Abstract
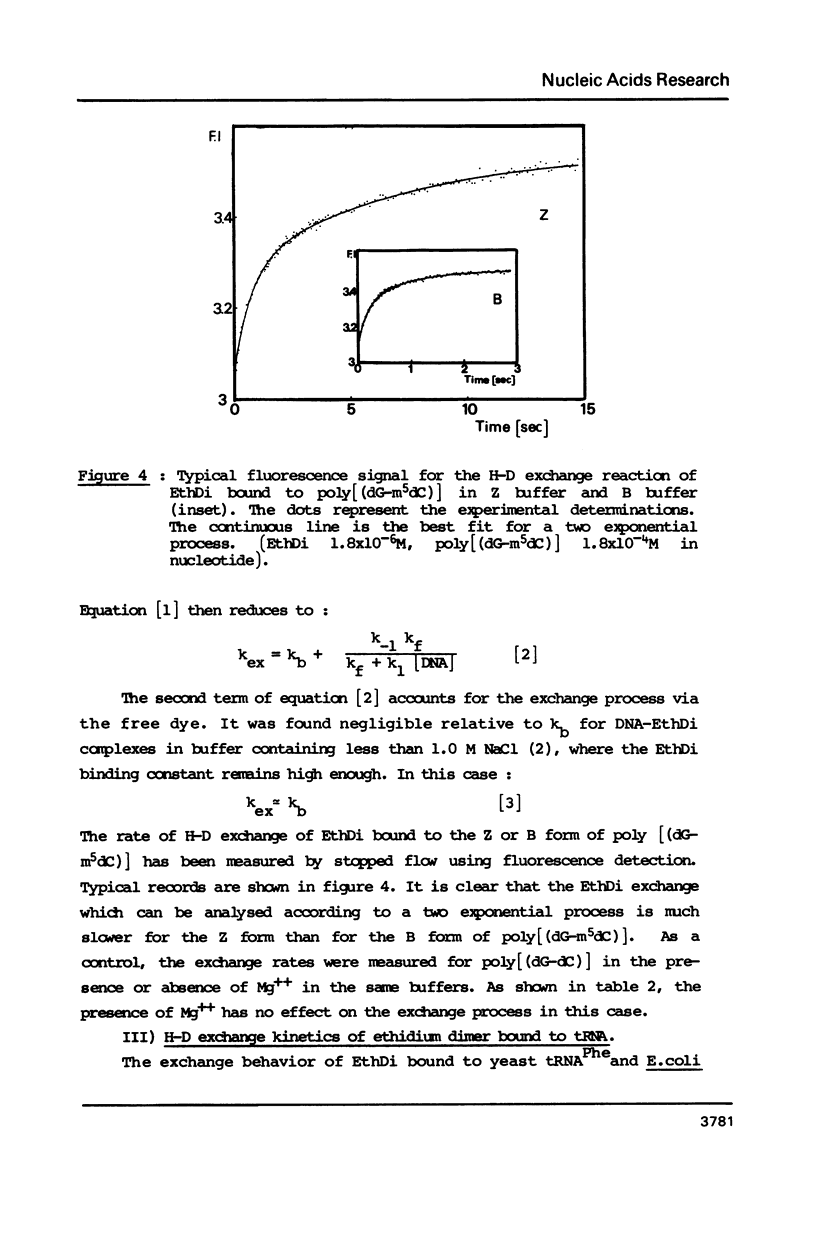
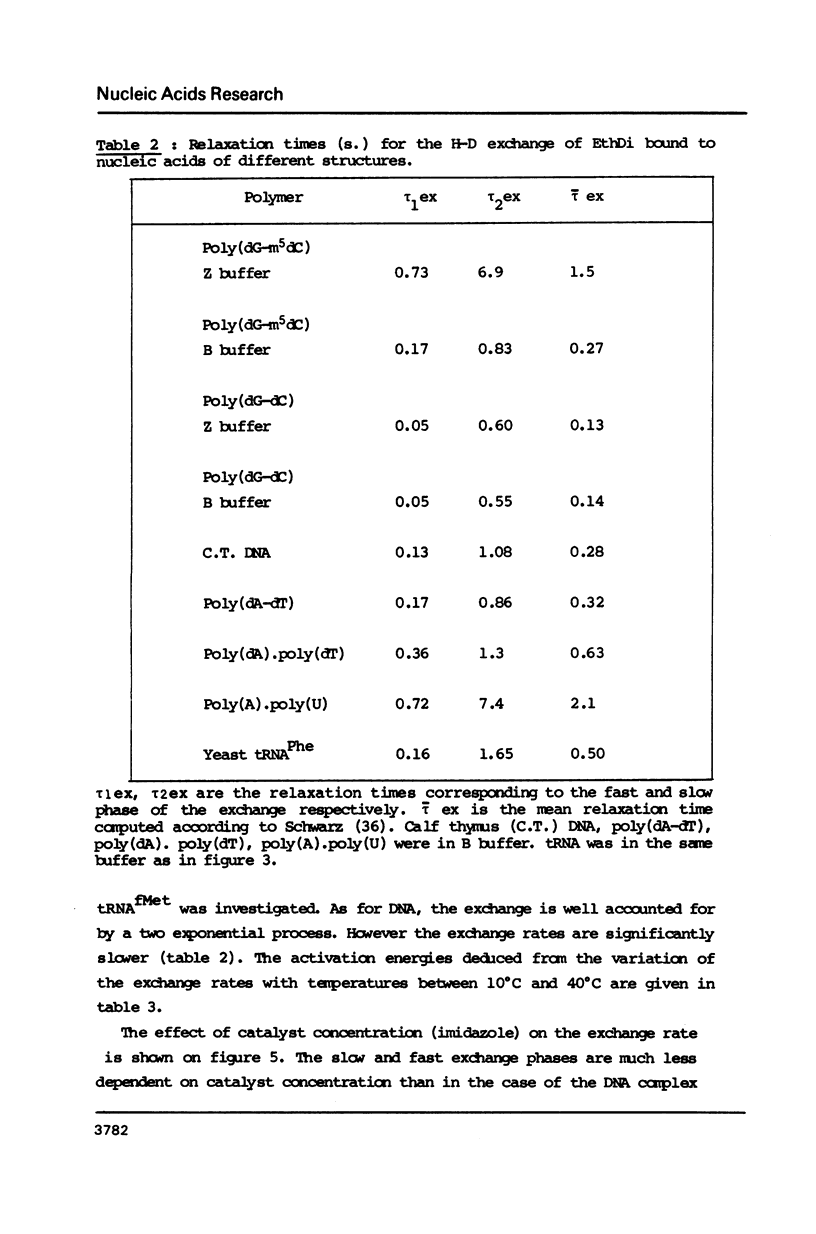
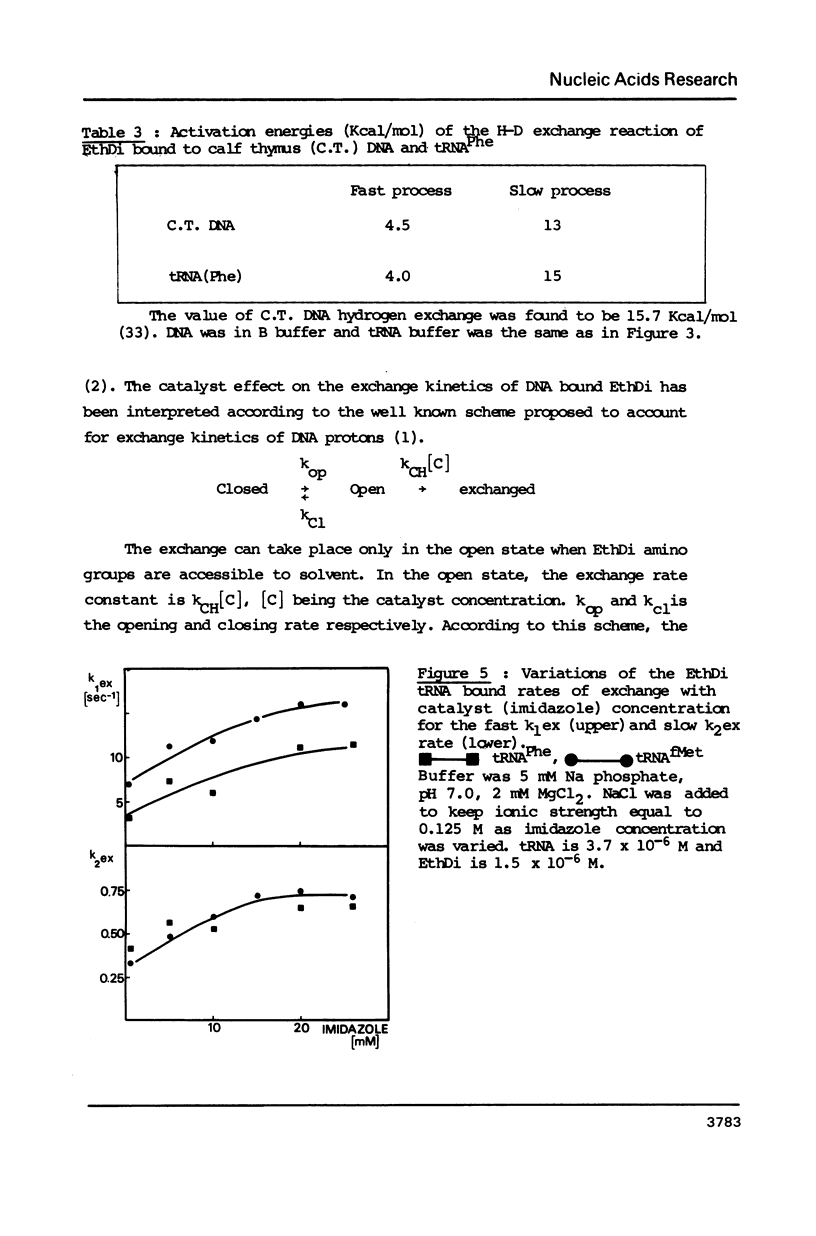
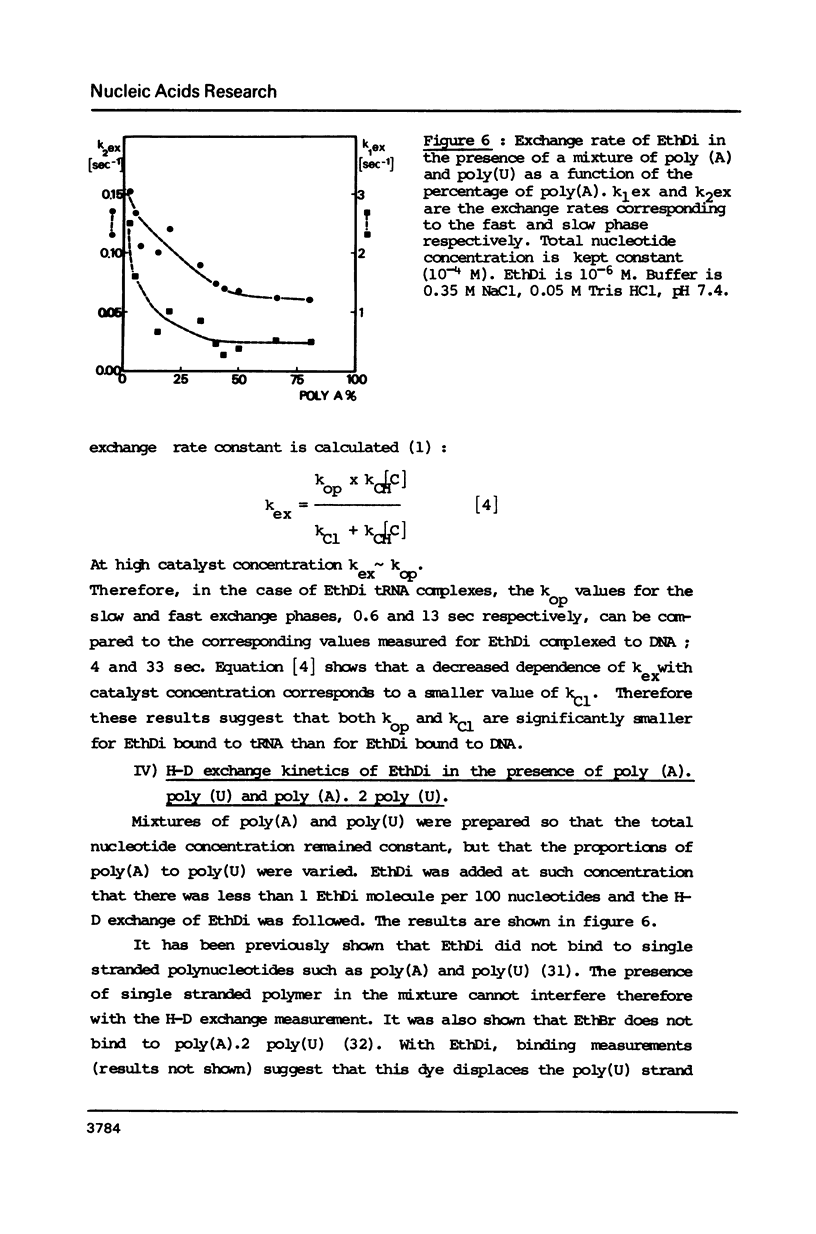
Ethidium dimer is shown to bind by intercalation, almost equally well, to the B and Z form of poly[(dG-m5dC)].poly[(dG-m5dC)], whereas the ethidium monomer shows a strong preference for the B form. The hydrogen-deuterium (H-D) exchange kinetics of the ethidium dimer bound to the B and Z form of poly [(dG-m5dC)].poly[(dG-m5dC)] could then be compared. The kinetics of the H-D exchange were strikingly slower when the dye was bound to Z DNA as compared to B DNA. The exchange kinetics were also modified when ethidium dimer was bound to tRNA and to a triple stranded structure. It is proposed that a dynamic fluctuation at the level of the nucleic acid could modulate the dynamic fluctuation at the level of the bound ligand.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delbarre A., Gourevitch M. I., Gaugain B., Le Pecq J. B., Roques B. P. 1H NMR study of an ethidium dimer poly(dA-dT) complex: evidence of a transition between bis and monointercalation. Nucleic Acids Res. 1983 Jul 11;11(13):4467–4482. doi: 10.1093/nar/11.13.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagravère C., Malfoy B., Leng M., Laval J. Ring-opened alkylated guanine is not repaired in Z-DNA. 1984 Aug 30-Sep 5Nature. 310(5980):798–800. doi: 10.1038/310798a0. [DOI] [PubMed] [Google Scholar]
- Markovits J., Ramstein J., Roques B. P., Le Pecq J. B. Dynamic structure of DNA complexes. Fluorometric measurement of hydrogen-deuterium exchange kinetics of dna-bound ethidium dimer and acridine-ethidium dimer. Biochemistry. 1983 Jun 21;22(13):3231–3237. doi: 10.1021/bi00282a030. [DOI] [PubMed] [Google Scholar]
- Markovits J., Roques B. P., Le Pecq J. B. Ethidium dimer: a new reagent for the fluorimetric determination of nucleic acids. Anal Biochem. 1979 Apr 15;94(2):259–264. doi: 10.1016/0003-2697(79)90357-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Pardi A., Morden K. M., Patel D. J., Tinoco I., Jr Kinetics for exchange of the imino protons of the d(C-G-C-G-A-A-T-T-C-G-C-G) double helix in complexes with the antibiotics netropsin and/or actinomycin. Biochemistry. 1983 Mar 1;22(5):1107–1113. doi: 10.1021/bi00274a018. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstein J., Buckingham R. H. Tritium exchange on transfer RNA: slowly exchanging protons sensitive to a change in the dihydrouridine stem. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1567–1571. doi: 10.1073/pnas.78.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Reinhardt C. G., Roques B. P., Le Pecq J. B. Binding of bifunctional ethidium intercalators to transfer RNA. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1376–1385. doi: 10.1016/0006-291x(82)91402-4. [DOI] [PubMed] [Google Scholar]
- Shafer R. H., Brown S. C., Delbarre A., Wade D. Binding of ethidium and bis(methidium)spermine to Z DNA by intercalation. Nucleic Acids Res. 1984 Jun 11;12(11):4679–4690. doi: 10.1093/nar/12.11.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P., Paoletti J., Le Pecq J. B. Decay of fluorescence emission anisotropy of the ethidium bromide-DNA complex. Evidence for an internal motion in DNA. Proc Natl Acad Sci U S A. 1970 Feb;65(2):417–421. doi: 10.1073/pnas.65.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



