Abstract
The development of imaging technologies that have sufficient specificity and sensitivity to enable early, accurate detection of cancer and response to therapy has long been a goal in oncology. Various radiological techniques have been used for diagnosis and surveillance of disease recurrence and imaging has revolutionised oncology. However, despite the widespread use of technologies, the ability of currently available imaging methods to facilitate early detection, precise characterisation, and accurate localisation of maligant disease could be improved. The simultaneous use of two or more techniques, contrast reagents, signalling methods, or the coupling of agent and tissue properties to achieve so-called multiplexed imaging is a promising approach. In this review, we provide a broad overview of current and emerging multiplexed, imaging technologies.
Introduction
Imaging technologies and radiological techniques have been used for cancer detection, diagnosis and surveillance of disease recurrence. These techniques include radiography, x-ray CT, MRI, single-photon-emission CT (SPECT), PET, and ultrasonography. Imaging has revolutionised oncology; however, despite widespread use of these technologies, the ability of currently available methods to facilitate the early detection, precise characterisation, and accurate localisation of malignant disease could be improved. For example solid tumours smaller than 1 cm3 (about 109 cancer cells) cannot be detected. Achieving early and accurate diagnosis clearly requires detection of cancer far earlier in the course of disease.1 Multiplexed imaging that provides data sets from a single imaging session offers great potential for improving cancer detection and treatment.
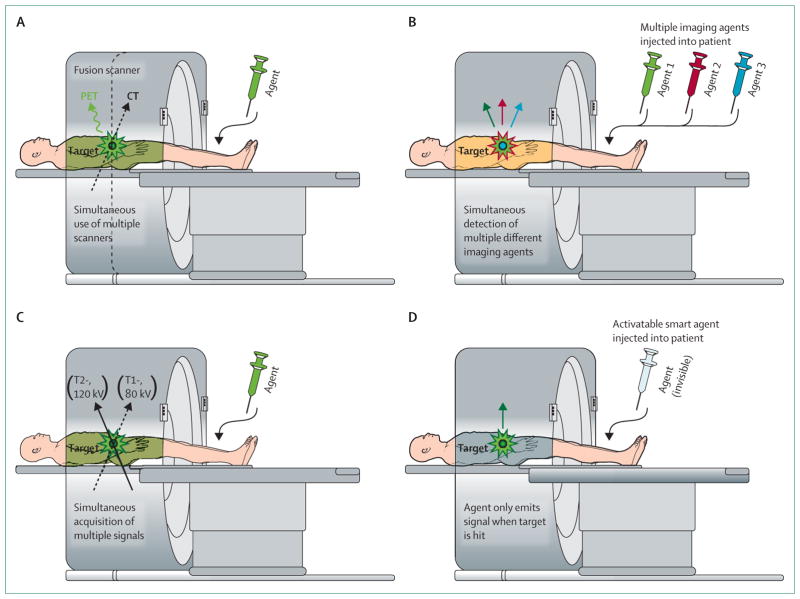
Four general strategies for multiplexed imaging have been proposed (figure 1, table). In multimodality imaging, such as combined PET and CT, two (or more) distinct imaging techniques are used simultaneously or consecutively. Multicolour contrast agents with distinct energies (colours) can be simultaneously imaged with a single technique, SPECT has been used in this manner and can simultaneously detect two imaging agents (ie, 99mTc and 201Tl). Multiple signal collection uses a single technique to detect and interpret multiple signals obtained with distinct signalling technologies, such as T1, T2, and diffusion-weighted MRI or dual energy x-ray CT. Finally, activatable, so called, smart reagents use molecular targeting and intracellular processing to generate signals that provide information on both tumour location and molecular profile.
Figure 1. Four categories of multiplexed imaging.
Multimodality (A) such as PET with CT, MR with optical, or nuclear with optical imaging. Multicolour (B) such as multiple energy single-photon-emission CT or multicolour optical image. Multisignalling (C) such as T1 and T2-weighted MRI, dual energy x-ray CT, or fluorescence intensity with lifetime fusion signalling acquisition. Activatable sensing probes (D) such as target-cell specific smart contrast agents.
Table 1.
Multiplexed imaging applications and advantages over single modalities
| Clinical applications | Preclinical applications | Advantages | |
|---|---|---|---|
| Multimodality | PET–CT, PET–MRI: cancer detection and monitoring response to therapy and disease recurrence2–10 | Optical–CT: evaluation of metastatic bone disease11 Dual MRI–optical contrast agent: lymphatic mapping and sentinel lymph node biopsy12 |
PET–CT: improved spatial resolution; superimposed functional and anatomical data; improved detection and characterisation of lesions PET–MRI: high tissue resolution without ionising radiation13,14 Nuclear–optical, MRI–optical: quantitative information and real-time anatomical localisation for presurgical mapping and surgery assistance15,16 |
| Multicolour contrast agents | Multiple gamma emitting contrast agents: localisation of parathyroid adenomas and medullary thyroid cancer; 17–19 Cardiac nuclear imaging: cardiac function and detection of ischaemia by detecting blood perfusion and damaged myocardium20–23 |
Multiple optical probes: in-vivo detection and differentiation of multiple tumour types expressing distinct surface marker;24 simultaneous visualisation of multiple lymphatic basins15 | Multiple gamma/optical contrast agents: simultaneous localisation, quantification and characterisation of disease Multiple optical probes: probes easily synthesised with similar pharmacokinetics; in-vivo molecular profiling of tissue |
| Multiple signal collection | Whole body T1,T2 and diffusion weighted MRI: tumour characterisation, evaluation of tumour microenvironment, and monitoring response to therapy;25,26 improved detection of cancer in liver, bone, and brain;27–29 staging of haematological diseases, such as multiple myeloma and lymphoma29 | Fluorescence intensity and lifetime imaging: localisation of HER2 positive tumours and their tumour oxygenation status30,31 | Whole body T1-T2 and diffusion weighted MRI: radiation-free alternative for surveillance of patients with multiple follow-up exams with accurate localisation29 Fluorescence intensity and lifetime imaging: combined target-specific detection and information of tumour microenvironment |
| Activatable smart reagents | None currently clinically implemented | Enzyme-specific activatable probes: selective detection of cancerous tissue32 Target cell-specific activatable probes: highly selective detection of target viable cancer cells33–35 |
Metabolic characterisation of tissue; improved sensitivity and specificity; minimal background signal, high tumour/ background ratio; differentiation of viable from non-viable cancer cells in real-time |
Multiplexed imaging strategies aim to overcome the limitations of individual conventional techniques. Existing technologies may be broadly categorised into mainly anatomical and mainly molecular imaging techniques. These techniques share an inverse relationship between spatial resolution and their sensitivity to subtle disease changes. Anatomical imaging technologies include CT, MRI, and ultrasound. These methods are limited, because they can detect only morphological changes in tumour size and structure. By contrast, molecular imaging techniques offer the ability to detect molecular and cellular changes before anatomical changes can be detected. Although extremely promising, available molecular techniques are often limited by depth of signal penetration as well as poor spatial resolution. The most widely used functional techniques include PET and SPECT. Although these techniques provide information about tissue characterisation and biological activity, anatomical and spatial resolutions are limited.
The most desired improvements in cancer imaging include earlier detection of disease, improved accuracy in molecular characterisation of tumours and the tumour microenvironment, and earlier detection of response to therapy. To achieve these important imaging objectives, the limitations of individual technologies must be minimised and new innovations developed. Towards this end, the simultaneous use of two or more techniques, contrast reagents, signalling methods, or the coupling of agent and tissue properties to achieve multiplexed imaging is a promising approach. For example, combining PET with CT simultaneously yields data regarding tissue metabolism and structural anatomy. As a parallel example in molecular imaging, a conventional, so called “always-on” probe is combined with a smart probe, an agent, which emits a signal only after binding to the target molecules on the cancer cells. The quantitative pharmacokinetics depicted by the always-on probe, coupled with the intracellular signal activation and molecular targeting capacity of the smart probe combine to improve sensitivity and specificity.16 In this review, we provide a broad overview of multiplexed imaging technologies, ranging from those now available to those under development for oncological imaging.
Multiple modalities
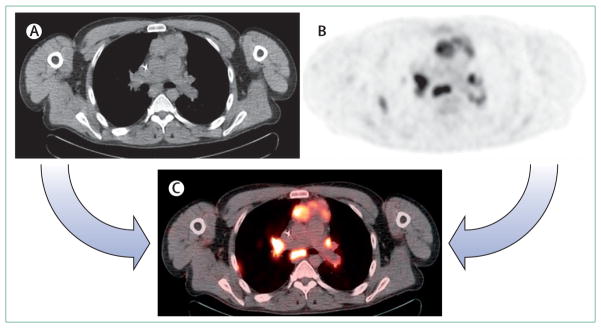
The most widely used multiplexed imaging technology combines the exquisite anatomical CT, with the physiological imaging data of PET, providing both functional and anatomic information36 (figure 1A). Within oncology, combination PET and CT is used for cancer screening, diagnosis, staging and monitoring response to therapy.2–4 Combined PET and CT is more sensitive and specific than alone.5 There is a synergistic advantage, because the attenuation correction needed for PET can also be derived more accurately and efficiently from CT data. This makes combined imaging 25–30% faster than PET alone, with standard attenuation correction methods. This enables a substantially higher patient throughput and a total examination time of less than 30 min.5 For instance, the assessment of lung cancer is among the most important applications of whole-body 18-fluoro-deoxyglucose ([18F]FDG) PET, and combination with CT adds important information to the clinical assessment as a result of better lesion identification and localisation and fewer missed lesions (figure 2). Additionally, combined PET and CT might improve diagnosis and disease management in colorectal cancer, lymphoma, and head and neck tumours.5–10
Figure 2. PET, CT, and PET–CT images of a patient with multiple mediastinal and hilar lymph node metastasis.
The lesions are difficult to identify with x-ray CT scan (A). The anatomical locations of lesions are difficult to be defined with 18-fluorodeoxyglucose ([18F]FDG) PET (B). PET–CT image can easily identify all lesions and their anatomical locations (C).
Combining PET with CT reduces the limitations of PET imaging alone, such as poor spatial resolution and difficulty in differentiating tumour from infection or inflammation. Similarly, combined PET and MRI is currently being developed.13,14 PET with MRI offers the advantage of providing high tissue resolution, without contrast agents or additional ionising radiation. This is an important advantage for patients with renal impairment because of superior tissue contrast without imaging agents. It is also an advantage for imaging of the urinary tract, because the high concentration of excreted iodine contrast agent, which hampers the appropriate attenuation correction with contrast-enhanced CT combined with PET, is avoided.37,38 MRI provides high spatial resolution without ionising radiation. MRI is superior to CT for the detection of liver, bone marrow, and CNS lesions,39,40 and combined PET and MRI may prove better than PET with CT in the diagnosis and management of cancers within these anatomical regions. With this in mind, magnetic resonance based attenuation correction methods are being developed by several groups.41–45
A promising area of research is the fusion of two or more molecular imaging techniques. There are two general approaches to achieving multiplexed molecular imaging. The first is coupling molecular imaging with a conventional method that is primarily anatomical. This combination offers the advantage of providing the anatomical resolution of conventional imaging with the specific molecular information capabilities of molecular imaging. The clinical potential of this combination includes, combining optical imaging with CT for the assessment of metastatic bone disease11 and with nuclear imaging for longitudinal imaging of tumour hypoxia.46 A second approach is the creation of individual molecular imaging probes, so called multimodal contrast agents, that can be viewed by several different imaging techniques. An advantage of this approach is the limitation of contrast exposure to a single agent rather than multiple agents, as in the case of combined molecular and anatomical approaches such as PET–CT. Applications currently under investigation include MRI with optical lymphatic contrast agents. This combination uses MRI to produce a map of the lymphatic drainage and sentinel node draining from a tumour as well as three-dimensional anatomical information, while real-time fluorescence imaging provides intraoperative imaging guidance during node sampling12 (webappendix). Another promising combination of MRI and an optical probe is a pancreas specific quantum dot with both optical and magnetic resonance capabilities, to improve the detection and localisation of pancreatic cancer.47
When creating a combination probe, the sensitivity of signal detection in each component technique must be taken into consideration and adequately adjusted to properly balance the visualisation of each component. Combination of nuclear and optical imaging probes are attractive, because the sensitivity of these two methods is comparable, which reduces the need to adjust the ratio of each component to achieve signal balance. Nuclear and optical dual-modality contrast agents provide the advantage of static, yet quantitative information from the nuclear imaging probe while providing real-time dynamic information with optical fluorescence imaging.15,16 Whereas modality combination techniques such as PET–CT combine to compensate for the weakness of each individual component, the rationale behind dual-modality contrast agents is to design and create a synergistic probe that harnesses the strengths of each imaging technology for a specific clinical application.
Multiple colour reagents
Conventional imaging methods are restricted to the detection of solitary, monochromatic contrast agents and therefore provide only a single type of uniparametric data. One approach to increase the information is to use multiplex imaging with an additional imaging agent. Multi contrast agent applications include SPECT in the thyroidandparathyroid17–19 and cardiac scintigraphy,20–23,48–51 both of which can simultaneously detect two different imaging agents (ie, 99mTc, 123I, 111In, and 201Tl) with the energy resolution features of gamma cameras (figure 1B). These methods rely on the use of two different agents, each targeting a different process, and simultaneously acquiring data from each. Clinical applications include the assessment of acute cardiac ischaemia with 201Tl to identify viable myocardium through patent blood flow in combination with 111In-labelled myosin antibody to identify exposed myosin fibres in damaged myocardium.20–23 Although promising, these advances have limited spatial resolution and do not allow the recon struction of high-resolution images, as can be achieved using conventional methods. Unfortunately, imaging with multiple radiolabelled antibodies faces similar challenges. It takes several days from the time the agent is injected to achieve sufficient tumour binding for imaging. Because of differing pharmacokinetics and physical half-lives of distinct radionuclides, a single optimised imaging time that is successful for both agents might be hard to define. Therefore the combinations of isotopes that can be used for multiple energy scintigraphy are limited.
Optical imaging is the best adapted imaging method for multiplexed imaging. By the use of sensitive optical cameras with spectral filters, two or more optical agents can be differentiated on the basis of their differing emission wavelengths.24 Differentiation can also be made by the unaided eye if the wavelength of the emitted light is within the visible spectrum.52 Spectral resolution not only simultaneously detects more than one target, but the background signal can also be minimised by calculating and adjusting for unwanted fluorescence signals derived from unbound reagents in circulation or that of host tissues (eg, autofluorescence).53 One advantage of optical imaging is that various easily synthesised, organic fluorophores have similar chemical profiles but different emission spectra. Therefore, unlike radionuclides, many different reagents can be produced with distinct emission profiles but have comparable pharmacokinetics and biological half-lives. Successful laboratory applications of multicolour optical imaging include simultaneous in-vivo visualisation of multiple tumours, each with different molecular targets, using a cocktail injection of three different near-infrared fluorescence-labelled humanised monoclonal antibodies.24 Such experiments show that targeted probes with multiple-contrast optical agents can create an easily interpreted visible signal profile that enable in-vivo characterisation of multiple molecular phenotypes of tumours. Additionally, by use of optically labelled nanoparticles in a murine model, at least five different lymphatic basins in the head and neck region can be simultaneously and separately depicted.15 Although these innovations have yet to be translated to clinical settings, they show the potential for multicolour imaging to improve the molecular characterisation of cancer, as well as the simultaneous depiction of several anatomical structures.
Multiple signal collection
Multiplexed imaging with multiple signal collection relies on capturing two or more distinct signals that are generated by individual tissues (figure 1C). For example, dual energy x-ray CT uses the different x-ray absorption of distinct chemical compositions in various tissues at two separate photon energies to characterise processes such as iodine-calcium separation(eg, tumours, arterial plaque),54–56 renal-stone composition, and perfusion and local blood volume in organs and tumours.57,58 Of the available imaging technologies, MRI offers perhaps the greatest ability to harness differences in molecular composition with multiple signal collections. Magnetic resonance scanners can detect serial signals from protons (T1 and T2 weighted imaging), and carbohydrates or water (diffusion weighted signals), and differences in the physical state of protons in each fraction of tissue.25 Clinical uses of multiplexed technology include whole-body MRI for the assessment of cancer treatment, tumour characterisation, investigation of tumour microenvironment and assessment of tumour viability after cancer therapy.25,26 Combined signal collection enables virtual combination PET with MRI, in which T1 or T2 weighted images are used for anatomical localisation of lesions. Diffusion weighted MRI is used to generate [18F]FDG-PET-like images of the body. Promising oncological applications include detection of metastatic disease, especially distant disease and tumours with known predilection for the liver, bone or brain.27–29 Whole-body diffusion weighted MRI also has efficacy as a whole-body bone-marrow screening technique and is highly accurate in the detection of skeletal metastases and staging of haematological diseases, such as multiple myeloma and lymphoma.29 Furthermore, whole-body diffusion-weighted MRI is comparable to [18F] FDG-PET combined with CT in the assessment of metastatic disease in some cancers.59 Because MRI does not require exposure of patients to ionising radiation and can provide [18F]FDG-PET-like functional imaging without contrast agents, this technology is especially useful as a radiation-free alternative for the surveillance of patients with multiple follow-up exams.29
Multisignal molecular imaging relies on extracting multiple types of data from a single source. For example, the signal from fluorescent probes can be measured in terms of energy, signal intensity, and fluorescence lifetime. The signal intensity can serve as a measure of fluorophore concentration, therefore providing semi quantitative local concentration data. In comparison fluorescence lifetime is dependent on physical conditions, such as temperature and pH, it therefore provides insight into the micro-environment.30,31 A potential clinical application is the creation of an optical imaging probe capable of localisation of HER2-positive cells within the body, which could be achieved by conjugating AlexaFlore750, a near-infrared fluorophore, with trastuzumab, an antibody against the HER2 receptor. Tumour imaging can be achieved by measuring fluorescence intensity, while fluorescence lifetime provides an estimation of oxygenation and the degree of hypoxia within the tumour microenvironment.60 Therefore, by extrapolating multiple types of data from a single probe, not only can the presence of a tumour be determined, but also important qualitative data on tumour conditions can be recorded.
Activatable smart agents
Conventional contrast agents, such as those previously discussed are always on, meaning they emit a signal whether they are bound to a target or not. As a consequence, there is usually substantial non-specific background signal to be overcome. One approach is to ensure that such agents are low in molecular weight and therefore rapidly cleared. Rapid clearance decreases the amount available for specific binding. Activatable smart probes are an emerging technology that combines molecular targeting with physico chemical probe activation to detect disease with low background signal, even if the clearance is slow (figure 1D). For example, monoclonal antibodies are very promising because of their specificity and resulting high target concentrations, but have the undesirable feature of prolonged clearance leading to high background signal with always-on agents.61 Two approaches might improve sensitivity and specificity of imaging probes, both of which involve enhancing the ratio of target to background by maximising the signal from the target, and minimising the background signal.62 Research has focused on maximising the signal from the target and less attention has been paid to reducing the background signal. Recently, however, innovations in MRI63 and optical imaging32 probes have led to the development of smart probes that produce higher target to background ratios than conventional imaging methods by reducing the background signal from unbound probes. The activatable smart probes are designed to generate a signal only after being bound and processed by the target tissue32 or cancer cells.33 Several signal activation strategies have recently been investigated, although none have yet been approved for clinical use. Among the approaches, pH-activatable targeted imaging probes enable selective detection of viable cancer cells in real-time with minimum background fluorescence signal.34 One of the main strengths of this technology is the measurement of cancer cell viability. Because the probes lose signal when they leak out of the cells and into the more neutral conditions of the extracellular environment, cellular integrity can be assessed. Thus, viable cancer cells can be distinguished from dead or apoptotic cells.
Non-invasive, in-vivo optical imaging with activatable technology can be achieved with near-infrared emission range agents. Such probes include indocyanine-green, the only near-infrared fluorophore approved by the US Food and Drug Administration (FDA) for clinical use. The absorption and emission peaks of indocyanine-green (780 nm and 820 nm, respectively) provide adequate tissue penetration for non-invasive imaging and has lower associated autofluorescence. Indocyanine-green-labelled activatable humanised EGFR antibodies, which are approved by FDA, do not fluoresce. However, upon target binding and internalisation by tumour cells expressing HER1 or HER2, the fluorescence intensity of the probe is regained, thus selectively imaging tissues with specific molecular profiles.35 Indocyanine-green coupled to FDA-approved humanised monoclonal IgG, represents a promising activatable probe that is on the verge of clinical use. Antibodies conjugated with indocyanine-green enable detection and characterisation of tumours in vivo with high specificity and minimum background signal. Activatable probes might significantly improve the specificity and sensitivity of cancer imaging.
Combining multiplexed strategies
Combination of the different multiplexed imaging strategies might further improve imaging. For example, combining multicolour fluorescence imaging with radio nuclide imaging provides both qualitative (optical) and quantitative (radionuclide) assessment (figure 3). Combining multiplexed strategies not only provides qualitative and quantitative advantages, but also compensates for the respective weaknesses of each modality,15 such as low temporal and spatial resolution of radionuclide imaging and poor depth penetration of optical imaging. Useful combinations include multicolour imaging with a cocktail of optical probes or multimodal imaging with activatable agents (figure 4). These combinations are synergistic, offering superior sensitivity with improved specificity and superior spatial-temporal resolution with precise pharmacokinetic characterisation, respectively. These developments could signal the beginning of an era of multiplexed imaging.
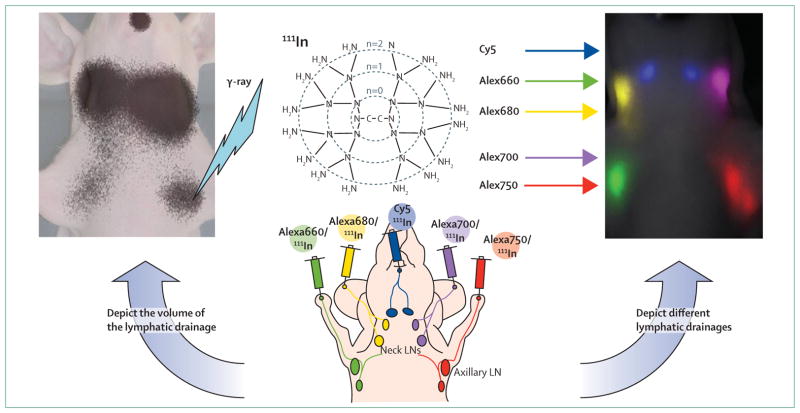
Figure 3. Multicolour, dual-modality lymphatic imaging agents.
Nuclear imaging with nuclear 111In can depict quantitative lymphatic drainage from the injection site to the draining lymph nodes to identify the primary sentinel lymph node (left). Near-infrared fluorescence imaging depicts different lymphatic drainages, with distinct colours to determine the individual lymphatic drainage from several different sites in the body (right). LN=lymph node.
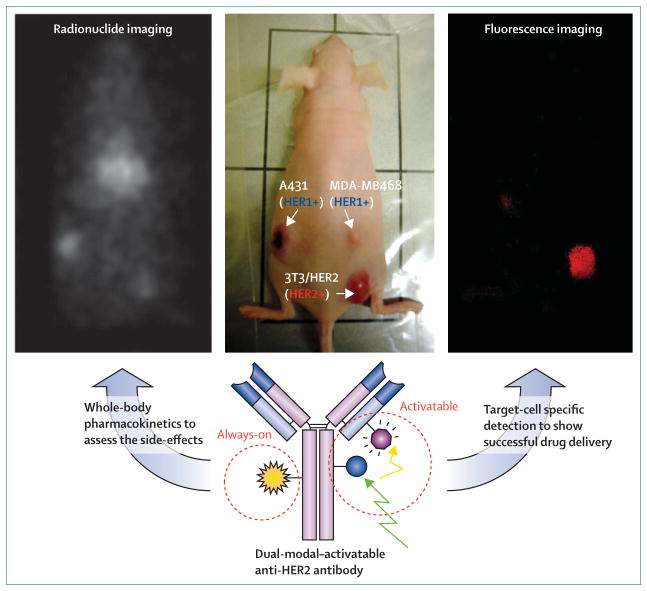
Figure 4. Nuclear imaging with optical imaging multimodality, activatable imaging agent.
Nuclear imaging with 111In can show the quantitative pharmacokinetics of the agent (left), illustrating the distribution of injected reagent. This might predict the drug side-effects at the off-target site in the body. Activatable fluorescence imaging can only depict the tumour expressing the target (specific antigen) with minimum background signal (right). This shows the successful target-cell binding, followed by the processing and initiation of the reagent signal at the target cancer cells.
Conclusion
By combining existing modalities and taking new innovations from the bench to the clinic, the goals of early cancer detection, molecular characterisation of tumours and the tumour microenvironment, monitoring of therapy response, and improved treatment will ideally be achieved.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Contributors
HK designed the concept. All authors were involved in the reference search, writing, and the final approval of the paper.
Conflicts of interest
The authors declared no conflicts of interest.
Contributor Information
Hisataka Kobayashi, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Michelle R Longmire, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Mikako Ogawa, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Peter L Choyke, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Satomi Kawamoto, Department of Radiology, Johns Hopkins University, Baltimore, MD, USA.
References
- 1.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–21. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50:88–99. doi: 10.2967/jnumed.108.054205. [DOI] [PubMed] [Google Scholar]
- 3.Ford EC, Herman J, Yorke E, Wahl RL. 18F-FDG PET/CT for Image-Guided and Intensity-Modulated Radiotherapy. J Nucl Med. 2009;50:1655–65. doi: 10.2967/jnumed.108.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mawlawi O, Townsend DW. Multimodality imaging: an update on PET/CT technology. Eur J Nucl Med Mol Imaging. 2009;36 (suppl 1):S15–29. doi: 10.1007/s00259-008-1016-6. [DOI] [PubMed] [Google Scholar]
- 5.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–22. doi: 10.1148/radiol.2382041977. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj A, Cook GJ, Hansell DM. PET/CT in non-small cell lung cancer staging-promises and problems. Clin Radiol. 2007;62:97–108. doi: 10.1016/j.crad.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Eschmann SM, Bitzer M, Paulsen F, et al. The benefit of functional-anatomical imaging with [18F]fluorodeoxyglucose utilizing a dual-head coincidence gamma camera with an integrated X-ray transmission system in non-small cell lung cancer. Nucl Med Commun. 2004;25:909–15. doi: 10.1097/00006231-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gilman MD, Aquino SL. State-of-the-Art FDG-PET imaging of lung cancer. Semin Roentgenol. 2005;40:143–53. doi: 10.1053/j.ro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50 (suppl 1):31S–42S. doi: 10.2967/jnumed.108.057216. [DOI] [PubMed] [Google Scholar]
- 10.Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–19. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 11.Kaijzel EL, Snoeks TJ, Buijs JT, van der Pluijm G, Lowik CW. Multimodal imaging and treatment of bone metastasis. Clin Exp Metastasis. 2009;26:371–79. doi: 10.1007/s10585-008-9217-8. [DOI] [PubMed] [Google Scholar]
- 12.Koyama Y, Talanov VS, Bernardo M, et al. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–71. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 13.Judenhofer MS, Wehrl HF, Newport DF, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–65. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 14.Pichler BJ, Judenhofer MS, Wehrl HF. PET/MRI hybrid imaging: devices and initial results. Eur Radiol. 2008;18:1077–86. doi: 10.1007/s00330-008-0857-5. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Koyama Y, Barrett T, et al. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano. 2007;1:258–64. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa M, Regino CA, Seidel J, et al. Dual-modality molecular imaging using antibodies labeled with activatable fluorescence and a radionuclide for specific and quantitative targeted cancer detection. Bioconjug Chem. 2009;20:2177–84. doi: 10.1021/bc900362k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBiles M, Lambert AT, Cote MG, Kim SY. Sestamibi parathyroid imaging. Semin Nucl Med. 1995;25:221–34. doi: 10.1016/s0001-2998(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 18.Neumann DR, Obuchowski NA, Difilippo FP. Preoperative 123I/99mTc-sestamibi subtraction SPECT and SPECT/CT in primary hyperparathyroidism. J Nucl Med. 2008;49:2012–17. doi: 10.2967/jnumed.108.054858. [DOI] [PubMed] [Google Scholar]
- 19.Shih WJ, Stipp V, Magoun S, Ain KB, Pulmano C. Medullary thyroid carcinoma imaged by Tc-99m MIBI SPECT and Tl-201 chloride/Tc-99m pertechnetate subtraction SPECT. Clin Nucl Med. 1996;21:213–17. doi: 10.1097/00003072-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Antunes ML, Johnson LL, Seldin DW, et al. Diagnosis of right ventricular acute myocardial infarction by dual isotope thallium-201 and indium-111 antimyosin SPECT imaging. Am J Cardiol. 1992;70:426–31. doi: 10.1016/0002-9149(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LL, Lerrick KS, Coromilas J, et al. Measurement of infarct size and percentage myocardium infarcted in a dog preparation with single photon-emission computed tomography, thallium-201, and indium 111-monoclonal antimyosin Fab. Circulation. 1987;76:181–90. doi: 10.1161/01.cir.76.1.181. [DOI] [PubMed] [Google Scholar]
- 22.Johnson LL, Seldin DW, Keller AM, et al. Dual isotope thallium and indium antimyosin SPECT imaging to identify acute infarct patients at further ischemic risk. Circulation. 1990;81:37–45. doi: 10.1161/01.cir.81.1.37. [DOI] [PubMed] [Google Scholar]
- 23.Schoeder H, Topp H, Friedrich M, Jatzkewitz A, Roser M. Thallium and indium antimyosin dual-isotope single-photon emission tomography in acute myocardial infarction to identify patients at further ischaemic risk. Eur J Nucl Med. 1994;21:415–22. doi: 10.1007/BF00171416. [DOI] [PubMed] [Google Scholar]
- 24.Koyama Y, Barrett T, Hama Y, Ravizzini G, Choyke PL, Kobayashi H. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia. 2007;9:1021–29. doi: 10.1593/neo.07787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 26.Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002;16:407–22. doi: 10.1002/jmri.10176. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs MA, Pan L, Macura KJ. Whole-body diffusion-weighted and proton imaging: a review of this emerging technology for monitoring metastatic cancer. Semin Roentgenol. 2009;44:111–22. doi: 10.1053/j.ro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–82. [PubMed] [Google Scholar]
- 29.Schmidt GP, Reiser MF, Baur-Melnyk A. Whole-body MRI for the staging and follow-up of patients with metastasis. Eur J Radiol. 2009;70:393–400. doi: 10.1016/j.ejrad.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Ramanujan VK, Zhang JH, Biener E, Herman B. Multiphoton fluorescence lifetime contrast in deep tissue imaging: prospects in redox imaging and disease diagnosis. J Biomed Opt. 2005;10:051407. doi: 10.1117/1.2098753. [DOI] [PubMed] [Google Scholar]
- 31.Elson D, Requejo-Isidro J, Munro I, et al. Time-domain fluorescence lifetime imaging applied to biological tissue. Photochem Photobiol Sci. 2004;3:795–801. doi: 10.1039/b316456j. [DOI] [PubMed] [Google Scholar]
- 32.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–17. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 33.Hama Y, Urano Y, Koyama Y, et al. A target cell-specific activatable fluorescence probe for in vivo molecular imaging of cancer based on a self-quenched avidin-rhodamine conjugate. Cancer Res. 2007;67:2791–99. doi: 10.1158/0008-5472.CAN-06-3315. [DOI] [PubMed] [Google Scholar]
- 34.Urano Y, Asanuma D, Hama Y, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–09. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–72. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bybel B, Brunken RC, Shah SN, Wu G, Turbiner E, Neumann DR. PET and PET/CT imaging: what clinicians need to know. Cleve Clin J Med. 2006;73:1075–87. doi: 10.3949/ccjm.73.12.1075. [DOI] [PubMed] [Google Scholar]
- 37.Antoch G, Freudenberg LS, Egelhof T, et al. Focal tracer uptake: a potential artifact in contrast-enhanced dual-modality PET/CT scans. J Nucl Med. 2002;43:1339–42. [PubMed] [Google Scholar]
- 38.Ay MR, Zaidi H. Assessment of errors caused by X-ray scatter and use of contrast medium when using CT-based attenuation correction in PET. Eur J Nucl Med Mol Imaging. 2006;33:1301–13. doi: 10.1007/s00259-006-0086-6. [DOI] [PubMed] [Google Scholar]
- 39.Domingues RC, Carneiro MP, Lopes FC, Domingues RC, da Fonseca LM, Gasparetto EL. Whole-body MRI and FDG PET fused images for evaluation of patients with cancer. AJR Am J Roentgenol. 2009;192:1012–20. doi: 10.2214/AJR.08.1498. [DOI] [PubMed] [Google Scholar]
- 40.Lauenstein TC, Semelka RC. Emerging techniques: whole-body screening and staging with MRI. J Magn Reson Imaging. 2006;24:489–98. doi: 10.1002/jmri.20666. [DOI] [PubMed] [Google Scholar]
- 41.Beyer T, Weigert M, Quick HH, et al. MR-based attenuation correction for torso-PET/MR imaging: pitfalls in mapping MR to CT data. Eur J Nucl Med Mol Imaging. 2008;35:1142–46. doi: 10.1007/s00259-008-0734-0. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann M, Pichler B, Scholkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36 (suppl 1):S93–104. doi: 10.1007/s00259-008-1007-7. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann M, Steinke F, Scheel V, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49:1875–83. doi: 10.2967/jnumed.107.049353. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Moller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–26. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 45.Zaidi H, Montandon ML, Slosman DO. Magnetic resonance imaging-guided attenuation and scatter corrections in three-dimensional brain positron emission tomography. Med Phys. 2003;30:937–48. doi: 10.1118/1.1569270. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann S, Stiehl DP, Honer M, et al. Longitudinal and multimodal in vivo imaging of tumor hypoxia and its downstream molecular events. Proc Natl Acad Sci USA. 2009;106:14004–09. doi: 10.1073/pnas.0901194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yong KT. Mn-doped near-infrared quantum dots as multimodal targeted probes for pancreatic cancer imaging. Nanotechnology. 2009;20:015102. doi: 10.1088/0957-4484/20/1/015102. [DOI] [PubMed] [Google Scholar]
- 48.Berman DS, Kiat H, Van Train K, Friedman JD, Wang FP, Germano G. Dual-isotope myocardial perfusion SPECT with rest thallium-201 and stress Tc-99m sestamibi. Cardiol Clin. 1994;12:261–70. [PubMed] [Google Scholar]
- 49.Kiat H, Germano G, Friedman J, et al. Comparative feasibility of separate or simultaneous rest thallium-201/stress technetium-99m-sestamibi dual-isotope myocardial perfusion SPECT. J Nucl Med. 1994;35:542–48. [PubMed] [Google Scholar]
- 50.Matsunari I, Kanayama S, Yoneyama T, et al. Electrocardiographic-gated dual-isotope simultaneous acquisition SPECT using 18F-FDG and 99mTc-sestamibi to assess myocardial viability and function in a single study. Eur J Nucl Med Mol Imaging. 2005;32:195–202. doi: 10.1007/s00259-004-1668-9. [DOI] [PubMed] [Google Scholar]
- 51.Weinmann P, Faraggi M, Moretti JL, Hannequin P. Clinical validation of simultaneous dual-isotope myocardial scintigraphy. Eur J Nucl Med Mol Imaging. 2003;30:25–31. doi: 10.1007/s00259-002-0995-y. [DOI] [PubMed] [Google Scholar]
- 52.Kosaka N, Ogawa M, Sato N, Choyke PL, Kobayashi H. In vivo real-time, multicolor, quantum dot lymphatic imaging. J Invest Dermatol. 2009;129:2818–22. doi: 10.1038/jid.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett T, Koyama Y, Hama Y, et al. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007;13:6639–48. doi: 10.1158/1078-0432.CCR-07-1119. [DOI] [PubMed] [Google Scholar]
- 54.Brockmann C, Jochum S, Sadick M, et al. Dual-energy CT angiography in peripheral arterial occlusive disease. Cardiovasc Intervent Radiol. 2009;32:630–37. doi: 10.1007/s00270-008-9491-5. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Gilkeson RC, Fei B. Automatic 3D-to-2D registration for CT and dual-energy digital radiography for calcification detection. Med Phys. 2007;34:4934–43. doi: 10.1118/1.2805994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran DN, Straka M, Roos JE, Napel S, Fleischmann D. Dual-energy CT discrimination of iodine and calcium: experimental results and implications for lower extremity CT angiography. Acad Radiol. 2009;16:160–71. doi: 10.1016/j.acra.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Thieme SF, Johnson TR, Lee C, et al. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol. 2009;193:144–49. doi: 10.2214/AJR.08.1653. [DOI] [PubMed] [Google Scholar]
- 58.Pontana F, Faivre JB, Remy-Jardin M, et al. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15:1494–504. doi: 10.1016/j.acra.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Ohno Y, Koyama H, Onishi Y, et al. Non-small cell lung cancer: whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology. 2008;248:643–54. doi: 10.1148/radiol.2482072039. [DOI] [PubMed] [Google Scholar]
- 60.Hassan M, Riley J, Chernomordik V, et al. Fluorescence lifetime imaging system for in vivo studies. Mol Imaging. 2007;6:229–36. [PMC free article] [PubMed] [Google Scholar]
- 61.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 62.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–89. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louie AY, Huber MM, Ahrens ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–25. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]