it has limited availability worldwide, and is not licensed in the United States. It also has the perceived disadvantages of high total cost, and that patients are required to attend the nuclear medicine departments on two separate occasions, and to be exposed to a low-dose radiation (0.25 mSv).
The concentration of the bile-acid intermediate, serum 7α-hydroxy-4-cholesten-3-one (C4), in the peripheral blood is an alternative test. C4 estimates the rate of hepatic bile acid synthesis rate and strongly correlates with the activity of cholesterol 7-hydroxylase (CYP7A1), which is the rate-limiting enzyme of bile acid synthesis.20, 21, 22 Several studies have shown a negative relationship between SeHCAT retention and C4, and compared their use in the diagnosis of BAD in patients with chronic diarrhea.23, 24, 25 Serum C4 has been measured by high-performance liquid chromatography, and in one study concentrations >35 ng/ml had positive and negative predictive values of 71% and 94%, respectively, for an abnormal SeHCAT retention of less than 10% and similar values for a response to bile acid sequestrants.24 A tandem mass spectrometry assay for C4 has recently been developed and used in patients with IBS-D,26 further establishing C4 as an alternative to SeHCAT.
Another possible screening test is to measure fibroblast growth factor 19 (FGF19) in blood. Recent evidence suggested that the underlying pathophysiology of primary BAD is an abnormal hormonal regulation of bile acid synthesis by FGF19 leading to overproduction.27, 28 FGF19 is produced in the terminal ileum in response to bile acid uptake and then travels to the liver via the portal vein, where it suppresses bile acid synthesis. We have shown significantly lower fasting levels of FGF19 in patients with BAD compared with healthy controls, associated with high levels of C4, indicating increased bile acid synthesis.27
The aims of this study were to evaluate the relationship between serum FGF19 and C4 in a large cohort of patients investigated for diarrhea and to estimate the sensitivity and specificity of FGF19 as a diagnostic tool.
METHODS
Subjects
Fasting blood samples were collected from consecutive patients with chronic diarrhea in Edinburgh and the surrounding areas of South East Scotland, from July 2008 to March 2009, as part of a routine clinical referral service for C4 measurements. These patients have been included in a larger group recently reported.29 The decision to refer for C4 assay was made by the referring clinician as part of the investigation for chronic diarrhea, usually defined as more than three watery stools per day for more than 3 months. These subjects did not routinely have SeHCAT testing, as C4 was used instead in this region. Subjects were asked to avoid alcohol for at least 12 h before the test and were not taking bile acid sequestrants. Serum was separated; samples were stored at −20 °C within 24 h for C4 assay and then refrozen and retrieved for a later batch analysis of FGF19. Group 1 patients (n=119) were referrals who had unequivocally normal C4 levels (reference range 4–28 ng/ml). Group 2 (n=139) were diagnosed as having possible BAD based on high C4 measurements (>28 ng/ml). This group included patients with any of the causes of BAD (type 1, 2, or 3 BAM) based on the diagnosis on referral for C4 testing. Data were analyzed to compare the relationship between FGF19 and C4 in both of these groups and in the three types of BAD.
Laboratory assays
C4 measurements were determined using a high-performance liquid chromatography method with a prior solid-phase extraction as previously described.20, 24 Although the original reference range quoted was 5–35 ng/ml,24 this was subsequently revised, using a new commercial standard from Steraloids (Newport, RI). Further details are as described recently.29
Serum FGF19 was measured on stored frozen samples from both of these groups by quantitative sandwich enzyme-linked immunosorbent assay, using a commercially available kit (FGF19 Quantikine ELISA kit, Cat No. DF1900; R&D Systems, Minneapolis, MN), and following the manufacturer's instructions. This assay requires 100 μl of serum (or plasma) and is linear between 16 and 1,000 pg/ml (∼ 42 pmol/l). The intra-assay and inter-assay coefficients of variance are about 4–6% (manufacturer's data, confirmed in our laboratory). All serum samples were assayed in duplicate.
Statistical analysis
As data in all the groups were not normally distributed, results are expressed as median values. Non-parametric statistical analyses were used, including Mann–Whitney U-test to compare the groups. Correlations were evaluated by Spearman's correlation coefficient (rs). Receiver-operating characteristic analysis was performed using the software from Analyse-it (Leeds, UK).
RESULTS
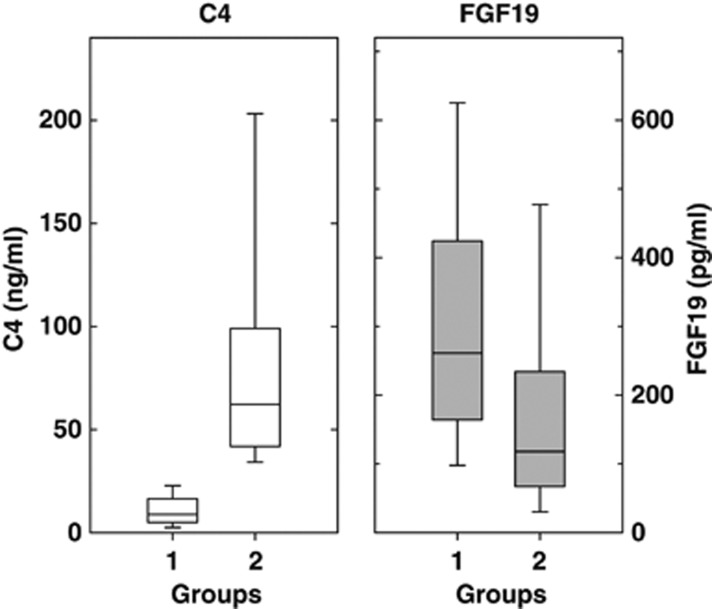
There were no significant differences between the two patient groups in age (mean 49.5 years in group 1 and 51.5 years in group 2) or M:F ratio (females 66% and 71%, respectively). Figure 1 illustrates the variability of C4 and FGF19. As the two groups were defined by C4, there was no overlap in these values. The median serum FGF19 values differed significantly between the groups (Mann–Whitney U-test, P<0.0001), with higher values in group 1 than in group 2.
Figure 1.
Fasting C4 and fibroblast growth factor 19 (FGF19) levels within each group of patients investigated for chronic diarrhea. C4 is shown on the left and FGF19 on the right. In each part of the figure, the median, 25th, and 75th quartiles (boxes) and the 10th and 90th percentiles (whiskers) are shown. Group 1 comprises 119 patients with C4≤28 ng/ml. Group 2 comprises 139 patients with C4>28 ng/ml.
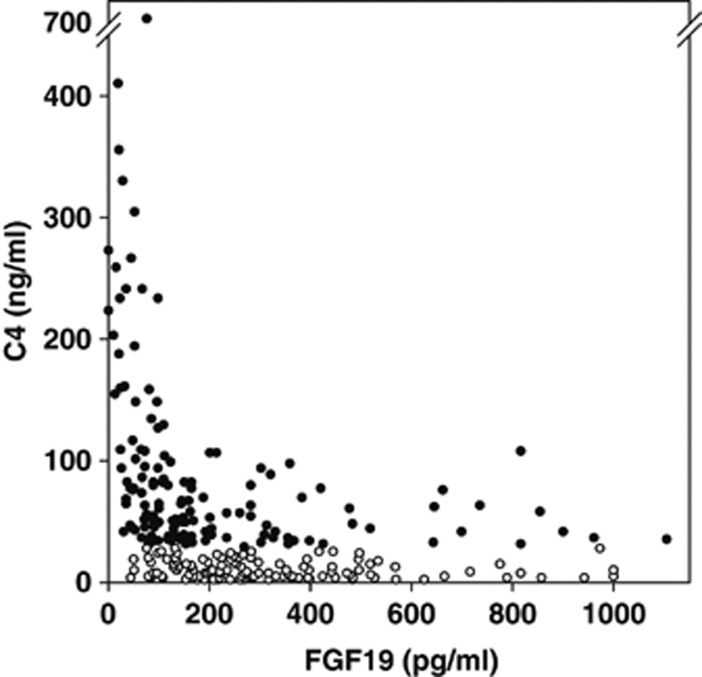
There was a highly significant inverse relationship overall between FGF19 and C4 levels (rs=−0.64, P<0.001, Spearman's rank correlation); that is, higher C4 levels were associated with lower FGF19 values (Figure 2). When the groups were studied separately, this relationship was much weaker in group 1 patients, with normal C4, than in group 2, with raised C4 (Table 1).
Figure 2.
Inverse correlation between C4 and fibroblast growth factor 19 (FGF19). Fasting C4 and FGF19 values are shown for individual patients. Open symbols are patients in group 1 with C4 ≤28 ng/ml. Solid symbols are patients in group 2 with C4 >28 ng/ml.
Table 1. C4 and FGF19 in patients presenting with chronic diarrhea.
| Subject group | No. of patients in the group |
C4 (ng/ml) |
FGF19 (pg/ml) |
Correlation |
|||
|---|---|---|---|---|---|---|---|
| Median | Interquartile range | Median | Interquartile range | rs | P | ||
| Group 1 | 119 | 9 | 5–17 | 261 | 164–424 | −0.16 | 0.04 |
| Group 2 | 139 | 62 | 42–99 | 118 | 67–234 | −0.51 | 0.0001 |
| BAD 1 (all) | 55 | 83 | 53–160 | 76 | 35–145 | −0.63 | <0.0001 |
| With IR | 27 | 117 | 77–250 | 50 | 26–99 | −0.43 | 0.01 |
| Without IR | 28 | 68 | 50–97 | 109 | 68–189 | −0.61 | 0.001 |
| BAD 2 | 43 | 48 | 34–65 | 166 | 98–359 | −0.30 | 0.03 |
| BAD 3 | 35 | 53 | 39–86 | 148 | 91–321 | −0.26 | 0.07 |
BAD, bile acid diarrhea; C4, serum 7α-hydroxy-4-cholesten-3-one; FGF19, fibroblast growth factor 19; IR, ileal resection.
Group 1: patients with chronic diarrhea and normal fasting C4 values (≤28 ng/ml). Group 2: patients with possible BAD based on abnormal C4 (>28 ng/ml). The three BAD types are defined in the text. The patients in the BAD 1 group are further analyzed as those with or without ileal resections (IR). Correlation coefficients (rs, Spearman's rank) and their significances (P) are shown.
Information was available for 133 of the 139 patients in group 2 regarding associated disorders and the possible type of BAD (Table 1). Fifty-five patients had ileal disease or resection as a possible cause (type 1), and in these, the 27 with ileal resection were also analyzed separately and compared with the 28 without resection. Thirty-five patients had a variety of conditions, including post cholecystectomy diarrhea, small-bowel bacterial overgrowth, and celiac disease, suggesting type 3 secondary BAD. Forty-three patients with no obvious cause, that is primary BAD (type 2), accounted for 31% of the total.
The lowest median levels of FGF19 were seen in the subgroup diagnosed with type 1 BAD, who also had the highest C4 values. These values differed significantly between the three types of BAD (Kruskal–Wallis H test, P<0.0001), with significantly lower FGF19 and higher C4 values in type 1 BAD (ileal disease) compared with either of the other subgroups, which did not differ significantly from each other. The patients with type 1 BAD due to ileal resection had the most extreme values, with a median C4 of 117 ng/ml and a median FGF19 of 50 pg/ml. The inverse relationships between C4 and FGF19 were significant in types 1 and 2, but not in type 3 (Table 1).
We attempted to define values for FGF19, which would predict values of C4. Using a cut off for FGF19 at ≤145 pg/ml to indicate a diagnosis of BAD (as suggested in the recent study27), the sensitivity and specificity were 58% and 79%, respectively, to detect C4 at >28 ng/ml, as used to originally define the groups. Table 2 also shows the figures obtained for the detection of higher values of C4 (35, 48, and 60 ng/ml), which may improve the specificity of C4 in the diagnosis of patients with possible BAD.25, 26 The results are also shown for a higher value of FGF19 (200 pg/ml) and at two values for the three separate types of BAD.
Table 2. Sensitivities and specificities for FGF19 to detect abnormal C4 levels.
| FGF19 (pg/ml) | C4 value (ng/ml) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| All patients | |||||
| ≤145 | >28 | 58 | 79 | 76 | 62 |
| ≤145 | >35 | 62 | 77 | 71 | 70 |
| ≤145 | >48 | 67 | 73 | 57 | 81 |
| ≤145 | >60 | 74 | 72 | 50 | 88 |
| ≤200 | >28 | 71 | 67 | 72 | 66 |
| ≤200 | >60 | 81 | 58 | 42 | 88 |
| Type 1 BAD | |||||
| ≤145 | >28 | 76 | 79 | 63 | 88 |
| ≤200 | >60 | 93 | 62 | 42 | 97 |
| Type 2 BAD | |||||
| ≤145 | >28 | 40 | 79 | 40 | 78 |
| ≤200 | >60 | 64 | 64 | 15 | 95 |
| Type 3 BAD | |||||
| ≤145 | >28 | 49 | 79 | 40 | 84 |
| ≤200 | >60 | 60 | 63 | 15 | 94 |
BAD, bile acid diarrhea; C4, serum 7α-hydroxy-4-cholesten-3-one; FGF19, fibroblast growth factor 19; PPV, positive predictive value; NPV, negative predictive value.
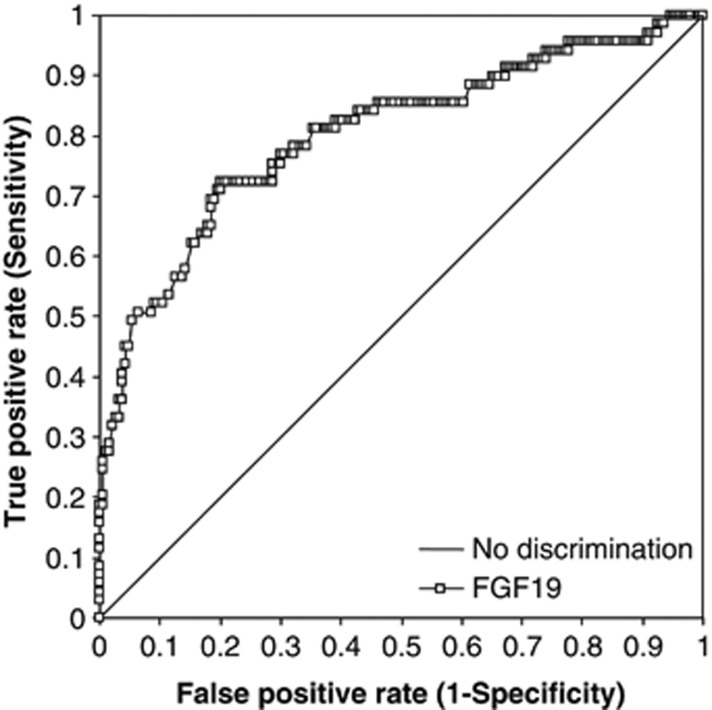
We performed a receiver-operating characteristic analysis (Figure 3) looking at the predictive values of FGF19 for a cutoff value of C4 at >60 ng/ml. Seventy-two patients (28%) out of the total of 258 were positive at this value. The area under the curve was 0.80 (95% confidence interval 0.74–0.87). The sensitivity and specificity were 74% and 72%, respectively, for the FGF19 cutoff at 145 pg/ml, but marginally better, at 75% and 71%, using a FGF19 cutoff of 148 pg/ml.
Figure 3.
Receiver-operating characteristic analysis for fibroblast growth factor 19 (FGF19) detection of C4 >60 ng/ml. True-positive and false-positive rate data are shown for FGF19 in 258 patients (square symbols). The line of no discrimination is also shown.
DISCUSSION
This study has looked at the relationship between the two blood markers, C4 and FGF19, which have been suggested to have roles in diagnosing BAD. As this condition is currently underdiagnosed and specific treatments exist, the development of reliable and cheap methods for screening and diagnosis is important.
The current diagnostic tests for BAD are SeHCAT and C4; these are reliable but time-consuming for either the patient (SeHCAT) or the laboratory (C4). Their relationship has been established in several studies. Eusufzai et al.23 were the first to show a significant positive correlation between the two tests, in a study of 28 patients with chronic diarrhea. Brydon et al.24 evaluated SeHCAT and serum C4 in 164 patients with chronic diarrhea; they found positive and negative predictive values for serum C4 (>35 ng/ml) of 74% and 98%, respectively, for a SeHCAT 7d retention value of <10%. Sauter et al.25 determined a reference range of C4 in 106 normal subjects between 6 and 48 ng/ml. Including 23 patients with chronic diarrhea, they found a sensitivity of 90% and a specificity of 79% for a SeHCAT 7d retention of <10%, with a positive predictive value of 73% and a negative predictive value of 92%.
Measurement of C4 using the high-performance liquid chromatography method is a fairly time-consuming process and is not generally available. Only one center in the UK offers this as a routine clinical test. Other methods have been developed for use elsewhere.26, 30 Camilleri et al.26 used a liquid chromatography tandem mass spectrometry assay for C4, finding an interquartile range of 9.5–29.2 ng/ml in 111 healthy volunteers, and elevated median levels in patients with ileal resection or disease, which, as in our study, were greater than that found in patients with IBS-D. This method allows a faster analysis for C4 (14 min per sample) and potentially could increase the availability of C4 assays. Reference ranges for C4 will need to be confirmed in other laboratories that offer this assay and confirmed from time to time.
A plausible mechanism of BAM in the ileum has existed for type 1 and 3 BAD. However, the precise mechanism underlying the physiological process in primary BAD (type 2) has been poorly understood until recently, when a mechanism of impaired negative feedback of bile acid synthesis resulting in an overproduction of bile salts has been proposed.27, 28 The hormone FGF19 is at the heart of this model; reduced serum levels have been demonstrated in patients with BAD compared with healthy normal volunteers. The concept of utilizing FGF19 as a potential tool to diagnose BAD has been suggested. When a new potential test is considered, it must be analyzed against another test of routine and reliable use.
This present study has shown significant differences in FGF19 and C4 in groups of patients with chronic diarrhea, confirming recent findings.27 The significant inverse relationship between FGF19 and C4 suggests an important homeostatic relationship between bile acid synthesis and its control. Low FGF19 levels are associated with high C4 levels, and conversely, subjects with high FGF19 values have low C4 values, representing low bile acid synthesis. The most marked differences, and the best inverse correlation, occurs in those with type 1 BAD, secondary to ileal disease, but these relationships are also found in patients with primary type 2 BAD, with no other obvious disease. Patients with ileal resection have the lowest FGF19 values, with a median of 50 pg/ml, lower than those found in the other type 1 patients with ileal inflammation. Another study is in progress, which will relate these levels to the precise length of ileum resected, giving a better idea of the areas of the distal small intestine capable of FGF19 production. The high C4 levels in this group indicate the maximal rate of hepatic bile acid synthesis in the absence of FGF19 feedback inhibition and in the presence of reduced bile acid reabsorption.
The values in the patients with primary type 2 BAD are less extreme, with lower levels of C4 and less reduction in FGF19, and can be harder to differentiate from the control patients in group 1, with normal C4 values. As this study is a laboratory study, using stored serum samples, validated therapeutic response data are not available, so we cannot say what proportion of patients respond to bile acid sequestrants at different C4 and FG19 values. This is a limitation of the present study and further work is in progress in a prospective series where response can be assessed and also related to SeHCAT values. However, an idea of the values found in primary type 2 BAD, where all patients had a response to bile acid sequestrants, can be obtained from our previous retrospective series.27
This inverse pattern of FG19 and C4 accounts for the majority of patients in our cohort. There are, however, groups of patients that do not fit this pattern. Both values were found to be low, representing false positives for FGF19 (<145 pg/ml), in 25 out of 119 patients who had low, normal values of C4 below 28 ng/ml. This increased to 53 of 186 patients at a higher C4 cutoff level of 60 ng/ml. These patients would not be considered to have BAD on C4 criteria. Conversely, both values were high, representing false-negative results for FGF19 (>145 pg/ml), in 19 of the 72 patients with C4 values >60 ng/ml. What other factors could affect C4 and FGF19 so that the usual inverse relationship is lost?
Several conditions are reported to elevate the level of C4, including chronic liver disease, alcohol intake, and hyperlipidemia,24, 31, 32 and these may have not been fully accounted for in our cohort of patients. The timing of blood collection may have influenced the C4 and FGF19 values. Diurnal variation of C4 has been well described,32, 33, 34 with C4 levels showing two peaks during the day, and values 2 to 4 × higher than the initial 0900-h values occurring 3–4 h later.33 Although other groups have identified only one peak of bile acid synthesis (this discrepancy may be related to complicated indirect techniques and small numbers),34 there are still differences in levels during the day. A recent review of 1390 patients from South East Scotland over a 3-year period confirmed that serum C4 levels were significantly higher at 1200–1300 h (median 25, range 12–49 ng/ml) compared with the results at 0900–1000 h (median 17, range 9–35 ng/ml).29
FGF19 levels also vary throughout the day and are increased following meals, with a time course that differs from C4.35 In this present study, patients were required to be fasted for the blood sampling, but in practice, this may not have been the case for patients arriving after midday, or may have varied in different hospitals. There may also have been a loss of serum C4 from the collected sample if left for a period of time, which would result in an overall lower-than-expected C4 concentration. SeHCAT testing, which integrates bile acid loss over a 7-day period, does not have these drawbacks of sample timing and processing, but clearly involves more time commitment from the patient to have two scans. Interpretation of C4 and FGF19 values will need to ensure that the time of sampling and the fasting state is considered.
Fasting FGF19 has been shown to be reduced in the metabolic syndrome.36 Our recent studies in patients undergoing bariatric surgery show that grossly obese individuals have significantly lower fasting serum FGF19 levels when compared with nonobese controls.37 A number of patients in our study with normal, low C4 and low FGF19 levels may well be obese, but data regarding height and weight were not recorded for this study. BMI has been shown to be significantly higher in patients with idiopathic BAM compared with healthy controls.38 We postulate that there may be a correction factor for an increased BMI, which will need to be established in future studies. However, in a recent large study, no relationship of fasting FGF19 was found in normal subjects with BMI, plasma triglycerides, or glucose, and unlike C4, did not vary by gender.39 FGF19 has also been demonstrated to be raised in extrahepatic cholestasis40 and is synthesized in the biliary tree.41
We speculate that a similar phenotype of primary BAD with increased bile acid synthesis could occur if normal levels of FGF19 mRNA are transcribed in the terminal ileum, when problems exist further along the pathway. For example, there may be impaired processing, release, or breakdown of the FGF19 protein, or there may be an impaired response at the level of the hepatocyte, related to the FGF19 receptor, FGFR4, or the coreceptor, β-Klotho. This model would be similar to that seen to produce type 2 diabetes, with insulin resistance and impaired receptor function. Studies have shown that mice lacking FGFR4 have an increased bile acid pool size and increased expression of cholesterol 7-hydroxylase.42 Genetic variants in β-Klotho and FGFR4 have recently been shown to be associated with colonic transit in patients with IBS-D.43 Further work is needed to identify the relevance of these plausible factors in primary BAD.
This study has shown, in a large population of patients being investigated for chronic diarrhea, that there is an inverse correlation between FGF19 and C4. Possible discriminating FGF19 values have been investigated. The overall negative predictive value of FGF19 value of >145 ng/ml for a C4 >60 ng/ml is 88%, and this seems to be a reasonable starting point to explore the use of FGF19 as a simple test to exclude the diagnosis of BAD. Patients with FGF19 values above this are unlikely to have BAD, and are unlikely to benefit from C4 testing, but those with values below this should be investigated with C4 and/or SeHCAT testing, where available. However, the positive predictive values, particularly for idiopathic, primary BAD (type 2), are poor. There are a number of compounding factors that will need additional study to improve the predictive values. However, FGF19 is cheaper and simpler than the other tests and so is ideal for use as an initial screening test in patients with chronic diarrhea. This may confer an advantage over current test strategies and increase the recognition of BAD more widely, encouraging the use of effective therapy with bile acid sequestrants in a sizeable proportion of chronic diarrhea patients.
Study Highlights
Acknowledgments
SP was funded by the Bardhan Research & Education Trust. We also acknowledge support from the UK NIHR Biomedical Research Centre funding scheme.
Guarantor of the article: Julian R.F. Walters, MA, MB, FRCP.
Specific author contributions: Study design: S.P. and J.W.; assays: G.B., T.D., S.P.; analysis: S.P. and J.W.; review of draft: all authors.
Financial support: S.P. was funded by the Bardhan Research & Education Trust.
Potential competing interests: None.
References
- Pattni SS, Walters JRF. Recent advances in the understanding of bile acid malabsorption. Br Med Bull. 2009;92:79–93. doi: 10.1093/bmb/ldp032. [DOI] [PubMed] [Google Scholar]
- Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K, Huott PA, Vongkovit P, et al. Cl- secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J Clin Invest. 1989;84:945–953. doi: 10.1172/JCI114257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely SJ, Scharl MM, Bertelsen LS, et al. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007;292:G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- Bajor A, Ung KA, Ohman L, et al. Indirect evidence for increased mechanosensitivity of jejunal secretomotor neurones in patients with idiopathic bile acid malabsorption. Acta Physiol (Oxf) 2009;197:129–137. doi: 10.1111/j.1748-1716.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- Walters JRF, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol. 2010;3:349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009;49:1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy. Gastroenterology. 1967;52:752–757. [PubMed] [Google Scholar]
- Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol. 1986;15:567–582. [PubMed] [Google Scholar]
- Thaysen EH, Pedersen L. Idiopathic bile acid catharsis. Gut. 1976;17:965–970. doi: 10.1136/gut.17.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Forbes A, Green J, et al. Guidelines for the investigation of chronic diarrhoea 2nd edition. Gut. 2003;52 (Suppl 5:v1–15. doi: 10.1136/gut.52.suppl_5.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC, Thompson WG. Bowel disorders. Am J Gastroenterol. 2010;105:775–785. doi: 10.1038/ajg.2010.69. [DOI] [PubMed] [Google Scholar]
- Khalid U, Lalji A, Stafferton R, Andreyev J. Bile acid malabsorption: a forgotten diagnosis. Clin Med. 2010;10:124–126. doi: 10.7861/clinmedicine.10-2-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlake L, A′Hern R, Thomas K, Walters JRF, Andreyev HJN. Systematic review: the prevalence of idiopathic bile acid malabsorption (I-BAM) as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome (IBS) Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- Walters JR. Defining primary bile acid diarrhea: making the diagnosis and recognizing the disorder. Expert Rev Gastroenterol Hepatol. 2010;4:561–567. doi: 10.1586/egh.10.54. [DOI] [PubMed] [Google Scholar]
- Boyd GS, Merrick MV, Monks R, Thomas IL. Se-75-labeled bile acid analogs, new radiopharmaceuticals for investigating the enterohepatic circulation. J Nucl Med. 1981;22:720–725. [PubMed] [Google Scholar]
- Bajor A, Kilander A, Sjovall H, Rudling M, Ung KA. The bile acid turnover rate assessed with the (75)SeHCAT test is stable in chronic diarrhoea but slightly decreased in healthy subjects after a long period of time. Dig Dis Sci. 2008;53:2935–2940. doi: 10.1007/s10620-008-0256-4. [DOI] [PubMed] [Google Scholar]
- Axelson M, Aly A, Sjovall J. Levels of 7 alpha-hydroxy-4-cholesten-3-one in plasma reflect rates of bile acid synthesis in man. FEBS Lett. 1988;239:324–328. doi: 10.1016/0014-5793(88)80944-x. [DOI] [PubMed] [Google Scholar]
- Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusufzai S, Axelson M, Angelin B, Einarsson K. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut. 1993;34:698–701. doi: 10.1136/gut.34.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:117–123. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- Sauter GH, Munzing W, von RC, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JRF, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Mangelsdorf DJ, Kliewer SA. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release. Clin Gastroenterol Hepatol. 2009;7:1151–1154. doi: 10.1016/j.cgh.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon WG, Culbert P, Kingstone K, et al. An evaluation of the use of serum 7-alpha-hydroxycholestenone as a diagnostic test of bile acid malabsorption causing watery diarrhea. Can J Gastroenterol. 2011;25:319–323. doi: 10.1155/2011/701287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Yamashita K, Numazawa M, et al. Highly sensitive quantification of 7alpha-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J Lipid Res. 2007;48:458–464. doi: 10.1194/jlr.D600032-JLR200. [DOI] [PubMed] [Google Scholar]
- Axelson M, Mork B, Sjovall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett. 1991;281:155–159. doi: 10.1016/0014-5793(91)80382-d. [DOI] [PubMed] [Google Scholar]
- Duane WC. Abnormal bile acid absorption in familial hypertriglyceridemia. J Lipid Res. 1995;36:96–107. [PubMed] [Google Scholar]
- Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Pooler PA, Duane WC. Effects of bile acid administration on bile acid synthesis and its circadian rhythm in man. Hepatology. 1988;8:1140–1146. doi: 10.1002/hep.1840080530. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Stejskal D, Karpisek M, Hanulova Z, Stejskal P. Fibroblast growth factor-19: development, analytical characterization and clinical evaluation of a new ELISA test. Scand J Clin Lab Invest. 2008;68:501–507. doi: 10.1080/00365510701854967. [DOI] [PubMed] [Google Scholar]
- Kuganolipava S, Dew T, Pattni SS, et al. Rapid normalisation of fasting serum fibroblast growth factor (FGF) 19 following bariatric surgery. Gut. 2009;58 (Suppl II:A121. [Google Scholar]
- Sadik R, Abrahamsson H, Ung K-A, Stotzer P-O. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med. 2011;270:580–588. doi: 10.1111/j.1365-2796.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2011;55:575–583. doi: 10.1002/hep.24702. [DOI] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- Wong BS, Camilleri M, Carlson PJ, et al. A Klotho-beta variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]