Summary
Levels of HIV-1 RNA in endocervical specimens fluctuate with the menstrual cycle, suggesting that cell-free HIV-1 levels may vary during the cycle, which could influence infectivity. Here, we examined daily changes in endocervical HIV-1–infected cells during 1 cycle. There were significant positive associations between the number of days from the luteinizing hormone surge and the number of HIV-1 DNA copies/swab (P = 0.001) and the number of total cells/swab (P < 0.001) in endocervical specimens. These data suggest that sampling of cell-associated endocervical HIV-1 increases after the periovulatory period, which could result in increased exposure to HIV-1–infected cells during sexual contact.
Keywords: cervical, genital shedding, HIV-1, HIV-1 DNA, hormones, infectivity
Hormonal contraceptives have been implicated as cofactors for HIV-1 acquisition and genital virus shedding.1 Recently, we reported cyclic fluctuations in HIV-1 RNA levels in endocervical specimens, implying that normal hormonal fluctuations during the menstrual cycle have an impact on genital virus sampling, shedding, or both.2 In this study, HIV-1 RNA levels were significantly associated with the number of days from the midcycle luteinizing hormone (LH) surge and were minimal at midcycle and increased during the luteal phase. Similar patterns of HIV-1 RNA shedding in the female genital compartment have been reported,3,4 although others have found no association.5,6 It is unclear whether increased HIV-1 RNA levels reflect changes in the number of infected cells or increased viral expression in some infected cells. This distinction may be relevant to the risk of infectivity, given recent data suggesting a stronger association for HIV-1–infected cell number than HIV-1 RNA level in the context of vertical transmission.7 Infected cells may also play a critical role in sexual transmission of HIV-1, although this relation has not yet been examined. One previous analysis found no association between the menstrual cycle and HIV-1 DNA detection;8 however, to date, there has not been a quantitative analysis of changes in HIV-1–infected cell numbers during the menstrual cycle. Here, we examined the relation between the menstrual cycle and daily changes in the number of HIV-1–infected cells in endocervical secretions.
METHODS
Laboratory Analysis
The endocervical specimens used in this study are the same as those used previously for analysis of daily HIV-1 RNA levels.2 Specimens were provided each day by 17 women during 1 menstrual cycle.8 This cohort was restricted to women who had not used hormonal contraceptives during the 6 months before the start of the study. Specimens were provided from the first day of menses through the day before the start of the next menses; the median cycle length was 28 days (range: 22 to 37 days). The median age for the study participants was 31 years (range: 25 to 43 years). Urine was analyzed daily, beginning from days 8 to 10 of each participant’s cycle, to ascertain the midcycle LH surge. At each daily visit, participants were asked to report douching and sexual activity during the previous 24 hours. Douching with soap or detergent was reported by 5 women at a median frequency of 0% of daily visits (range: 0% to 31%).2 Unprotected sex was reported by 14 women at a median frequency of 10% of daily visits (range: 0% to 33%). At enrollment and at weekly screening visits, participants were screened and treated for sexually transmitted diseases and other conditions of the genital tract, as described previously.8 At enrollment three participants were diagnosed with Trichomonas vaginalis, 1 participant with Neisseria gonorrhoeae, and 1 participant with syphilis; no participants were diagnosed with Chlamydia trachomatis. At weekly screening visits, Candida species were detected at least once in 8 participants, bacterial vaginosis was detected at least once in 13 participants, and 5 participants had a vaginal polymorphonuclear leukocyte (PMNL) count > 5/1000 × field at least once.2,8 Four participants had a cervical PMNL count > 30/1000 × field at least once.2 The overall median serum HIV-1 RNA level was 26,690 copies/mL (range: 17–305,400 copies/mL). Informed consent was obtained from study participants, and Human Subjects Review Committees at the Fred Hutchinson Cancer Research Center, the University of Washington, and the University of Nairobi approved the research protocol.
Endocervical specimens had been stored in liquid nitrogen between our analyses of HIV-1 RNA2 and DNA. Recovery of HIV-1 proviruses after a freeze-thaw cycle was validated (data not shown) using mock genital samples consisting of mixtures of uninfected (CEMX174) and HIV-1 infected (ACH2) cells pipetted onto a Dacron swab and stored as described.2 For the current study, up to 400 µL (two fifths) of sample volume was subjected to DNA purification using a QiaAmp 96 DNA Blood Kit (Qiagen, Valencia, CA). DNA was eluted in 100 µL of water, and 10 µL of this eluate was used for each HIV-1 real-time polymerase chain reaction (PCR). Genomic DNA was extracted from low-copy (consisting of mixtures of uninfected [HeLa] and HIV-1–infected [ACH2] cells) and negative (consisting of uninfected cells alone) mock genital swabs in each batch of DNA extractions for actual endocervical specimens to control for HIV-1 low copy recovery and contamination, respectively, as described.9 Mock swab and endocervical specimen DNA were also subjected to real-time PCR in the same batch.
The total number of cells in 451 endocervical specimens was quantified using β-actin Detection Reagents (Applied Biosystems, Foster City, CA) as described.10 Levels of HIV-1 DNA, as a marker for HIV-1 infected cells, were quantified in each specimen using a real-time HIV-1 PCR assay,10 with minor changes in reaction conditions.9 This assay is linear at copy levels between 1 and 100.9 The sequence specificity of the HIV- 1 primers and probe for detection of the HIV-1 strains present in this cohort was confirmed by sequencing the region of pol that is amplified by the assay in genital HIV-1 proviruses isolated from a subset of study participants (n = 7, data not shown).
An HIV-1 pol real-time reaction was considered positive if the assay readout was ≥1.0 or if the assay readout was between 0.5 and 1.0 and the specific PCR product could be visualized on a gel. All other reactions were considered negative. Samples were tested in duplicate and were considered acceptable if the values obtained for the 2 reactions were within 5-fold of each another. Samples for which discordant results were obtained were retested. To identify these samples, negative reactions were assigned a value of 0.5. Of 33 discordant reaction pairs, 5 were discordant after repeat testing, and in these cases, all 4 PCR assays of that sample were used to calculate HIV-1 DNA copies.
Endocervical specimens were defined as positive if at least 1 HIV-1 pol real-time PCR was positive. To increase the level of cell sampling in specimens in which HIV-1 proviruses were not detected in either of the duplicate PCR assays, negative specimens were subjected to 2 additional PCR assays. Based on this criterion, 158 specimens were retested, and 40 were positive after subsequent PCR assays. The ratio of HIV-1–infected cells to uninfected cells was calculated by summing the total of HIV-1 proviral copies (rounded to the nearest whole integer) in each acceptable PCR assay performed and dividing this sum by the total number of cellular genomes sampled in all acceptable PCR assays performed per sample. If no HIV-1 proviral copies were detected, the sum was assigned a level of 0.5. These ratios were expressed as the number of HIV-1–infected cells per 106 cells. Thus, for calculation of these ratios, we assumed that each provirus resided in a different cell.
HIV-1 pol reactions for some specimens were spiked with a low copy internal positive control template.11 For 1 of 366 specimens, this control failed to amplify in at least 2 reactions. This specimen was omitted, leaving 450 observations.
Statistical Methods
The correlation between frequency of detection of HIV-1 DNA and the median log10 HIV-1 RNA level was assessed using the Spearman rank correlation coefficient. Generalized estimating equations (STATA, version 7.0; Stata Corp., College Station, TX) with a Gaussian link were used to estimate all other associations, and values for missing observations were interpolated, as described previously.2 Data from all specimens (with the exception of 1 specimen in which the positive control PCR failed) collected over the duration of the study were used in statistical analyses, although some of these observations are not presented in Figure 1 because of the availability of fewer than 14 specimens for certain days. Statistical analyses were performed using actual log10 values for HIV-1 DNA copies and total cells/swab, HIV-1 DNA copies/106 cells, and HIV-1 RNA copies/swab (shown in Fig. 1). Figure 2 presents the change in each of these parameters from the level present on day 0 so to allow normalization of HIV-1 DNA and HIV-1 RNA levels across participants (these parameters varied by as many as 3 orders of magnitude between individuals; Table 1). To address whether the relation between the number of days from the LH surge and HIV-1 DNA copies/swab or HIV-1 DNA copies/106 cells was influenced by low cell recovery, and thus low recovery of HIV-1 DNA copies, we performed additional analyses in which we restricted each model to include only observations for specimens with more than 10,000 cells. These analyses gave similar results. We also included the total number of cells/swab in each final model that included HIV-1 DNA copies/swab or HIV-1 DNA copies/106 cells as outcomes.
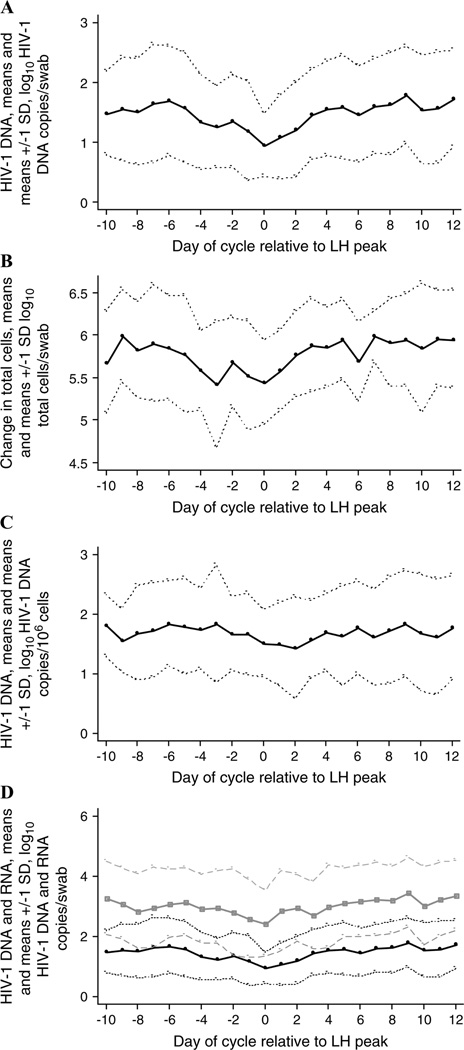
FIGURE 1.
Shedding of HIV-1 DNA and total cells in endocervical secretions during the menstrual cycle. The log10 HIV-1 DNA level, log10 cell number, or log10 RNA level is presented. Data are summarized as daily means (solid lines) and means ± 1 SD (dotted lines for HIV-1 DNA and cell number; dashed lines for HIV-1 RNA) for the 16 participants who exhibited an LH surge.8 Only days on which there were at least 14 observations8 are shown. A, Log10 HIV-1 DNA copies/swab. B, Log10 total cell number/swab. C, Log10 HIV-1 DNA copies/106 cells. D, Log10 HIV-1 DNA copies/swab (black lines) and log10 HIV-1 RNA level/swab (gray lines).
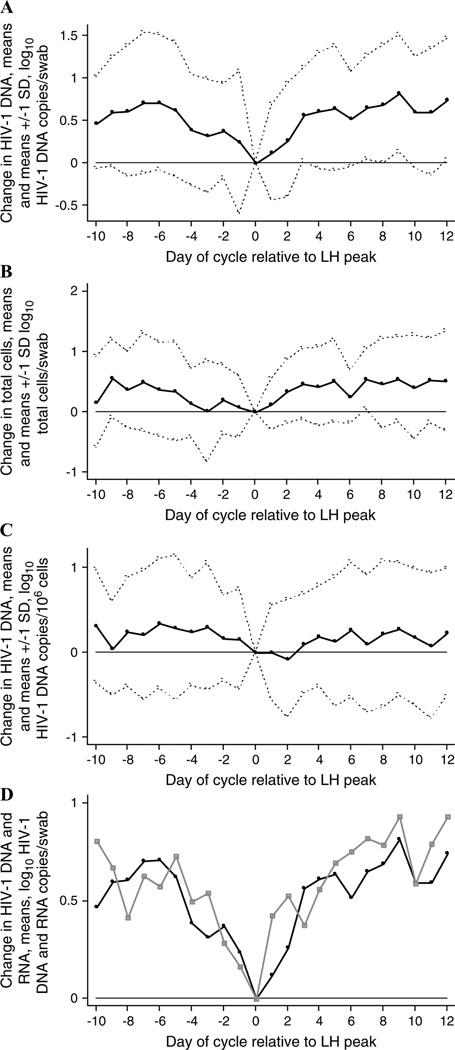
FIGURE 2.
Change in HIV-1 DNA and total cells in endocervical secretions during the menstrual cycle. The change in log10 HIV-1 DNA level, log10 cell number, or log10 RNA level from the level present on the day of the LH surge (day 0) is presented. Data are summarized as daily means (solid lines, A–D) and means ± 1 SD (dotted lines, A–C) for the 16 participants who exhibited an LH surge.8 Only days on which there were at least 14 observations8 are shown. A, Change in log10 HIV-1 DNA copies/swab. B, Change in log10 total cell number/swab. C, Change in log10 HIV-1 DNA copies/106 cells. D, Change in log10 HIV-1 DNA copies/swab (black line) and change in log10 HIV-1 RNA level/swab (gray line).
TABLE 1.
Summary of Endocervical HIV-1 DNA Data for the 17 Participants
| Participant* | Median Endocervical HIV-1 RNA Load Copies/Swab |
Median Endocervical HIV-1 DNA Load Copies/Swab |
Endocervical Samples Positive for HIV-1 DNA (%) |
|---|---|---|---|
| 1 | 243 | <6 | 28 |
| 2 | 15,332 | 331 | 96 |
| 3 | <18 | <6 | 21 |
| 4 | 395 | <6 | 23 |
| 5 | 388 | 10 | 44 |
| 6 | 160 | 6 | 52 |
| 7 | <18 | <6 | 24 |
| 8 | 22,218 | 75 | 93 |
| 9 | 4333 | 25 | 83 |
| 10 | 2110 | 175 | 100 |
| 11 | 4559 | 69 | 88 |
| 12 | 268 | 38 | 83 |
| 13 | 3582 | 254 | 97 |
| 14 | 3578 | 75 | 96 |
| 15 | 23,238 | 513 | 97 |
| 16 | 71,098 | 125 | 96 |
| 17 | 141,445 | 2150 | 100 |
| Overall | 69 | 88 |
Participants are listed in the same order as has been published previously.8
RESULTS
The number of total cells/swab, as indicated by copies of β-actin, was variable (median = 912,011 cells/swab, range: 2512 to 10,000,000 cells/swab). The median number of cells in endocervical swabs that was cumulatively tested for HIV-1 DNA was 85,967 (range: 199 to 808,841 cells/swab). HIV-1 DNA was detected in 326 (72%) of 450 samples. Within participants, the frequency of detection of HIV-1 DNA in endocervical samples ranged from 21% to 100%, with an overall median detection frequency within women of 88% (see Table 1). The overall median number of HIV-1 DNA copies/swab was 69 (range: < 6 to 2150 HIV-1 DNA copies/swab). Participants with higher median endocervical HIV-1 RNA levels had higher frequencies of HIV-1 DNA detection (r = 0.96; P < 0.0001).
HIV-1 DNA copies in endocervical swabs were variable over the menstrual cycle, with the minimum average log10 HIV-1 DNA copies/swab occurring at midcycle (see Figs. 1A, 2A). Consistent with this observation, there was a significant positive association between the number of days from the LH surge and log10 HIV-1 DNA copies/swab (regression coefficient [RC] = 0.04, 95% confidence interval [CI]: 0.01 to 0.06; P = 0.001).
Specimens with higher numbers of total cells possessed higher numbers of HIV-1 DNA: there was a significant positive association between the log10 number of total cells/swab and the log10 number of HIV-1 DNA copies/swab (RC = 0.48, 95% CI: 0.35 to 0.62; P < 0.001). The number of total cells/swab also seemed to decline at midcycle (see Figs. 1B, 2B); thus, we examined whether there was a relation between the number of days from the LH surge and the total number of cells/swab. We found a significant positive association between the number of days from the LH surge and the log10 number of total cells/swab (RC = 0.03, 95% CI: 0.01 to 0.05; P < 0.001).
To determine whether the number of days from the LH surge was associated with the number of endocervical HIV-1 DNA copies beyond what might be attributable to changes in the number of cells shed in endocervical secretions, we examined the ratios of HIV-1 DNA copies to total cells. When plotted by day of the menstrual cycle, the mean number of log10 HIV-1 DNA copies/106 cells was also lower at midcycle (see Figs. 1C, 2C).
We tested whether the number of days from the LH surge was associated with the number of HIV-1 DNA copies/106 cells. We included log10 total cells/swab as a covariate in each model that evaluated HIV-1 DNA copies/106 cells as an outcome, because we observed a significant negative association between log10 total cells/swab and log10 HIV-1 DNA copies/106 cells (data not shown) in univariate analysis. This negative association may have been attributable to potentially inflated values for the number of HIV-1 DNA copies/106 cells for specimens in which few total cells were present, and the HIV-1 DNA copy number was assigned a value of 0.5 because of lack of detection. In multivariate analyses, there was a significant positive association between the number of days from the LH surge and log10 HIV-1 DNA copies/106 cells, after adjusting for log10 total cells/swab (RC = 0.02, 95% CI: 0.0001 to 0.04; P = 0.05). Similarly, the number of days from the LH surge was significantly associated with log10 HIV-1 DNA copies/swab, adjusted for log10 total cells/swab (RC = 0.02, 95% CI: 0.001 to 0.04; P = 0.05).
The pattern of HIV-1 DNA and total cell fluctuations in endocervical specimens observed here is similar to the pattern of cell-free and cell-associated HIV-1 RNA changes we reported previously,2 in that there is a minimal level at midcycle (see Figs. 1D, 2D). We examined whether HIV-1 DNA copies or total cell numbers in endocervical secretions predicted HIV-1 RNA levels in this compartment. In a multivariate model, there was a significant positive association (RC = 0.73, 95% CI: 0.47 to 0.99; P < 0.001) between log10 HIV-1 DNA copies/swab and log10 HIV-1 RNA levels/swab. By contrast, changes in total cells/swab did not account for fluctuations in HIV-1 RNA levels, because log10 total cells/swab was not a significant independent predictor of HIV-1 RNA in this model.
Changes in HIV-1 DNA copies did not fully explain the relation between the number of days from the LH surge and log10 HIV-1 RNA levels.2 In a multivariate model that included log10 HIV-1 DNA copies/swab, log10 total cells/swab, and all covariates that were considered in our previous analysis of HIV-1 RNA shedding (serum HIV-1 RNA level, presence of menses, unprotected sex in the previous 24 hours, douching in the previous 24 hours, and number of days from the LH surge), there was a significant positive relation between the number of days from the LH surge and the log10 level of endocervical HIV-1 RNA (RC = 0.02, 95% CI: 0.002 to 0.05; P = 0.03). In this model, the positive relation between log10 HIV-1 DNA copies/swab and log10 HIV-1 RNA copies/swab remained highly significant (RC = 0.55, 95% CI: 0.40 to 0.70; P < 0.001).
DISCUSSION
This study is the first quantitative analysis of endocervical HIV-1–infected cell levels during the menstrual cycle. We found that the number of days from the LH surge was significantly associated with the number of HIV-1–infected cells, as quantified by HIV-1 DNA copies, suggesting that HIV-1–infected cell numbers in endocervical specimens are low at midcycle. We observed a similar association between the LH surge and the total cell number collected per swab specimen. Together, these results suggest that increases in the number of HIV-1–infected cells after the LH surge are attributable to increased levels of total cells, including a higher proportion that are HIV-1–positive.
Cyclic changes in the endocervical epithelium may explain these data. Endocervical secretions can increase in level at midcycle and become thicker and more scant during the luteal phase.12,13 These changes could potentially lead to a “washout” of cellular debris, resulting in a decreased number or concentration of cells in endocervical secretions that coincides with the periovulatory period, an increase in sampling of cells on the swab during the luteal phase, or both.
We adjusted the number of HIV-1–infected cells/swab for total cells/swab. Still, we observed a significant positive association between the number of days from the LH surge and HIV-1–infected cells. We cannot rule out the possibility that hormonal changes during the menstrual cycle have an impact on target cell availability or susceptibility to infection. These issues could only be addressed by typing the cell populations in endocervical specimens, which was not possible using the frozen swabs collected for this study. Nevertheless, the magnitude of the change in HIV-1 DNA copies from midcycle to the late luteal phase was subtle after adjusting for total cell number, implying that these effects are perhaps modest.
A previous qualitative analysis of HIV-1–infected cell shedding in the same cohort examined here did not find a significant relation between the menstrual cycle and detection of endocervical HIV-1–infected cells.8 This discrepancy is likely attributable to a lack of quantitative data on HIV-1 DNA copy number and a low frequency of HIV-1 DNA detection (46% vs. 72%), which presumably resulted from the limit of detection of the assay used to detect HIV-1 DNA (250 copies/swab vs. 6 copies/swab in this study). Indeed, the overall median number of HIV-1 DNA copies/swab was 69 in the current study, consistent with the lower frequency of detection observed previously.
Endocervical HIV-1–infected cell and total cell sampling during the menstrual cycle follows a similar pattern as that previously observed for endocervical HIV-1 RNA levels. Here, we found that fluctuations in HIV-1 RNA could be partly explained by changes in HIV-1–infected cell numbers. Our analyses imply that increased recovery of HIV-1–infected cells, in part, is correlated with increased levels of HIV-1 RNA in endocervical secretions after the LH surge. The significance of this association is unclear, however, because our methods for sampling HIV-1–infected cells and HIV-1 RNA did not allow us to distinguish between actively or latently infected cells or between cell-free and cell-associated HIV-1 RNA. We cannot rule out the possibility that changes in HIV-1–infected cells and RNA levels are attributable to cyclic changes in the level of endocervical secretions during the menstrual cycle.
In summary, we observed lower numbers of HIV-1 DNA copies during the periovulatory period and increased numbers of DNA copies after the LH surge. An increased number of HIV-1 DNA copies are likely a result of fluctuations in the total number of cells recovered in endocervical secretions. In turn, the increase in the number of HIV-1–infected cell levels after the LH surge partly, but not entirely, contributed to fluctuations in HIV-1 RNA sampling. Regardless of the determinants of HIV-1 RNA or infected cell fluctuations during the menstrual cycle, the data presented here suggest that virus levels, including cell-free and cell-associated virus and, by extension, infectivity,14 may increase as menses is approached.
ACKNOWLEDGMENTS
The authors thank S. Holte for advice and helpful discussions; C. Rousseau for establishing the HIV-1 pol real-time PCR assay; B. Chohan and S. Emery for technical advice and assistance; the research staff at Coast Provincial General Hospital and the Ganjoni Clinic in Mombasa, Kenya; and J. Bwayo, J. Ndinya-Achola, and the Nairobi HIV/Sexually Transmitted Diseases Research Project for the continued collaborations and interactions that make this research possible. They are also indebted to the women who contributed their time and effort by participating in this research.
Supported by National Institutes of Health (NIH) grants AI38518 and AI39996, NIH predoctoral fellowship GM62819-02 (S. Benki), and the Poncin Scholarship Fund (S. Benki).
REFERENCES
- 1.Benki S, McClelland RS, Overbaugh J. Risk factors for human immunodeficiency virus type-1 acquisition in women in Africa. J Neurovirol. 2005;11(Suppl 1):58–65. [PubMed] [Google Scholar]
- 2.Benki S, Mostad SB, Richardson BA, et al. Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J Infect Dis. 2004;189:2192–2201. doi: 10.1086/421298. [DOI] [PubMed] [Google Scholar]
- 3.Reichelderfer PS, Kovacs A, Wright DJ, et al. The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis. 2002;186:726–728. doi: 10.1086/342051. [DOI] [PubMed] [Google Scholar]
- 4.Money DM, Arikan YY, Remple V, et al. Genital tract and plasma human immunodeficiency virus viral load throughout the menstrual cycle in women who are infected with ovulatory human immunodeficiency virus. Am J Obstet Gynecol. 2003;188:122–128. doi: 10.1067/mob.2003.65. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva JM, Ellerbrock TV, Lennox JL, et al. The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis. 2002;185:170–177. doi: 10.1086/338447. [DOI] [PubMed] [Google Scholar]
- 6.Goulston C, Stevens E, Gallo D, et al. Human immunodeficiency virus in plasma and genital secretions during the menstrual cycle. J Infect Dis. 1996;174:858–861. doi: 10.1093/infdis/174.4.858. [DOI] [PubMed] [Google Scholar]
- 7.Tuomala RE, O’Driscoll PT, Bremer JW, et al. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J Infect Dis. 2003;187:375–384. doi: 10.1086/367706. [DOI] [PubMed] [Google Scholar]
- 8.Mostad SB, Jackson S, Overbaugh J, et al. Cervical and vaginal shedding of human immunodeficiency virus type 1- infected cells throughout the menstrual cycle. J Infect Dis. 1998;178:983–991. doi: 10.1086/515665. [DOI] [PubMed] [Google Scholar]
- 9.Benki S, McClelland RS, Emery S, et al. Quantification of genital human immunodeficiency virus type 1 (HIV-1) DNA in specimens from women with low plasma HIV-1 RNA levels typical of HIV-1 nontransmitters. J Clin Microbiol. 2006;44:4357–4362. doi: 10.1128/JCM.01481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 12.Ferin M, Jewelewicz R, Warren M. The Menstrual Cycle: Physiology, Reproductive Disorders, and Infertility. New York: Oxford University Press; 1993. pp. 67–68. [Google Scholar]
- 13.Ferenczy A, Wright TC. Anatomy and histology of the cervix. In: Kurman R, editor. Blaustein’s Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag; 2002. pp. 185–201. [Google Scholar]
- 14.Baeten JM, Overbaugh J. Measuring the infectiousness of persons with HIV-1: opportunities for preventing sexual HIV-1 transmission. Current HIV Research. 2003;1:69–86. doi: 10.2174/1570162033352110. [DOI] [PubMed] [Google Scholar]