Abstract
Background
Cocaine is a cause of intracerebral hemorrhage (ICH), but there are no large studies that have characterized the location, pathology, and outcome of patients with cocaine-associated ICH.
Methods
We performed a retrospective analysis of all patients admitted to our stroke service from 2004 to 2007 who had non-traumatic ICH and urine drug screens positive for cocaine and compared them with similar patients who had a negative drug screen for cocaine.
Results
We identified 45 patients with cocaine-associated ICH and 105 patients with cocaine-negative ICH. There were no significant differences in age or gender but there was a significantly higher incidence of African-American patients in the cocaine positive group. Cocaine-associated ICH patients had higher admission blood pressures, significantly more subcortical hemorrhages, and higher rates of intraventricular hemorrhage (IVH) compared to patients with cocaine-negative ICH. Cocaine-positive patients had worse functional outcome, defined as an mRS>3 at the time of discharge (OR 4.90, 95% CI 2.19–10.97), and were less likely to be discharged home or to inpatient rehab. Patients with cocaine-associated ICH were nearly 3 times more likely to die during their acute hospitalization when compared to cocaine-negative patients.
Conclusion
Recent cocaine ingestion is associated with hemorrhages that occur more frequently in subcortical locations, have a higher risk of IVH, and carry a poor prognosis compared to patients with cocaine-negative, spontaneous ICH.
Keywords: Intracerebral hemorrhage, cocaine, outcome
Intracerebral hemorrhage (ICH) is an uncommon CNS complication of cocaine use. While prior case series have suggested that the etiologies for ICH in cocaine users include rapid rises in blood pressure1, aneurysmal rupture1–3, and vasculitis4–6, these studies have been limited by small sample size. To our knowledge, no large, systematic studies have characterized the pathology and prognosis of patients with cocaine-associated ICH. We compared the demographics, location and outcome of cocaine-associated ICH with cocaine-negative, spontaneous ICH in patients admitted to our stroke center.
Methods
We conducted a retrospective chart review of all ICH patients admitted to our stroke service from 2004 to 2007 for whom cocaine was marked as an exposure on admission. Recent exposure was defined as a positive urine drug screen (UDS) for cocaine metabolites at the time of hospital presentation. Other substances of abuse were also captured. A control group was also collected, consisting of all patients who presented to our service with non-traumatic, spontaneous ICH during the same time frame in which a urine drug screen was obtained and found to be negative for cocaine. All patients admitted to our stroke service with spontaneous ICH undergo a UDS if they are younger than 50 years old, have no history of hypertension or dementia, report a history of illicit drug use or have a history of substance abuse.
Baseline demographics, admission blood pressure, NIHSS scores, GCS, ICH scores (GCS, age >80 yo, infratentorial origin, ICH volume >30 ml, presence of intraventricular blood), echocardiogram reports, neuroimaging results, urine drug screen results, and various lab measurements (creatine kinase, troponin, glucose levels) were collected.
All patients had frequent vital sign monitoring, including an ECG and 24 hours of telemetry. Cardiac and cerebrovascular imaging and lipid and glucose levels were obtained as part of routine practice. Early outcomes were evaluated at hospital discharge using the modified Rankin Scale (mRS) score and discharge disposition. ICH characteristics, results of cardiac and vascular imaging, and outcomes in ICH patients with negative toxicology screens were compared to patients with positive toxicology for cocaine metabolites, admitted to our stroke service during the same time period. We also report the incidence of untreated hypertension, blood pressure at initial presentation, evidence of chronic hypertension (defined as left ventricular hypertrophy on echocardiography), and cardiac sources of emboli. The primary outcome of interest was the mRS (modified Rankin score) scale at discharge. Poor outcome was defined as a discharge mRS of 4–6.
Statistical analyses were performed using SPSS 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean +/− standard deviation when the distribution was normal and median with range for non-normal distributions. The mRS and NIHSS were reported as the median with IQR. Categorical variables were analyzed using Chi Square and Fisher’s exact tests where appropriate.
Results
Of 3,241 stroke patients admitted to our stroke service during the study period, 132 (4.1%) tested positive for cocaine metabolites on UDS. Of those patients, 45 (34%) had ICH. Of the 45 cocaine positive ICH patients, 6 tested positive for other illicit drugs (5 for marijuana, 1 for marijuana and amphetamines). They were included in the analysis. During the same time period, 105 patients were identified who had a spontaneous ICH and were found to have a negative urine drug screen for cocaine metabolites. Table 1 depicts the baseline demographics of cocaine positive versus cocaine negative ICH patients. Compared to ICH patients that both denied a history of polysubstance use and were found to have a negative urine drug screen, cocaine positive patients presenting with ICH were more likely to be African American (69% vs. 44%) and less likely to be Hispanic (11% vs. 28%, p=0.022). There was no significant difference in median age, gender, baseline hypertension, or rate of untreated hypertension.. Patients using cocaine had a significantly higher median admission DBP [121 (100–126) vs. 110 (107–141), p=0.024] and showed a trend toward higher median SBP [216 (158–241) vs. 204 (154–228), p=0.066]. Cocaine positive patients presented with more severe ICH [NIHSS 18 (8–25) vs. 13 (6–20), p=0.014] (Table 2). While the median ICH volume in cocaine users (18cc) was larger than that of non-cocaine users (12cc), this difference was not statistically significant (p=.329). The median ICH score was significantly higher in cocaine positive patients [2 (1–3) vs. 1 (0–2), p<0.0001] (Table 2).
Table 1.
Demographics of cocaine positive vs. cocaine negative ICH patients
| Cocaine + | Cocaine − | p value | |
|---|---|---|---|
|
| |||
| Age, median (min-max), yrs | 50 (33–69) | 51 (34–67) | .361 |
| IQR=(43–54) | IQR=(44–56.5) | ||
|
| |||
| Gender, % male | 60.0% | 67.6% | .369 |
|
| |||
| Race, % | .022 | ||
| African American (AA) | 68.9% | 43.8% | |
| Hispanic | 11.1% | 27.6% | |
| Caucasian | 20.0% | 24.8% | |
| Asian | 0% | 3.8% | |
|
| |||
| NIHSS, median (min-max) | 18 (0–41) | 13 (0–40) | .014 |
| IQR=(8–25) | IQR=(6–20 | ||
|
| |||
| h/o HTN, % | 84.6% | 76.7% | .322 |
|
| |||
| Current BP Med Use, % | 34.4% | 21.6% | .167 |
|
| |||
| Untreated HTN, % | 45.2% | 50.0% | .651 |
|
| |||
| Admission SBP, median (min-max) | 216 (132–287) | 204 (105–287) | .066 |
| IQR=(158–241) | IQR=(154–228) | ||
|
| |||
| Admission DBP, median (min-max) | 121 (74–199) | 110 (51–189) | .024 |
| IQR=(100–126) | IQR=(107–141) | ||
|
| |||
| Glucose | 149.5 (81–596) | 134 (89–399) | .239 |
| IQR=(127–178) | IQR=(113–178) | ||
|
| |||
| CK | 201 (48–3263) | 187 (34–3362) | .683 |
| IQR=(120–413) | IQR=(123–324) | ||
|
| |||
| CK-Mb | 4.2 (1.8–37) | 3.5 (1.1–35) | .155 |
| IQR=(3.0–6.8) | IQR=(2.5–5.9) | ||
|
| |||
| Troponin | 0.01 (.01–.66) | 0.01 (.01–.50) | .035 |
| IQR=(.01–.03) | IQR=(.01–.01) | ||
|
| |||
| Dilated LV, % | 7.4% | 2.4% | .558 |
|
| |||
| Low EF, % (<50%) | 14.3% | 9.8% | .706 |
|
| |||
| Shunt, % | 9.5% | 0% | .111 |
|
| |||
| LVH on Echo, % | 93.3% | 79.5% | .168 |
Table 2.
ICH characteristics of cocaine positive vs. cocaine negative patients
| Cocaine + | Cocaine− | p value | |
|---|---|---|---|
|
| |||
| Volume cc3, median (min-max) | 18 (2–126) | 12 (1–113) | .329 |
| IQR=(8–38) | IQR=(8.5–31) | ||
|
| |||
| ICH score, median (min-max) | 2 (0–4) | 1 (0–5) | <.0001 |
| IQR=(1–3) | IQR=(0–2) | ||
|
| |||
| GCS, median (min-max) | 9 (3–15) | 15 (3–15) | <.0001 |
| IQR=(3.8–14) | IQR=(11–15) | ||
|
| |||
| Location, % | |||
| Basal ganglia | 42.2% | 45.7% | .020 |
| Thalamus | 17.8% | 16.2% | |
| Lobar | 11.1% | 26.7% | |
| Brainstem | 20.0% | 4.8% | |
| Cerebellum | 4.4% | 5.7% | |
| Primary IVH | 4.4% | 1.0% | |
|
| |||
| IVH, % | 71.1% | 39.0% |
<.0001 OR=3.84, 95%CI= 1.81–8.17 |
While an unequal distribution of brainstem hemorrhages were noted in the cocaine positive group as shown in Table 2 (20% v. 5%), use of cocaine was not directly correlated with ICH location (r=.007, p=0.9) or with ICH volume (r=0.08, p=0.3). Cocaine use was, however, found to directly correlate with the occurrence of IVH (r=.30, p<.0001). Among patients with ICH associated with cocaine, they were nearly 4 times more likely to have IVH compared to ICH patients who did not have a recent cocaine exposure (OR 3.84, 95% CI 1.81–8.17, p<.0001). About half (24/45) of the cocaine positive patients with ICH had an arteriogram (CTA, MRA, or DSA); 2 of the 24 patients had an AVM (8.3%) and 1 patient had findings consistent with Moya Moya syndrome (4.2%). None were found to have vasculitis.
Cocaine positive patients had worse functional outcome at the time of discharge on mRS [5 (4–6) vs. 3 (2–5), p<0.0001] and were less likely to be discharged home or to inpatient rehab (31% vs. 62%, p=0.002) (Table 3). Patients with cocaine associated ICH were nearly 3 times more likely to die during their acute hospitalization when compared to cocaine negative patients (OR 2.71, 95% CI 1.18–6.25, p=.017). After controlling for variables that were significantly different between the two groups - NIHSS on admission, GCS, IVH, and ventriculostomy, cocaine use remained a significant independent predictor of poor outcome (OR 3.21, 95% CI 1.03–10.01, p=.044).
Table 3.
Outcome of cocaine positive vs. cocaine negative ICH patients
| Cocaine + | Cocaine− | p value | |
|---|---|---|---|
|
| |||
| Ventriculostomy, % | 33.3% | 16.2% | .019 |
|
| |||
| Hemicraniectomy, % | 11.1% | 2.9% | .053 |
|
| |||
| Aspiration pneumonia, % | 17.8% | 7.6% | .084 |
|
| |||
| Hospital acquired UTI, % | 11.1% | 7.6% | .532 |
|
| |||
| Length of Stay (d), median (min-max) | 10 (0–72) | 6 (1–74) | .015 |
| IQR=(6.5–19.5) | IQR=(4–14) | ||
|
| |||
| mRS on discharge, median (min-max) | 5 (1–6) | 3 (0–6) | <.0001 |
| IQR=(4–6) | IQR=(2–5) | ||
|
| |||
| Poor outcome (mRS 4–6) on discharge, % | 77.3% | 41.0% |
<.0001 OR 4.90,95% CI 2.19–10.97 |
|
| |||
| Discharge disposition, % | .002 | ||
| Home or inpatient rehab | 31.1% | 61.9% | |
| SNF/LTAC/nursing home | 35.6% | 23.8% | |
| Dead/Hospice | 33.3% | 14.3% | |
|
| |||
| Death, % | 31.1% | 14.3% |
.017 OR=2.71,95%CI= 1.18–6.25 |
Discussion
To our knowledge, this study involves the largest series of patients with cocaine associated intracerebral hemorrhage and presents an opportunity to better understand the demographics, pathophysiology, location, and outcome of cocaine-related ICH. Consistent with prior studies of cocaine users, we found a male predominance and higher proportion of African-Americans as compared to other ethnic groups7. The age of our cohort (range 33–69) is consistent with prior studies but includes some of the oldest patients ever to be reported and concords with prior work showing that the elderly use cocaine and develop CNS complications8.
In contrast to prior studies reporting a lobar location, cocaine-associated intracerebral hemorrhage in our cohort was predominately subcortical9. There was a significantly higher incidence of brainstem ICH in cocaine positive patients when compared with cocaine-negative patients. In agreement with other studies1, hypertension may have contributed to the underlying pathology in patients with cocaine associated ICH given the high admission blood pressure and lesion location. The rate of vascular malformations in our cohort of cocaine users was lower than rates previously cited3, 10. This may be confounded by the fact that only half of our patients had angiographic imaging. However, our findings are in agreement with small studies that found no evidence for vascular anomalies as the predominant cause of cocaine-associated ICH11–13. Therefore, cocaine-related ICH may not be a necessary indication for angiography in patients with pre-existing hypertension and subcortical ICH. On the other hand, the literature suggests that a lobar location in patients with cocaine-related ICH may be associated with an AVM or an aneurysm14, 15. For those patients who did undergo angiography in our study, there was also no evidence for vasculitis, another purported pathology for cocaine-associated ICH5. No other etiologies were found in our patients, including bleeding diatheses or coagulopathies.
The clinical presentation of cocaine-associated ICH in our sample was more severe than cocaine-negative ICH, as measured by admission neurological deficits on the NIHSS score and the ICH score. Cocaine-positive hemorrhages had a substantially higher rate of intraventricular extension, leading to higher ICH scores. The risk of mortality doubled in cocaine positive patients when compared with cocaine negative patients. The high morbidity and mortality rate may be due to a combination of factors including brainstem location, IVH, and chronic hypertension; however, we cannot exclude the possibility that the intrinsic pharmacokinetic properties of the cocaine itself may also contribute to higher morbidity and mortality in ICH. Worse prognosis in cocaine users could also be related to socio-economic factors, outside of the direct action of the cocaine, such as poor general medical care including suboptimal compliance with, or access to, antihypertensive therapy.
There was a higher rate of death in cocaine users who developed IVH when compared to patients with non-cocaine IVH, although this result did not prove statistically significant. Small sample size may be preventing us from detecting a significant association between IVH and outcome, but prior studies have already shown that IVH is an independent predictor of worse outcome16. We investigated further the high rate of IVH in cocaine-associated ICH and found there was a moderate correlation between cocaine use and the occurrence of IVH. Prior work has shown that volume and location of the hemorrhage are associated with the likelihood of intraventricular extension16; however, there was no correlation in this study between cocaine use and hemorrhage location or between cocaine use and hematoma volume. The underlying mechanisms that predispose cocaine exposure to lead to IVH remains unclear and will require further investigation.
This study has several limitations including its retrospective nature and lack of angiographic studies on all patients. Not all of our patients with spontaneous hemorrhage undergo a urine drug screen, which may result in a sampling and/or selection bias when comparing cocaine-positive with cocaine-negative patients. The proven cocaine negative group limited the age of the patients studied since not all elderly patients undergo a toxicology screen at our center but this age cutoff makes it unlikely that other etiologies of spontaneous hemorrhage such as amyloid angiopathy was a predominant cause in the cocaine-negative patients. As the average age was slightly above 50 years old in the cocaine negative controls, there may be inherent bias about which patients over 50 undergo a toxicology screen. DNR status on admission was not captured and therefore we cannot rule out the possibility of a self-fulfilling prophecy accounting for worse outcomes in the cocaine positive patients. In addition, we did not collect the type of cocaine used, quantity used, route of administration, or frequency of use which prior reports have suggested may affect the likelihood of having ICH versus ischemic stroke10. As we focused only on intracerebral hemorrhage, we cannot comment on the etiologies of subarachnoid hemorrhage after cocaine use. Patients with large AVMs, apparent on baseline CT scan, would have been admitted to our neurosurgery service; thus, the rate of underlying vascular anomalies in this cohort may be underrepresented.
In summary, recent cocaine ingestion is associated with large, subcortical intracerebral hemorrhage, often with intraventricular extension and high morbidity and mortality. The severity of cocaine-related ICH, in comparison with spontaneous ICH not associated with cocaine, may be due in large part to the occurrence of IVH and brainstem location but further studies are needed to substantiate this possibility. The mechanisms of cocaine-induced IVH also require further study.
Figure 1.
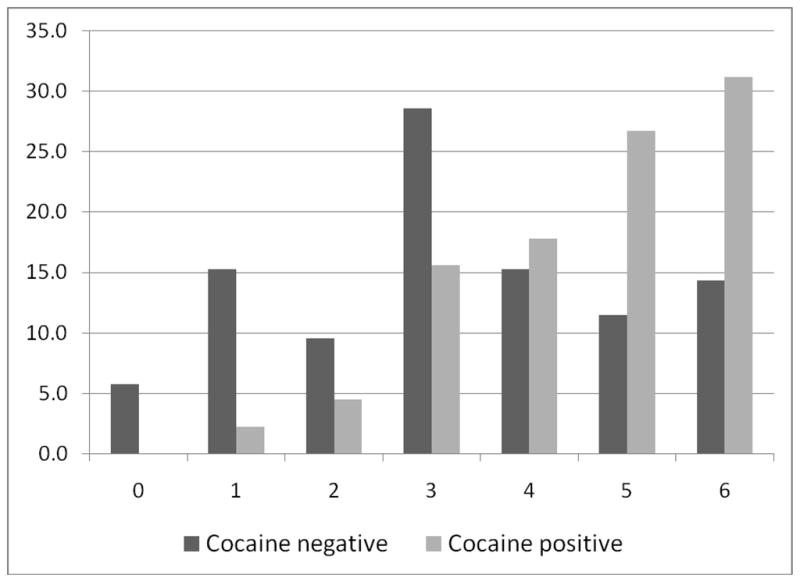
The distribution of mRS scores at hospital discharge in patients with ICH who were either cocaine positive or cocaine negative on urine drug screen. y-axis represents percent.
Acknowledgments
This work was supported by AHA Award 0475008N (SIS), NIH training grant T32NS04712, and P50 NS044227.
References
- 1.Kibayashi K, Mastri AR, Hirsch CS. Cocaine induced intracerebral hemorrhage: Analysis of predisposing factors and mechanisms causing hemorrhagic strokes. Hum Pathol. 1995;26:659–663. doi: 10.1016/0046-8177(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 2.McEvoy AW, Kitchen ND, Thomas DG. Lesson of the week: Intracerebral haemorrhage in young adults: The emerging importance of drug misuse. Bmj. 2000;320:1322–1324. doi: 10.1136/bmj.320.7245.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daras M, Tuchman AJ, Koppel BS, Samkoff LM, Weitzner I, Marc J. Neurovascular complications of cocaine. Acta Neurol Scand. 1994;90:124–129. doi: 10.1111/j.1600-0404.1994.tb02691.x. [DOI] [PubMed] [Google Scholar]
- 4.Morrow PL, McQuillen JB. Cerebral vasculitis associated with cocaine abuse. J Forensic Sci. 1993;38:732–738. [PubMed] [Google Scholar]
- 5.Tapia JF, Schumacher JM. Case 27-1993—a 32-year-old man with the sudden onset of a right-sided headache and left hemiplagia and hemianesthesia. The New England Journal of Medicine- Case Records of the Massachusetts General Hospital. 1993;329:117–124. doi: 10.1056/NEJM199307083290209. [DOI] [PubMed] [Google Scholar]
- 6.Toffol GJ, Biller J, Adams HP., Jr Nontraumatic intracerebral hemorrhage in young adults. Arch Neurol. 1987;44:483–485. doi: 10.1001/archneur.1987.00520170013014. [DOI] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration OoAS. DAWN Series D-27, DHHS Publication No (SMA) 05–4023. Vol. 2003 Rockville, MD: 2005. Drug abuse warning network, 2003: Area profiles of drug-related mortality. [Google Scholar]
- 8.Williams ONJ, Brust JCM. Stroke associated with cocaine abuse: No longer just a problem of the young. Neurology. 2006:66. [Google Scholar]
- 9.EL-Mitwalli A, Malkoff MD. Intracerebral hemorrhage. The Internet Journal of Emergency and Intensive Care Medicine. 2001:5. [Google Scholar]
- 10.Levine SR, Brust JC, Futrell N, Brass LM, Blake D, Fayad P, Schultz LR, Millikan CH, Ho KL, Welch KM. A comparative study of the cerebrovascular complications of cocaine: Alkaloidal versus hydrochloride--a review. Neurology. 1991;41:1173–1177. doi: 10.1212/wnl.41.8.1173. [DOI] [PubMed] [Google Scholar]
- 11.Nalls G, Disher A, Daryabagi J, Zant Z, Eisenman J. Subcortical cerebral hemorrhages associated with cocaine abuse: Ct and mr findings. J Comput Assist Tomogr. 1989;13:1–5. doi: 10.1097/00004728-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal SK, Williams V, Levine SR, Cassin BJ, Garcia JH. Cocaine-associated intracranial hemorrhage: Absence of vasculitis in 14 cases. Neurology. 1996;46:1741–1743. doi: 10.1212/wnl.46.6.1741. [DOI] [PubMed] [Google Scholar]
- 13.Nolte KB, Brass LM, Fletterick CF. Intracranial hemorrhage associated with cocaine abuse: A prospective autopsy study. Neurology. 1996;46:1291–1296. doi: 10.1212/wnl.46.5.1291. [DOI] [PubMed] [Google Scholar]
- 14.Toffol GJ, Biller J, Adams HP, Jr, Smoker WR. The predicted value of arteriography in nontraumatic intracerebral hemorrhage. Stroke. 1986;17:881–883. doi: 10.1161/01.str.17.5.881. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs IG, Roszler MH, Kelly JK, Klein MA, Kling GA. Cocaine abuse: Neurovascular complications. Radiology. 1989;170:223–227. doi: 10.1148/radiology.170.1.2909100. [DOI] [PubMed] [Google Scholar]
- 16.Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, Gonzales NR, Illoh K, Noser EA, Grotta JC. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70:848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


