Treatment with doxycycline leads to improvement of filarial lymphedema independent of active infection (ie, patients positive or negative for circulating filarial antigen). Therefore, doxycycline (200 mg/d for 6 weeks) should be considered for patients with stage 1–3 lymphedema to improve morbidity management.
Abstract
Background. The aim of this study was to determine whether improvement of filarial lymphedema (LE) by doxycycline is restricted to patients with ongoing infection (positive for circulating filarial antigen [CFA]), or whether the majority of CFA-negative patients with LE would also show a reduction in LE severity.
Methods. One hundred sixty-two Ghanaian participants with LE stage 1–5 (Dreyer) were randomized blockwise into 2 groups (CFA positive or negative) and allocated to 3 treatment arms of 6 weeks: (1) amoxicillin (1000 mg/d), (2) doxycycline (200 mg/d), or (3) placebo matching doxycycline. All groups received standard hygiene morbidity management. The primary outcome was reduction of LE stages. Secondary outcomes included frequency of acute attacks and ultrasonographic assessment of skin thickness at the ankles. Parameters were assessed before treatment and after 3, 12, and 24 months.
Results. Doxycycline-treated patients with LE stage 2–3 showed significant reductions in LE severity after 12 and 24 months, regardless of CFA status. Improvement was observed in 43.9% of doxycycline-treated patients, compared with only 3.2% and 5.6% in the amoxicillin and placebo arms, respectively. Skin thickness was correlated with LE stage improvement. Both doxycycline and amoxicillin were able to reduce acute dermatolymphangioadenitis attacks.
Conclusions. Doxycycline treatment improves mild to moderate LE independent of ongoing infection. This finding expands the benefits of doxycycline to the entire population of patients suffering from LE. Patients with LE stage 1–3 should benefit from a 6-week course of doxycycline every other year or yearly, which should be considered as an improved tool to manage morbidity in filarial LE.
Clinical Trials Registration. ISRCTN 90861344.
Worldwide, 40 million persons are disabled owing to filariasis-related morbidity, with 15 million suffering from lymphedema (LE) or elephantiasis and 25 million with hydrocele. Filarial LE is caused by infection with the lymphatic filarial nematodes, Wuchereria bancrofti, Brugia malayi, and Brugia timori and occurs most commonly in the legs. Patients experience a gradual and progressive development in the severity of clinical LE, graded 1–7 (Supplementary Figure 1), leading to severe disability, loss of productivity, and social stigmatization [1]. Patients with LE also experience “acute attacks” or acute dermatolymphangioadenitis, arising through secondary microbial infection acquired through lesions in the skin [2].
The goals of the Global Programme to Eliminate Lymphatic Filariasis (GPELF) are (1) to interrupt transmission by reduction of microfilaremia levels using mass drug administration (MDA) of filaricidal drugs and (2) to provide morbidity management for those who suffer from clinical manifestations associated with lymphatic filariasis (LF) [3–5]. The first goal has been successfully approached in areas having used diethylcarbamazine and albendazole for mass drug treatment for ≥5 years but has been met less successfully in Africa, where ivermectin and albendazole have been administered [6]. During the first 10 years of the GPELF, prevention of new morbidity has been impressive, with an estimated 22 million persons protected from LF infection and disease, accounting for economic savings of US $24.2 billion [4]. However, the goal to reduce existing morbidity associated with chronic filarial disease has not scaled up as rapidly as MDA, with only 33% of endemic countries introducing morbidity management in the first decade of the GPELF [1]. Current morbidity management strategies rely on improving hygiene and skin care of affected limbs, with limb elevation, exercise, and the use of topical antibiotics and antifungal creams, which reduces the frequency of acute attacks and can help arrest the development of LE [3]. We have shown elsewhere that 6 weeks of doxycycline results in a significant amelioration of LE severity in patients with active infection of W. bancrofti [7].
The present study was designed to address whether these benefits of a 6-week course of doxycycline (200 mg/d) could be extended to patients negative for circulating filarial antigen (CFA). In addition, we tested to determine whether a similar course of amoxicillin was also effective against LE. Our results demonstrate that doxycycline improves mild to moderate LE over 2 years. Importantly, CFA-negative patients also demonstrated significant improvement in LE, showing that the activity of doxycycline is not confined to patients with active infection. These results strongly promote the use of doxycycline as a new strategy for improved morbidity management of LF.
MATERIALS AND METHODS
The trial was approved by the Committee on Human Research, Publication and Ethics at the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. The study conformed to the principles of the Declaration of Helsinki 1964 (amended most recently in 2004). The trial registration number is ISRCTN 90861344.
Participants and Study Area
This trial was conducted in 21 endemic communities in the Nzema East and Ahanta West districts in the western region of Ghana [7–9], where MDA was started in 2001. Written informed consent was obtained from all participants. Eligible individuals were adults of both sexes aged 18–60 years with LE stage 1–5 according to the staging scheme by Dreyer [5], with a minimum body weight of 40 kg and no requirement for chronic medication. Exclusion criteria included LE stage 6 or 7; abnormal gamma-glutamyltransferase, alanine aminotransferase, or creatinine levels; pregnancy; breast-feeding; intolerance to doxycycline or amoxicillin; and alcohol or drug abuse. A clinician (S. M.) experienced in the symptoms of LE examined all consenting patients and performed the staging as well as ultrasonography at pretreatment and follow-up examinations.
Randomization and Masking
This was a randomized trial; the design was placebo controlled for the comparison of doxycycline and placebo, with an additional open amoxicillin arm. Randomization was carried out using computer-generated software. Blinding (masking) was ensured by exclusion of study personnel performing clinical or laboratory analyses from randomization or tablet distribution. To avoid an influence on results caused by the open amoxicillin arm, the personnel administering the treatment (A. B. D. and Y. M. D.) did not take part in follow-up examinations. A total of 162 patients were randomly allocated into 3 treatment arms: (1) amoxicillin, (2) doxycycline, and (3) placebo treatment, each with 54 patients. Before randomization, participants were stratified into those with and those without active infection (CFA positive and CFA negative). These 2 subgroups were separately randomized, resulting in 6 final treatment groups (Figure 1, Supplementary Figure 2A and B).
Figure 1.
Participant flow. Flow chart of all assessed volunteers (circulating filarial antigen positive and negative). Abbreviations: CFA, circulating filarial antigen; USG, ultrasonography.
Treatment and Follow-up
Participants received doxycycline (2 100-mg capsules daily), matching placebo, or amoxicillin (2 500-mg tablets daily) for a total of 6 weeks. Treatment was directly observed daily. Adverse events were recorded in the case report forms, and patients were seen by the trial clinician (A. B. D.). Two rounds of single-dose ivermectin and albendazole were distributed during the clinical trial within the scope of the annual MDA. Follow-up time points were 3, 12, and 24 months after treatment onset.
Foot Care Training and Monitoring of Hygiene Measures
All patients received soap, towels, and plastic bowls for washing their legs and were trained in foot hygiene, according to the booklet “New Hope” for persons with LE [5].
LE Staging
Staging of LE was performed according to the “Basic Lymphedema Management” guidelines established by G. Dreyer and colleagues [5] (Supplementary Figure 1). To allow comparisons of LE staging in patients with either one or both legs affected, the following approach was employed: (1) if only one leg showed LE, this leg was analyzed; (2) if both legs were affected, the leg with the lower stage is reported; (3) if both legs were affected showing equal stages, either the left or the right leg was analyzed after computer-generated randomization.
Monitoring of Acute Attacks
Acute attacks were defined as pain, lymph node swelling (femoral and/or inguinal), fever, and peeling of the skin on the affected leg after the resolving of the febrile attack (Supplementary Figure 3). The history regarding acute attacks was evaluated based on booklets kept by the patients and questionnaires filled out by the research team at every visit. Each study participant was asked in detail about the number and duration of attacks, fever, lymph node swelling, and peeling of the skin to differentiate general pain in affected legs from genuine acute attacks.
Microfilaremia, Antigenemia and Vascular Endothelial Growth Factors
For quantification of microfilariae in the blood the Whatman Nucleopore filter method was applied using 10-mL samples of night blood before treatment and at follow-up. Levels of W. bancrofti CFA were determined using the TropBio enzyme-linked immunosorbent assay (ELISA) test (TropBio) [7]. Levels of vascular endothelial growth factor (VEGF) C and soluble VEGF receptor (VEGFR) 3 were measured from plasma samples using Quantikine or DuoSet ELISA kits, respectively (R&D systems).
Circumference Measurement of Legs
Leg circumferences were determined using a tape measure. Measurements were performed at 10 cm from the large toe and 12, 20, and 30 cm from the sole of the foot, as described elsewhere [10, 11]. Averages of the 4 measurements were determined before treatment and at follow-up.
Ultrasound Examinations of the Ankles
Ultrasonography was performed between 2 and 7 PM using a SonoSite 180 PLUS hand-carried ultrasound system (SonoSite) equipped with a 38-mm 5–10-MHz linear-array transducer [12]. Patients were scanned sitting with stretched legs and feet perpendicular to the legs. The transducer was positioned at the top of the malleolus (ankle) and kept at a 90° angle to the skin surface in transverse sections. The head of tibia or fibula had to be visible, and the malleolus had to appear as a sharp line (Supplementary Figure 1) to permit reproducibility. The thickness of the tissue (subcutis, dermis, and epidermis) was measured from the malleolus to the skin surface. Lateral and medial malleoli of both legs were measured before treatment and at follow-up.
Statistical Analysis
To compare between the treatment arms, the intraindividual differences in staging or measurements between the respective follow-up and pretreatment findings were first calculated. Kruskal-Wallis tests followed by Mann-Whitney U tests or Fisher's exact test were used to compare between the treatment arms. The Wilcoxon signed rank test or the McNemar test were chosen for comparing pretreatment with follow-up results in one treatment arm. Correlations were done using the Spearman rank test. Differences between the treatment arms in the survival curve (Kaplan-Meier) were analyzed with the log-rank test.
To confirm the results of the per-protocol analyses (patients who completed the treatment and were present for the respective follow-up visit) an intention-to-treat (ITT) analysis was carried out with all patients who started treatment (missing values were filled in using the "last observation carried forward" method). Analyses were done using PASW statistics 18.0 software (IBM) and SAS 9.2 software (SAS).
RESULTS
Participants
From a recruitment pool of 205 patients with LE, 162 patients were stratified according to CFA status (Figure 1, Supplementary Figure 2A and B). At the start of treatment, 21 patients abandoned participation, although they had explicitly agreed to participate and had been randomized, and 13 participants stated that they had not given correct information about age or breast-feeding on recruitment. Along with these 34 patients, another 9 dropped out during treatment for reasons unrelated to the study drugs.
Baseline Data
Of the 119 patients who completed the treatment, 46 (39%) were CFA positive and 73 (61%) were CFA negative. Most of the patients had LE stage 2 (44.5%) or 3 (47.9%). The mean age was 47.7 ± 10.8 years. CFA-negative patients had on average a longer history of LE development than CFA-positive patients (P = .029). There was no difference among the treatment arms regarding compliance with MDA (Table 1).
Table 1.
Baseline Data
| Variable | Total | Doxycycline | Amoxicillin | Placebo | P Value |
|---|---|---|---|---|---|
| Patients (male/female), No. | 119 (34/85) | 46 (10/36) | 35 (9/26) | 38 (15/23) | .198a |
| CFA positive | 46 (17/29) | 19 (5/14) | 13 (6/7) | 14 (6/8) | .471a |
| CFA negative | 73 (17/56) | 27 (5/22) | 22 (3/19) | 24 (9/15) | .153a |
| LE stage 1 | 2 (0/2) | 1 (0/1) | 1 (0/1) | 0 | |
| LE stage 2 | 53 (16/37) | 20 (5/15) | 18 (4/14) | 15 (7/8) | |
| LE stage 3 | 57 (15/42) | 23 (4/19) | 15 (5/10) | 19 (6/13) | |
| LE stage 4 | 1 (0/1) | 1 (0/1) | 0 | 0 | |
| LE stage 5 | 6 (3/3) | 1 (1/0) | 1 (0/1) | 4 (2/2) | |
| Age, mean ± SD, years | 47.7 ± 10.8 | 46.1 ± 11.6 | 51.3 ± 9.1 | 46.3 ± 10.9 | .045b |
| CFA positive | 49.7 ± 12.1 | 45.5 ± 13.9 | 56.0 ± 5.6 | 49.4 ± 12.1 | .045b |
| CFA negative | 46.5 ± 9.8 | 46.5 ± 9.9 | 48.6 ± 9.7 | 44.5 ± 9.9 | .304b |
| Duration of LE, mean ± SD, years | 13.8 ± 12.3 | 13.7 ± 11.8 | 14.6 ± 14.9 | 13.3 ± 10.5 | .893b |
| CFA positive | 11.5 ± 12.7 | 9.3 ± 10.2 | 12.2 ± 16.5 | 13.7 ± 12.3 | .475b |
| CFA negative | 15.3 ± 11.9 | 16.9 ± 11.9 | 16.0 ± 14.1 | 13.0 ± 9.5 | .554b |
| MDA/total, No. (%) | |||||
| 2006 | 87/117 (74) | 31/46 (67.4) | 28/34 (82.4) | 28/37 (75.7) | .329a |
| 2007 | 85/115 (74) | 30/44 (68.2) | 27/34 (79.4) | 28/37 (75.7) | .535a |
| 2008 | 75/109 (69) | 25/41 (61.0) | 24/31 (77.4) | 26/37 (70.3) | .347a |
Abbreviations: CFA, circulating filarial antigen; LE, lymphedema; MDA, mass drug administration (ivermectin plus albendazole); SD, standard deviation.
a Fisher's exact test.
b Kruskal-Wallis test.
Primary Outcome Analysis
LE Staging
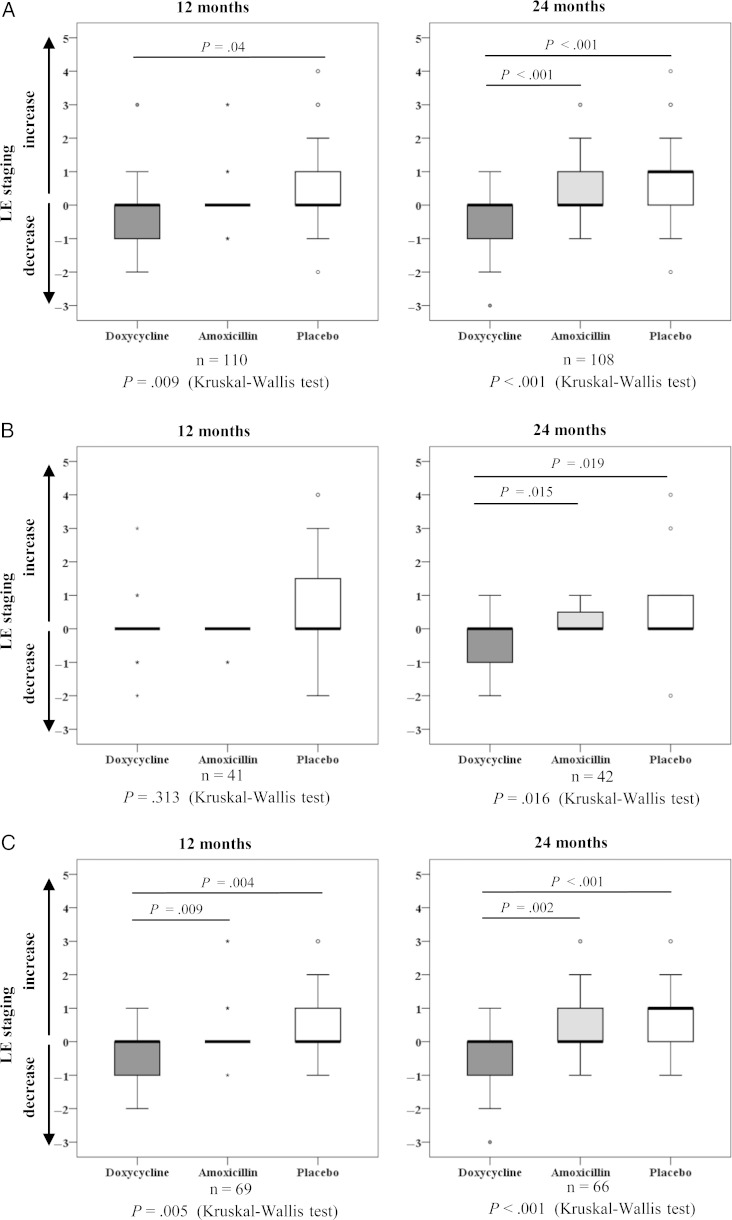
Figures 2A–C show changes in LE stages before treatment compared with 12 and 24 months after treatment. The affected legs of patients in the doxycycline group reverted to a lower stage at 12 and 24 months (P = .002; Table 2), whereas they progressed to a higher stage in the amoxicillin and placebo groups (P = .012 and P = .001 respectively). Comparing all groups at 24 months (Figure 2A), there was a difference between doxycycline and amoxicillin groups (P < .001) and between doxycycline and placebo groups (P < .001). The ITT analysis confirmed these results (Supplementary Table 2, Supplementary Figure 4). However, although there was no significant difference between amoxicillin and placebo groups at 24 months in the per-protocol analysis, a better outcome in the amoxicillin group compared with placebo could be detected in the ITT analysis (P = .034). Because 92.4% of the patients had LE stage 2 or 3, additional per-protocol and ITT analyses were carried out to follow these group of patients only, confirming the overall analyses (Supplementary Tables 3 and 4).
Figure 2.
Lymphedema (LE) staging. Differences in LE severity at 12 and 24 months compared with pretreatment severity is shown for each treatment group, according to per-protocol analysis. A, Box plots for all patients. B, C, Differences for the circulating filarial antigen (CFA)–positive (B) and CFA-negative (C) subgroups. For all figure parts, 0 denotes no change in LE stage, changes to <0 denote a decrease to lower LE stages, and changes to >0 denote an increase to higher LE stages. Abbreviation: LE, lymphedema.
Table 2.
Lymphedema Staging: Descriptive Statistics (Per-Protocol Analysis)
| Pretreatment | 3-Month Follow-up | 12-Month Follow-up | 24-Month Follow-up | |
|---|---|---|---|---|
| Treatment A: doxycycline (200 mg/d) | ||||
| CFA positive and negative | ||||
| Patients, No. | 46 | 44 | 45 | 41 |
| LE stage, mean ± SD (range) | 2.6 ± 0.7 (1–5) | 2.6 ± 0.8 (1–6) | 2.4 ± 1.2 (0–6) | 2.2 ± 1.2 (0–6) |
| LE stage, median (25th; 75th percentile) | 3 (2; 3) | 3 (2; 3) | 2 (2; 3) | 2 (1; 3) |
| Pa | .48 | .067 | .002 | |
| Subgroup: CFA positive before treatment | ||||
| Patients, No. | 19 | 19 | 19 | 17 |
| LE stage, mean ± SD (range) | 2.6 ± 0.8 (1–5) | 2.6 ± 1.1 (1–6) | 2.6 ± 1.6 (0–6) | 2.2 ± 1.4 (0–6) |
| LE stage, median (25th; 75th percentile) | 3 (2; 3) | 3 (2; 3) | 3 (2; 3) | 2 (1; 3) |
| Pa | .564 | .861 | .058 | |
| Subgroup: CFA negative before treatment | ||||
| Patients, No. | 27 | 25 | 26 | 24 |
| LE stage, mean ± SD (range) | 2.6 ± 0.7 (2–4) | 2.5 ± 0.7 (1–4) | 2.2 ± 0.9 (0–4) | 2.1 ± 1.0 (0–4) |
| LE stage, median (25th; 75th percentile) | 3 (2; 3) | 2 (2; 3) | 2 (2; 3) | 2 (1.25; 3) |
| Pa | .18 | .02 | .013 | |
| Treatment B: amoxicillin (1000 mg/d) | ||||
| CFA positive and negative | ||||
| Patients, No. | 35 | 34 | 31 | 31 |
| LE stage, mean ± SD (range) | 2.5 ± 0.7 (1–5) | 2.5 ± 0.7 (1–6) | 2.7 ± 1.3 (1–6) | 3.0 ± 1.4 (1–6) |
| LE stage, median (25th; 75th percentile) | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 2 (2; 4) |
| Pa | 1.0 | .38 | .012 | |
| Subgroup: CFA positive before treatment | ||||
| Patients, No. | 13 | 13 | 11 | 12 |
| LE stage, mean ± SD (range) | 2.4 ± 0.7 (1–3) | 2.4 ± 0.7 (1–3) | 2.3 ± 0.8 (1–3) | 2.7 ± 1.0 (1–4) |
| LE stage, median (25th; 75th percentile) | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 2.5 (2; 3.75) |
| Pa | 1.0 | .157 | .083 | |
| Subgroup: CFA negative before treatment | ||||
| Patients, No. | 22 | 21 | 20 | 19 |
| LE stage, mean ± SD (range) | 2.6 ± 0.7 (2–5) | 2.6 ± 1.0 (1–6) | 2.9 ± 1.5 (1–6) | 3.2 ± 1.6 (1–6) |
| LE stage, median (25th; 75th percentile) | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 2 (2; 5) |
| Pa | 1.0 | .129 | .04 | |
| Treatment C: placebo | ||||
| CFA positive and negative | ||||
| Patients, No. | 38 | 36 | 34 | 36 |
| LE stage, mean ± SD (range) | 2.8 ± 0.8 (2–5) | 3.0 ± 1.3 (1–6) | 3.4 ± 1.6 (0–6) | 3.6 ± 1.5 (0–6) |
| LE stage, median (25th; 75th percentile) | 3 (2; 3) | 3 (2; 3) | 3 (2; 5) | 3 (3; 4.75) |
| Pa | .206 | .02 | .001 | |
| Subgroup: CFA positive before treatment | ||||
| Patients, No. | 14 | 13 | 11 | 13 |
| LE stage, mean ± SD (range) | 2.9 ± 1.2 (2–5) | 3.1 ± 1.4 (2–6) | 3.7 ± 2.0 (0–6) | 3.6 ± 2.0 (0–6) |
| LE stage, median (25th; 75th percentile) | 2.5 (2; 3.5) | 3 (2; 4) | 3 (2; 6) | 3 (2; 6) |
| Pa | .317 | .141 | .168 | |
| Subgroup: CFA negative before treatment | ||||
| Patients, No. | 24 | 23 | 23 | 23 |
| LE stage, mean ± SD (range) | 2.8 ± 0.7 (2–5) | 2.9 ± 1.2 (1–6) | 3.3 ± 1.4 (2–6) | 3.6 ± 1.2 (2–6) |
| LE stage, median (25th; 75th percentile) | 3 (2; 3) | 3 (2; 3) | 3 (2; 3) | 3 (3; 4) |
| Pa | .317 | .046 | .001 |
Abbreviations: CFA, circulating filarial antigen; LE, lymphedema; SD, standard deviation.
a P value for comparison between staging before treatment and at follow-up time point (Wilcoxon signed rank test).
Doxycycline was able to improve conditions in 36.6% of patients and halt progression in 58.5%; in 4.9% the legs became worse (Table 3). This improvement was significantly superior to that seen with amoxicillin or placebo (P < .001). In contrast, only 3.2% of patients treated with amoxicillin showed improvement; 67.7% had a lack of progression and 29% showed deterioration.
Table 3.
Lymphedema Staging 24 Months After Treatment
| Patients, No. (%) |
|||
|---|---|---|---|
| Treatment | Improvement | Lack of Progression | Progression |
| Doxycycline | 15/41 (36.6) | 24/41 (58.5) | 2/41 (4.9) |
| Amoxicillin | 1/31 (3.2) | 21/31 (67.7) | 9/31 (29.0) |
| Placebo | 2/36 (5.6) | 14/36 (38.9) | 20/36 (55.6) |
P < .001a for the comparison of all 3 groups. P < .001a for doxycycline vs amoxicillin and doxycycline vs placebo; P= .042a for amoxicillin vs placebo.
a Fisher's exact test.
To compare the performance of the 2 antibiotics in patients with or without active infection, the treatment arms had been divided into subgroups of CFA-positive and CFA-negative patients. In both subgroups a significant difference in LE stages between the treatment arms could be detected at 24 months (P = .016 and P < .001, respectively; Figures 2B and C). In the CFA-negative subgroup, LE stage amelioration was significantly more frequent in the doxycycline arm than in the amoxicillin (P = .009) and placebo (P = .004) arms after just 12 months, and this trend was confirmed in both subgroups after 24 months. There was no difference between amoxicillin and placebo in either subgroup at 12 and 24 months.
Secondary Outcome Analyses
Acute Attacks
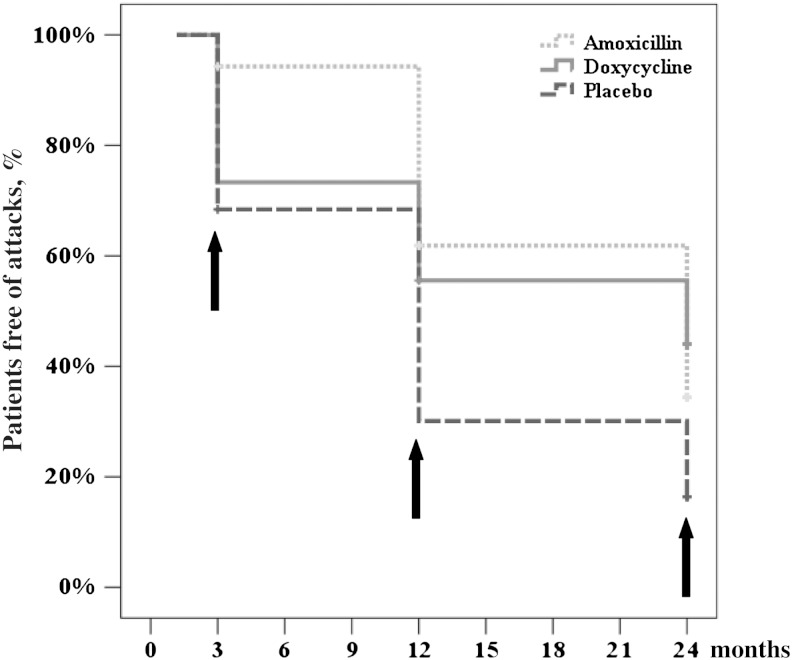
At 3 months, 2 patients in the amoxicillin arm (5.7%) reported acute attacks since treatment end, compared to 12 (26.7%) in the doxycycline (P = .018) and 12 (31.6%) in the placebo arm (P = .007; Figure 3, Supplementary Table 5). No attacks until 24 months were reported by 46.1% in the doxycycline, 32.3% in the amoxicillin, and 16.7% in the placebo arm. With regard to the whole observation period a difference could be verified between the doxycycline and placebo arms (P = .015) and between the amoxicillin and placebo arms (P = .006) but not between the doxycycline and amoxicillin arms.
Figure 3.
Kaplan-Meier curves showing occurrence of acute attacks after treatment end for each treatment arm. Arrows denote follow-up time points. The following significant differences between the 3 treatment arms were detected at 3 months: amoxicillin versus doxycycline (P = .018) and amoxicillin versus placebo (P = .007); at 12 months: doxycycline versus placebo (P = .012) and amoxicillin versus placebo (P = .007); and at 24 months: doxycycline versus placebo (P = .007).
Ultrasound Examinations of the Ankles
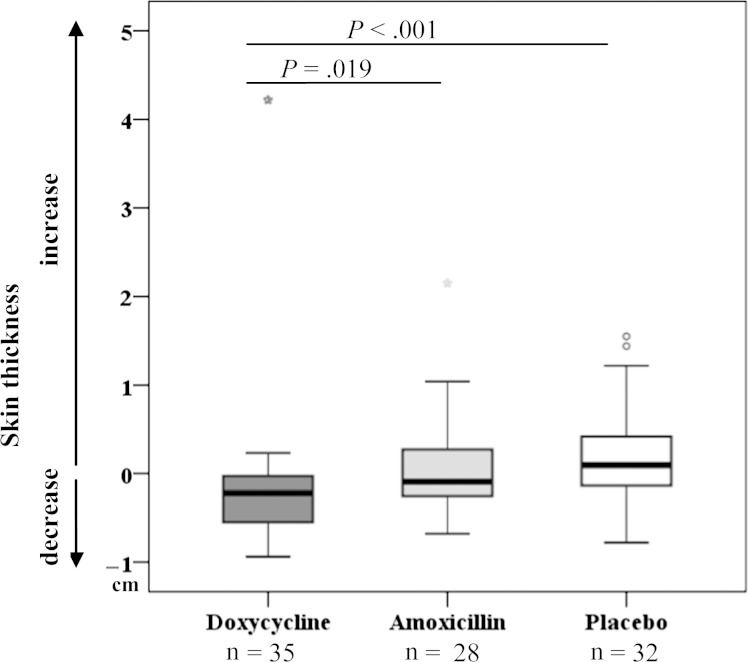
There was a significant decrease in skin thickness in doxycycline-treated patients after 12 and 24 months (P = .008 and P = .001, respectively; Supplementary Table 6) but no change in the amoxicillin- or placebo-treated patients. At 24 months, skin thickness was significantly more reduced in the doxycycline arm than in the amoxicillin arm (P = .019) or the placebo arm (P < .001; Figure 4). The measurement showed a positive correlation with the pretreatment LE severity (r = 0.719; P < .001), and, in contrast to the circumference measurement, it was possible to determine a positive correlation between thickness and LE stage 2 years after treatment (r = 0.555; P < .001).
Figure 4.
Reduction in skin thickness at the ankles, as analyzed by ultrasound. Box plots show differences in skin thickness at the ankles at 24 months compared to pretreatment (P = .001 for overall difference between the 3 treatment arms; Kruskal-Wallis test).
Vascular Endothelial Growth Factors
Regarding soluble VEGFR-3, a significant decrease between pretreatment and 24-month values could be detected in doxycycline- and amoxicillin-treated patients (P = .001 and P = .009, respectively), in contrast to placebo-treated patients. At 24 months, the difference between doxycycline and placebo arms was significant (P = .048), suggesting a superior effect for doxycycline (Supplementary Figure 5). The measurements of VEGF-C showed no significant difference between treatment groups.
Circumference Measurement of Legs
There was no significant difference between the 3 treatment arms regarding the circumference of legs when all patients where analyzed, nor when we limited the analysis to LE stage 2 and 3 (data not shown).
Foot Care and Hygiene Measures
Of 105 patients present at 24 months, 34 showed improved hygiene and only 15 showed worsened hygiene. Most of the patients with improvement were in the placebo group (41.2%), whereas 40% of patients with worsened hygiene were in the amoxicillin group (Supplementary Table 7).
Adverse Events
The total number of adverse events was equally distributed among the treatment arms. No serious adverse events occurred during treatment.
DISCUSSION
The major outcome of this trial is that a 6-week course of doxycycline, in addition to basic morbidity management through hygiene, led to a significant improvement in the severity of LE in both patients with and those without active infection. A similar course of amoxicillin failed to cause such long-term improvement in LE severity, although it did lead to fewer acute attacks and an initial improvement in LE severity, which, however, was not sustained. These findings extend the benefit of doxycycline therapy to the majority of affected patients with LE, independent of their infection status, and offers the opportunity to broaden the scope of care for improved morbidity management in LF.
The improvement in LE severity was observed in patients with LE stage 1–3, but not in those with LE stage 4 or 5, suggesting that doxycycline is primarily active against early LE stages and that its benefits do not extend to stages in which extensive fibrotic tissue growth, skin thickening, and restructuring have already occurred. However, because patients with higher-stage LE were few (stage 4–5) or not included in this study (stage 6–7), additional trials would be needed to extend a benefit of doxycycline to those patients. The improvements observed in patients without active infection suggest that the therapeutic activity of doxycycline is not restricted to its known anti-Wolbachia or macrofilaricidal activities.
The lack of progression of LE and the reduction in acute dermatolymphangioadenitis attacks, especially during the first 3 months after begin of amoxicillin treatment, suggest that there may be 2 distinct benefits from treatment, the first related to reduction in bacterial load achieved by both doxycycline and amoxicillin and the other related to doxycycline's additional nonantibiotic mode of action, which includes the inhibition of inflammation, angiogenesis, proteolysis, and apoptosis [13–18]. Soluble VEGFR-3 is a marker found in a previous study [7] to be elevated in patients with LE compared with microfilaremic asymptomatic patients with LF. Its greater decrease in the doxycycline group, confirming earlier results, underscores the inhibition of angiogenesis by doxycycline. Therefore, should the activity of doxycycline be independent of the antifilarial or antibiotic mode of action, then doxycycline treatment may be further explored in nonfilarial LE. Amoxicillin may be given to halt the progression of LE in patients who cannot take doxycycline, such as pregnant women.
Acute attacks were reduced in all arms as anticipated based on the known effects of basic LE management through hygiene [5]. Over the whole observation period, both amoxicillin and doxycycline groups showed a reduction in the frequency of acute attacks, consistent with the secondary bacterial etiology of acute attacks [2].
Scaling up the implementation of basic LE morbidity management and increasing the scope of care are key priorities for GPELF during the second decade of the program [1]. The addition of doxycycline as a complementary therapy may, through demonstration of improved outcomes, encourage both national and local efforts to implement morbidity management and increase MDA compliance and may also lead to more rapid and sustained improvements in morbidity management. The additional benefits of doxycycline regarding hydrocele [19] and its macrofilaricidal activity in patients with active infection [7, 20, 21] would further enhance its effectiveness in morbidity management. The use of doxycycline in patients with LE would not be subject to the same constraints that prevent its use in MDA, and contraindications in children (usually >9 years old when LE begins) and pregnant women may be manageable in patients with LE. Tolerability and adverse events were the same in all treatment arms, which, together with our experience in all previous trials [7, 19–22], shows that doxycycline is well tolerated and safe.
In conclusion, this trial clearly demonstrates that doxycycline is beneficial in reverting or halting the progression of early stages of filarial LE, regardless of whether there is still active infection. These findings lead us to recommend that individuals with filarial LE stage 1–3 should take a course of doxycycline (200 mg/d) for 6 weeks every other year, or maybe even yearly, and that doxycycline should be considered as a new tool to improve morbidity management of LE.
Supplementary Material
Notes
Acknowledgments. We thank Dr Susanne Deininger and Kathrin Arndts for measuring immunological parameters and Dr Laura Layland for critical reading of the manuscript.
Financial support. This work was supported by the Volkswagen Foundation, Hannover, Germany (grant I/81 306 and I/84 159 “Communicable Diseases in Sub-Saharan Africa: From the African Bench to the Field”) and as part of the A-WOL consortium by the Liverpool School of Tropical Medicine, through a grant from the Bill and Melinda Gates Foundation (grant 39284).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. GPELF progress report 2000-2009 and strategic plan 2010–2020. Available at: http://www.who.int/neglected_diseases/integrated_media/integrated_media_urogenital_lf_2011/en/index.html. Accessed 23 May 2012. [Google Scholar]
- 2.Dreyer G, Medeiros Z, Netto MJ, Leal NC, de Castro LG, Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans R Soc Trop Med Hyg. 1999;93:413–7. doi: 10.1016/s0035-9203(99)90140-2. [DOI] [PubMed] [Google Scholar]
- 3.Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi: 10.1186/1475-2883-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addiss D. The 6th Meeting of the Global Alliance to Eliminate Lymphatic Filariasis: a half-time review of lymphatic filariasis elimination and its integration with the control of other neglected tropical diseases. Parasit Vectors. 2010;3:100. doi: 10.1186/1756-3305-3-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyer G, Addiss D, Dreyer P, Noroes J. Basic lymphoedema management: treatment and prevention of problems associated with lymphatic filariasis. Hollis, NH: Hollis Publishing; 2002. [Google Scholar]
- 6.Dreyer G, Noroes J, Amaral F, et al. Direct assessment of the adulticidal efficacy of a single dose of ivermectin in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1995;89:441–3. doi: 10.1016/0035-9203(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 7.Debrah AY, Mand S, Specht S, et al. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2:e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunyo SK, Nkrumah FK, Simonsen PE. Single-dose treatment of Wuchereria bancrofti infections with ivermectin and albendazole alone or in combination: evaluation of the potential for control at 12 months after treatment. Trans R Soc Trop Med Hyg. 2000;94:437–43. doi: 10.1016/s0035-9203(00)90135-4. [DOI] [PubMed] [Google Scholar]
- 9.Gbakima AA, Appawu MA, Dadzie S, et al. Lymphatic filariasis in Ghana: establishing the potential for an urban cycle of transmission. Trop Med Int Health. 2005;10:387–92. doi: 10.1111/j.1365-3156.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy RK, Kumaraswami V, Suma TK, Rajan K, Radhakuttyamma G. A double-blind, placebo-controlled study of the efficacy of oral penicillin, diethylcarbamazine or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis. Ann Trop Med Parasitol. 1999;93:367–77. doi: 10.1080/00034989958366. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Lymphatic Filariasis: the disease and its control. Tech Rep Ser. 1992 821:1–71. [PubMed] [Google Scholar]
- 12.Mand S, Marfo-Debrekyei Y, Dittrich M, Fischer K, Adjei O, Hoerauf A. Animated documentation of the filaria dance sign (FDS) in bancroftian filariasis. Filaria J. 2003;2:3. doi: 10.1186/1475-2883-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130–45. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Soory M. A role for non-antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: a literature review. Open Dent J. 2008;2:5–12. doi: 10.2174/1874210600802010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fainaru O, Adini I, Benny O, et al. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22:3728–35. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 17.Fainaru O, Hornstein MD, Folkman J. Doxycycline inhibits vascular leakage and prevents ovarian hyperstimulation syndrome in a murine model. Fertil Steril. 2009;92:1701–5. doi: 10.1016/j.fertnstert.2008.08.129. [DOI] [PubMed] [Google Scholar]
- 18.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 19.Debrah AY, Mand S, Marfo-Debrekyei Y, et al. Reduction in levels of plasma vascular endothelial growth factor-A and improvement in hydrocele patients by targeting endosymbiotic Wolbachia sp. in Wuchereria bancrofti with doxycycline. Am J Trop Med Hyg. 2009;80:956–63. [PubMed] [Google Scholar]
- 20.Mand S, Pfarr K, Sahoo PK, et al. Macrofilaricidal activity and amelioration of lymphatic pathology in bancroftian filariasis after 3 weeks of doxycycline followed by single-dose diethylcarbamazine. Am J Trop Med Hyg. 2009;81:702–11. doi: 10.4269/ajtmh.2009.09-0155. [DOI] [PubMed] [Google Scholar]
- 21.Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet. 2005;365:2116–21. doi: 10.1016/S0140-6736(05)66591-9. [DOI] [PubMed] [Google Scholar]
- 22.Hoerauf A, Volkmann L, Hamelmann C, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–3. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.