Abstract
The appearance of heme, an organic ring surrounding an iron atom, in evolution forever changed the efficiency with which organisms were able to generate energy, utilize gasses and catalyze numerous reactions. Because of this, heme has become a near ubiquitous compound among living organisms. In this review we have attempted to assess the current state of heme synthesis and trafficking with a goal of identifying crucial missing information, and propose hypotheses related to trafficking that may generate discussion and research. The possibilities of spatially organized supramolecular enzyme complexes and organelle structures that facilitate efficient heme synthesis and subsequent trafficking are discussed and evaluated. Recently identified players in heme transport and trafficking are reviewed and placed in an organismal context. Additionally, older, well established data are reexamined in light of more recent studies on cellular organization and data available from newer model organisms.
Heme is an iron containing prosthetic group in many proteins and plays a critical role in various biological processes such as electron transport, gas synthesis and sensing, xenobiotic detoxification, signal transduction, microRNA processing, and circadian clock control [1–4]. In metazoans, heme is synthesized via a conserved eight-step biosynthetic pathway. The last step of heme biosynthesis, the insertion of ferrous iron into the protoporphyrin IX ring, occurs inside the mitochondrial matrix. However, target hemoproteins such as guanylyl cyclases, catalases, cytochrome P450 and certain transcription factors are present in extra-mitochondrial compartments including the cytoplasm, peroxisomes, the secretory pathway and the nucleus [5–8]. As an iron-containing amphipathic porphyrin, free heme can catalyze the production of reactive oxygen species and intercalate into lipid bilayers [9, 10]. Thus, heme is unlikely to diffuse freely within the cell but instead, specific molecules and pathways must exist to facilitate heme delivery to distinct cellular destinations.
Although the enzymes for heme biosynthesis and its regulation are well-characterized in metazoans, significant knowledge gaps persist [6, 11]. Even less well defined are the pathways for transport and trafficking of heme and its intermediates. A comprehensive survey of the possible mechanisms for heme trafficking based upon known pathways for membrane trafficking and interorganellar transfer of metabolites has been covered in detail elsewhere [6–8]. The current review highlights scientific gaps in our understanding of heme synthesis and trafficking in metazoans and offers plausible models which can be experimentally tested. Given the significant differences in tetrapyrrole metabolism in photosynthetic organisms, readers are referred to insightful, comprehensive reviews elsewhere [11–15].
Heme biosynthesis
Aminolevulinate synthesis
The first committed step in heme biosynthesis is the formation of 5-aminolevulinate (ALA) [14, 16–19]. In the metazoa the enzyme ALA synthase (ALAS) (E.C. 2.3.1.37) catalyzes the condensation of glycine with succinyl CoA to form ALA and CO2. ALAS is a homodimeric, pyridoxal phosphate-containing enzyme that is a member of the large and well-characterized α-oxoamine synthase family. At present only the crystal structure of ALAS from the bacterium Rhodobacter capsulatus has been determined [20], but it is clear that the mature eukaryotic ALAS is highly similar to the bacterial enzyme except that it possesses an additional carboxyl-terminal sequence of approximately 25 residues. Much is known about the mammalian enzyme from kinetic and site-directed mutagenesis studies [17]. In vertebrates, two isozymes of ALAS exist, one specific for differentiating erythroid cells (ALAS-2 or ALAS-E) and the other expressed in all other cell types (ALAS-1 or ALAS-N). The genes for ALAS, as for all heme biosynthetic enzymes, are nuclear, although the final destination for ALAS is the mitochondrion. Both ALAS-1 and -2 possess mitochondrial targeting sequences that are cleaved as part of the translocation of ALAS into the mitochondrial matrix [21–23].
Synthesis of the monopyrrole, porphobilinogen
Once ALA is produced by ALAS it is exported out of the mitochondrial matrix (see below) to reach the second pathway enzyme, porphobilinogen synthase (PBGS) (E.C. 4.2.1.24) (previously named ALA dehydratase) (Fig 1) [24]. This enzyme catalyzes the condensation of two molecules of ALA to form one molecule of the monopyrrole PBG. The cytoplasmically located homo-octomer can best be considered a tetramer of homodimers with one divalent metal atom per subunit [16, 24]. In humans and yeast, this metal is zinc while in bacteria one may also find magnesium. Four metal atoms are essential for catalysis and four are involved in stabilization of tertiary structure. These zinc ions may be replaced by lead in lead poisoning resulting in an inactive enzyme. The protein has been crystallized from multiple sources and kinetically characterized (see [[14, 25]). The Jaffe group has shown that PBGS exists in alternate quaternary structures named morpheeins [26]. The morpheeins represent a dynamic change in oligomerization of PBGS between the high activity octomer and a low activity hexamer. This change in quaternary structure is the basis of allosteric regulation of PBGS. However, given that ALAS is considered rate-limiting to heme synthesis, the role of allosteric regulation at PBGS is something of an enigma.
Figure 1.
The mammalian heme biosynthetic pathway. The diagram presents the enzymatic steps and structures of intermediates in the pathway from ALA to heme. Steps that occur in the mitochondrion are enclosed in the dashed box and those outside the box are present in the cytosol. Regions highlighted in red show the site of chemical change catalyzed by the enzyme listed. Synthesis of ALA from glycine and succinyl CoA, which is the first committed step and occurs in the mitochondrion, is not shown. Abbreviations are as in the text.
Assembly of the linear tetrapyrrole, hydroxymethylbilane
Following formation of PBG, the enzyme hydroxymethylbilane synthase (HMBS, previously called PBG deaminase or PBGD) (E.C. 2.5.1.61) catalyzes the head to tail synthesis of four PBG molecules to form the linear tetrapyrrole hydroxymethylbilane (HMB) and releases four molecules of ammonium [24]. Partly because a decrease in HMBS activity leads to the human disease acute intermittent porphyria (AIP) [27, 28], this enzyme became the early focus of much attention by researchers. HMBS has been purified from a variety of sources and the crystal structures of the Escherichia coli [29] and human [30] enzymes have been determined. The cytoplasmically-located monomer is synthesized as an apoprotein that in its first complete catalytic cycle synthesizes a covalently-bound hexameric linear polypyrrole. From this the distal linear tetrapyrrole HMB is cleaved resulting in formation of the holoenzyme HMBS with a covalently bound dipyrromethane which serves as a cofactor for future turnovers and the product HMB. HMB is chemically reactive and will spontaneously cyclize to form uroporphyrinogen I in the absence of the next pathway enzyme. Uroporphyrinogen I cannot be converted into protoporphyrin IX.
Cyclization of the tetrapyrrole to form uroporphyrinogen
Conversion of HMB to the physiological uroporphyrinogen III isomer requires the action of uroporphyrinogen synthase (UROS) (E.C. 4.2.1.75) [24]. The reaction which is catalyzed without a cofactor is the “flipping” or inversion of the final, or D, ring of HMB followed by cyclization to yield the III isomer of uroporphyrinogen. UROS is a monomeric protein whose structure has been determined for both human [31] and Thermus thermophilus [32]. Its structure is unusual since it is composed of two distinct domains connected by a flexible linker region. Crystal structures have been obtained for a variety of domain orientations suggesting that the molecule is highly flexible in solution [31, 32].
Decarboxylation of uroporphyrinogen
The final cytoplasmic enzyme in the pathway is uroporphyrinogen decarboxylase (UROD) (E.C. 4.1.1.37) [33]. This homodimeric enzyme has been crystallized [34] and its catalytic mechanism well studied [35]. Each subunit contains an independent active site and the enzyme contains no cofactors or metal ions. UROD catalyzes the decarboxylation of the four pyrrole acetic acid side chains of uroporphyrinogen to yield the four methyl pyrrole side chains of coproporphyrinogen and four molecules of CO2. UROD will utilize both uroporphyrinogen I and III and in the presence of high concentrations of substrate, UROD will decarboxylate randomly [24]. However, it is believed that in situ the reaction starts with the decarboxylation of the D ring acetate and proceeds sequentially in a clockwise fashion (i.e. D, A, B, C) [34, 35].
Formation of the vinyl groups of protoporphyrinogen IX
Coproporphyrinogen III produced by UROD is transported into the mitochondrial intermembrane space where the antepenultimate enzyme coproporphyrinogen oxidase (CPOX) (E.C. 1.3.3.3) is located [36, 37]. This protein is synthesized in the cytoplasm with an unusually long (~120 amino acid) mitochondrial leader sequence that targets it to the inner membrane space [21]. The mature protein is a homodimer without bound cofactor. The structures of both human [38] and yeast (which is a cytoplasmic protein) [39] CPOX have been solved and were found to possess unique folds. The reaction catalyzed is an unusual oxidative decarboxylation of the A and B ring propionates to yield the vinyl groups of protoporphyrinogen IX. CPOX will utilize only the coproporphyrinogen III isomer and proceeds in a stepwise fashion that requires two molecules of molecular oxygen and generates two molecules of CO2. The reaction catalyzed by CPOX has been extensively studied both experimentally and in silico, but at present no definitive mechanism has been identified [40, 41].
Oxidation of the porphyrinogen to protoporphyrin IX
The penultimate step is the oxidation of protoporphyrinogen IX to protoporphyrin IX. This is catalyzed by protoporphyrinogen oxidase (PPOX) (E.C. 1.3.3.4) which requires three molecules of molecular oxygen and generates three molecules of hydrogen peroxide [36, 37]. Crystal structures of the plant [42], bacterial [43, 44], and human [45] enzymes have been determined and show that the protein is a homodimer with one noncovalently bound FAD per subunit. PPOX is synthesized in the cytoplasm in its mature size and is translocated to the outer surface of the inner mitochondrial membrane via a mechanism that requires an internal mitochondrial targeting sequence [21, 46, 47]. Interestingly, the active site is proposed to be situated in the middle of a tunnel that passes through the protein.
Insertion of iron to form protoheme IX
The terminal step of heme synthesis is the insertion of ferrous iron into the protoporphyrin macrocycle to produce protoheme IX (heme). This is catalyzed by the enzyme ferrochelatase (FECH) (E.C. 4.99.1.1) [48]. In metazoans this enzyme is synthesized in the cytoplasm as a preprotein and is translocated to the mitochondrial matrix where it is associated with the inner mitochondrial membrane. The mature, processed protein is a homodimer with each subunit possessing a [2Fe-2S] cluster [49]. Similar [2Fe-2S] clusters are not found in plant FECHs, but are found in some bacteria [48, 50]. There is no evidence to suggest that the cluster participates directly in catalysis, but recent studies suggest that it may serve as a sensor of mitochondrial matrix redox or membrane potential (D. Shah et al., unpublished data). The crystal structures of human FECH with and without substrate and product have been determined and it has been shown that the molecule undergoes considerable active site remodeling during catalysis [18, 51].
Synthesis and localization of metazoan heme biosynthetic enzymes
Enzymes of heme biosynthesis are nuclear encoded and cytoplasmically synthesized. The overall reaction to synthesize heme in metazoan cells is: 8 glycine + 8 succinyl CoA + 5 O2 + Fe2+ → Heme + 8 CoA + 4 NH4 + 14 CO2 + 3 H2O2 + 2 H+ + 11 H2O. Of the products, 8 CoA, 8 CO2, 2 H+ and heme are generated in the mitochondrial matrix, and 2 CO2 + 3 H2O2 + and 2 H2O are generated in the mitochondrial inner membrane space. All remaining products are cytosolic. As noted above, five pathway proteins possess either organic co-factors or essential metals. To date no pathway enzymes has been shown to be glycosylated and only mouse liver FECH has been reported to be phosphorylated [52], albeit on residues that that appear to be in inaccessible sites within the protein. Interestingly these investigators also reported that phosphorylated FECH is located on the mitochondrial outer membrane, something never reported by any other group previously.
Regulation of the pathway is tissue and cell specific, subject to modulation by a myriad of developmental and environmental pressures through diverse transcriptional factors, and is beyond the scope of the current review [18, 53, 54]. In general, however, synthesis of the first committed intermediate, ALA, is considered rate-limiting. Among red blood cell containing metazoans there are clear distinctions between regulation of heme synthesis in non-erythroid cells (so-called housekeeping heme synthesis) and in differentiating erythroid precursor cells. The housekeeping ALAS-1 gene is regulated by a diverse factors frequently associated with xenobiotic, hormone and drug metabolism, and is subject to tissue-specific regulation [53, 55]. ALAS-1 has an estimated half-life of only a few hours and may be significantly induced in some cells [19, 56]. ALAS-1 is present in erythroid precursor cells before they begin erythroid differentiation, but the gene is turned off when ALAS-2 is turned on as these cells begin to synthesize heme for hemoglobin. Thus ALAS-1 cannot substitute for a deficiency of ALAS-2 [57, 58].
ALAS-2 is transcriptionally regulated by erythroid-specific factors such as GATA-1 and possesses an iron regulatory element (IRE) located in the mRNA 5’ untranslated region that allows for translational regulation by cellular iron concentration [55, 59, 60]. As with similarly regulated systems, such as for ferritin synthesis, the IRE binds the iron-free form of the iron regulatory protein (IRP) thereby preventing translation of the ALAS-2 message when cellular iron concentrations are insufficient for optimal heme synthesis. Thus, maximal ALAS-2 activity requires gene induction by erythroid-specific transcription factors as well as sufficient cellular iron levels to support heme synthesis. Studies by Schranzhofer et al. [61] with differentiating erythroblasts demonstrated that ALAS-2 translational regulation becomes “uncoupled” from that of other IRE-regulated proteins such as transferrin receptors (which are increased, not decreased during erythropoiesis) and ferritin (whose protein levels do not increase). This is reasonable during the later accelerated hemoglobinization phase of erythropoiesis since under “standard” IRE-IRP regulation elevated iron would diminish transferrin receptor expression and increase ferritin synthesis, both of which would limit iron availability for heme synthesis. Additionally it was postulated that during maximal hemoglobinization, ALAS-2 mRNA increases disproportionately to IRP synthesis thus circumventing the IRE-IRP regulatory mechanism. It was suggested that the “kiss and run” hypothesis [62], which proposes direct vesicle mediated transfer of iron to mitochondria, may offer a possible means to circumvent this potential problem [63]. In this hypothesis, during terminal erythropoiesis, vesicular iron acquired by receptor mediated endocytosis is targeted directly to the mitochondrion, thereby bypassing the cytosolic iron pool and the IRE-IRP system [63].
Multiple studies and reviews have presented data suggesting that pathway enzymes after ALAS are in excess, although relative enzyme amounts vary considerably [1, 28, 53]. However, these calculations are based upon in vitro assays of individual enzymes under what are considered optimal conditions and then extrapolated back to intact cell conditions. There are no comprehensive studies that examine the induction of biosynthetic pathway enzymes other than ALAS-1 in nontransformed nonerythroid cells, but the fact that all of the pathway genes possess diverse transcription factor binding sites is compatible with regulation at multiple sites [57, 64–72]. Indeed, in cancer cells it is clear that at least CPOX and FECH are up and down regulated, respectively, and this is the basis for the effectiveness of photodynamic therapy [73, 74]. It has been demonstrated that there is up-regulation of all pathway enzymes during erythroid differentiation (see [73]). Even so, current dogma relegates pathway regulation during erythropoiesis to ALAS-2 alone [18, 54]. Recent data may cause a reevaluation of this model since it has been discovered that a mutation in the carboxyl-terminus of ALAS-2, which results in a hyperactive enzyme, causes an accumulation of free protoporphyrin as is found in the disease erythropoietic protoporphyria (EPP) [75]. The name currently given to this disorder is X-linked protoporphyria (XLPP) to reflect the X chromosome location of ALAS-2. This disorder is, thus, similar, but not identical, to that observed when ALAS-2 is overproduced in Irp2−/− mice [76]. The observation of protoporphyrin accumulation in XLPP is particularly interesting since levels of FECH are generally assumed to be present in considerable excess in the cell. Indeed, normal EPP occurs only when FECH levels drop to approximately one-fourth of normal [77]. This suggests that either the erythroid pathway is designed so that the amount of FECH (or iron transport mechanisms for heme synthesis) available is normally three- to four-fold above the maximum possible levels of ALAS-2, or that additional regulatory steps or limiting mechanisms exist at the end of the pathway that we currently do not recognize or understand.
For both ALAS-1 and ALAS-2 one finds alternative mRNA splice variants. For ALAS-1 two known splice variants occur in the untranslated region of exon 1 [78]. These variants possess altered sensitivity to heme-mediated decay of the message. For human ALAS-2 one splice variant has been described in which exon 4 is absent [79]. This variant represents 35–45% of total ALAS-2 mRNA in the cell and encodes a protein with slightly reduced activity. This variant ALAS-2 is translocated into the mitochondrial matrix where it was shown to interact with succinyl CoA synthetase (SUCLG1), just as the full length enzyme does, and contributes to erythroid heme synthesis. Interestingly, both ALAS-1 and -2 possess three heme regulatory motifs (HRMs) composed of a canonical Cys-Pro sequence [62]. Two HRMs are found in the targeting leader sequence and the third is near the amino terminus of the mature processed ALAS [21]. While such motifs have recently been shown to serve as a redox sensitive switch for heme oxygenase-2 [51], there is no evidence to suggest such a role for these motifs in ALAS. Indeed, evidence from multiple groups has shown that heme binds to the ALAS HRMs in vitro [22] and in vivo [21, 23], and a series of mutagenesis experiments clearly demonstrated the significance of all three ALAS HRMs in the heme responsive translocation of the protein into mitochondria in vivo [21]. While such heme regulation of apoprotein translocation into the mitochondria of nonerythroid cells can easily be rationalized, a similar occurrence in differentiating erythroid cells where massive quantities of heme are being synthesized in a relatively short period of time is less easily justified. Several significant pieces of information that would address this issue are lacking. Among these is an identification of the fate of both cytoplasmic ALAS precursor apoprotein and any heme bound to the HRMs. The possibility that the HRM-containing leader peptide or apoprotein HRM serves as a heme chaperone for globin assembly is intriguing, but no data exist to support or rule out this proposition.
While two distinct genes on separate chromosomes exist for ALAS-1 and -2, only single genes exist for the remaining pathway enzymes. However, for PBGS, HMBS, UROS and FECH, housekeeping vs. erythroid specific promoter regions drive expression that leads to tissue-specific alternate mRNA splicing. Only the HMBS alternate splice variants, however, give rise to isoenzymes of HMBS with alternative amino-terminal segments [66]. Here the mRNA of the erythroid form of HMBS skips exon 1 and starts with the noncoding exon 2. The translation start site employed for the erythroid HMBS is in exon 3. The housekeeping HMBS mRNA starts with exon 1, which contains an internal start site and coding region, and skips the non-coding exon 2. The result is that the housekeeping HMBS has an additional 17 amino acid residues at the amino terminus that are lacking in the erythroid form. Human PBGS possesses two non-coding exons, 1A and 1B [67]. The translational start site for housekeeping PBGS mRNA is exon 1A while erythroid PBGS mRNA starts in exon 1B. Thus, an additional 5’ untranslated region is present in the mRNA for the housekeeping variant that is lacking in the erythroid splice variant. Since these variants are in the noncoding region, the housekeeping and erythroid forms of the PBGS enzyme are identical. Splice variants also exist for UROS [64]. The gene for UROS possesses spatially distinct erythroid and housekeeping promoters with the erythroid promoter elements located in the intron between exons 1 and 2. This promoter drives transcription from an initiation site in exon 2. The housekeeping promoter region is present upstream of exon 1 and drives transcription from an initiation site in exon 1. However, since exon 1 is non-coding, both housekeeping and erythroid UROS proteins are identical. For mouse FECH there appears to be an alternate splice site that gives rise to additional 3’ untranslated nucleotides [80].
While the existence of splice variants is not a new observation, any experimentally supported rationale for a physiological role for these variants is lacking. However, recent studies on the role of RNA in the spatial regulation of protein synthesis [81] and/or in stabilization of multiprotein complexes [82] raise the intriguing possibility that the alternative splicing for mammalian housekeeping vs. erythroid forms of PBGS, HMBS and UROS are not random, but may serve for sequestration of mRNA primed for function.
It has been proposed that during the course of normal erythropoiesis, heme synthesis in developing erythroid cells overproduces heme to an extent that it is toxic to the cell unless it is exported by the plasma membrane heme transporter Feline Leukemia Virus subtype C Receptor (FLVCR) (see below) [83]. While it is clear that disabling mutations in FLVCR result in cell death, presumably due to the toxic effects of excessive heme, it seems counterintuitive that evolution would have designed such intricate regulatory mechanisms for erythroid heme synthesis (ie, IRE-IRP, erythroid specific transcription factors and complex iron supply regulatory schemes) that would permit excessive synthesis of heme necessitating heme export. Indeed, given the “cost” of heme synthesis, it seems more likely that the “excessive” heme produced is a planned synthesis of heme by erythroid cells (which must be considered the ultimate heme synthesizing factories in the body) for orderly export and transit to other organs and cells whose heme synthesizing capabilities may be physiologically limited. The presence of specific heme carriers, such as hemopexin [84], and the observation that exogenous administration of heme to human porphyria patients downregulates heme synthesis, and is subsequently utilized for hemoproteins by the patient receiving the infusion [27], provide support for this hypothesis.
Possible role of supramolecular protein assemblies in heme synthesis
The chemical intermediates in tetrapyrrole synthesis are relatively reactive and can be cytotoxic. The physiological proof of this statement is amply demonstrated by the fact that a deficiency in any one of the pathway enzymes results in a clinically distinct disease in animals [28, 53, 77], and inhibition of some later steps can result in photo-induced death of plants or microorganisms. Other than a defect in ALAS-2 which results in X-linked sideroblastic anemia, the clinical manifestations of these disorders, named porphyrias, are generally not anemia, but result from the accumulation of pathway intermediates upstream from the genetic block. In the case of early pathway enzyme deficiencies, the disorders are neurological in nature and are thought to result from the accumulation of ALA. For the enzymatic steps after the synthesis of the first tetrapyrrole, the most profound symptoms of the disorders are cutaneous in nature due to the chemical and photo-reactivity of the tetrapyrrole intermediate. These diseases and their molecular basis have been well studied and frequently reviewed by others (see [27, 28, 53, 77]).
Of significance to the current review is the intracellular distribution of heme synthesis pathway enzymes. The first and last three enzymes are cytoplasmically synthesized and translocated into the mitochondrion while PBGS, HMBS, UROS and UROD are all located in the cytoplasm [16, 24]. As discussed above, the first and last enzymes, ALAS [17, 21–23] and FECH [37, 48], are translocated to the mitochondrial matrix where their pre-proteins are proteolytically processed and assembled with appropriate cofactors while CPOX [85] and PPOX [86] are both homodimers located in the inner mitochondrial membrane space. The translocation of all four mitochondrially-located enzymes has been studied to varying extents, but only ALAS has been found to have any identifiable heme-responsive translocation regulatory motifs [21] and no experimental data exist to suggest that there is coordinate regulation of either synthesis or translocation of these enzymes.
Given the reactivity of the pathway intermediates it is highly unlikely that the intracellular concentrations of substrate or products attain the µM concentrations of the measured enzyme Kms. Thus, it is reasonable to assume that “free intermediates” do not exist in the cell although various porphyrin intermediates are routinely found in the extracellular medium of mammals. This topic was first approached experimentally in the 1980s when data were presented in support of the hypothesis that the terminal three pathway enzymes, which are all mitochondrial membrane-associated, form at least a transient complex to facilitate the transfer of intermediates [37, 86, 87]. Biochemical data clearly demonstrated that while obligate, tight substrate channeling such as occurs in tryptophan synthesis does not exist, under normal circumstances equilibration of products/substrates with the bulk medium does not occur. Data gleaned from PPOX and FECH crystal structures provides good support for possible interactions [36, 51].
Other than data for the terminal, membrane-associated enzymes, there exists little experimental evidence to support multienzyme complexes of earlier pathway enzymes. For the first step, an interaction of ALAS-2 with SUCLG1 on the inner mitochondrial membrane has been demonstrated by two groups [79, 88]. With the recent identification of SLC25A38 as the putative glycine/ALA transporter [89], it is reasonable to anticipate the existence of transient complexes between ALAS, SUCLG1, and SLC25A38 on the mitochondrial inner membrane (Fig. 2). The possibility for a stable, rigid complex for these and most other heme synthesis enzymes seems unlikely given that they possess active site pockets with a single entrance/exit (see [14]). The necessity for substrate(s) and product(s) to enter and exit via a single route would require movement of one enzyme between donor and acceptor molecules in the complex, much as cytochrome c physically cycles between electron donors and acceptors. The only enzyme for which this may not be the case is PPOX which appears to have a channel through the protein with the active site located within this feature [42, 43, 45].
Figure 2.
Proposed model for components involved in heme synthesis. Details are discussed in the text and where specific data exist for a particular component, its name is shown. Components that are currently unidentified experimentally are denoted with “?”. Abbreviations and conventions are given in the text.
For the synthesis of uroporphyrinogen III from PBG, it has long been thought that close proximity of HMBS and UROS would be probable given the chemical reactivity of the linear tetrapyrrolic intermediate, hydroxymethylbilane. Unfortunately at present no data exist to support the presence of a multienzyme complex involving PBGS and HMBS. Given that ALA must be exported out and coproporphyrinogen imported into the mitochondria, logic would suggest that not only PBGS and HMBS, but also UROS and UROD exist in a supramolecular complex that is spatially close to the mitochondrion (Fig. 2). To date, high resolution microscopic studies identifying the intracellular distribution of these enzymes is lacking. Approaches that rely upon cell disruption and physical isolation of complexes or in vitro reconstitution of complexes from isolated components assume a strength of protein-protein interaction in solution that may not exist and may not be necessary in the highly concentrated cytosolic milieu. One intriguing possibility is that PBGS, which forms a large complex best categorized as a tetramer of homodimers, could, because of its size, serve as a scaffold for a multienzyme complex. The observation that PBGS can also assume a hexameric form [14, 26] may hint at its plastic role as a supramolecular assembly nucleation site. Resolution of these issues will require masterful research, but the results will be illuminating.
The presence and nature of multienzyme complexes involving the terminal three membrane-associated enzymes is considerably advanced over what is known, or not known, about the earlier pathway enzymes, but is still extremely rudimentary. Little is known about possible interactions between CPOX and PPOX other than substrate channeling experiments [87]. However, because of spatial and chemical arguments, the possibility for interaction seems highly likely. Radiolabeling experiments employing isolated mitochondrial fragments clearly support transient interactions in situ, although reconstitution of this process with purified components was not accomplished, which suggests that additional participants other than just CPOX, PPOX and FECH are required. In silico structural studies show that an interaction across the inner mitochondrial membrane to transport protoporphyrin from PPOX to FECH is feasible and highly likely [42] (Fig 3). Interestingly, in the docked PPOX-FECH complex, the opening of the active site tunnels of the PPOX dimer not only coincide with the position of the openings to the active site pockets of the dimeric FECH, but are spatially juxtaposed to surface-bound porphyrin molecules observed in some FECH crystal structures [18, 90, 91]. The observation that binding of substrate and product induces changes in the surface contour and charge distribution around the active site pocket opening of FECH [18, 51] provide an explanation for how PPOX and post-catalytic heme-accepting proteins recognize the appropriate form of FECH with which to interact. Given the need to acquire coproporphyrinogen from the cytosol and movement of at least some heme out of the mitochondrion, the existence of a complex of an outer membrane coproporphyrinogen transporter, CPOX, PPOX, the inner membrane iron transporter mitoferrin (Mtf1) [92], FECH and a heme chaperone/outer membrane heme transporter at a mitofilin-mediated junction between outer and inner mitochondrial membranes [47] is an intriguing possibility.
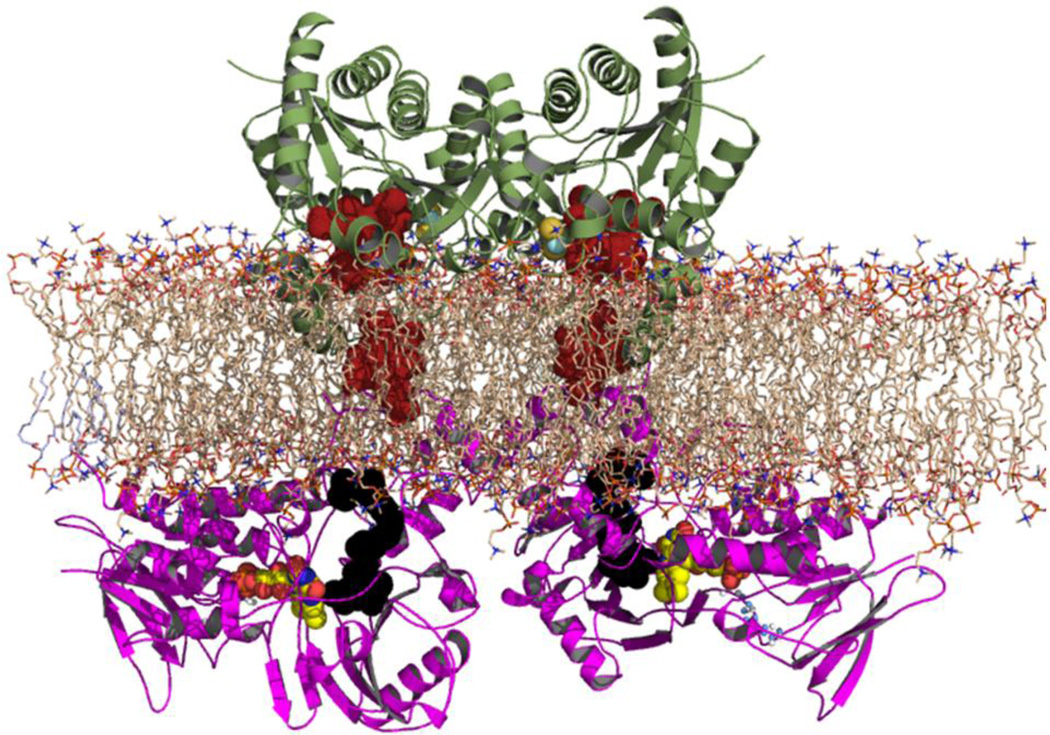
Figure 3.
Cartoon of proposed interaction between protoporphyrinogen oxidase (PPOX) and FECH across the inner mitochondrial membrane. The initial PPOX-FECH docking model was published by Koch et al. [42] for plant PPOX and human FECH. The current model was derived from PDB files 3NKS (PPOX) and 2QD1 (FECH). FECH is colored green and PPOX is colored violet. Porphyrin atoms are depicted as red colored solid spheres and the PPOX inhibitor is shown in solid black. Phospholipids are presented as sticks that are colored wheat. Of note is that the crystal structures of FECH with bound porphyrin possess one protoporphyrin bound in the active site and a second one associated with the outer edge of the active site lip. This is proposed to be the entry route of porphyrin into the active site pocket [18, 90]. It is proposed that FECH and PPOX interact transiently across the membrane to facilitate transfer of protoporphyrin to FECH. Following this transfer FECH undergoes spatial alterations that are proposed to lower the affinity between FECH and PPOX so that their interaction ceases thereby allowing FECH to interact with MTF1 and later a heme accepting protein.
Three significant issues remain unanswered: i) transport of coproporphyrinogen into the mitochondrion, ii) mitochondrial supply of iron for heme synthesis, and iii) transport of heme away from its site of synthesis. With regard to transport of coproporphyrinogen III a series of studies propose that ABCB6, a mitochondrial outer membrane ATP-dependent transporter, is responsible for coproporphyrinogen transport (see below) [93, 94]. At present, however, this claim must be viewed with skepticism since only the transport of the fully conjugated, planar macrocycle coproporphyrin, not the physiologically relevant non-planar coproporphyrinogen, has ever been experimentally demonstrated. However, given that ABCB6 has been observed to be one of a relatively few genes induced at high levels during erythropoiesis [95], it seems likely that it plays some role in erythropoiesis. Given the size of coproporphyrinogen, the possibility that it transits the outer membrane via a porin rather than a specific transporter cannot be ruled out by current data.
Although the coordinate regulation of tetrapyrrole synthesis and iron metabolism must exist to prevent their inappropriate accumulation, there have been limited studies that address this issue at either the genetic or molecular level. The role of the iron responsive element system [45] and the cellular machinery for iron sulfur cluster assembly has garnered significant interest [96], and our knowledge of whole body iron trafficking is relatively mature [97]. However, only recently has there been significant advancement in identifying participants essential to the iron supply for heme synthesis. This came with the identification of Mtf1 as the mitochondrial inner membrane iron transporter that supplies iron to FECH for heme synthesis [92]. Mtf1 is responsible for iron transport for heme synthesis during erythropoiesis, but the presence of another inner membrane transport protein, Abcb10, is required to stabilize Mtf1 [98]. The exact role that Abcb10 plays has yet to be elucidated. Mtf1 has been found to form a complex with FECH which might make possible a direct transfer of transported ferrous iron for heme synthesis to FECH (Figure 4) [98]. However, the actual site of iron entry for FECH remains undefined experimentally. One model proposes that mammalian FECH acquires iron from Mtf1 or frataxin via the extended π-helix of FECH described by Medlock et al. [91] and enters the active site via the main “mouth”[99, 100]. However, this model is based upon the structure of a single monomer of FECH, not the physiologically significant homodimer, and the putative membrane binding surface in the model is, in fact, the dimer interface. The crystal structures of dimeric human FECH are consistent with a spatial orientation where the π-helix unwinds into the membrane and not back up into the matrix. All available data suggest that the helix extension is related to heme release and in the absence of product there is nothing to stabilize the extended helix. Additionally the simultaneous exit of heme and entrance of iron via the same path is not supported by structural or kinetic studies [101, 102]. Alternatively, structural and site directed mutagenesis data obtained for human FECH along with its orientation relative to the inner membrane are all consistent with iron entering the active site of FECH via a solvent-filled channel whose entrance is on the rear of the enzyme. It will be of interest to learn the molecular details of this process and how it is regulated.
Figure 4.
Model of FECH and mitoferrin in the inner mitochondrial membrane. Human FECH is shown as a cartoon colored green with bound protoporphyrin in red spheres. A structure of mitoferrin 1 is currently not available, but it will be highly homologous to the structure of another inner mitochondrial solute transporter, the ATP/ADP transporter, whose structure has been solved [246] and is shown as a cartoon in red. Both proteins are oriented in a phospholipid bilayer (wheat colored). No structure of ABCB10 is available and the structures of known ABC transporters are so diverse as to make estimates of a structure model of ABCB10 not possible. The model is shown to demonstrate that interactions between FECH and MTF1 are possible given the relative sizes and spatial orientations in the membrane.
Transit of heme away from its site of synthesis remains an unexplored territory. In vitro, the release of heme from the enzyme post-metallation is the rate-limiting step [102]. This probably reflects the absence of the native heme acceptor a cell-free assay. Given that heme is utilized throughout the cell in a variety of compartments, it is clear that multiple and specific heme chaperones exist. For transit from the mitochondrion to other membranous compartments it appears likely that transfer occurs at direct contact points rather than by diffusion through the cytoplasm [5, 103], but even that requires first moving heme from FECH to the site of transfer between membranes. As yet unknown is what molecule first accepts heme from FECH and whether there is a single unique protein that interacts with FECH or multiple pathway-specific proteins. Given its location on the inner membrane, it would seem that for respiratory cytochrome maturation direct transfer from FECH to cytochrome assembly machinery is possible although definitive data are still lacking. For cytoplasmic hemoproteins, including hemoglobin and myoglobin, there are no identified players. Putative heme/porphyrin binding/carrier proteins have been suggested and even biochemically characterized [65, 104], but none of these is supported by data as in vivo participants in heme trafficking (see below).
Heme transport and trafficking
Heme transport in yeast
Heme uptake molecules have been identified in the pathogenic yeast Candida albicans. Weissman et al. (2002) first revealed that C. albicans can utilize heme and hemoglobin through a pathway that is distinct from iron uptake pathways [105]. Heme uptake in C. albicans exhibits a rapid initial binding phase followed by a slower uptake phase [106]. It was subsequently discovered by a genome-wide screen that Rbt51 and Rbt5 are involved in heme uptake in C. albicans [107]. RBT5 is highly induced by iron limitation and its deletion in C. albicans impairs the utilization of heme. Both Rbt51 and Rbt5 are glycosylphosphatidylinositol (GPI)-anchored proteins mainly localized to the plasma membrane, suggesting that they might function as receptors for heme and hemoglobin [108]. After being taken up by Rbt5/Rbt51, heme and hemoglobin are delivered to the vacuole through an endocytosis-mediated pathway [108]. Heme is then degraded by HO. The heme oxygenase enzyme CaHMX1 of C. albicans was identified and characterized both in vivo and in vitro [106, 109]. The expression of CaHMX1 is positively regulated by heme and hemoglobin [110].
In contrast to fungal pathogens, the budding yeast Saccharomyces cerevisiae utilizes exogenous heme very poorly. This indicates that S. cerevisiae lacks high-affinity heme transport systems. However, under conditions of heme starvation or hypoxia, an energy-dependent pathway for heme uptake has been detected [111]. Protoporphyrin uptake gene 1 (PUG1) was identified by microarray analysis on S. cerevisiae grown under heme starvation conditions [112]. Overexpression of PUG1 resulted in decreased cellular heme levels and increased protoporphyrin IX (PPIX) content in both wild type and heme-deficient strains. However, heme uptake was not affected in PUG1Δ strain [112]. Taken together, these data suggest that Pug1 is involved in PPIX influx and heme efflux. The mechanism and components of the yeast heme uptake system remain unknown.
Heme transport and detoxification in insects
Adult hematophagous arthropods ingest enormous amount of blood in a single meal [113]. Accordingly, hemoglobin is undoubtedly the sole major source of iron for blood-feeding insects. In the mosquito Aedes aegypti, >98% of iron in the insect body and the eggs come from heme [114]. However, free heme is toxic to cells because it can cause lipid peroxidation. To minimize the toxicity of the heme released by hemoglobin digestion, these insects have evolved efficient strategies to excrete, transport, and sequester heme.
The first defense against free heme occurs in the insect’s gut. According to an inductively coupled plasma mass spectrometry (ICP-MS) study in A. aegypti, 87% of total ingested heme iron was excreted by the end of the first gonotrophic cycle [114]. In addition, insects have specialized structures in the gut to sequester and detoxify heme. In the lumen of the intestine, ferrous heme can be converted into ferric heme and aggregate into an insoluble structure called hemozoin [115]. Hemozoin has been identified in the malaria parasite Plasmodium falciparum, the parasitic worm Schistosoma mansoni, the parasitic protozoan Haemoproteus columbae, and the kissing bug Rhodnius prolixus [115, 116]. In R. prolixus, the hemozoin in the lumen of midgut is the first defense against toxicity from free heme [117]. Inhibition of hemozoin formation by chloroquine leads to increased levels of free heme and increased lipid peroxidation [118]. In vitro studies further confirmed that, compared with free heme, hemozoin generated fewer free radicals, caused less lipid peroxidation, and did not lead to the lysis of red blood cells [119].
Instead of forming hemozoin, the mosquito A. aegypti has a layer of peritrophic matrix covering the intestinal epithelium. This structure separates intestinal cells from the food and it has been shown to be associated with high levels of heme after feeding [120]. Devenport et al. (2006) identified a heme-binding protein, A. aegypti intestinal mucin 1, in the peritrophic matrix that might be one of the major molecules required for heme sequestration [121].
Heme uptake has been characterized at the cellular level by monitoring fluorescent hemoglobin conjugates or heme analogs in Boophilus microplus. In contrast to most eukaryotes and even the kissing bug R. prolixus, the cattle tick B. microplus lacks the heme biosynthetic pathway [117]. Hemoglobin is taken up by specialized digestive cells in the midgut through receptor-mediated endocytosis [122]. After the endosomes fuse with primary lysosomes, hemoglobin is degraded [123]. The released heme is sequestered in specialized intracellular membrane-bound organelles called hemosomes [113, 122]. Hemosomes provide a sequestration mechanism for heme and prevent it from forming free radicals. After absorption, a fraction of heme in the digestive cells is translocated into the open circulatory system hemocoel. It has been shown that several heme-binding lipoproteins may play a role in the transport and sequestration of heme in the hemolymph. The major hemolymph protein in B. microplus has been characterized as a heme lipoprotein (HeLp). The gene encoding HeLp is expressed in both male and female ticks after host-attachment and blood feeding [124]. The protein contains two molecules of heme and has the capacity to bind six more heme molecules [118]. By injecting HeLp labeled with 55Fe-heme into the hemocoel, a quick drop of the radioactivity in hemolymph and a simultaneous increase of 55Fe-heme within oocytes was observed, suggesting that HeLp might play a role in delivering heme across tissues [118]. In the American dog tick Dermacentor variabilis the homolog of HeLp, hemolymph carrier protein (CP), is also the major hemolymph protein and has been suggested to play a role in sequestering heme [124–126]. In vitro assays indicated that HeLp/CP-bound heme induced less oxidative damage to phospholipids than free heme [127]. These data suggest that HeLp/CP may be involved in the sequestration and transport of heme.
In ticks and other insects, the major yolk protein vitellogenin has heme-binding activity. Binding of heme by vitellogenin strongly inhibited heme-induced lipid peroxidation [119]. After blood feeding, vitellogenin is primarily produced in the fat body and midgut of female ticks and is then transferred to developing oocytes [126, 128]. Therefore, insect vitellogenin may play a role in transferring heme and other nutrients to eggs [119, 126]. In the egg bound heme is released when vitellogenin is degraded by endopeptidases such as vitellin-degrading cysteine endopeptidase, Boophilus yolk cathepsin and tick heme-binding aspartic proteinase [129, 130]. Besides HeLp/CP and vitellogenin, other proteins such as Rhodnius heme-binding protein may bind heme and decrease toxicity of free heme in insect hemolymph [131, 132].
Cellular heme transport in animals
Heme import
It has been long thought that duodenal enterocytes internalize heme through a receptor-mediated endocytic pathway. Existence of heme receptors or heme uptake proteins has been shown in the microvilli of upper small intestine, cultured enterocytes and non-intestinal cells [133, 134]. In a search for putative heme transporters HRG-1 (Heme- Responsive Gene-1, systematically named solute carrier 48A1, SLC48A1) was identified in a screen for genes regulated by heme in the roundworm Caenorhabditis elegans [135]. This worm is a heme auxotroph and must, therefore, absorb heme from the environment and transport it throughout the organism [136]. This nutritional requirement for heme negates several of the variables, including feedback regulation of heme biosynthesis and non-heme porphyrin transport, that have historically confounded the mammalian heme transport field. In worms, the HRG proteins hrg-1 and hrg-4 are transmembrane permeases and depletion of either one disrupts heme sensing and leads to abnormal responses to heme analogs [135].
Transient knockdown of the zebrafish ortholog, hrg-1, leads to profound erythropoietic defects and ectopic expression of hrg-1 in murine erythroleukemia (MEL) cells leads to accumulation of heme analogs [135]. In addition, heme-dependent transport across the plasma membrane was observed in Xenopus oocytes expressing worm and human hrg-1. Only one hrg-1 gene is present in the human genome, whereas worms have three additional hrg-1 paralogs [137]. This indicates that worms might have evolved redundant heme acquisition pathways since they lack the ability to synthesize heme.
HRG-1 interacts with heme in a low pH-dependent manner, suggesting that it may function in relatively acidic microenvironments such as the endolysosome [135]. HRG-1 has been shown to interact with the c subunit of the vacuolar proton ATPase (V-ATPase) pump and enhances endosomal acidification [138]. It is important to note that the directionality of heme import from the plasma membrane is topologically identical to heme import from an intracellular vesicular compartment as heme still travels from the exoplasmic space into the cytoplasm.
Human HRG-1 mRNA is highly expressed in the brain, kidney, heart and skeletal muscle and moderately expressed in the liver, lung, placenta and small intestine [135]. HRG-1 was also recently identified as a target of the heme-dependent transcription factor BACH1 in microarray expression analysis and ChIP-Seq experiments, further reinforcing its potential relevance to heme metabolism in mammalian cells [139]. Bach1 is a basic leucine zipper transcription factor shown to bind heme [140]. Bach1-Maf heterodimers bind to Maf recognition elements (MAREs) and repress the expression of target genes such as globins and heme oxygenase 1 (HO-1) [140–142]. Heme can interact with Bach1 and de-repress transcription. Mechanistic studies using a yeast system suggest that the heme is translocated across membranes by human and worm HRG-1 proteins by conserved amino acids positioned on the exoplasmic, cytoplasmic, and transmembrane regions [137]. Thus, given HRG1’s 1) ability to transport heme, 2) regulation of synthesis by heme in worms and BACH1 in mammalian cells, 3) distribution in cell types relevant to heme and iron metabolism, and 4) localization to endolysosomes, along with the anemic phenotype of the zebrafish knockdown, and heme transport in MEL cells and Xenopus oocytes, one would predict that HRG1 is a compelling candidate for a heme transporter relevant to macrophage, intestinal and red blood cell heme metabolism.
Heme export
The existence of a heme exporter has been speculated for two reasons: 1) efflux may be one of the main mechanisms for heme detoxification, since the accumulation of excess heme is highly toxic to the cells; 2) efflux may facilitate intercellular heme transfer and heme iron recycling. For example, when macrophages phagocytose senescent red blood cells and degrade hemoglobin to release heme, a portion of the iron is exported from macrophages as intact heme-iron [143].
One proposed heme exporter is FLVCR (FLVCR1) which belongs to the major facilitator superfamily [144]. Suppression of FLVCR by the virus FLV-C in feline embryonic fibroblasts resulted in significantly increased cellular heme content, while ectopic expression of FLVCR in renal epithelial cells reduced the intracellular heme levels [144]. This result was further confirmed by heme export assays using a fluorescent heme analog and 55Fe-heme in renal epithelial and K562 cells. FLVCR is highly expressed in hematopoietic cells, and heme efflux mediated by FLVCR is essential for erythroid differentiation [144, 145]. Cats infected with FLVC developed pure red cell aplasia in which erythroid progenitor cells failed to mature from burst-forming units into the colony-forming-units erythroid cells. No erythropoiesis was observed in FLVCR knock-out mice, and these mice died at mid-gestation [146]. Deletion of FLVCR also perturbed the heme efflux from macrophages and therefore blocked the recycling of heme and iron from senescent red blood cells [146].
Is the mechanism for heme transport across membranes by HRG-1-related proteins also shared by FLVCR? A recent report showed that FLVCR2 imported heme in mammalian cells and may be the basis for the vascular defect in Fowler Syndrome [147]. Although heme import by FLVCR2 was not observed in the yeast heme import assays alongside HRG-1, given the high degree of homology between FLVCR2 and the heme effluxer FLVCR1, it is possible that FLVCR2 may be a exporting heme via intracellular compartments [137]. Heme export by FLVCR1 is facilitated by hemopexin which binds to a 69 amino acid peptide (residues 132–201) residing between two exoplasmic loops and two transmembrane domains [44]. Given the similarity in membrane topology and conservation of several strategically placed, heme-binding ligands in the exoplasmic and cytoplasmic loops between FLVCR1 and FLVCR2, it is possible that these proteins may have similar functional mechanisms.
The ABC transporter ABCG2, also named as BCRP, was originally identified as a drug resistance protein in breast cancer cells. Krishnamurthy et al. (2004) showed that heme interacts with ABCG2 by using hemin-agarose pull-down assays [148]. PPIX levels in the erythrocytes of ABCG2-null mice were ten times higher than those in wild type mice [149] suggesting that ABCG2 might function as an exporter for porphyrin compounds. However, no evidence has directly confirmed that ABCG2 can export heme (see Contradictions below).
Intracellular heme transport
Whether synthesized within cells or taken up from the environment, heme in all eukaryotic cells has to be translocated across membrane barriers for either storage and sequestration, or utilization and incorporation into hemoproteins. The molecules and the mechanisms involved in intracellular heme trafficking remain poorly understood. ABCB6 is postulated to play a role in heme transport between mitochondria and the cytoplasm. ABCB6 was initially identified as a mammalian ortholog for yeast ATM1, a mitochondrial iron transporter important for Fe-S cluster biogenesis [150]. However, Krishnamurthy et al. (2006) proposed that ABCB6 was more likely a porphyrin/heme transporter in mitochondria [94]. In cells expressing ABCB6, 55Fe-heme was readily transported into mitochondria from the cytoplasm in an energy-dependent manner. The physiological significance of a mitochondrial heme importer is of question given that the mitochondrion is the site of the terminal steps of heme synthesis. Another tetrapyrrole compound, coproporphyrin III, competed with ABCB6 for heme binding and inhibited heme uptake into mitochondria. Its puzzling that ABCB6 is present in the C. elegans genome even though worms do not synthesize heme implying that the true substrate for ABCB6 is still unclear.
More recently, two molecular weight forms of ABCB6 of 79 kDa and 104 kDa were identified [151]. Using specific ABCB6 antibodies, it was shown that while the light form localized to the mitochondrial outer membrane, the heavy form predominantly resided on the plasma membrane. Overexpression of the plasma membrane form of ABCB6 reduced the cellular accumulation of another tetrapyrrolic compound pheophorbide A but not heme. It is possible that the two ABCB6 forms have distinct functions at different sub-cellular locations, but further studies are required to pinpoint their physiological roles in the cell.
Contradictions
Heme carrier protein (HCP1) was initially identified from a suppression subtractive hybridization screen using hypotransferrinaemic mice [152]. Expression of HCP1 in Xenopus oocytes and HeLa cells resulted in 2–3 fold increase in heme uptake with an apparent Km of 125 µM [152]. However, HCP1 was eventually demonstrated to be essential for folate transport in the intestine when it was found to be mutated in patients with congenital folate deficiency; HCP1 has a high affinity for folate (Km ~1.3 µM at pH 5.5) [153]. Whether Hcp1 also contributes to the absorption of heme in the intestine is uncertain at this time [154].
Although BCRP can export heme from cells, BCRP has broad substrate specificity and can mediate the efflux of a large number of natural and unnatural substrates, and for that reason has been called a “multidrug resistance” protein [148] Furthermore, null alleles in BCRP in humans result in the “Junior” blood system group that has no apparent clinical phenotype other than transfusion incompatibility [155]. Likewise, humans with null mutations in ABCB6 have no apparent defects in erythropoiesis but instead produce a new blood group system named Langereis, suggesting that at least a portion of ABCB6 resides on the plasma membrane of red blood cells [155]. Furthermore, a L811V allele in humans cause ocular coloboma, a developmental defect in the closure of the optic fissure [156]. Although genetic experiments in mice clearly reveal a hematological defect consistent with the role for FLVCR1 as a heme transporter, recent work describes a mitochondrial isoform of FLVCR1 that may mediate heme export from mitochondria into the cytoplasm, suggesting that the FLVCR1 null phenotype may be due to cytosolic heme deficiency, not accumulation [157].
Heme transfer between organelles
Just as multiprotein assemblies, for example between CPOX, PPOX, and FECH, may form to facilitate transport of intermediates during heme biosynthesis, similar complexes may play a role in exporting heme from the mitochondrial matrix. The dynamin-like protein (DLP) mitofilin has been shown to tether the christae-folds of the inner mitochondrial membrane to the outer membrane, establishing the appropriate mitochondrial morphology [158, 159]. These contacts between the IM and OM would serve as favorable location for the proposed CPOX-PPOX-FECH complex, as well as for proteins regulating the mobilization of heme out of the mitochondria.
Delivery of iron-loaded transferrin to the mitochondria has been observed, implying contiguity between mitochondria and the endocytic pathway [63]. Conversely, vesicles derived from mitochondrial membranes (MDV) have been shown to carry selected cargo to peroxisomes and lysosomes [160–162]. These observations hint at the possibility that heme may be carried out of the mitochondria in MDVs. This would allow highly regulated transport of heme to target compartments, all the while shielding the cytoplasm from potentially cytotoxic heme.
Recent studies have also alluded to physical connections between the ER and mitochondria, raising the possibility that mitochondrial components can directly move into the ER. Firstly, the yeast proteins Mmm1, Mdm10, Mdm12, and Mdm34 have been identified in a complex that tethers mitochondria to the ER membrane [163]. This ER-Mitochondria Encounter Structure (ERMES) has been hypothesized to serve as an interface for exchange of materials between the ER and mitochondria [164–167]. Secondly, the dynamin-like GTPase mitofusin, originally known for its role in mitochondrial fusion and fission, has been shown to fuse the ER and outer mitochondrial membrane at specific contact points [158, 159]. After crossing the inner mitochondrial membrane using an as-yet-unknown mechanism, heme would be able to move directly into the ER lumen at these contact points between the two organelles.
Heme binding proteins
Free heme has peroxidase activity and the iron in heme can generate reactive oxygen species through Fenton reactions. In addition, as a small lipophilic molecule, free heme can easily intercalate with, and disrupt the lipid bilayers of cell membranes. Therefore, heme-binding proteins or heme chaperones may be required to prevent the toxicity associated with free heme. The heme-binding proteins may function to sequester or transport heme.
Cytoplasmic heme binding proteins
Glutathione S-transferases (GSTs) catalyze the conjugation of glutathione to various electrophilic substrates and play essential roles in xenobiotic detoxification. Besides their enzymatic functions, GSTs have also been known for their ability to bind a variety of ligands in the cytoplasm. In fact, GSTs were first identified in mammalian liver as “ligandins” that can selectively bind steroids, bilirubin and organic anions [168]. Subsequent studies showed that GSTs interact with heme and porphyrins [169–171].
In malaria parasites and helminths, GSTs are highly abundant in the cytoplasm. A GST from P. falciparum (Pf-GST) is capable of interacting with heme [172]. This Pf-GST was subsequently shown to contain both high- and low-affinity heme binding sites [173]. In the rodent malaria parasite, Plasmodium berghei, heme but not PPIX can inhibit GST activities [174]. Furthermore, an inverse correlation between heme levels and GST activities has been shown both in vitro and in P. berghei [174, 175]. Rossum et al. (2005) discovered a novel GST from the blood-feeding helminth Haemonchus contortus (Hc-GST-1) that is able to interact with heme [176]. The homologous protein in Ancylostoma caninum, Ac-GST-1, also binds heme [177]. These GSTs have been postulated to play critical roles in the detoxification and transport of heme [172, 176, 178].
A murine 22 kDa protein, p22HBP, was identified as a cytosolic heme-binding protein ubiquitously expressed in various tissues with high levels in the liver [65]. In mouse erythroleukemia (MEL) cells, p22HBP was induced during erythroid differentiation and knock-down of the gene resulted in reduced heme content in MEL cells. In addition to heme, the mouse and human protein binds other porphyrin compounds such as PPIX [65, 71, 104]. It was revealed by a structural analysis that a hydrophobic cleft was responsible for tetrapyrrole binding in p22HBP [104]. Two homologous proteins in Arabidopsis thaliana, cHBP1 and cHBP2, were also found to bind tetrapyrroles reversibly in vitro [179]. These two HBPs may play similar roles in different plant tissues since cHBP1 is highly expressed in leaves whereas the highest level of cHBP2 is detected in roots [179]. The murine protein SOUL has 27% sequence identity to p22HBP. Early studies on SOUL reported it to be a dimer in the absence of heme and a hexamer in the presence of heme [180, 181]. More recently, however, crystal structure, surface plasmon resonance and NMR studies by Ambrosi [182] clearly demonstrated that SOUL is a monomer and that it does not bind heme. SOUL was shown to possess a Bcl-2 homology 3 (BH3) domain and current evidence suggests that it plays a role in apoptosis and/or oxidative stress and not heme trafficking.
HBP23 belongs to the peroxiredoxin family of peroxidases and is also called mouse stress-inducible 23 kDa protein or proliferation-associated gene product. The protein was originally identified from rat liver using chromatography on hemin-agarose and was shown to have a high binding affinity for heme [183]. On the protein surface, two hydrophobic regions, both containing histidine residues, might be responsible for heme binding [184]. The protein is highly expressed in the cytoplasm of the liver and also present in kidney, spleen, small intestine, and heart. Heme, PPIX, and other metalloporphyrins are able to stimulate HBP23 expression in rat primary hepatocytes [185]. Furthermore, incubation of rat liver HBP23 with heme inhibited its antioxidant activity [186].
Nitric oxide synthases (NOS) are soluble hemoproteins which catalytically convert L-arginine to citrulline and nitric oxide (NO). Mammals have three NOS - endothelial (eNOS), inducible (iNOS), and neuronal (nNOS). NOS form homodimers and its activity is dependent on heme insertion. Studies from the Stuehr group have suggested that NO blocks heme insertion into extramitochondrial hemoproteins without changes in intracellular heme levels [187, 188]. Interestingly, heme insertion into iNOS has been reported to be mediated by GAPDH, a glycolytic enzyme [189]. Although GAPDH has been traditionally implicated as a cytoplasmic housekeeping protein, potential new roles are emerging for this protein in the nucleus (DNA repair), cytoplasm (membrane fusion and cytoskeletal remodeling) and mitochondria (cell survival) [190]. It has been reported that heme insertion into NOS is facilitated by the molecular chaperone HSP90; the ATPase activity of HSP90 in the presence of as of yet unidentified heme chaperone, postulated to be GAPDH, is essential for heme insertion into apo-iNOS [191], or nNOS [192]. However, it’s important to note that HSP90 is a highly abundant (1–2% of total cellular protein) and promiscuous protein that localizes to the nucleus, mitochondria and even the extracellular matrix, and associates with a wide variety of client proteins with low stability or exposed hydrophobic surfaces [193].
Fatty acid binding proteins are known to be able to bind heme [194, 195]. However, the cellular functions of heme-binding activities in these cytosolic proteins are still unclear.
Extracellular heme binding proteins
Heme and hemoglobin are released into the plasma during the destruction of senescent erythrocytes and enucleation of erythroblasts. Under pathological conditions such as hemoglobinopathies, trauma, and infections, more severe intravascular hemolysis is induced. To prevent tissues from experiencing heme toxicity and to increase the recycling of heme iron, mammalian cells secrete specific molecules to bind heme and hemoglobin in the circulation.
Haptoglobin (hapto- “bind to”), primarily synthesized by hepatocytes, is a plasma glycoprotein with hemoglobin-binding capacity. There are three major subtypes of haptoglobin [196] all of which can form soluble complexes in an equimolar ratio with hemoglobin dimers (Kd ~10−12 M) [197–199]. Haptoglobin-hemoglobin complexes bind to the CD163 receptor on the surface of monocytes and macrophages and these complexes are subsequently endocytosed [200]. Receptors for the haptoglobin-hemoglobin complex also exist in hepatocytes and hepatoma cell lines [201, 202]. After entering the cells, iron is released by heme degradation, whereas the remaining protein complex is degraded by lysosomes [203–205]. Physiologically, binding of haptoglobin reduces the loss of hemoglobin and heme iron [205, 206].
Hemopexin is a heme-binding plasma protein that binds heme with high affinity (Kd ~10−13 M) [207]. Hemopexin-heme complexes are endocytosed in response to the binding with LRP/CD91 (LDL receptor-related protein, or CD91) in a variety of cells including hepatocytes, macrophages, and syncytiotrophoblasts [208]. Unlike haptoglobin, hemopexin is recycled back into the circulation after the release of heme during endocytosis [208, 209]. In response to heme overload, hemopexin null mice exhibited increased oxidative stress and altered regulation of HO-1 as well as ferritin, suggesting critical roles of hemopexin in heme detoxification [210].
Human serum albumin (HSA), a 66 kDa protein, binds a wide variety of proteins as well as heme (Kd ~10−8 M) [211, 212]. The crystal structure shows that a hydrophobic cleft in one of its three sub-domains binds heme. Three basic residues at the entrance to this cleft form charge pair interactions with the propionate side chains of heme, and the iron in heme is coordinated by a tyrosine residue [213].
Two additional serum proteins, high-density and low-density lipoproteins, bind heme faster than both hemopexin and HSA with an affinity that is higher than that of HSA for heme (Kd ~10−11 M) [211, 214]. It is thought that this rapid binding is critical to prevent damage by heme during the initial release of heme and provides a buffer period for hemopexin and HSA to steadily but tightly bind heme. Eventually, hemopexin and HSA remove all but a residual amount of heme from the lipoproteins [214].
Intercellular heme transport
Genetic screens in C. elegans identified over 200 Heme Responsive Genes (HRGs). Dietary heme is transported into the worm intestine by membrane-bound permeases, HRG-1 and HRG-4 [135, 137]. However tissues such as muscle, neurons, hypodermal cells, and embryos are dependent upon intestinal heme to fulfill their metabolic requirements, so some mechanism for intercellular transport of heme must exist since C. elegans lacks heme synthesizing capability. Consistent with this concept HRG-3, a peptide secreted by the mother’s intestine, functions to transport maternal heme to developing oocytes and ensures that sufficient heme is available to sustain embryonic development [215]. HRG-3 deficiency results either in death during embryogenesis or in developmental arrest immediately post hatching – phenotypes that are fully suppressed by maternal but not zygotic hrg-3 expression. HRG-3 binds both ferrous and ferric heme with a stoichiometry of two protomers to one heme [215]. These results imply that HRG-3 may function as an intercellular chaperone to mobilize maternal heme and deliver it to extra-intestinal tissues and oocytes. However, questions remaining unanswered are 1.) the nature of the internalization mechanism for HRG-3-heme complex into oocytes, 2.) the identity of specific cell surface receptors for HRG-3 on extra-intestinal tissues, and 3.) the mechanism and regulation of heme release from HRG-3 within the embryo.
Although an intercellular heme transport system is to be expected in worms which are heme auxotrophs, an intercellular pathway for heme distribution may also exist in vertebrates based on the following experimental evidence. Firstly, cell culture studies with human colon-derived Caco-2 cells and mouse macrophages reveal that a portion of heme derived from dietary sources or senescent red blood cells is released into the blood stream as an intact metalloporphyrin [143, 216]. Secondly, human patients with acute attacks of porphyrias are administered heme intravenously as an effective therapeutic treatment which results in lowering the accumulation of heme synthesis intermediates and a concomitant increase in liver heme-dependent enzyme activities indicating that infused heme can be utilized by peripheral tissues [28, 217]. Thirdly, even though knockout of the heme synthesis pathway in mice is embryonic lethal, homozygous embryos survive at least till E3.5 suggesting the existence of heme stores [218, 219]. Lastly, zebrafish embryos with loss-of-function mutations in heme synthesis genes can survive from 10–25 days post fertilization, because these embryos may either contain maternal-derived mRNA for heme synthesis enzymes or directly deposited maternal heme [114, 220]. Together, these results lead us to propose the existence of a functional intercellular pathway to facilitate the targeted delivery and redistribution of heme during vertebrate development. Consistent with this notion, FLVCR1 and HRG-1 may function to redistribute heme during organogenesis and development. Indeed, FLVCR1 is expressed substantially in the yolk sac and placenta, perhaps to mobilize maternal heme for fetal development [146, 221].
Parasitic worms and heme
Parasitic worms are a major cause of chronic infections in humans, farm animals and plants. More than a quarter of the world’s population is affected by one or more of the 20 most common parasites including Ascaris, Ancylostoma, Trichuris and Schistosome [222, 223]. In addition, animal parasites and plant parasites cause enormous economic loss in agricultural production every year. Traditional drugs are becoming more and more ineffective in helminthic control as drug-resistant nematodes are already prevalent in humans and other animals [224, 225].
Heme and iron play essential roles in development, reproduction and pathogenicity of parasitic worms. For example, iron supplement stimulates the growth of the fluke S. mansoni in vitro [226]. Due to the fact that free iron is tightly controlled in animal hosts and S. mansoni ingests huge amount of blood, it is highly possible that these parasites acquire iron predominantly in the form of heme from the blood [227]. In the hookworm, Ancylostoma ceylanicum, host iron status has been shown to be a key mediator of pathogenicity. Challenged animal hosts supplied with iron-restricted diet had a significant reduction in the intestinal load of worms [228].
In the intestine of the canine hookworm A. caninum, hemoglobin is degraded by a cascade of proteolytic reactions involving aspartic proteases (APR-1), cysteine proteases and metalloproteases [229]. Vaccination of dogs with APR-1 to generate neutralizing antibodies dramatically reduced the hookworm burdens, fecal egg counts, as well as host blood loss, after challenging with A. caninum larvae [177].
Recently, Rao et al. discovered that parasitic worms including Strongyloides, Ancylostoma, Haemonchus, Trichuris and Ascaris are unable to synthesize heme de novo [136]. Analysis of the genomes for these nematodes indicates that they encode abundant hemoproteins such as globins, cytochrome P450s, catalases and guanylate cyclases. Therefore, worms must have developed an efficient heme uptake system to meet their nutritional requirement and a heme storage system to store essential but cytotoxic heme. In addition, since none of the cells have the ability to synthesize heme, an intercellular heme transport system is required to mobilize heme from intestinal cells – the site of absorption – to other cell types including neurons, muscles, hypodermis, and developing embryos. Thus, worm-specific molecules involved in heme homeostasis, especially in heme uptake, could be potential drug targets for helminthic control.
Heme Reductases
Metalloreductases have been shown to be essential for the uptake of metals. For example, duodenal cytochrome b (Dcytb) and six transmembrane epithelial antigen of the prostate 3 (Steap3) were identified as ferric reductases that were associated with efficient iron uptake into cells [230–232]. Subsequent studies found that Dcytb and proteins in the Steap family also function as cupric reductases [233, 234].
Studies have indicated that hemin (heme with an oxidized iron atom) has to be reduced for its covalent attachment to apocytochrome c [235, 236]. The Cyc2p protein is postulated to function as a heme reductase, using NAD(P)H as a cofactor, in the intermembrane space (IMS) of the yeast mitochondria [237, 238]. Cyc2p functions in concert with cytochrome heme lyases for covalent attachment of heme to apocytochromes c and c1. Given that heme synthesis occurs within the mitochondrial matrix and FECH produces reduced heme, the model for heme reduction in the IMS by Cyc2p would suggest that the heme is oxidized in that compartment by an unknown mechanism.
In Gram-negative bacteria, cytochrome c synthetase CcmF was recently proposed to function as a quinol:heme oxidoreductase [236]. In addition, the lipocalin α1-microglobulin also has the ability to reduce hemin in cytochrome c and methemoglobin [216]. However, plasma membrane-associated hemin reductases that might play a role in hemin uptake remain elusive. Another possible candidate heme reductase is HRG-2, a type Ia membrane protein, which localizes to the ER in the C. elegans hypodermis [239]. Worms deficient in HRG-2 show abnormal distribution of soluble hemoproteins and altered gene expression of several cytochromes. However, direct evidence for a reductase activity was not demonstrated because of the inability to isolate purified protein. Measurement of heme reductase activity is in crude cell extracts is nontrivial and fraught with technical hindrances, not the least of which are identifying the source of reducing power (enzyme and cofactor) or the nature of potential heme donor and/or acceptor. Nevertheless, the topology of HRG-2 is identical to that of microsomal cytochrome P450s and is reminiscent of the heme-binding protein Dap1p in yeast and its human ortholog PGRMC1 which can interact with cytochrome P450s and increase their activities by an as of yet poorly characterized heme delivery system [240, 241].
Conclusion
Because of its brilliant color and near ubiquitous presence in biology, heme has long been an object of scientific inquiry. The relationship between clinical symptoms of a disease and specific biochemical defects was first recognized with the porphyrias. The identification and characterization of enzymes of the heme biosynthetic pathway has a history of over 60 years, yet information on the intracellular movement of heme and heme precursors along with how heme is eventually assembled into hemoproteins is lacking. Interestingly this field now seems to be following the lead set by researchers who focus on metal transport over the past two decades and defined components necessary for transport and sequestration of physiologically relevant metals. With the availability of advanced genetic, biophysical, microscopic and biochemical tools it is hoped that the pathways and molecules in porphyrin and heme trafficking are soon identified and result in a holistic model for porphyrin and metal metabolism.
Table 1.
Heme/porphyrin transporters
| Protein name | Proposed Function | Location of function | Reference |
|---|---|---|---|
| HRG-1 (SLC48A1) | Transport heme | Vesicles | [135] |
| FLVCR (MFSD7B) | Export heme | RBC plasma membrane | [144] |
| PCFT (formerly HCP1) | Import heme? | Plasma membrane | [152, 153] |
| ABCG2 (BCRP) | Export heme | RBC plasma membrane | [148] |
| ABCB6 | Transport coproporphyrinogen III | Plasma membrane, Mitochondria outer membrane, Golgi | [94] |
| ABC-me (M-ABC2/ABCB10) | Transport heme intermediates | IM | [242, 243] |
| SLC25A38 | Transport glycine and ALA | IM | [89] |
| Pug1p | Exports heme and imports PPIX in yeast | Yeast PM | [112] |
Table 2.
Heme binding proteins
| Protein Name | Proposed Function | Location of function | Reference |
|---|---|---|---|
| HRG-3 | Bind heme | Extracellular in C. elegans | [215] |
| HRG-2 | Bind heme | Hypodermis in C. elegans | [239] |
| FABP | Bind heme/tetrapyrroles | Cytosol | [194] |
| GST | Bind heme/tetrapyrroles | Cytosol | [169] |
| HBP23 | Bind heme/tetrapyrroles | Cytosol | [183] |
| SOUL/p22HBP family | Bind heme/tetrapyrroles | Cytosol | [181, 244] |
| Haptoglobin | Bind extracellular Hb | Blood | [199] |
| Hemopexin | Bind extracellular heme | Blood | [207] |
| HDL & LDL | Bind extracellular heme | Blood | [214] |
| HeLp | Bind extracellular heme | Hemolymph in insects | [118, 124] |
| Vitellogenin | Bind heme for heme transfer | Extracellular in insects | [119, 126, 128] |
| GAPDH | Heme insertion into iNOS | Cytosol | [189] |
| HSP90 | Heme insertion into iNOS/nNOS | Cytosol | [191, 192] |
| Cyc2p | Heme reductase | Mitochondria IMS | [237, 238] |
| Dap1p/PGRMC1 | Heme transfer to Cytochrome P450 | ER but also detected in cytosol, PM and secretory pathway | [240, 241, 245] |
| Dcytb | Ferric reductase | Enterocytes | [231] |
| Human serum albumin | Bind extracellular heme | Blood | [211] |
Highlights.
Enzymology and regulation of heme biosynthesis
Supramolecular protein assemblies in heme synthesis
Heme transport and trafficking
Intracellular and intercellular heme transport
Heme-binding proteins
Acknowledgements
This work is supported by funding from the National Institutes of Health (R01DK74797 and R01DK85035 to I.H.) and the Roche Foundation for Anemia Research (I.H.). H.A.D. has been supported by funding from the National Institutes of Health (DK32303). We thank T. Dailey and T. Korolnek for helpful discussions and critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ponka P. Cell biology of heme. Am. J. Med. Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbα, a Heme Sensor That Coordinates Metabolic and Circadian Pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 3.Tsiftsoglou AS, Tsamadou AI, Papadopoulou LC. Heme as key regulator of major mammalian cellular functions: Molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- 5.Schultz IJ, Chen C, Paw BH, Hamza I. Iron and porphyrin trafficking in heme biogenesis. J. Biol. Chem. 2010;285:26753–26759. doi: 10.1074/jbc.R110.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem. Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamza I. Intracellular trafficking of porphyrins. ACS Chem Biol. 2006;1:627–629. doi: 10.1021/cb600442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Severance S, Chen C, Hamza I. Eukaryotic Heme Trafficking. In: Kadish KM, Smith KM, Guilard R, editors. Handbook of Porphyrin Science, vol. 15: Biochemistry of Tetrapyrroles. World Scientific Publishing Co.; 2011. pp. 1–48. [Google Scholar]
- 9.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 1991;64:648–655. [PubMed] [Google Scholar]
- 10.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ. The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci. 2010;15:488–498. doi: 10.1016/j.tplants.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Masuda T, Fujita Y. Regulation and evolution of chlorophyll metabolism. Photochem Photobiol Sci. 2008;7:1131–1149. doi: 10.1039/b807210h. [DOI] [PubMed] [Google Scholar]
- 13.Zappa S, Li K, Bauer CE. The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus. Adv. Exp. Med. Biol. 2010;675:229–250. doi: 10.1007/978-1-4419-1528-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layer G, Reichelt J, Jahn D, Heinz DW. Structure and function of enzymes in heme biosynthesis. Protein Sci. 2010;19:1137–1161. doi: 10.1002/pro.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka R, Kobayashi K, Masuda T. Tetrapyrrole Metabolism in Arabidopsis thaliana. Arabidopsis Book. 2011;9:e0145. doi: 10.1199/tab.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hunter GA, Ferreira GC. Molecular enzymology of 5-aminolevulinate synthase, the gatekeeper of heme biosynthesis. Biochim. Biophys. Acta. 2011;1814:1467–1473. doi: 10.1016/j.bbapap.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medlock AE, Carter M, Dailey TA, Dailey HA, Lanzilotta WN. Product release rather than chelation determines metal specificity for ferrochelatase. J. Mol. Biol. 2009;393:308–319. doi: 10.1016/j.jmb.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May BK, Bawden MJ. Control of heme biosynthesis in animals. Semin. Hematol. 1989;26:150–156. [PubMed] [Google Scholar]
- 20.Astner I, Schulze JO, van den Heuvel J, Jahn D, Schubert WD, Heinz DW. Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans. EMBO J. 2005;24:3166–3177. doi: 10.1038/sj.emboj.7600792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dailey TA, Woodruff JH, Dailey HA. Examination of mitochondrial protein targeting of haem synthetic enzymes: in vivo identification of three functional haem-responsive motifs in 5-aminolaevulinate synthase. Biochem. J. 2005;386:381–386. doi: 10.1042/BJ20040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lathrop JT, Timko MP. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993;259:522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi K, Hayashi N, Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J. Biol. Chem. 1980;255:1746–1751. [PubMed] [Google Scholar]
- 24.Schubert HL, Erskine PT, Cooper JB. 5-Aminolaevulinic Acid Dehydratase, Porphobilinogen Deaminase and Uroporphyrinogen III Synthase. In: Warren MJ, Smith AG, editors. Tetrapyrroles: Birth, Life and Death. Austin, Texas: Landes Bioscience; 2009. pp. 43–73. [Google Scholar]
- 25.Erskine PT, Senior N, Awan S, Lambert R, Lewis G, Tickle IJ, Sarwar M, Spencer P, Thomas P, Warren MJ, Shoolingin-Jordan PM, Wood SP, Cooper JB. X-ray structure of 5-aminolaevulinate dehydratase, a hybrid aldolase. Nat. Struct. Biol. 1997;4:1025–1031. doi: 10.1038/nsb1297-1025. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe EK, Lawrence SH. Allostery and the dynamic oligomerization of porphobilinogen synthase. Arch. Biochem. Biophys. 2011 doi: 10.1016/j.abb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann. Intern. Med. 2005;142:439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 28.Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 29.Louie GV, Brownlie PD, Lambert R, Cooper JB, Blundell TL, Wood SP, Warren MJ, Woodcock SC, Jordan PM. Structure of porphobilinogen deaminase reveals a flexible multidomain polymerase with a single catalytic site. Nature. 1992;359:33–39. doi: 10.1038/359033a0. [DOI] [PubMed] [Google Scholar]
- 30.Gill R, Kolstoe SE, Mohammed F, Al DBA, Mosely JE, Sarwar M, Cooper JB, Wood SP, Shoolingin-Jordan PM. Structure of human porphobilinogen deaminase at 2.8 A: the molecular basis of acute intermittent porphyria. Biochem. J. 2009;420:17–25. doi: 10.1042/BJ20082077. [DOI] [PubMed] [Google Scholar]
- 31.Mathews MA, Schubert HL, Whitby FG, Alexander KJ, Schadick K, Bergonia HA, Phillips JD, Hill CP. Crystal structure of human uroporphyrinogen III synthase. EMBO J. 2001;20:5832–5839. doi: 10.1093/emboj/20.21.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert HL, Phillips JD, Heroux A, Hill CP. Structure and mechanistic implications of a uroporphyrinogen III synthase-product complex. Biochemistry. 2008;47:8648–8655. doi: 10.1021/bi800635y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoolingin-Jordan PM. The Biosynthesis of Coproporphyrinogen III. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook, vol. 12. San Diego: Academic Press; 2003. pp. 33–92. [Google Scholar]
- 34.Phillips JD, Whitby FG, Kushner JP, Hill CP. Structural basis for tetrapyrrole coordination by uroporphyrinogen decarboxylase. EMBO J. 2003;22:6225–6233. doi: 10.1093/emboj/cdg606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis CA, Jr, Wolfenden R. Uroporphyrinogen decarboxylation as a benchmark for the catalytic proficiency of enzymes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17328–17333. doi: 10.1073/pnas.0809838105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhtar M. Coproporphyrinogen III and Protoporphyrinogen IX Oxidase. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook, vol. 12. San Diego: Academic Press; 2003. pp. 75–92. [Google Scholar]
- 37.Dailey HA. Conversion of Coproporphyrinogen to Protoheme in Higher Eukaryotes and Bacteria: Terminal Three Enzymes. In: Dailey HA, editor. Biosynthesis of Heme and Chlorophylls. New York: McGraw-Hill; 1990. pp. 123–161. [Google Scholar]
- 38.Lee DS, Flachsova E, Bodnarova M, Demeler B, Martasek P, Raman CS. Structural basis of hereditary coproporphyria. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14232–14237. doi: 10.1073/pnas.0506557102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips JD, Whitby FG, Warby CA, Labbe P, Yang C, Pflugrath JW, Ferrara JD, Robinson H, Kushner JP, Hill CP. Crystal structure of the oxygen-dependant coproporphyrinogen oxidase (Hem13p) of Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:38960–38968. doi: 10.1074/jbc.M406050200. [DOI] [PubMed] [Google Scholar]
- 40.Lash TD. The enigma of coproporphyrinogen oxidase: how does this unusual enzyme carry out oxidative decarboxylations to afford vinyl groups? Bioorg. Med. Chem. Lett. 2005;15:4506–4509. doi: 10.1016/j.bmcl.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Silva PJ, Ramos MJ. Computational characterization of the substrate-binding mode in coproporphyrinogen III oxidase. J Phys Chem B. 2011;115:1903–1910. doi: 10.1021/jp110289d. [DOI] [PubMed] [Google Scholar]
- 42.Koch M, Breithaupt C, Kiefersauer R, Freigang J, Huber R, Messerschmidt A. Crystal structure of protoporphyrinogen IX oxidase: a key enzyme in haem and chlorophyll biosynthesis. EMBO J. 2004;23:1720–1728. doi: 10.1038/sj.emboj.7600189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corradi HR, Corrigall AV, Boix E, Mohan CG, Sturrock ED, Meissner PN, Acharya KR. Crystal structure of protoporphyrinogen oxidase from Myxococcus xanthus and its complex with the inhibitor acifluorfen. J. Biol. Chem. 2006;281:38625–38633. doi: 10.1074/jbc.M606640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin X, Sun L, Wen X, Yang X, Tan Y, Jin H, Cao Q, Zhou W, Xi Z, Shen Y. Structural insight into unique properties of protoporphyrinogen oxidase from Bacillus subtilis. J. Struct. Biol. 2010;170:76–82. doi: 10.1016/j.jsb.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Qin X, Tan Y, Wang L, Wang Z, Wang B, Wen X, Yang G, Xi Z, Shen Y. Structural insight into human variegate porphyria disease. FASEB J. 2011;25:653–664. doi: 10.1096/fj.10-170811. [DOI] [PubMed] [Google Scholar]
- 46.Morgan RR, Errington R, Elder GH. Identification of sequences required for the import of human protoporphyrinogen oxidase to mitochondria. Biochem. J. 2004;377:281–287. doi: 10.1042/BJ20030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von und zu Fraunberg M, Nyroen T, Kauppinen R. Mitochondrial targeting of normal and mutant protoporphyrinogen oxidase. J. Biol. Chem. 2003;278:13376–13381. doi: 10.1074/jbc.M300151200. [DOI] [PubMed] [Google Scholar]
- 48.Dailey HA, Dailey TA. Ferrochelatase. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook, vol. 12. San Diego: Academic Press; 2003. pp. 93–122. [Google Scholar]
- 49.Wu CK, Dailey HA, Rose JP, Burden A, Sellers VM, Wang BC. The 2.0 A structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 2001;8:156–160. doi: 10.1038/84152. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd M, Dailey TA, Dailey HA. A new class of [2Fe-2S]-cluster-containing protoporphyrin (IX) ferrochelatases. Biochem. J. 2006;397:47–52. doi: 10.1042/BJ20051967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dailey HA, Wu CK, Horanyi P, Medlock AE, Najahi-Missaoui W, Burden AE, Dailey TA, Rose J. Altered orientation of active site residues in variants of human ferrochelatase. Evidence for a hydrogen bond network involved in catalysis. Biochemistry. 2007;46:7973–7979. doi: 10.1021/bi700151f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaino M, Ishigaki M, Ohgari Y, Kitajima S, Masaki R, Yamamoto A, Taketani S. Dual mitochondrial localization and different roles of the reversible reaction of mammalian ferrochelatase. FEBS J. 2009;276:5559–5570. doi: 10.1111/j.1742-4658.2009.07248.x. [DOI] [PubMed] [Google Scholar]
- 53.Kappas A, Sassa S, Galbraith RA, Nordmann Y. The Porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 1995. pp. 2103–2159. [Google Scholar]
- 54.May BK, Dogra SC, Sadlon TJ, Bhasker CR, Cox TC, Bottomley SS. Molecular regulation of heme biosynthesis in higher vertebrates. Prog. Nucleic Acid Res. Mol. Biol. 1995;51:1–51. doi: 10.1016/s0079-6603(08)60875-2. [DOI] [PubMed] [Google Scholar]
- 55.Dierks P. Molecular Biology of Eukaryotic 5-Aminolevulinate Synthase. In: Dailey HA, editor. Biosynthesis of Heme and Chlorophylls. New York: McGraw-Hill; 1990. pp. 201–233. [Google Scholar]
- 56.Tian Q, Li T, Hou W, Zheng J, Schrum LW, Bonkovsky HL. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011;286:26424–26430. doi: 10.1074/jbc.M110.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lake-Bullock H, Dailey HA. Biphasic ordered induction of heme synthesis in differentiating murine erythroleukemia cells: role of erythroid 5-aminolevulinate synthase. Mol. Cell. Biol. 1993;13:7122–7132. doi: 10.1128/mcb.13.11.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin X, Dailey HA. Erythroid 5-aminolevulinate synthase is required for erythroid differentiation in mouse embryonic stem cells. Blood Cells. Mol. Dis. 1998;24:41–53. doi: 10.1006/bcmd.1998.0169. [DOI] [PubMed] [Google Scholar]
- 59.Bhasker CR, Burgiel G, Neupert B, Emery-Goodman A, Kuhn LC, May BK. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J. Biol. Chem. 1993;268:12699–12705. [PubMed] [Google Scholar]
- 60.Melefors O, Goossen B, Johansson HE, Stripecke R, Gray NK, Hentze MW. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J. Biol. Chem. 1993;268:5974–5978. [PubMed] [Google Scholar]
- 61.Schranzhofer M, Schifrer M, Cabrera JA, Kopp S, Chiba P, Beug H, Mullner EW. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 1995;14:313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheftel AD, Zhang A-S, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- 64.Aizencang G, Solis C, Bishop DF, Warner C, Desnick RJ. Human uroporphyrinogen-III synthase: genomic organization, alternative promoters, and erythroid-specific expression. Genomics. 2000;70:223–231. doi: 10.1006/geno.2000.6373. [DOI] [PubMed] [Google Scholar]
- 65.Blackmon JB, Dailey TA, Lianchun X, Dailey HA. Characterization of a human and mouse tetrapyrrole-binding protein. Arch. Biochem. Biophys. 2002;407:196–201. doi: 10.1016/s0003-9861(02)00471-x. [DOI] [PubMed] [Google Scholar]
- 66.Grandchamp B, De Verneuil H, Beaumont C, Chretien S, Walter O, Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur. J. Biochem. 1987;162:105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- 67.Kaya AH, Plewinska M, Wong DM, Desnick RJ, Wetmur JG. Human delta-aminolevulinate dehydratase (ALAD) gene: structure and alternative splicing of the erythroid and housekeeping mRNAs. Genomics. 1994;19:242–248. doi: 10.1006/geno.1994.1054. [DOI] [PubMed] [Google Scholar]
- 68.Magness ST, Tugores A, Brenner DA. Analysis of ferrochelatase expression during hematopoietic development of embryonic stem cells. Blood. 2000;95:3568–3577. [PubMed] [Google Scholar]
- 69.Magness ST, Tugores A, Diala ES, Brenner DA. Analysis of the human ferrochelatase promoter in transgenic mice. Blood. 1998;92:320–328. [PubMed] [Google Scholar]
- 70.Romana M, Dubart A, Beaupain D, Chabret C, Goossens M, Romeo PH. Structure of the gene for human uroporphyrinogen decarboxylase. Nucleic Acids Res. 1987;15:7343–7356. doi: 10.1093/nar/15.18.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi S, Taketani S, Akasaka JE, Kobayashi A, Hayashi N, Yamamoto M, Nagai T. Differential regulation of coproporphyrinogen oxidase gene between erythroid and nonerythroid cells. Blood. 1998;92:3436–3444. [PubMed] [Google Scholar]
- 72.Tugores A, Magness ST, Brenner DA. A single promoter directs both housekeeping and erythroid preferential expression of the human ferrochelatase gene. J. Biol. Chem. 1994;269:30789–30797. [PubMed] [Google Scholar]
- 73.Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71:6040–6050. doi: 10.1158/0008-5472.CAN-11-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahashi K, Ikeda N, Nonoguchi N, Kajimoto Y, Miyatake S, Hagiya Y, Ogura S, Nakagawa H, Ishikawa T, Kuroiwa T. Enhanced expression of coproporphyrinogen oxidase in malignant brain tumors: CPOX expression and 5-ALA-induced fluorescence. Neuro Oncol. 2011;13:1234–1243. doi: 10.1093/neuonc/nor116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC, Puy H. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet. 2008;83:408–414. doi: 10.1016/j.ajhg.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooperman SS, Meyron-Holtz EG, Olivierre-Wilson H, Ghosh MC, McConnell JP, Rouault TA. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elder GH, Gouya L, Whatley SD, Puy H, Badminton MN, Deybach JC. The molecular genetics of erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand) 2009;55:118–126. [PubMed] [Google Scholar]
- 78.Roberts AG, Elder GH. Alternative splicing and tissue-specific transcription of human and rodent ubiquitous 5-aminolevulinate synthase (ALAS1) genes. Biochim. Biophys. Acta. 2001;1518:95–105. doi: 10.1016/s0167-4781(01)00187-7. [DOI] [PubMed] [Google Scholar]
- 79.Cox TC, Sadlon TJ, Schwarz QP, Matthews CS, Wise PD, Cox LL, Bottomley SS, May BK. The major splice variant of human 5-aminolevulinate synthase-2 contributes significantly to erythroid heme biosynthesis. Int. J. Biochem. Cell Biol. 2004;36:281–295. doi: 10.1016/s1357-2725(03)00246-2. [DOI] [PubMed] [Google Scholar]
- 80.Chan RY, Schulman HM, Ponka P. Expression of ferrochelatase mRNA in erythroid and non-erythroid cells. Biochem. J. 1993;292(Pt 2):343–349. doi: 10.1042/bj2920343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lecuyer E, Yoshida H, Krause HM. Global implications of mRNA localization pathways in cellular organization. Curr. Opin. Cell Biol. 2009;21:409–415. doi: 10.1016/j.ceb.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 82.Hyman AA, Brangwynne CP. Beyond stereospecificity: liquids and mesoscale organization of cytoplasm. Dev Cell. 2011;21:14–16. doi: 10.1016/j.devcel.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Khan AA, Quigley JG. Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim. Biophys. Acta. 2011;1813:668–682. doi: 10.1016/j.bbamcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shipulina N, Smith A, Morgan WT. Heme binding by hemopexin: evidence for multiple modes of binding and functional implications. J. Protein Chem. 2000;19:239–248. doi: 10.1023/a:1007016105813. [DOI] [PubMed] [Google Scholar]
- 85.Elder GH, Evans JO. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem. J. 1978;172:345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreira GC, Andrew TL, Karr SW, Dailey HA. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J. Biol. Chem. 1988;263:3835–3839. [PubMed] [Google Scholar]
- 87.Proulx KL, Woodard SI, Dailey HA. In situ conversion of coproporphyrinogen to heme by murine mitochondria: terminal steps of the heme biosynthetic pathway. Protein Sci. 1993;2:1092–1098. doi: 10.1002/pro.5560020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furuyama K, Sassa S. Interaction between succinyl CoA synthetase and the hemebiosynthetic enzyme ALAS-E is disrupted in sideroblastic anemia. J. Clin. Invest. 2000;105:757–764. doi: 10.1172/JCI6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guernsey DL, Jiang H, Campagna DR, Evans SC, Ferguson M, Kellogg MD, Lachance M, Matsuoka M, Nightingale M, Rideout A, Saint-Amant L, Schmidt PJ, Orr A, Bottomley SS, Fleming MD, Ludman M, Dyack S, Fernandez CV, Samuels ME. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat. Genet. 2009;41:651–653. doi: 10.1038/ng.359. [DOI] [PubMed] [Google Scholar]
- 90.Medlock A, Swartz L, Dailey TA, Dailey HA, Lanzilotta WN. Substrate interactions with human ferrochelatase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1789–1793. doi: 10.1073/pnas.0606144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medlock AE, Dailey TA, Ross TA, Dailey HA, Lanzilotta WN. A pi-helix switch selective for porphyrin deprotonation and product release in human ferrochelatase. J. Mol. Biol. 2007;373:1006–1016. doi: 10.1016/j.jmb.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, Paw BH. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 93.Krishnamurthy P, Schuetz JD. The role of ABCG2 and ABCB6 in porphyrin metabolism and cell survival. Curr Pharm Biotechnol. 2011;12:647–655. doi: 10.2174/138920111795163995. [DOI] [PubMed] [Google Scholar]
- 94.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 95.Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, Baughman JM, Paradkar PN, Kingsley PD, Culotta VC, Kaplan J, Palis J, Paw BH, Mootha VK. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009;10:119–130. doi: 10.1016/j.cmet.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lill R, Kispal G. Maturation of cellular Fe-S proteins: an essential function of mitochondria. Trends Biochem. Sci. 2000;25:352–356. doi: 10.1016/s0968-0004(00)01589-9. [DOI] [PubMed] [Google Scholar]
- 97.Sheftel AD, Mason AB, Ponka P. The long history of iron in the Universe and in health and disease. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbagen.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen W, Dailey HA, Paw BH. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood. 2010;116:628–630. doi: 10.1182/blood-2009-12-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hunter GA, Ferreira GC. Identification and characterization of an inhibitory metal ion-binding site in ferrochelatase. J. Biol. Chem. 2010;285:41836–41842. doi: 10.1074/jbc.M110.174243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunter GA, Al-Karadaghi S, Ferreira GC. Ferrochelatase: The Convergence of the Porphyrin Biosynthesis and Iron Transport Pathways. J Porphyr Phthalocyanines. 2011;15:350–356. doi: 10.1142/S108842461100332X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davidson RE, Chesters CJ, Reid JD. Metal ion selectivity and substrate inhibition in the metal ion chelation catalyzed by human ferrochelatase. J Biol Chem. 2009;284:33795–33799. doi: 10.1074/jbc.M109.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoggins M, Dailey HA, Hunter CN, Reid JD. Direct measurement of metal ion chelation in the active site of human ferrochelatase. Biochemistry. 2007;46:8121–8127. doi: 10.1021/bi602418e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang AS, Sheftel AD, Ponka P. Intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. Blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- 104.Dias JS, Macedo AL, Ferreira GC, Peterson FC, Volkman BF, Goodfellow BJ. The first structure from the SOUL/HBP family of heme-binding proteins, murine P22HBP. J. Biol. Chem. 2006;281:31553–31561. doi: 10.1074/jbc.M605988200. [DOI] [PubMed] [Google Scholar]
- 105.Weissman Z, Shemer R, Kornitzer D. Deletion of the copper transporter CaCCC2 reveals two distinct pathways for iron acquisition in Candida albicans. Mol. Microbiol. 2002;44:1551–1560. doi: 10.1046/j.1365-2958.2002.02976.x. [DOI] [PubMed] [Google Scholar]
- 106.Santos R, Buisson N, Knight S, Dancis A, Camadro JM, Lesuisse E. Haemin uptake and use as an iron source by Candida albicans: role of CaHMX1-encoded haem oxygenase. Microbiology. 2003;149:579–588. doi: 10.1099/mic.0.26108-0. [DOI] [PubMed] [Google Scholar]
- 107.Weissman Z, Kornitzer D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol. Microbiol. 2004;53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- 108.Weissman Z, Shemer R, Conibear E, Kornitzer D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol. Microbiol. 2008;69:201–217. doi: 10.1111/j.1365-2958.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- 109.Kim D, Yukl ET, Moenne-Loccoz P, Montellano PR. Fungal heme oxygenases: Functional expression and characterization of Hmx1 from Saccharomyces cerevisiae and CaHmx1 from Candida albicans. Biochemistry. 2006;45:14772–14780. doi: 10.1021/bi061429r. [DOI] [PubMed] [Google Scholar]
- 110.Pendrak ML, Chao MP, Yan SS, Roberts DD. Heme oxygenase in Candida albicans is regulated by hemoglobin and is necessary for metabolism of exogenous heme and hemoglobin to alpha-biliverdin. J. Biol. Chem. 2004;279:3426–3433. doi: 10.1074/jbc.M311550200. [DOI] [PubMed] [Google Scholar]
- 111.Protchenko O, Rodriguez-Suarez R, Androphy R, Bussey H, Philpott CC. A screen for genes of heme uptake identifies the FLC family required for import of FAD into the endoplasmic reticulum. J. Biol. Chem. 2006;281:21445–21457. doi: 10.1074/jbc.M512812200. [DOI] [PubMed] [Google Scholar]
- 112.Protchenko O, Shakoury-Elizeh M, Keane P, Storey J, Androphy R, Philpott CC. Role of PUG1 in inducible porphyrin and heme transport in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:859–871. doi: 10.1128/EC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lara FA, Lins U, Paiva-Silva G, Almeida IC, Braga CM, Miguens FC, Oliveira PL, Dansa-Petretski M. A new intracellular pathway of haem detoxification in the midgut of the cattle tick Boophilus microplus: aggregation inside a specialized organelle, the hemosome. J. Exp. Biol. 2003;206:1707–1715. doi: 10.1242/jeb.00334. [DOI] [PubMed] [Google Scholar]
- 114.Wang H, Zhou Q, Kesinger JW, Norris C, Valdez C. Heme regulates exocrine peptidase precursor genes in zebrafish. Exp Biol Med (Maywood) 2007;232:1170–1180. doi: 10.3181/0703-RM-77. [DOI] [PubMed] [Google Scholar]
- 115.Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J. Inorg. Biochem. 2008;102:1288–1299. doi: 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 116.Chen MM, Shi L, Sullivan DJ., Jr Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and beta-hematin. Mol. Biochem. Parasitol. 2001;113:1–8. doi: 10.1016/s0166-6851(00)00365-0. [DOI] [PubMed] [Google Scholar]
- 117.Braz GR, Coelho HS, Masuda H, Oliveira PL. A missing metabolic pathway in the cattle tick Boophilus microplus. Curr. Biol. 1999;9:703–706. doi: 10.1016/s0960-9822(99)80312-1. [DOI] [PubMed] [Google Scholar]
- 118.Maya-Monteiro CM, Daffre S, Logullo C, Lara FA, Alves EW, Capurro ML, Zingali R, Almeida IC, Oliveira PL. HeLp, a Heme Lipoprotein from the Hemolymph of the Cattle Tick Boophilus microplus. J. Biol. Chem. 2000;275:36584–36589. doi: 10.1074/jbc.M007344200. [DOI] [PubMed] [Google Scholar]
- 119.Logullo C, Moraes J, Dansa-Petretski M, Vaz IS, Masuda A, Sorgine MH, Braz GR, Masuda H, Oliveira PL. Binding and storage of heme by vitellin from the cattle tick, Boophilus microplus. Insect Biochem. Mol. Biol. 2002;32:1805–1811. doi: 10.1016/s0965-1748(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 120.Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJ. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem. Mol. Biol. 2002;32:517–523. doi: 10.1016/s0965-1748(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 121.Devenport M, Alvarenga PH, Shao L, Fujioka H, Bianconi ML, Oliveira PL, Jacobs-Lorena M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- 122.Lara FA, Lins U, Bechara GH, Oliveira PL. Tracing heme in a living cell: hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J. Exp. Biol. 2005;208:3093–3101. doi: 10.1242/jeb.01749. [DOI] [PubMed] [Google Scholar]
- 123.Donohue KV, Khalil SM, Sonenshine DE, Roe RM. Heme-binding storage proteins in the Chelicerata. J. Insect Physiol. 2009;55:287–296. doi: 10.1016/j.jinsphys.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 124.Donohue KV, Khalil SM, Mitchell RD, Sonenshine DE, Roe RM. Molecular characterization of the major hemelipoglycoprotein in ixodid ticks. Insect Mol. Biol. 2008;17:197–208. doi: 10.1111/j.1365-2583.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 125.Gudderra NP, Neese PA, Sonenshine DE, Apperson CS, Roe RM. Developmental profile, isolation, and biochemical characterization of a novel lipoglycoheme-carrier protein from the American dog tick, Dermacentor variabilis (Acari: Ixodidae) and observations on a similar protein in the soft tick, Ornithodoros parkeri (Acari: Argasidae) Insect Biochem. Mol. Biol. 2001;31:299–311. doi: 10.1016/s0965-1748(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 126.Gudderra NP, Sonenshine DE, Apperson CS, Roe RM. Tissue distribution and characterization of predominant hemolymph carrier proteins from Dermacentor variabilis and Ornithodoros parkeri. J. Insect Physiol. 2002;48:161–170. doi: 10.1016/s0022-1910(01)00160-3. [DOI] [PubMed] [Google Scholar]
- 127.Maya-Monteiro C, Alves L, Pinhal N, Abdalla D, Oliveira P. HeLp, a heme-transporting lipoprotein with an antioxidant role. Insect Biochem. Mol. Biol. 2004;34:81–88. doi: 10.1016/j.ibmb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 128.Thompson DM, Khalil SMS, Jeffers LA, Sonenshine DE, Mitchell RD, Osgood CJ, Michael Roe R. Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem. Mol. Biol. 2007;37:363–374. doi: 10.1016/j.ibmb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 129.Pohl PC, Sorgine MH, Leal AT, Logullo C, Oliveira PL, Vaz Ida S, Jr, Masuda A. An extraovarian aspartic protease accumulated in tick oocytes with vitellin-degradation activity. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008;151:392–399. doi: 10.1016/j.cbpb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Seixas A, Leal AT, Nascimento-Silva MC, Masuda A, Termignoni C, da Silva Vaz I., Jr Vaccine potential of a tick vitellin-degrading enzyme (VTDCE) Vet. Immunol. Immunopathol. 2008;124:332–340. doi: 10.1016/j.vetimm.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Oliveira PL, Kawooya JK, Ribeiro JM, Meyer T, Poorman R, Alves EW, Walker FA, Machado EA, Nussenzveig RH, Padovan GJ, et al. A heme-binding protein from hemolymph and oocytes of the blood-sucking insect, Rhodnius prolixus. Isolation and characterization. J. Biol. Chem. 1995;270:10897–10901. doi: 10.1074/jbc.270.18.10897. [DOI] [PubMed] [Google Scholar]
- 132.Machado EA, Oliveira PL, Moreira MF, de Souza W, Masuda H. Uptake of Rhodnius heme-binding protein (RHBP) by the ovary of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 1998;39:133–143. doi: 10.1002/(SICI)1520-6327(1998)39:4<133::AID-ARCH1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 133.Latunde-Dada GO, Simpson RJ, McKie AT. Recent advances in mammalian haem transport. Trends Biochem. Sci. 2006;31:182–188. doi: 10.1016/j.tibs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 134.Wyllie JC, Kaufman N. An electron microscopic study of heme uptake by rat duodenum. Lab. Invest. 1982;47:471–476. [PubMed] [Google Scholar]
- 135.Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M, Hamza I. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan X, Protchenko O, Philpott CC, Hamza I. Topologically conserved residues direct heme transport in HRG-1-related proteins. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Callaghan KM, Ayllon V, O'Keeffe J, Wang Y, Cox OT, Loughran G, Forgac M, O'Connor R. Heme-binding protein HRG-1 is induced by insulin-like growth factor I and associates with the vacuolar H+-ATPase to control endosomal pH and receptor trafficking. J. Biol. Chem. 2010;285:381–391. doi: 10.1074/jbc.M109.063248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, Lehrach H, Yaspo ML. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J. Biol. Chem. 2011;286:23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol. Cell. Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mense SM, Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell. Res. 2006;16:681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- 142.Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, Hattori T, Shirato K, Igarashi K, Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 143.Knutson M, Oukka M, Koss L, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 145.Quigley JG, Burns CC, Anderson MM, Lynch ED, Sabo KM, Overbaugh J, Abkowitz JL. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. [PubMed] [Google Scholar]
- 146.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 147.Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, Tailor CS. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol. Cell. Biol. 2010;30:5318–5324. doi: 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 149.Jonker J, Buitelaar M, Wagenaar E, van der Valk M, Scheffer G, Scheper R, Plosch T, Kuipers F, Elferink R, Rosing H, Beijnen J, Schinkel A. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, Miyazaki M, Nakajima N, Iwanaga T, Yokoyama Y, Shibata T, Seino S. MTABC3, a novel mitochondrial ATP-binding cassette protein involved in iron homeostasis. J. Biol. Chem. 2000;275:17536–17540. doi: 10.1074/jbc.275.23.17536. [DOI] [PubMed] [Google Scholar]
- 151.Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, Szakacs G, Akiyama S, Gottesman MM. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443–9452. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- 152.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 153.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 154.Laftah AH, Latunde-Dada GO, Fakih S, Hider RC, Simpson RJ, McKie AT. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1) Br. J. Nutr. 2009;101:1150–1156. doi: 10.1017/S0007114508066762. [DOI] [PubMed] [Google Scholar]
- 155.Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, Tanaka M, Deybach JC, Puy H, Le Gall M, Sureau C, Pham BN, Le Pennec PY, Tani Y, Cartron JP, Arnaud L. ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat. Genet. 2012;44:170–173. doi: 10.1038/ng.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L, He F, Bu J, Liu X, Du W, Dong J, Cooney JD, Dubey SK, Shi Y, Gong B, Li J, McBride PF, Jia Y, Lu F, Soltis KA, Lin Y, Namburi P, Liang C, Sundaresan P, Paw BH, Li DY, Phillips JD, Yang Z. ABCB6 Mutations Cause Ocular Coloboma. Am J Hum Genet. 2012;90:40–48. doi: 10.1016/j.ajhg.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiabrando D, Mercurio S, Marro S, Fagoonee S, Messana E, Turco E, Silengo L, Altruda R, Tolosano E. FLVCRb: a Mitochondrial FLVCR Isoform Important for Erythropoiesis. Blood; ASH. 2011;4243 [Google Scholar]
- 158.John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JML, Rangell L, Bennett MJ, Zha J. The Mitochondrial Inner Membrane Protein Mitofilin Controls Cristae Morphology. Mol. Biol. Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wong ED, Wagner JA, Scott SV, Okreglak V, Holewinske TJ, Cassidy-Stone A, Nunnari J. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J. Cell Biol. 2003;160:303–311. doi: 10.1083/jcb.200209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schumann U, Subramani S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008;18:253–256. doi: 10.1016/j.tcb.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-Selected Transport from the Mitochondria to Peroxisomes Is Mediated by Vesicular Carriers. Curr. Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 162.Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 2012;22:135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 163.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-Mitochondria Tethering Complex Revealed by a Synthetic Biology Screen. Science. 20019 doi: 10.1126/science.1175088. 1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kornmann B, Walter P. ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J. Cell Sci. 2010;123:1389–1393. doi: 10.1242/jcs.058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Swayne TC, Zhou C, Boldogh IR, Charalel JK, McFaline-Figueroa JR, Thoms S, Yang C, Leung G, McInnes J, Erdmann R, Pon LA. Role for cER and Mmr1p in anchorage of mitochondria at sites of polarized surface growth in budding yeast. Curr. Biol. 2011;21:1994–1999. doi: 10.1016/j.cub.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McBride HM. Mitochondrial-ER tethering: the inheritance of a functional unit. Curr. Biol. 2011;21:R949–R951. doi: 10.1016/j.cub.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 168.Litwack G, Ketterer B, Arias IM. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971;234:466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- 169.Harvey JW, Beutler E. Binding of heme by glutathione S-transferase: a possible role of the erythrocyte enzyme. Blood. 1982;60:1227–1230. [PubMed] [Google Scholar]
- 170.Tipping E, Ketterer B, Christodoulides L, Enderby G. The interactions of haem with ligandin and aminoazo-dye-binding protein A. Biochem. J. 1976;157:461–467. doi: 10.1042/bj1570461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tipping E, Ketterer B, Koskelo P. The binding of porphyrins by ligandin. Biochem. J. 1978;169:509–516. doi: 10.1042/bj1690509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Harwaldt P, Rahlfs S, Becker K. Glutathione S-transferase of the malarial parasite Plasmodium falciparum: characterization of a potential drug target. Biol. Chem. 2002;383:821–830. doi: 10.1515/BC.2002.086. [DOI] [PubMed] [Google Scholar]
- 173.Hiller N, Fritz-Wolf K, Deponte M, Wende W, Zimmermann H, Becker K. Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition. Protein Sci. 2006;15:281–289. doi: 10.1110/ps.051891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Srivastava P, Puri SK, Kamboj KK, Pandey VC. Glutathione-S-transferase activity in malarial parasites. Trop. Med. Int. Health. 1999;4:251–254. doi: 10.1046/j.1365-3156.1999.00387.x. [DOI] [PubMed] [Google Scholar]
- 175.Platel DF, Mangou F, Tribouley-Duret J. Role of glutathione in the detoxification of ferriprotoporphyrin IX in chloroquine resistant Plasmodium berghei. Mol. Biochem. Parasitol. 1999;98:215–223. doi: 10.1016/s0166-6851(98)00170-4. [DOI] [PubMed] [Google Scholar]
- 176.van Rossum AJ, Jefferies JR, Rijsewijk FA, LaCourse EJ, Teesdale-Spittle P, Barrett J, Tait A, Brophy PM. Binding of hematin by a new class of glutathione transferase from the blood-feeding parasitic nematode Haemonchus contortus. Infect. Immun. 2004;72:2780–2790. doi: 10.1128/IAI.72.5.2780-2790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Loukas A, Bethony JM, Mendez S, Fujiwara RT, Goud GN, Ranjit N, Zhan B, Jones K, Bottazzi ME, Hotez PJ. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med. 2005;2:e295. doi: 10.1371/journal.pmed.0020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Senjo M, Ishibashi T, Imai Y. Purification and characterization of cytosolic liver protein facilitating heme transport into apocytochrome b5 from mitochondria. Evidence for identifying the heme transfer protein as belonging to a group of glutathione S-transferases [published erratum appears in J Biol Chem 1986 Mar 5;261(7):3469)] J. Biol. Chem. 1985;260:9191–9196. [PubMed] [Google Scholar]
- 179.Takahashi S, Ogawa T, Inoue K, Masuda T. Characterization of cytosolic tetrapyrrole-binding proteins in Arabidopsis thaliana. Photochem. Photobiol. Sci. 2008;7:1216–1224. doi: 10.1039/b802588f. [DOI] [PubMed] [Google Scholar]
- 180.Sato E, Sagami I, Uchida T, Sato A, Kitagawa T, Igarashi J, Shimizu T. SOUL in Mouse Eyes Is a New Hexameric Heme-Binding Protein with Characteristic Optical Absorption, Resonance Raman Spectral, and Heme-Binding Properties†. Biochemistry. 2004;43:14189–14198. doi: 10.1021/bi048742i. [DOI] [PubMed] [Google Scholar]
- 181.Zylka MJ, Reppert SM. Discovery of a putative heme-binding protein family (SOUL/HBP) by two-tissue suppression subtractive hybridization and database searches. Mol. Brain Res. 1999;74:175–181. doi: 10.1016/s0169-328x(99)00277-6. [DOI] [PubMed] [Google Scholar]
- 182.Ambrosi E, Capaldi S, Bovi M, Saccomani G, Perduca M, Monaco HL. Structural changes in the BH3 domain of SOUL protein upon interaction with the anti-apoptotic protein Bcl-xL. Biochem J. 2011;438:291–301. doi: 10.1042/BJ20110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Iwahara S-I, Satoh H, Song D-X, Webb J, Burlingame AL, Nagae Y, Muller-Eberhard U. Purification, characterization, and cloning of a heme-binding protein (23 kDa) in rat liver cytosol. Biochemistry. 1995;34:13398–13406. doi: 10.1021/bi00041a017. [DOI] [PubMed] [Google Scholar]
- 184.Hirotsu S, Abe Y, Okada K, Nagahara N, Hori H, Nishino T, Hakoshima T. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12333–12338. doi: 10.1073/pnas.96.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Immenschuh S, Nell C, Iwahara S-i, Katz N, Muller-Eberhard U. Gene Regulation of HBP 23 by Metalloporphyrins and Protoporphyrin IX in Liver and Hepatocyte Cultures. Biochem. Biophys. Res. Commun. 1997;231:667–670. doi: 10.1006/bbrc.1997.6166. [DOI] [PubMed] [Google Scholar]
- 186.Ishii T, Kawane T, Taketani S, Bannai S. Inhibition of the thiol-specific antioxidant activity of rat liver MSP23 protein by hemin. Biochem. Biophys. Res. Commun. 1995;216:970–975. doi: 10.1006/bbrc.1995.2715. [DOI] [PubMed] [Google Scholar]
- 187.Albakri QA, Stuehr DJ. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996;271:5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- 188.Waheed SM, Ghosh A, Chakravarti R, Biswas A, Haque MM, Panda K, Stuehr DJ. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 2010;48:1548–1558. doi: 10.1016/j.freeradbiomed.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chakravarti R, Aulak KS, Fox PL, Stuehr DJ. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18004–18009. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell. Signal. 2011;23:317–323. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–2060. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Billecke SS, Draganov DI, Morishima Y, Murphy PJ, Dunbar AY, Pratt WB, Osawa Y. The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase. J. Biol. Chem. 2004;279:30252–30258. doi: 10.1074/jbc.M403864200. [DOI] [PubMed] [Google Scholar]
- 193.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 194.Vincent SH, Muller-Eberhard U. A protein of the Z class of liver cytosolic proteins in the rat that preferentially binds heme. J. Biol. Chem. 1985;260:14521–14528. [PubMed] [Google Scholar]
- 195.Stewart JM, Slysz GW, Pritting MA, Muller-Eberhard U. Ferriheme and ferroheme are isosteric inhibitors of fatty acid binding to rat liver fatty acid binding protein. Biochem. Cell Biol. 1996;74:249–255. doi: 10.1139/o96-026. [DOI] [PubMed] [Google Scholar]
- 196.Smithies O, Walker N. Genetic control of some serum proteins in normal humans. Nature. 1955;176:1265–1266. doi: 10.1038/1761265a0. [DOI] [PubMed] [Google Scholar]
- 197.Okazaki T, Yanagisawa Y, Nagai T. Analysis of the affinity of each haptoglobin polymer for hemoglobin by two-dimensional affinity electrophoresis. Clin. Chim. Acta. 1997;258:137–144. doi: 10.1016/s0009-8981(96)06468-6. [DOI] [PubMed] [Google Scholar]
- 198.Tong Y, Guo M. Bacterial heme-transport proteins and their heme-coordination modes. Arch. Biochem. Biophys. 2009;481:1–15. doi: 10.1016/j.abb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J. Structure of haptoglobin and the haptoglobin-hemoglobin complex by electron microscopy. J. Mol. Biol. 1984;174:319–341. doi: 10.1016/0022-2836(84)90341-3. [DOI] [PubMed] [Google Scholar]
- 200.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman H-J, Law SKA, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 201.Kino K, Tsunoo H, Higa Y, Takami M, Hamaguchi H, Nakajima H. Hemoglobin-haptoglobin receptor in rat liver plasma membrane. J. Biol. Chem. 1980;255:9616–9620. [PubMed] [Google Scholar]
- 202.Okuda M, Tokunaga R, Taketani S. Expression of haptoglobin receptors in human hepatoma cells. Biochim. Biophys. Acta. 1992;1136:143–149. doi: 10.1016/0167-4889(92)90249-b. [DOI] [PubMed] [Google Scholar]
- 203.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 204.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem. J. 2008;411:123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 205.Fagoonee S, Gburek J, Hirsch E, Marro S, Moestrup SK, Laurberg JM, Christensen EI, Silengo L, Altruda F, Tolosano E. Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 2005;166:973–983. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 1996;42:1589–1600. [PubMed] [Google Scholar]
- 207.Hrkal Z, Vodrázka Z, Kalousek I. Transfer of Heme from Ferrihemoglobin and Ferrihemoglobin Isolated Chains to Hemopexin. Eur. J. Biochem. 1974;43:73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- 208.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 209.Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin. Chim. Acta. 2001;312:13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 210.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin Prevents Endothelial Damage and Liver Congestion in a Mouse Model of Heme Overload. Am. J. Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Adams P, Berman M. Kinetics and mechanism of the interaction between human serum albumin and monomeric haemin. Biochem. J. 1980;191:95–102. doi: 10.1042/bj1910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dockal M, Carter DC, Ruker F. The three recombinant domains of human serum albumin. Structural characterization and ligand binding properties. J. Biol. Chem. 1999;274:29303–29310. doi: 10.1074/jbc.274.41.29303. [DOI] [PubMed] [Google Scholar]
- 213.Zunszain P, Ghuman J, Komatsu T, Tsuchida E, Curry S. Crystal structural analysis of human serum albumin complexed with hemin and fatty acid. BMC Struct. Biol. 2003;3:6. doi: 10.1186/1472-6807-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miller YI, Shaklai N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta - Molecular Basis of Disease. 1998;1454:153–164. doi: 10.1016/s0925-4439(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 215.Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145:720–731. doi: 10.1016/j.cell.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Allhorn M, Klapyta A, Akerstrom B. Redox properties of the lipocalin alpha1-microglobulin: reduction of cytochrome c, hemoglobin, and free iron. Free Radic. Biol. Med. 2005;38:557–567. doi: 10.1016/j.freeradbiomed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 217.Bonkovsky HL, Healey JF, Lourie AN, Gerron GG. Intravenous heme-albumin in acute intermittent porphyria: evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am. J. Gastroenterol. 1991;86:1050–1056. [PubMed] [Google Scholar]
- 218.Magness ST, Maeda N, Brenner DA. An exon 10 deletion in the mouse ferrochelatase gene has a dominant-negative effect and causes mild protoporphyria. Blood. 2002;100:1470–1477. doi: 10.1182/blood-2001-12-0283. [DOI] [PubMed] [Google Scholar]
- 219.Okano S, Zhou L, Kusaka T, Shibata K, Shimizu K, Gao X, Kikuchi Y, Togashi Y, Hosoya T, Takahashi S, Nakajima O, Yamamoto M. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells. 2010;15:77–89. doi: 10.1111/j.1365-2443.2009.01366.x. [DOI] [PubMed] [Google Scholar]
- 220.Dooley KA, Fraenkel PG, Langer NB, Schmid B, Davidson AJ, Weber G, Chiang K, Foott H, Dwyer C, Wingert RA, Zhou Y, Paw BH, Zon LI. montalcino, A zebrafish model for variegate porphyria. Exp. Hematol. 2008;36:1132–1142. doi: 10.1016/j.exphem.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chiabrando D, Tolosano E. Diamond Blackfan Anemia at the Crossroad between Ribosome Biogenesis and Heme Metabolism. Adv Hematol. 2010;2010:790632. doi: 10.1155/2010/790632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109(Pt 3):373–387. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 223.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fuller V, Lilley C, Urwin P. Nematode resistance. New Phytol. 2008;180:27–44. doi: 10.1111/j.1469-8137.2008.02508.x. [DOI] [PubMed] [Google Scholar]
- 225.Jasmer DP, Goverse A, Smant G. Parasitic Nematode Interactions with Mammals and Plants. Annu Rev Phytopathol. 2003;41:245–270. doi: 10.1146/annurev.phyto.41.052102.104023. [DOI] [PubMed] [Google Scholar]
- 226.Clemens LE, Basch PF. Schistosoma mansoni: effect of transferrin and growth factors on development of schistosomula in vitro. J. Parasitol. 1989;75:417–421. [PubMed] [Google Scholar]
- 227.Correa Soares JB, Maya-Monteiro CM, Bittencourt-Cunha PR, Atella GC, Lara FA, d'Avila JC, Menezes D, Vannier-Santos MA, Oliveira PL, Egan TJ, Oliveira MF. Extracellular lipid droplets promote hemozoin crystallization in the gut of the blood fluke Schistosoma mansoni. FEBS Lett. 2007;581:1742–1750. doi: 10.1016/j.febslet.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 228.Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Dietary iron content mediates hookworm pathogenesis in vivo. Infect. Immun. 2006;74:289–295. doi: 10.1128/IAI.74.1.289-295.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Williamson AL, Lecchi P, Turk BE, Choe Y, Hotez PJ, McKerrow JH, Cantley LC, Sajid M, Craik CS, Loukas A. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J. Biol. Chem. 2004;279:35950–35957. doi: 10.1074/jbc.M405842200. [DOI] [PubMed] [Google Scholar]
- 230.McKie AT. The role of Dcytb in iron metabolism: an update. Biochem. Soc. Trans. 2008;36:1239–1241. doi: 10.1042/BST0361239. [DOI] [PubMed] [Google Scholar]
- 231.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 232.Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wyman S, Simpson RJ, McKie AT, Sharp PA. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008;582:1901–1906. doi: 10.1016/j.febslet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 235.Nicholson DW, Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc. Natl. Acad. Sci. U. S. A. 1989;86:4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Richard-Fogal CL, Frawley ER, Bonner ER, Zhu H, San Francisco B, Kranz RG. A conserved haem redox and trafficking pathway for cofactor attachment. EMBO J. 2009;28:2349–2359. doi: 10.1038/emboj.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Corvest V, Murrey DA, Hirasawa M, Knaff DB, Guiard B, Hamel P. The Flavoprotein Cyc2p, a Mitochondrial Cytochrome C Assembly Factor, Is a NAD(P)H-Dependent Heme Reductase. Mol. Microbiol. 2012 doi: 10.1111/j.1365-2958.2012.07981.x. [DOI] [PubMed] [Google Scholar]
- 238.Bernard DG, Quevillon-Cheruel S, Merchant S, Guiard B, Hamel PP. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J. Biol. Chem. 2005;280:39852–39859. doi: 10.1074/jbc.M508574200. [DOI] [PubMed] [Google Scholar]
- 239.Chen C, Samuel TK, Krause M, Dailey HA, Hamza I. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by Heme Responsive Gene-2. J. Biol. Chem. 2012 doi: 10.1074/jbc.M111.307694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143–149. doi: 10.1016/j.cmet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 241.Mallory JC, Crudden G, Johnson BL, Mo C, Pierson CA, Bard M, Craven RJ. Dap1p, a heme-binding protein that regulates the cytochrome P450 protein Erg11p/Cyp51p in Saccharomyces cerevisiae. Mol. Cell. Biol. 2005;25:1669–1679. doi: 10.1128/MCB.25.5.1669-1679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shirihai O, Gregory T, Yu C, Orkin S, Weiss M. ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. EMBO J. 2000;19:2492–2502. doi: 10.1093/emboj/19.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang F, Hogue DL, Liu L, Fisher CL, Hui D, Childs S, Ling V. M-ABC2, a new human mitochondrial ATP-binding cassette membrane protein. FEBS Lett. 2000;478:89–94. doi: 10.1016/s0014-5793(00)01823-8. [DOI] [PubMed] [Google Scholar]
- 244.Taketani S, Adachi Y, Kohno H, Ikehara S, Tokunaga R, Ishii T. Molecular Characterization of a Newly Identified Heme-binding Protein Induced during Differentiation of murine Erythroleukemia Cells. J. Biol. Chem. 1998;273:31388–31394. doi: 10.1074/jbc.273.47.31388. [DOI] [PubMed] [Google Scholar]
- 245.Szczesna-Skorupa E, Kemper B. Progesterone receptor membrane component 1 inhibits the activity of drug-metabolizing cytochromes P450 and binds to cytochrome P450 reductase. Mol. Pharmacol. 2011;79:340–350. doi: 10.1124/mol.110.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]