Abstract
Recent results from both human epidemiological and experimental studies with animals suggest that intake of non-caloric sweeteners may promote, rather than protect against, weight gain and other disturbances of energy regulation. However, without a viable mechanism to explain how consumption of non-caloric sweeteners can increase energy intake and body weight, the persuasiveness of such results has been limited. Using a rat model, the present research showed that intake of non-caloric sweeteners reduces the effectiveness of learned associations between sweet tastes and postingestive caloric outcomes (Experiment 1) and that interfering with this association may impair the ability of rats to regulate their intake of sweet, but not nonsweet, high-fat and high-calorie food (Experiment 2). The results support the hypothesis that consuming noncaloric sweeteners may promote excessive intake and body weight gain by weakening a predictive relationship between sweet taste and the caloric consequences of eating.
According to recent figures released from the Calorie Control Council, an international association representing the low-calorie and diet food and beverage industry, approximately 194 million people in the United States, or about 64% of the population, consumed low-calorie, sugar-free, foods and beverages such as diet sodas, reduced calorie desserts, and sugar substitutes in 2007. This compares to approximately 78 million consumers of these products in 1986 (Calorie Control Council, 2009). It may be that a significant portion of these consumers turned to sweet, non-calorie or reduced-calorie foods and beverages as a means of combating weight gain. Indeed, a position statement of the American Dietetic Association (2004) stated that “….consumers who want the taste of sweetness without added energy may select non-caloric sweeteners to assist in the management of weight, diabetes, and other chronic diseases”.
That would seem to be common sense. Foods and beverages have fewer calories when added sugars are replaced by non-caloric sweeteners. With other things held constant, reducing caloric intake will produce weight loss. Indeed, the idea that replacing calorically-dense sugars with non-caloric sweeteners not only fails to reduce, but could actually promote, energy intake and body weight gain might seem like nonsense. Nonetheless, a number of recent epidemiological studies have reported that consumption of products containing non-caloric sweetener is positively correlated with incidence of obesity, weight gain, Type II diabetes, cardiovascular disease, and other symptoms of the metabolic syndrome (Dhingra et al., 2007; Fowler et al., 2008; Fung et al., 2009; Lutsey et al., 2008). Such findings have met with strong public resistance from the manufacturers of those products and their representatives, often on the grounds that there is no viable mechanism to explain the result (Doheny, 2007). This was the case for a recent report that intake of diet soda was associated with overconsumption, metabolic dysfunction, and weight gain (Nettleton, Polak, Tracy, Burke, & Jacobs, 2009). The Calorie Control Council declared the findings to be “illogical” on the grounds that “It is physiologically impossible for foods and beverages without calories to cause weight gain” (Caloric Control Council, 2009). Based largely on this type of thinking, studies linking consumption of products containing non-caloric sweeteners to impaired energy regulation have also been publicly dismissed as being flawed methodologically (Parker-Pope, 2007) or as examples of “reverse causation” (Mattes & Popkin, 2009) in which weight gain, metabolic disease, etc., cause people to turn to non-caloric sweeteners as a means of combating these conditions.
The goal of the present research is to outline and test a physiologically-relevant mechanism that could explain how intake of food and fluids in which sweet taste is dissociated from normal caloric consequences could interfere with energy and body weight regulation. A well-known form of Pavlovian conditioning occurs when tastes (e.g., sweet, bitter) become conditioned stimuli (CSs) for postingestive (e.g., nutritive, gastric malaise) unconditioned stimuli (USs). As a result of this association, taste CSs come to evoke conditioned responses, which often take the form of changes in ingestive behavior (Sclafani, 1997; Welzl, D'Adamo, & Lipp, 2001).
Current physiological models of energy regulation suggest that tastes and other orosensory cues evoke not only intake and appetitive behavior, but also excite a variety of hormonal, neural, and metabolic conditioned responses that promote the efficient utilization of energy by anticipating and preparing for the arrival of nutrients in the gastrointestinal tract (Woods & Ramsay, 2000). One implication of these models is that the evocation of such physiological responses (Power & Schulkin, 2008), and thus the effectiveness of energy regulation, will be reduced under circumstances that degrade the ability of tastes to predict the occurrence of the caloric or nutritive postingestive consequences of eating (Davidson & Swithers, 2004; Swithers, Baker, & Davidson, 2009; Swithers & Davidson, 2005, 2008).
Previously, we reported that compared to rats with a history of consuming calorically-sweetened supplements, rats with a history of consuming substances sweetened with high intensity sweeteners (such as saccharin, Acesulfame Potassium, or Stevia) as supplements to their normal maintenance diet gain more weight, eat more, and are less able to demonstrate caloric compensation by reducing intake in a meal that follows a sweet-tasting high calorie premeal (see Swithers, Martin, & Davidson, 2010 for a review). We have hypothesized that because high-intensity sweeteners taste sweet, but do not provide an energetic postingestive US, consuming these substances might influence intake and body weight by weakening what we think is a strong predictive relationship between sweet taste and calories (Davidson & Swithers, 2004; Swithers & Davidson, 2008, 2009).
The purpose of Experiment 1 was to test the idea that intake of a noncaloric sweetener can weaken the ability sweet tastes to predict caloric postingestive outcomes. A longstanding idea in Pavlovian conditioning is that different stimuli compete for the associative control over behavior based on factors such as their salience and their relative validity as signals for occurrence of a US (e.g., Wagner, 1969; Rescorla & Wagner, 1972; Urushihara & Miller, 2009). Furthermore, recent research on preference conditioning provides clear evidence that such cue-competition is involved with learning about taste stimuli (Dwyer, Haslegrove, & Jones, in press). In view of these considerations, if consuming high-intensity sweeteners reduces the salience or the strength of the correlation between sweet tastes and energetic outcomes, this should also reduce the ability of sweet tastes to compete with other cues for association with that postingestive US.
Experiment 1 tested this prediction by giving different groups of rats either plain water or water sweetened with saccharin during an initial exposure phase. Next, both groups received training with two novel flavored solutions that were mixed with equal concentrations of either glucose or polycose. In the final test phase, intake of each flavored solution, presented without glucose or polycose, was recorded both for the group given saccharin and for group given only water during the initial exposure phase.
Glucose and polycose are isoenergetic and although rats prefer the taste of polycose to water, “poly” taste does not appear to be sweet to rodents (Bonacchi, Ackroff, & Sclafani, 2008; Treesukosol, Blonde, & Spector, 2009). Thus, with our procedure one flavor was trained in compound with a sweet taste (glucose) that was followed by caloric postingestive consequences and the other flavor was trained with a non-sweet taste (polycose) that was paired with the same or similar caloric US. If prior exposure to non-caloric saccharin weakens the ability of sweet taste to compete with a novel flavor for association with the caloric US, then rats exposed to saccharin should consume more of the flavor presented in compound with sweet-tasting glucose, compared to rats that had been exposed only to water. Training a different novel flavor in compound with polycose permitted us to assess the extent to which the effects of consuming the non-caloric sweetener during the exposure phase were specific to an association between sweet taste and calories. That is, prior exposure to sweet tasting saccharin should have little effect relative to prior water exposure on intake during testing of the novel flavor that had been trained with polycose because consuming a sweet taste without calories should do little to diminish the ability of “poly taste” to compete with a novel flavor for association with the caloric US.
Experiment 1
Methods
Subjects
The subjects were 32 naïve, adult, male, Sprague-Dawley rats that weighed 335-365 g upon arrival in the laboratory from Harlan Inc., Indianapolis. The care and treatment of all rats was reviewed and approved by the Purdue Animal Care and Use Committee as being consistent with the Guide for the Care and Use of Laboratory Animals. The rats were housed individually and were maintained under a 12:12h light:dark cycle with lights on at 0700 h daily. They had access to ad libitum food and water throughout the study, except where noted.
Procedures
Following a 14-day period during which each rat was handled daily and maintained on standard 5001 laboratory chow (testdiet.com), the rats were assigned to two groups of 16 rats each, matched on mean body weight. As illustrated in Table 1, Experiment 1 was conducted in 3 phases involving 1) initial exposure to a non-caloric sweet tasting solution or water, 2) compound training of novel flavors mixed in either a sweet or non-sweet caloric solution, and concluding with 3) a test in which intake of solutions containing the novel flavors without calories or sweeteners was recorded.
Table 1. Design of Experiment 1.
| Exposure | Training | Testing |
|---|---|---|
| Saccharin (Extinction of sweet taste-calorie association) |
Flavor A* – Glucose Flavor B* - Polycose |
Flavor A vs Flavor B |
| Water (Control) |
Flavor A* - Glucose Flavor B* - Polycose |
Flavor A vs Flavor B |
Grape flavor and cherry flavor counterbalanced with respect to nutrient (glucose, polycose).
During the exposure phase, for one group (Group Saccharin), 30 ml of a saccharin solution (0.3% saccharin in water) was placed in a 50 ml centrifuge tube with a sipper spout that was fastened to the front of the home cage. The second group (Group Water) was treated in the same way except that centrifuge tube contained 30 ml of water. The centrifuge tubes were fastened to the home cages of each rat at approximately 1400 h each day where they remained overnight before being removed at about 1400 h the following day. This procedure was repeated daily for 14 days. Because saccharin tastes sweet but has no calories, this procedure was expected to reduce the strength of the sweet taste-calorie association for rats exposed to saccharin, whereas this association should not be altered for the group that received only water during the exposure phase.
Next, all rats were trained with 30 ml of 0.05% grape- or cherry-flavored Kool-aid® solution mixed in water with either 10% glucose or 10% polycose. For half the rats in each group, grape flavor was presented with the glucose solution and cherry flavor was presented with the polycose solution. The identities of the flavors paired with each nutrient were reversed for the remaining rats. All rats were trained with both solutions, but only one of the two solutions was presented each day, with order of presentation alternating irregularly for 20 days (10 for each solution). The solutions were presented in centrifuge tubes that were fastened to the cages of each rat at approximately 1400 h each day using the same procedures as described above for the exposure phase. The study was conducted by two experimenters. The assignment of rats to receive saccharin or water exposure and to receive each combination of flavor and glucose or polycose during compound training was counterbalanced for each experimenter.
Finally, all rats were food-deprived for 24 h in preparation for testing which also began at about 1400 h. During testing, two centrifuge tubes were fastened, side-by-side to the front of each cage. Water bottles were removed during the test period. One centrifuge tube contained 30 ml of grape- and the other contained 30 ml of cherry-flavored Kool-aid®. Both flavors were presented without glucose or polycose. Each tube was weighed immediately prior to being placed on the cage front and was weighed again approximately 1, 2, and 4 hrs later. When the tubes were re-fastened to the cage front after the 1 and 2 hr weighing their relative positions on the front of each cage was alternated. The test was concluded after weighing the tubes at 4 h.
In this test we had two primary expectations. First, exposure to saccharin should weaken the correlation between sweet taste and calories and thereby reduce the ability of sweet taste to compete with the novel flavor paired with glucose. Therefore, intake of the glucose-paired flavor should be higher for the rats exposed to saccharin than for the rats given only water during the exposure phase. Second, exposure to saccharin should have no effect on the correlation between poly taste and calories because polycose is not sweet. Therefore, the amount of intake of the polycose-paired flavor during testing should not depend on whether the rats were exposed previously to saccharin or water. This outcome would indicate that the effects of exposure to saccharin are specific to sweet taste-calorie associations.
Results and Discussion
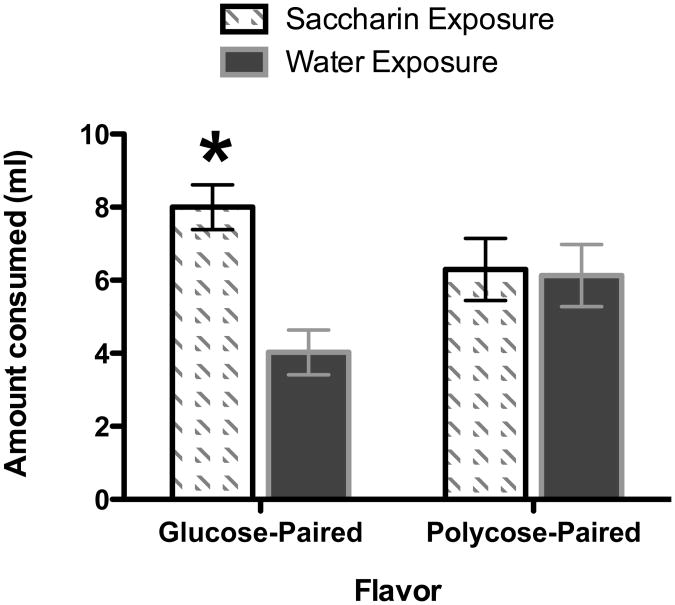
The results of the intake test (see Figure 1) showed that during the 4-hr test period rats that had previously been exposed to saccharin consumed more of the flavor that had previously been presented in compound with glucose compared to rats that had been exposed to water. In contrast, intake of the flavored solutions that had previously been paired with polycose did not depend on whether the rats had prior exposure to saccharin or water. A mixed-design analysis of variance with Exposure condition (saccharin or water), Flavor trained with glucose (grape or cherry) and Experimenter as between-subjects variables and Test Flavor (flavor paired with glucose or polycose in training) as a within-subjects factor was used to evaluate intake of the two flavored solutions during testing. This analysis yielded a significant main effect of Exposure Condition, F(1, 24) = 7.16, MSE = 7.18, p < .05, and more importantly, a significant Exposure Condition × Test Flavor interaction, F(1, 24) = 7.25, MSE = 6.03, p < .013. Post-hoc Newman-Keuls tests confirmed that the rats that were exposed to saccharin solution consumed significantly more of the test flavor that had been paired with glucose during training compared to the rats that had been exposed only to water (p < .01). In contrast, intake of the test flavor paired with polycose during training did not differ significantly dependent on whether the rats had previous experience with saccharin compared to only water (p >.63). Neither the main effects of Test Flavor or Experimenter achieved significance, Fs(1, 24) < 1. ANOVA also showed that the magnitude of the Exposure Condition × Test Flavor interaction failed to vary significantly as a function Experimenter, F(1, 24) =3.36, MSE, 6.00, p >.08, or Flavor trained with Glucose, F(1, 24) = 1.74, MSE = 6.00, p > .20.
Figure 1.
Mean amount consumed (± SEM) of the glucose-paired and polycose-paired solution during a 4-hr test for rats that received 0.3% saccharin solution or water during pre-training. *denotes significant difference between the saccharin and water pre-training conditions.
The results of Experiment 1 support the hypothesis that prior exposure to saccharin reduced the ability of sweet taste to signal a caloric postingestive outcome. As a result, the sweet taste of glucose was less able to compete for associative strength with a novel flavor when both the sweet taste and the flavor were paired with that caloric US. Exposure to water alone would not have been expected to impact the correlation between sweet taste and calories and thus would not be expected to alter the relative salience or predictive validity of sweet taste and novel flavor cues. Learning about the novel flavor paired with polycose in training was not reduced by prior exposure to saccharin. This outcome was also expected because experiencing a sweet taste without a caloric US should not influence the salience or validity of a poly taste to signal that caloric US because polycose does not appear to taste sweet to rodents (Treesukosol et al., 2009).
If one assumes that our rats entered Experiment 1 with no prior experience consuming sweet-tasting calories before they consumed saccharin, then reduced salience or associability of sweet taste with calories during subsequent training could be viewed as an example of latent inhibition, a phenomenon in which prior nonreinforced exposure to a CS retards the ability of that CS to become signal for a US later on (Lubow & Moore, 1959). On the other hand, sweet tasting substances containing calories are ubiquitous within the food environment and they are encountered by humans and other mammals beginning at an early age. Thus, the formation of associations between sweet taste CSs and postingestive caloric USs may occur even in the absence of explicit training within an experimental setting. For example, it is likely that our rats entered our Experiment 1 with sweet taste→calorie associations already formed, as result of pre-weaning experience or as a result of consuming sugars contained in their normal 5001 maintenance chow. In fact, Rescorla (2008) provided evidence consistent with the idea that sweet orosensory CSs possess associative value prior to the beginning of experimental training. He reported that when such CSs are subject to experimental training, their “initial non-zero value” modulates the effectiveness of a US in much the same way as other types of CSs that have been given explicit training with a US. The finding that sweet CSs affect training like pre-trained CSs is evidence that sweet tastes have pre-experimental value that could well be due to the prior formation of sweet-calorie associations.
Assuming our rats entered Experiment 1 with a sweet taste→calorie association already established, consuming saccharin may have weakened this association thereby reducing the extent to which sweet taste could block (e.g., Arcediano, Matute, & Miller, 1997; Kamin, 1969; Rescorla & Holland, 1982) the formation of an association between a novel flavor cue and the caloric US (e.g., Dwyer et al. in press: Balleine, Espinet, & Gonzalez, 2005; but also see Capaldi & Hunter, 2004). One way to reduce blocking by a previously trained CS is to present that CS without its US (Bills, Dopheide, Pineno, & Schachtman, 2006). Within this analysis, a consequence of reduced blocking by sweet taste would be increased conditioning of the novel flavor cue. This finding was obtained during testing in Experiment 1. While latent inhibition and decreased blocking may not encompass all of the potential explanations of the results of Experiment 1, they both identify a plausible mechanism by which intake of non-caloric sweeteners could reduce the capacity of sweet tastes to evoke physiological and behavioral responses that contribute to energy regulation.
Experiment 2
The results of Experiment 1 indicate that exposure to non-caloric sweeteners weakens the ability of sweet taste to signal the caloric postingestive consequences of eating, and that this reduction in associative control is specific to sweet tastes. As a result, disturbances in energy balance as a consequence of consuming non-caloric sweeteners would be expected to occur to the extent that animals are also consuming other foods which also taste sweet. That is, non-caloric sweeteners appear to impair the ability of a sweet taste to predict the delivery of calories, and if the maintenance diet of the animal also tastes sweet, this impairment will result in the overconsumption of the maintenance diet and/or a decrease in physiological responses related to utilization of that sweet-tasting maintenance diet. If the maintenance diet does not taste sweet, then consumption of the non-caloric sweetener would be expected to have minimal effects since there would be little consequence of the disruption of the predictive relationship between sweet tastes and calories. Experiment 2 tested this hypothesis.
Methods
Subjects
The subjects were 60 rats of the same description as those used in Experiment 1. Rats were maintained on a 14:10 light:dark cycle with lights on at 0200 h and off at 1600 h.
Procedure
All rats were initially placed on a plain, powdered, high fat (HF) diet (∼5.48 kcal/g, with ∼ 41% of calories provided by fat, 41% provided carbohydrate, and 18% provided by protein; testdiet.com cat. # 25312, modified diet 5012 with 4% starch and 16% peanut oil), for 7 days before being assigned to one of 6 groups matched on body weight (group means = 363 – 364 g). Two groups continued to receive the Plain HF diet throughout the experiment. Two other groups (HF + Glucose) received the HF diet to which 20% glucose was added (w/w; ∼5.18 kcal/g). The remaining two groups (HF + Polycose) received the HF diet to which 20% polycose was added (w/w; ∼5.18 kcal/g). Thus, for the Plain HF and HF+Polycose groups, the maintenance diet had minimal sweet taste, whereas for the HF+Glucose group, the maintenance diet had a strong sweet taste. In addition, while the HF diet had a different caloric density and macronutrient content than the HF+Polycose and HF+Glucose diets, the latter two diets were matched in both these respects.
On the same day that these modified HF diets were introduced, rats in all groups began receiving access to 30 g of a supplement of yogurt (Dannon low fat plain yogurt) 6 days per week in addition to their assigned diet and water. On 3 of the 6 days per week, plain, unsweetened yogurt (∼ 0.6 kcal/g) was provided. On the remaining 3 days, sweetened yogurt was provided. For one group of animals on each maintenance diet, the yogurt was sweetened with 20% glucose (w/w; ∼1.2 kcal/g); for this group of animals a sweet taste predicted an increase in the caloric density of the yogurt. For the remaining group of animals on each maintenance diet, the yogurt was sweetened with the high-intensity, non-caloric sweetener saccharin (0.3% w/w; ∼ 0.6 kcal/g); for this group, sweet taste did not predict an increase in the caloric content of the yogurt. Yogurt diets were placed into cages at approximately 1230 h daily and remained in the cage until the next day. Intake of the yogurt supplements, of the HF maintenance diet, and body weight were measured daily at approximately 1230 h. On the 7th day of each week, animals received their assigned maintenance diet and water alone (no yogurt supplements). The hypothesis was that animals in the non-predictive, saccharin-sweetened yogurt group would consume more maintenance diet and gain additional weight only when the maintenance diet tasted sweet (HF+Glucose group).
Results and Discussion
Body weight gain
Figure 2 shows that rats on all three maintenance diets gained weight during the 28-day diet period of testing. For the rats on the Plain HF and the HF+Polycose diets, this weight gain did not vary based on whether the yogurt supplement was sweetened with glucose or saccharin. In contrast, weight gain for rats maintained on the HF+Glucose diet was markedly greater for rats that received the saccharin-sweetened compared to the glucose-sweetened yogurt supplement over the last several days of the study. The weight gain data were analyzed using a 3 × 2 (Maintenance Diet × Yogurt Type) ANOVA, with Day of exposure (1-28) used as a repeated measure. This analysis yielded a significant Maintenance Diet × Yogurt Type × Day interaction, F(54,1458) = 1.55, MSE, = 22.0 p < .01. This interaction was further evaluated with separate ANOVAs and post-hoc Newman-Keuls tests for each maintenance diet. Neither the main effect of Yogurt Type, Fs(1,18) < 1, nor the interaction of Yogurt Type with Day, Fs(27, 486) < 1, achieved significance for either the Plain HF or the HF+Polycose maintenance diets. Only the main effect of Days achieved significance for rats on the Plain HF and HF+Polycose diet (smallest F(27, 486) = 259.29, MSE =23.2, p < .01 for the Plain HF diet). The main effects of Days, F(27, 486) = 328.91, MSE = 15.4, p < .01, Yogurt Type, F(1, 18) = 11.92, MSE = 1471.6, p < .001, and the Yogurt Type × Day interaction, F(27, 486) = 3.70, MSE = 15.4, p < .001, were significant for rats maintained on the HF+Glucose diet. Newman-Keuls tests confirmed that weight gain for rats given the supplement sweetened with saccharin was significantly greater compared to rats given the glucose-sweetened supplement on Days 14, 18, and 22-28.
Figure 2.
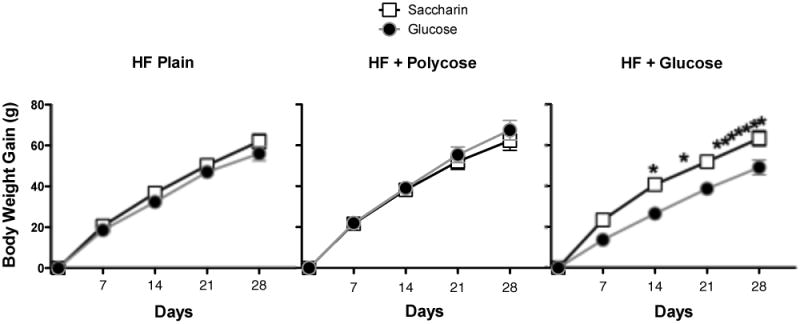
Mean body weight gain (± SEM) per day for rats that received yogurt sweetened with 0.3% saccharin or 20% glucose on some days and plain yogurt on other days as a function of type of high-fat (HF) maintenance diet: HF Plain (left panel); HF + 20% polycose (center panel) or HF + 20% glucose. *denotes significant difference between rats given saccharin-sweetened and glucose-sweetened yogurt
Comparing between diet groups, Figure 2 indicates that rats that received the yogurt supplements sweetened with glucose gained less weight if they were maintained on the HF+Glucose diet compared to the equicaloric HF+Polycose and the higher calorie Plain HF diets. An ANOVA evaluating these data obtained a significant main effect of Maintenance Diet, F(2, 27) = 5.75, MSE = 1821.0, p < .01, along with a significant main effect of Days, F(27, 279) = 369.37, MSE = 23.3, p < .01, and a significant Maintenance Diet × Days interaction, F(54, 279) = 2.99, p < .01. Newman-Keuls comparing each group revealed that only the difference in weight gain for the HF+Glucose compared to the HF+Polycose group achieved significance.
Food intake
Food intake (yogurt supplement plus maintenance diet expressed in kcals) was recorded each day for each rat. Figure 3 shows that for the rats maintained on the Plain HF and HF+Polycose diets, total kcals consumed per week during the entire experiment differed little as a function of whether they received the saccharin-sweetened or glucose-sweetened yogurt supplement along with the HF maintenance diet. In contrast, for rats maintained on the HF+Glucose diet, the rats that received the saccharin-sweetened yogurt consumed more total calories per week compared to the rats that were given yogurt sweetened with glucose. Mean cumulative intake per week was analyzed by an ANOVA using Maintenance Diet, Yogurt Type and Week (1-4) as factors. This ANOVA yielded a significant Maintenance Diet × Yogurt Type interaction, F(2, 48) = 4.71, MSE = 9022, p< .05. Neither the main effects of Maintenance Diet, F(2, 48) = 2.41, p > .10, nor Yogurt Type, F(1, 48) = 2.08, p > .15 were significant. The main effect of Week was significant, F(3, 144) = 24.10, MSE = 903, p < .01, but Week did not interact significantly with either Maintenance Diet, F(6, 144) < 1, or Yogurt Type F(3, 144) = 1.06, p > .36. Post-hoc Newman-Keuls tests showed that cumulative mean total caloric intake per week was significantly greater (p < .05) when the yogurt supplement was sweetened with saccharin compared to when it was sweetened with glucose only for rats that were maintained on the HF+Glucose diet.
Figure 3.
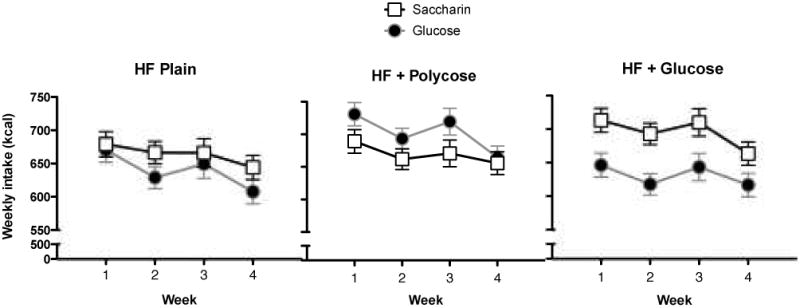
Mean kcals consumed (± SEM) per week (yogurt + maintenance diet) for rats that received yogurt sweetened with 0.3% saccharin or 20% glucose on some days and plain yogurt on other days as a function of type of high-fat (HF) maintenance diet: HF Plain (left panel); HF + 20% polycose (center panel) or HF + 20% glucose.
The data in Figure 3 also indicate that rats given the glucose sweetened yogurt supplement ate less if they were maintained on the HF+Glucose diet compared to the HF+Polycose diet. ANOVA comparing all diet groups that received the yogurt supplement sweetened with glucose obtained significant main effects of Maintenance Diet, F(2, 24) = 4.10, MSE = 13242, p < .05, and Week, F(3, 72) = 20.62, MSE = 740, p < .01. Newman-Keuls tests showed that rats maintained on the HF+Polycose diet ate significantly more overall than rats maintained on the HF+Glucose or Plain HF diet (ps < .05). Intake for these latter two groups did not differ significantly. This pattern of results indicates that rats maintained on a high fat diet are better able to regulate their intake and body weight when that diet is sweetened with glucose compared to when equicaloric, but nonsweet, polycose has been added. However, this advantage for rats on the HF+Glucose maintenance diet is eliminated if the rats also have consumed sweet, but noncaloric saccharin.
General Discussion
The results of the present research suggest that it may be time to re-examine common sense ideas about the effects of consuming non-caloric sweeteners on energy regulation. Experiment 1 provided clear evidence that experience consuming non-caloric saccharin weakens the ability of sweet taste to signal caloric consequences. This study exploited the idea that a manipulation that reduces the salience or predictive validity of a stimulus will also weaken the ability of that stimulus to compete with other relevant cues for association with a US. With its salience or predictive validity reduced, sweet taste would then be less able to compete with a novel flavor cue as signal for the occurrence of a caloric US produced by glucose, when both the flavor and sweet taste were presented in compound. The results confirmed this prediction and thus provided evidence that consuming saccharin weakens the ability of sweet taste to predict caloric outcomes (e.g., Davidson & Swithers, 2004; Swithers and Davidson, 2008).
Additionally, the results of Experiment 1 indicated that the effects of consuming saccharin were confined to the association between sweet taste and energetic outcomes. Based on this finding, we predicted that rats exposed to saccharin would overeat and gain more weight compared to rats exposed to glucose when they were required to regulate their intake of a sweet high-calorie diet, but would not be different compared to rats exposed to glucose in their ability to regulate intake of high-calorie but non-sweet diets. This pattern of results was obtained in Experiment 2. That is, consuming saccharin produced greater intake and body weight compared to consuming glucose for rats maintained on a sweet high-fat diet, but not for rats on a plain high-fat diet or an isocaloric (with respect to the sweet high-fat diet) non-sweet high fat diet mixed with polycose. The results of Experiment 2 cannot be dismissed as an example of reverse causation.
While consuming a saccharin-sweetened supplement was associated with greater food intake and body weight gain only for rats that were maintained on a sweetened high-fat diet, the results of Experiment 2 also showed that for rats given the yogurt supplement sweetened with glucose, maintenance on the HF+Glucose diet was accompanied by less food intake and weight gain compared to the equicaloric HF+Polycose diet. This difference between the HF+Glucose and HF+Polycose maintenance diets may mean that, compared to poly taste, sweet taste is an especially important contributor to efficient energy regulation. Consistent with this notion, sweet taste has been found to elicit stronger cephalic phase insulin release (CPIR) compared to polycose and to other nonsweet tastes (Tonosaki, et al, 2007). CPIR has also been described as a contributor to efficient energy regulation (e.g., Power & Schulkin, 2008). Nonetheless, whatever mechanism underlies the ability of rats maintained on a sweet HF diet to regulate intake and body weight, the present results indicate that the operation of that mechanism is compromised as a consequence of consuming saccharin.
The implications of our results for understanding the effects of non-caloric sweeteners on energy regulation in the complex human food environment remain to be investigated. However, there is little reason to assume that the learning mechanism we propose to underlie those effects is confined to rats in the laboratory. Similar mechanisms have been shown to operate across the phylogenetic continuum, from the simple sea slug (e.g., Aplysia Californicus) to humans (e.g., Arcediano et al., 1997; Baxter & Byrne, 2006), with a diverse array of stimuli and within a wide variety of response systems (see Domjan, 2005).
Furthermore, it is likely that one of the earliest associations formed by humans and other animals is that based on the signaling relationship between sweet taste in the mouth and the subsequent arrival and absorption of calories in the gut. This type of signaling relationship is thought to enable sweet taste to evoke physiological responses that anticipate and promote the efficient utilization of the energy contained in foods and fluids (Power & Schulkin, 2008; Woods & Ramsay, 2000). Therefore, if consuming non-caloric sweeteners weakens this relationship, the ability to regulate intake of sweet, high calorie foods and beverages could also be degraded.
At the minimum, our results should be viewed as cautionary about recommendations to substitute non-caloric sweeteners for caloric sweeteners as a method of weight control. More substantially, the present findings address the dismissive criticism that no viable mechanism to explain how consumption of high-intensity sweeteners could promote weight gain is available. Rather, our data suggest that the formation and modification of associations involving the orosensory properties of food and the postingestive consequences of eating merit greater attention in discussions of the etiology of obesity.
Acknowledgments
This research was supported by grants R01DK076078 and P01HD052112 from the National Institutes of Health.
References
- American Dietetic Association. Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners. Journal of the American Dietetic Association. 2004;104(2):255–275. doi: 10.1016/j.jada.2003.12.001. Erratum appears in J Am Diet Assoc. 2004 Jun;104(6):1013. [DOI] [PubMed] [Google Scholar]
- Arcediano F, Matute H, Miller RR. Blocking of Pavlovian conditioning in humans. Learning and Motivation. 1997;28(2):188–199. [Google Scholar]
- Balleine BW, Espinet A, Gonzalez F. Perceptual learning enhances retrospective revaluation of conditioned flavor preferences in rats. Journal of Experimental Psychology-Animal Behavior Processes. 2005;31(3):341–350. doi: 10.1037/0097-7403.31.3.341. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Byrne JH. Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning. Learning & Memory. 2006;13(6):669–680. doi: 10.1101/lm.339206. [DOI] [PubMed] [Google Scholar]
- Bills CH, Dopheide M, Pineno O, Schachtman TR. Effects of an extinguished CS on competition with another CS. Behavioural Processes. 2006;72(1):14–22. doi: 10.1016/j.beproc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Bonacchi KB, Ackroff K, Sclafani A. Sucrose taste but not polycose taste conditions flavor preferences in rats. Physiology & Behavior. 2008;95(1-2):235–244. doi: 10.1016/j.physbeh.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calorie Control Council. Calorie Control Council Response to “Diet Soda Intake and Risk of Incident Metabolic Syndrome and Type 2 Diabetes in the Multi-Ethnic Study of Atherosclerosis”. 2009 doi: 10.2337/dc08-1799. Retrieved January 21, 2010, from http://www.caloriecontrol.org/pressrelease/calorie-control-council-response-to-diet-soda-intake-and-risk-of-incident-metabolic-syn. [DOI] [PMC free article] [PubMed]
- Calorie Control Council. Calorie Control Council: Trends and statistics. 2009 Retrieved January 21, 2010, from http://www.caloriecontrol.org/press-room/trends-and-statistics.
- Capaldi ED, Hunter MJ. Taste and odor in conditioned flavor preference learning. Animal Learning & Behavior. 1994;22:355–365. [Google Scholar]
- Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. International Journal of Obesity & Related Metabolic Disorders. 2004;28(7):933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- Doheny K. Researchers Point Finger at Diet, Regular Sodas; Industry Officials Disagree WebMD. 2007 Retrieved January 21, 2010 from http://www.webmd.com/heart/metabolic-syndrome/news/20070723/1-daily-soda-may-boost-heart-disease.
- Domjan M. Pavlovian Conditioning: A Functional Perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Haselgrove M, Jones PM. Cue interactions in flavor preference learning: A configural analysis. Journal of Experimental Psychology: Animal Behavior Processes. 2010 doi: 10.1037/a0021033. in press. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ. Selective association and conditioning. In: Mackintosh J, Honig WK, editors. Fundamental Issues in Associative Learning. Halifax: Dalhousie University Press; 1969. pp. 42–64. [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced preexposure to the conditioned stimulus. Journal of Comparative and Physiological Psychology. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J, Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89(1):1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90(3):647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker-Pope T. Exploring a surprising link between obesity and diet soda Health Journal. New York: Wall Street Journal; 2007. [Google Scholar]
- Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50(2-3):194–206. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Conditioning of stimuli with nonzero initial value. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34(3):315–323. doi: 10.1037/0097-7403.34.3.315. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Holland PC. Behavioral studies of associative learning in animals. Annual Review of Psychology. 1982;33:265–308. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Sclafani A. Learned controls of ingestive behaviour. Appetite. 1997;29(2):153–158. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123(4):772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. Obesity: outwitting the wisdom of the body? Curr Neurol Neurosci Rep. 2005;5(3):159–162. doi: 10.1007/s11910-005-0041-0. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122(1):161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiology and Behavior. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki K, Hori Y, Shimizu Y, Tonosaki K. Relationships between insulin release and taste. Biomedical Research. 2007;28(2):79–83. doi: 10.2220/biomedres.28.79. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R855–865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Stimulus competition between a discrete cue and a training context: Cue competition does not result from the division of a limited resource. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:197–211. doi: 10.1037/a0013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR. Stimulus validity and stimulus selection in associative learning. In: Mackintosh NJ, Honing WK, editors. Fundamental issues in associative learning. Halifax Nova Scotia: Dalhousie Univ; 1969b. pp. 90–122. [Google Scholar]
- Welzl H, D'Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behavioural Brain Research. 2001;125(1-2):205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1-2):175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]